Abstract
Free full text

The Neuropeptide Alpha-Melanocyte–Stimulating Hormone Is Critical for Corneal Endothelial Cell Protection and Graft Survival after Transplantation
Abstract
Corneal transplantation is the most common form of tissue transplantation. The success of corneal transplantation mainly relies on the integrity of corneal endothelial cells (CEnCs), which maintain tissue transparency by pumping out excess water from the cornea. After transplantation, the rate of CEnC loss far exceeds that seen with normal aging, which can threaten sight. The underlying mechanisms are poorly understood. Alpha-melanocyte–stimulating hormone (α-MSH) is a neuropeptide that is constitutively found in the aqueous humor with both cytoprotective and immunomodulatory effects. The curent study found high expression of melanocortin 1 receptor (MC1R), the receptor for α-MSH, on CEnCs. The effect of α-MSH/MC1R signaling on endothelial function and allograft survival in vitro and in vivo was investigated using MC1R signaling-deficient mice (Mc1re/e mice with a nonfunctional MC1R). Herein, the results indicate that in addition to its well-known immunomodulatory effect, α-MSH has cytoprotective effects on CEnCs after corneal transplantation, and the loss of MC1R signaling significantly decreases long-term graft survival in vivo. In conclusion, α-MSH/MC1R signaling is critical for CEnC function and graft survival after corneal transplantation.
The cornea is the outermost transparent layer of the eye. Corneal diseases represent the fifth leading cause of blindness worldwide with approximately 4.5 million individuals being visually impaired due to loss of corneal clarity.1 In case of treatable corneal blindness, the diseased or damaged corneal layers can be replaced by healthy donated cadaveric corneal tissue (a corneal transplantation or graft). Although donor tissue accessibility varies around the world, corneal transplantation remains the most common form of solid tissue transplantation,2 with more than 85,000 cases performed annually in the United States alone.
Corneal endothelial cells (CEnCs), a monolayer of neural crest-derived cells on the inner corneal surface, are critical for maintaining tissue transparency.3,4 Human CEnCs have very low proliferative potential in vivo, and a gradual decline in endothelial cell density is observed during adulthood due to age-related cell death.5 Many factors, such as primary CEnC dystrophy, trauma, oxidative stress, and inflammation, can accelerate CEnC loss.6 If the endothelial cell density falls below the threshold level needed to maintain corneal dehydration and clarity, the cornea becomes edematous, and transplantation is needed to restore vision.3 However, even after transplantation surgery, the rate of CEnC loss still far exceeds that seen with normal aging, jeopardizing long-term graft survival.3,7, 8, 9 The mechanisms underlying the survival and function of these important cells are still not fully understood, and to date, none of the standard therapeutic strategies can successfully prevent CEnC loss after injury or transplantation. Thus, there is an urgent need for the development of new cytoprotective approaches that can prevent CEnC loss during tissue storage and after transplantation.10,11
Clinically, a concomitant decrease in corneal nerve density and CEnC numbers has been noted in various ocular conditions.12, 13, 14, 15, 16, 17, 18 Moreover, in full-thickness corneal transplantation, in which all the corneal nerves are cut in the mid-periphery, a progressive decline of CEnCs is seen even without any demonstrable intraocular inflammation or graft rejection.7,19,20 These clinical data suggest an important role of nerve-derived factors in maintaining CEnC survival in full-thickness corneal transplantation. Because there is no direct innervation of human CEnC,21,22 the hypothesized role of nerves on maintaining CEnCs is likely through secreted neuropeptides in these grafts.
The 13-amino acid–long neuropeptide α-melanocyte–stim-ulating hormone (α-MSH) is constitutively found in normal aqueous humor, which is the fluid filling the anterior chamber of the eye and is in direct contact with CEnCs. α-MSH has been shown to contribute to the immune privilege of the healthy mammalian eye.23,24 Through binding to different melanocortin receptors (MCRs) on various immune cells,24, 25, 26 α-MSH modulates both innate and adaptive immune responses.24 In fact, an in vivo mouse model study showed that local treatment with α-MSH may significantly reduce allorejection of orthotopic corneal transplants by modulating the immune response.27 However, several studies also report a direct cytoprotective function of α-MSH, mediated through the high-affinity melanocortin receptor 1 (MC1R) signaling.28, 29, 30, 31, 32, 33 The antiapoptotic/prosurvival effect of MC1R-mediated signaling pathway has been demonstrated in various cell types and animal disease models.28, 29, 30, 31, 32, 33 In the eye, α-MSH/MC1R pathway has been shown to protect retinal pigment epithelium cells from oxidative stress31 and photoreceptors from degeneration.34,35 More importantly, addition of α-MSH in the culture medium significantly reduces inflammatory cytokine- and oxidative stress-induced CEnC death and corneal edema in human donor corneas in eye banking.36 Although MCRs have also been found to be widely expressed by the ocular surface and adnexal tissue,37, 38, 39 the direct cytoprotective effect of α-MSH on CEnC survival after transplantation has not been identified.
To determine whether α-MSH/MC1R signaling has a direct cytoprotective effect, the ex vivo experiments were extended using murine and human CEnC lines and cornea-in-the-cup assays to an in vivo well-established murine orthotopic corneal transplantation model using syngeneic and allogeneic wild-type (WT) and MC1R signaling-deficient mice (Mc1re/e mice with a nonfunctional MC1R).29,40,41 Herein, the study shows, for the first time, that in addition to its well-known immunomodulatory effect, α-MSH has a pivotal cytoprotective effect on CEnCs, and loss of MC1R function in grafted donor corneas significantly decreases long-term graft survival.
Materials and Methods
Study Approval
All mice were housed in the animal vivarium of Schepens Eye Research Institute and treated according to the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research. The Institutional Animal Care and Use Committee of Schepens Eye Research Institute approved all animal procedures.
Corneal Endothelial Cell Lines and Animals
Immortalized human (hCEnC-21T) or mouse (mCEnC-P2) corneal endothelial cell lines42 were derived from Dr. Jurkunas’s lab (Schepens Eye Research Institute, Boston, MA). Eight-week–old male and female BALB/c and C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). B6.C-H2-Ab1bm12/KhEg-Mc1re-J/J MC1R heterozygous mice were purchased from the Jackson Laboratory (Bar Harbor, ME; Stock No:003625; recessive yellow Jackson) and bred in the authors’ animal facility to generate the homozygous line. The homozygous genotype (abbreviated in text and figures as Mc1re/e) was confirmed by genotyping (Transnetyx, Inc., Cordova, TN). In accordance with the Institutional Animal Care and Use Committee, the objective for small sample sizes was a power analysis using data that was previously obtained in the pilot studies.
Immunohistochemistry for MC1R
The murine corneas were collected from a WT C57BL/c mouse, and the human corneal tissue was excised in quarters (purchased from Eversight Eye Bank, Cleveland, OH). The murine and human corneal tissues and the cultured cell lines were fixed with paraformaldehyde 4% for 60 minutes and washed three times with phosphate-buffered saline (PBS) at room temperature. Cells were then permeabilized by incubation with 1% Triton X-100 (Sigma-Aldrich, St. Louis, MO) in PBS for 20 minutes at room temperature. Tissues were stained with a monoclonal mouse anti-human MC1R antibody (1:200, LS-B10498-200; LSBio, Seattle, WA) overnight at 4°C, and were then washed three times in PBS and incubated with goat anti-mouse IgM secondary antibody (1:200; A-21238, Thermo Fisher Scientific, Rockford, IL) for 1 hour (for whole tissues) or 30 minutes (for cell cultures). Flat mounts of the corneas were placed on glass slides with the endothelial layer facing up and cell cultures visualized in their plates (×100). Nuclear staining with DAPI was done to index cells, and the MC1R expression was detected using confocal microscopy (×100 to ×630 magnification; Leica TCS-SP8; Leica, Wetzlar, Germany). Notably, for excluding the cross reaction of the secondary antibody with the tissues, control cell cultures and tissues were only stained with the secondary antibody, which showed no positive staining.
Western Blot Analysis for MC1R
Western blot was performed as previously described.43 Briefly, both mice (mCEnC-P2) and human (hCEnC-21T) endothelial cell lines were harvested until 80% confluence. Cells were scraped from a T25 flask, incubated with 500 μL of cold radioimmunoprecipitation assay buffer (5 mmol/L Tris-HCl pH 6.8, 2 mmol/L MgCl2, 2 mmol/L EDTA, 65 mmol/L NaCl, 1% Triton X-100) containing 1× of TBP reducing agent (Bio-Rad, Hercules, CA) and 1× of Halt Protease inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA). The solution was incubated for 15 minutes at 4°C with gently shaking and centrifuged for 15 minutes at 14,000 × g. Soluble fraction (supernatant) was used to evaluate mice and human MC1R expression. Protein concentration of soluble fraction was determined according to Pierce method using Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Total protein (20, 10, and 2 μg per well) were used in an electrophoresis on a 1.5-mm, 4% to 12% gradient, SDS-polyacrylamide gel (Thermo Fisher Scientific) and blotted onto nitrocellulose membrane using the Trans-Blot Turbo system (Bio-Rad) for mixed protein for 7 minutes. The mouse small intestine tissue (SIT) lysate (No. PK-AB718-1408; PromoCell, Heidelberg, Germany) and A-375 human skin epithelial cell lysate (No. sc-3811; Santa Cruz Biotechnology, Dallas, TX) were used as positive controls for mouse and human MC1R, respectively. The blot was blocked using blocking solution Intercept (Li-Cor, Lincoln, NE) for 1 hour at room temperature. Blot was incubated with primary antibody over night at 4°C, washed three times with Trizma (buffer pH 7.4 with 0.1% Tween 20), and further incubated in a secondary antibody for 1 hour at room temperature. The bands were visualized using SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific). The dilution for rabbit anti-mMC1R (No. Ab180776; Abcam, Waltham, MA) was 1:1000, and for mouse anti-hMC1R (No. Ab230675; Abcam), 1:400. Dilution for secondary antibodies (horseradish peroxidase–conjugated) goat anti-rabbit (No. ab205718; Abcam) and goat anti-mouse (No. 405306; BioLegend, San Diego, CA) was 1:3000. All antibody dilutions were made using the blocking solution Intercept (Li-Cor).
Orthotopic Corneal Transplantation
A standard protocol for murine orthotopic corneal transplantation was followed.44,45 In brief, a 2-mm central corneal button was excised from donor mice and grafted onto a 1.5-mm recipient bed and secured with eight interrupted 11 to 0 nylon sutures (AB-0550S; MANI, Tochigi, Japan). All procedures were performed under anesthesia with intraperitoneal injection of ketamine (120 mg/kg) and xylazine (20 mg/kg). Postoperatively, buprenorphine (0.05 to 0.1 mg/kg) was injected subcutaneously to minimize pain. Corneal sutures were removed 7 days after surgery. Grafts that were opaque at postoperative week 2 were excluded from further analysis, as were eyes that underwent complications during or after surgery such as cataract, infection, intraocular hemorrhage, or synechiae. Although the half-life of α-MSH is short (only a few minutes), it exerts its effects via modulating the expression/translation of different genes via its melanocortin receptor family.46 Thus, using the authors’ previously published protocol,27 in the α-MSH–treated group, the mice received subconjunctival injection of 10 μL of 10−4 mol/L α-MSH (Sigma-Aldrich; diluted with sterile PBS) twice-per week after surgery for 8 weeks. The control group received a 10-μL subconjunctival injection of sterile PBS (sham) with the same schedule. The mice were followed for 8 weeks after transplantation. Transplanted corneas were evaluated weekly by a masked observer using slit lamp biomicroscopy to assess corneal opacity and neovascularization with a standardized grading system (ranges, 0 to 5+).44 At week 2, some of the transplanted corneas (n = 4) were harvested for ZO-1 and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining to measure CEnC density and the percentage of apoptotic (ZO-1–positive TUNEL-positive) cells.
Immunohistochemistry for ZO-1 and Apoptosis
Corneas were fixed and stained as previously reported.10,47 In brief, corneas were fixed in absolute ethanol for 20 minutes at room temperature, and then stained with a fluorescein isothiocyanate–conjugated ZO-1 monoclonal antibody (1:200; Thermo Fisher Scientific) overnight at 4°C. The corneas were then washed and permeabilized with 0.1% Triton-X (Sigma-Aldrich) in 0.1% sodium citrate for 10 minutes at room temperature. The TUNEL assay was performed using the In Situ Cell Death Detection Kit (Roche Diagnostics, Basel, Switzerland) according to the manufacturer's protocol. Flat-mounted corneas with the endothelium facing up were embedded with DAPI-containing mounting medium and were analyzed using confocal microscopy (×400 magnification; Leica TCS-SP5). Two images were taken from the central area of each cornea. Counting was assessed by an independent masked observer using ImageJ software version 1.52 (NIH, Bethesda, MD; https://imagej.nih.gov/ij/docs/faqs.html). As previously reported,10,47 live CEnCs were defined as ZO-1–positive TUNEL-negative cells, whereas apoptotic cells were defined as ZO-1–positive TUNEL-positive. The percentage of CEnC apoptosis in each experiment was calculated by dividing total number of ZO-1–positive TUNEL-positive cells by the total number of ZO-1–positive TUNEL-negative cells.
Flow Cytometry
Ipsilateral draining lymph nodes were collected after transplantation, and single-cell suspensions were prepared without digestion. Cells were filtered, suspensions were incubated with an Fc receptor blocking antibody (R&D Systems, Minneapolis, MN), and a Zombie UV fixable viability kit (BioLegend) was additionally added to label dead cells. After washing, cells were stained with different combination of antibodies from BioLegend or eBioscience (San Diego, CA). For T regulatory cells (Tregs), the authors used anti-CD3, anti-CD4, anti-CD25 for extracellular staining, and anti-FoxP3, anti–transforming growth factor-β1 (TGFβ1) and anti–IL-10 for intracellular staining without stimulation. For T helper type 1 (Th1) cells anti-CD3, anti-CD4 were used as above, and intracellular staining was performed with anti–IFN-γ previously stimulated with phorbol 12-myristate 13-acetate (PMA; 50 ng/mL; Sigma-Aldrich) and ionomycin (500 ng/mL; Sigma-Aldrich) for 6 hours, and GolgiStop (0.7 μL/100 μL medium; BD Biosciences, San Jose, CA) for 3 hours. For intracellular staining, cells were fixed, permeabilized, and then washed with appropriate buffers (eBioscience). Proper isotype controls and Fluorescence Minus One were used for all antibodies. Stained cells were analyzed using the LSR II flow cytometer (BD Biosciences, Franklin Lakes, NJ), and the results were analyzed using FlowJo software version 10.5.0 (FlowJo LLC, Ashland, OR).
Statistical Analysis
The Kaplan-Meier survival curve was used to determine graft survival, and the log-rank test was used to compare survival rates between the groups. The two-sided t-test or U-test was used for comparison of quantitative data between each 2 groups, as appropriate. One-way analysis of variance with Bonferroni correction was used to determine statistically significant differences between the means of three or more independent (unrelated) groups. Results are presented as means ± SEM. P < 0.05 was considered statistically significant.
Results
MC1R Expression in Corneal Endothelium
The expression of MC1R in human and murine corneal endothelium, and in cultured CEnC lines was first determined by fluorescent immunohistochemistry (Figure 1A). The immunofluorescence data were supported by the Western blot analysis showing expression of the MC1R in cultured murine and human CEnC cell lines and in murine corneal endothelium (Figure 1, B and C). Both human and murine CEnCs express abundant MC1R.

Corneal endothelial cells (CEnC) express high levels of the receptor for α-MSH. A: Immunostaining for α-MSH receptor, melanocortin receptor 1 (MC1R), on murine (mCEnC) and human (hCEnC) CEnC lines and primary CEnCs. Blue: DAPI, purple: MC1R. B and C: Representative Western blot bands of MC1R in murine and human CEnC lines (B) and in primary murine corneal endothelium lysates (C). SIT lysate: small intestine tissue lysate used as positive control for mouse MC1R (mMC1R). A-375: human skin epithelial cell lysate used as positive control for human MC1R (hMC1R). Scale bars = 50 μm.
Loss of α-MSH/MC1R Signaling Leads to Universal Graft Swelling and Failure Even in Syngeneic Transplantation
After corneal transplantation (grafting), functional donor CEnCs are critical for graft clarity and survival. CEnC loss and/or dysfunction after surgery thus presents as graft edema/swelling and loss of transparency (ie, increase in opacity), which results in graft failure and decreased survival. In syngeneic grafting, although there is non–antigen-specific inflammation as a result of the surgical procedure, there is no antigen-specific alloimmune response, thus leading to nearly universal acceptance and survival of the donor cornea. Donor corneas derived either from MC1R signaling-deficient (Mc1re/e) or WT C57BL/6 mice were grafted onto syngeneic WT C57BL/6 recipients (Figure 2A). The Mc1re/e mice, on a C57BL/6 background, have a frameshift mutation between exon 4 and 5, and lack a functional MC1R.29,41 At all time points post-transplantation, corneal graft opacity was significantly higher in the Mc1re/e-donor group compared with that in the WT-donor group (P = 0.0068, P = 0.0009, P = 0.0009, P = 0.0013, P = 0.002, P = 0.0016, and P = 0.0013, respectively) (Figure 2B). Although 100% graft survival was seen in the syngeneic WT-donor group up to 8 weeks, none of the transplants in the Mc1re/e-donor group remained transparent (ie, survived) beyond week 4 (hazard ratio, 0.02933; 95% CI, 0.005505–0.1563; P < 0.0001, log-rank Mantel-Cox test) (Figure 2C), suggesting that independent of allogenic immune modulation, α-MSH/MC1R signaling in the donor tissue is indispensable in the maintenance of graft transparency and survival.
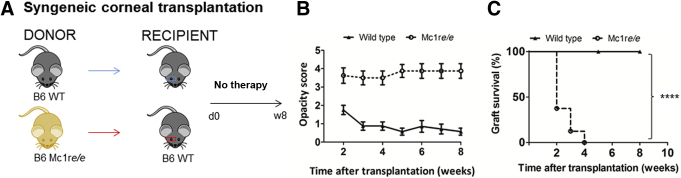
α-MSH/MC1R signaling deficiency leads to decreased graft survival of syngeneic corneal transplantation. A: Melanocortin receptor 1 signaling–deficient (Mc1re/e) or wild-type (WT) C57/BL6 donor corneas were transplanted into syngeneic C57BL/6 recipients. B: Loss of MC1R function significantly increased corneal opacity scores (ie, loss of transparency) post-transplantation at all time points. P = 0.0068, P = 0.0009, P = 0.0009, P = 0.0013, P = 0.002, P = 0.0016, and P = 0.0013, respectively (U-test). C: Survival curve of syngeneic corneal transplantation. n = 8 time points (B); n = 8 transplants (C). 


 P < 0.0001 log-rank test.
P < 0.0001 log-rank test.
Loss of α-MSH/MC1R Signaling Leads to CEnC Loss and Graft Failure in Allogeneic Corneal Transplantation
Allogeneic murine corneal transplantation was performed by grafting donor corneas derived from either Mc1re/e or WT C57BL/6 mice onto BALB/c recipients (Figure 3A). At all time points, graft opacity scores were lower in the grafts receiving WT-donor corneas compared with grafts receiving Mc1re/e-donor corneas (Figure 3B). Importantly, none of the grafts receiving donor corneas derived from Mc1re/e mice remained transparent (ie, survived) beyond week 4 (Figure 3, C and D), whereas 43% of grafts receiving donor corneas derived from WT mice maintained clarity and thus survived by week 8 (P = 0.005, log-rank Mantel-Cox test).
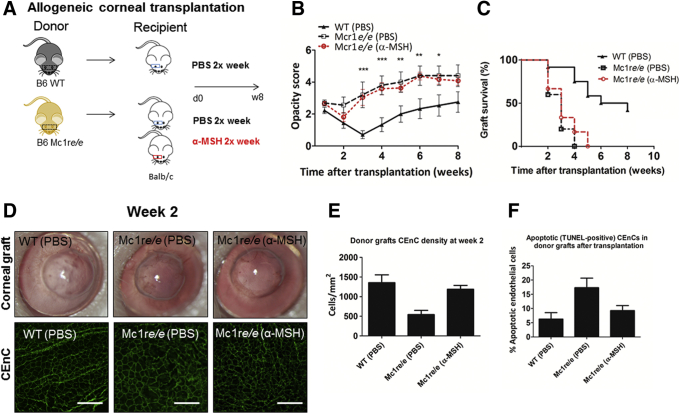
α-MSH/MC1R signaling deficiency leads to decreased graft survival and CEnC loss after allogeneic corneal transplantation. A: Allogeneic corneal transplantation was performed using donor corneas derived from MC1R signaling-deficient (Mc1re/e) or wild-type (WT) C57BL/6 mice. These corneas were transplanted into BALB/c recipient mice. After transplantation, subconjunctival injection of either α-MSH (10−4 mol/L) or phosphate-buffered saline (PBS) was given 2×/week for 8 weeks. B: α-MSH/MC1R signaling deficiency groups with and without α-MSH treatment had significantly increased graft opacity scores at 3, 4, 5, 6, and 7 weeks after transplantation, compared with WT. C: None of the grafts receiving donor corneas derived from Mc1re/e mice survived (ie, remained transparent) by week 5, regardless of subconjunctival α-MSH treatment. By contrast, 43% of PBS-treated (5/12) grafts receiving donor corneas derived from WT mice had survived by week 8 after transplantation; P < 0.0054 (log-rank test). D: Representative slit-lamp images (top panel) show corneal graft transparency. Confocal micrographs (lower panel) showing central CEnC stained for zonula occluden-1 (green) to visualize endothelial cell-to-cell junctions at week 2 post-transplantation. E: Bar graph shows significantly lower CEnC density in the Mc1e/e (PBS) group at 2 weeks after transplantation, when compared with WT or Mc1e/e (α-MSH) groups; P = 0.001 (one-way analysis of variance). F: Bar graph showing the percentages of apoptotic (TUNEL-positive) cells in donor grafts; P = 0.0164 (one-way analysis of variance). n = 8 donor grafts (E); n = 8 apoptotic grafts (F).  P < 0.05,
P < 0.05, 
 P < 0.01, and
P < 0.01, and 

 P < 0.001 (analysis of variance). Scale bars = 100 μm. TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling.
P < 0.001 (analysis of variance). Scale bars = 100 μm. TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling.
Subconjunctival α-MSH injection after WT donor transplantation leads to enhanced graft survival by suppressing the immune response to graft antigens using subconjunctival injections of α-MSH twice weekly.27 Furthermore, a recent in vitro pharmacokinetic study using rabbit albino eyes showed α-MSH diffusion across scleral tissue, but not corneal tissue (data not shown). Herein, α-MSH was administered to mice receiving Mc1re/e donor tissue to determine whether exogenous α-MSH can rescue these grafts. Mc1re/e grafts receiving α-MSH treatment had similar corneal opacity scores and survival rates compared with those receiving control PBS injections (Figure 3, A–C), suggesting that any effect of α-MSH on host immunity cannot circumvent the critical function of MC1R signaling in the donor cornea for its cytoprotective function on endothelial function, which is essential for graft survival.
CEnC loss after corneal transplantation was identified by immunohistochemistry (Figure 3D). At week 2 after transplantation, central CEnC density decreased from 3300 ± 58 cells/mm2 at baseline to 1356 ± 201 cells/mm2 in the WT-donor group, representing a 59% cell loss. By contrast, the Mc1re/e-donor group had an 84% cell loss with 542 ± 106 cells/mm2 remaining. In the Mc1re/e-donor group that received α-MSH injection, the central CEnC density was 1188 ± 98 cells/mm,2 representing a 64% cell loss (P = 0.001, one-way analysis of variance). Similarly, more apoptotic cells were observed in the Mc1re/e-donor grafts (α-MSH–treated, 9.25 ± 1.8%; PBS-treated, 17.30 ± 3.4%) (Figure 3E) compared with the WT-donor graft group (6.28 ± 2.2%; P = 0.0164, one-way analysis of variance). These data suggest that although local delivery of α-MSH to the Mc1re/e donors reduced CEnC loss and apoptosis, it was insufficient to preserve graft survival without functional MC1R in the donor tissue.
α-MSH Suppresses Alloimmunity Regardless of Donor Type (Mc1re/e or WT)
To evaluate the effect of α-MSH treatment on the initiation of host immune responses after allogeneic corneal transplantation, the ipsilateral draining lymph nodes were harvested at week 1 after transplantation. Flow cytometry was used to investigate frequencies of Tregs (CD4+FoxP3+) and CD3+CD4+IFN-γ+ T effector cells (Figure 4). Compared with Mc1re/e-donor group receiving PBS treatment, those receiving α-MSH treatment had higher mean fluorescence intensity of Tregs (Figure 4) (P = 0.0022). Moreover, frequencies of IL-10 in CD4+FoxP3+ Tregs were significantly increased in the α-MSH–treated group (3.4 ± 0.6%) compared with the PBS-treated WT-donor (1.0 ± 0.2%, P = 0.0068) or Mc1re/e-donor (1.0 ± 0.3%, P = 0.0086) groups. Similarly, the frequencies of TGFβ1 in CD4+FoxP3+ Tregs were significantly increased in the α-MSH–treated group (2.1 ± 0.1%) compared with the PBS-treated groups (WT, 0.7 ± 0.1%, P = 0.0001; and Mc1re/e, 0.9 ± 0.3%, P = 0.0115, respectively). In addition, the frequencies of IFN-γ+ effector T cells were comparable between WT- (3.2 ± 0.4%) and Mc1re/e-donor groups (3.8 ± 0.7%, P = 0.4675) receiving PBS treatment, but the Mc1re/e donor receiving α-MSH-treatment had significantly lower frequency (2.0 ± 0.3%, P = 0.0486, compared with the without Mc1re/e-donor α-MSH treatment). These data demonstrate that α-MSH treatment led to a concomitant increase in Treg function and decrease in T effector response, consistent with its known immunomodulatory effect. Importantly, however, such effect is insufficient to prevent CEnC loss or graft failure in donor tissues lacking MC1R signaling as seen in Figure 3, pointing toward that α-MSH/MC1R mediates corneal transplant clarity and function primarily via a nonimmune cytoprotective function.
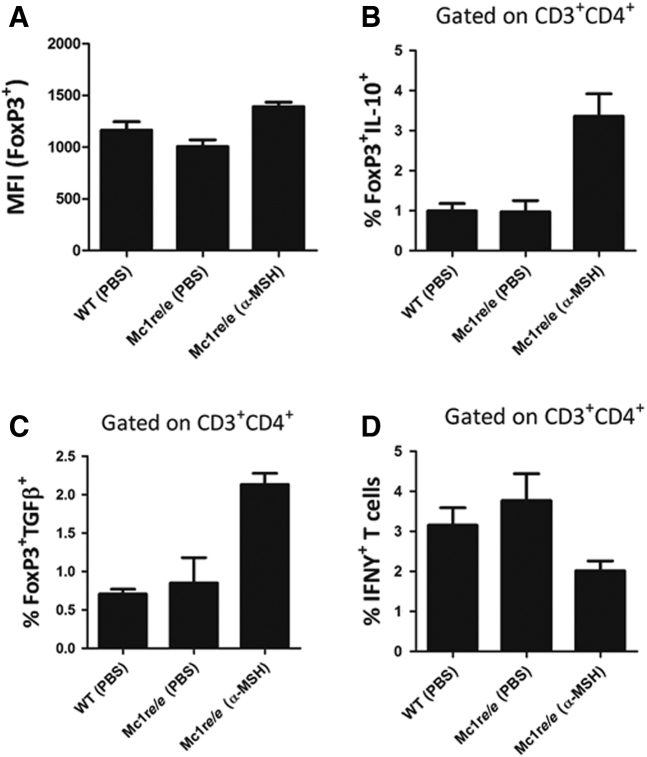
Subconjunctival injection of α-MSH increases IL-10 and TGF-β expression in CD4+FoxP3+ cells and decreases the frequencies of CD3+CD4+IFN-γ+ T cells in the draining lymph nodes 7 days after corneal transplantation. Compared with the phosphate-buffered saline (PBS)-treated groups, α-MSH treatment resulted in increases in the mean fluorescence intensity (MFI) of CD4+FoxP3+ cells P = 0.0451, P = 0.0022, respectively (2-sided t-test) (A); frequencies of FoxP3+IL-10+ cells, P = 0.0068, P = 0.0086, respectively (2-sided t-test) (B); and frequencies of FoxP3+TGF-β+ cells, P = 0.0001, P = 0.015, respectively (2-sided t-test) (C); and a decrease in frequencies of CD3+CD4+IFN-γ+ T cells, P = 0.0486 (2-sided t-test) (D). Data are expressed as means ± SEM. n = 4 mice/group.
Discussion
Despite significant advancements in donor cornea preservation and corneal transplant surgical techniques in the past decades, there has been no major breakthrough in the pharmacotherapy of preventing CEnC loss after transplantation for the last 60 years. Notably, 70% of CEnCs are lost over the first few years in successful grafts,48 and low CEnC density remains the principal cause of corneal graft failure, accounting for over 20% of current indications for corneal transplantation.49,50 Immune-based mechanisms prevent graft rejection by inducing immune tolerance.27,51, 52, 53 More recently, a role of neuropeptides in preserving CEnC has been identified.10,36 Herein, the study demonstrates, for the first time, that in addition to its well-known immunomodulatory effect,27 the neuropeptide α-MSH, via its receptor MC1R, is critically cytoprotective and plays a principal role in preventing tissue edema and graft failure.
α-MSH was originally isolated from the pituitary gland and considered to regulate skin pigmentation.23 Since then, MC1R expression has been described in various cell types including melanocytes,30,33,54 epithelial cells,29 and immune cells such as macrophages, dendritic cells, monocytes, neutrophils, B cells, CD8+ T cells, and natural killer cells.55, 56, 57 In the eye, α-MSH is a well-known constitutive immunomodulatory component of normal aqueous humor23,58, 59, 60, 61; it mediates induction of Tregs25 and suppresses IFN-γ production by Th1 cells.61 It also reduces innate immune responses by modulating the production of proinflammatory cytokines and chemokines62 and maturation of antigen-presenting cells.56 It is derived from proteolysis of proopiomelanocortin hormone; with retinal pigment epithelial cells, iris and ciliary body cells expressing the necessary enzymes to process a functional peptide, however, the exact source of α-MSH production in the eye is still not fully defined.24 This study provides novel evidence that in addition to the ocular surface epithelial cells,37, 38, 39 corneal endothelial cells express MC1R, the high-affinity receptor for α-MSH.
Prior in vivo evidence indicates that local treatment with α-MSH significantly reduces allorejection of orthotopic corneal transplants by modulating immune responses.27 After corneal transplantation, interactions between certain cytokines and cellular networks involving antigen-presenting cells, the interplay between IFNγ-producing Th1 effector cells and Tregs can determine ultimate graft fate.53 Host allosensitization is mediated by activated and mature antigen-presenting cells that migrate to lymphoid tissues where they prime T effector cells (eg, IFN-γ secreting Th1 cells), which are then recruited to the graft site.63 Donor CEnC apoptosis is primarily mediated by effector cells in a non–contact-dependent mechanism by apoptosis-inducing cytokine secretion, such as IFN-γ.64,65 By contrast, CD4+CD25+FoxP3+ Tregs are crucial for allograft survival.53 They play an important role in allospecific tolerance induction through several mechanisms (eg, inhibition of Th1 cell sensitization and their effector function by IL-10 and TGFβ1 immunomodulatory cytokine secretion).51,53,66
Consistent with the known immunomodulatory function of α-MSH, herein the present study indicates that local administration of α-MSH in the draining lymph nodes decreases frequencies of IFN-γ+ T cells and increases frequencies and expression (mean fluorescence intensity) of IL-10+ and TGFβ1+ Tregs. This suggests that α-MSH leads to early Treg activation and suppression of Th1 immunity. However, whereas local application of α-MSH clearly prolonged the survival of WT-donor grafts,27 survival of corneal grafts derived from MC1R signaling-deficient mice (Mc1re/e donors), regardless of α-MSH treatment, had significantly lower allograft survival (none of the grafts remained transparent by week 5). Loss of donor MC1R function did not impair the early immunomodulatory effect of α-MSH treatment on the host, but critically undermined the long-term fate of the graft, pointing toward a nonimmune cytoprotective aspect of α-MSH/MC1R function on endothelial function, which is essential for graft survival. This was further reflected by the studies showing that the Mc1re/e-donor group had lower CEnC densities and more apoptotic cells compared with the WT group. However, application of α-MSH to the Mc1re/e-donor group did reduce CEnC loss and apoptosis, compared with PBS treatment. This can be explained by one or more of the following mechanisms: i) an overall less inflammatory milieu in the graft site due to the anti-inflammatory effect of α-MSH; ii) α-MSH's effect on MCRs other than MC1R; and/or iii) the direct wound-healing effect of α-MSH on peripheral host CEnC (that were not grafted). Despite these mechanisms, α-MSH treatment was insufficient to overcome the detrimental effect of loss of MC1R signaling on the donor tissue.
In addition, using syngeneic corneal transplantation, an animal model that circumvents alloimmunity and enjoys almost universal graft acceptance in WT animals, the study showed significantly worse Mc1re/e-donor allograft opacity and survival compared with the WT-donor group. This confirms that loss of MC1R function contributes to increased CEnCs vulnerability to several nonspecific inflammatory stressors after injury. Therefore, we conclude that MC1R function in graft endothelial cells is indispensable for graft function and is likely related to the antiapoptotic effects of α-MSH.
These findings are consistent with the biological importance of the α-MSH/MC1R–signaling pathway in other tissues. Although a nonfunctional MC1R is not lethal under physiological conditions,41 the cytoprotective function is clearly revealed and unmasked from its immunomodulatory effect under various stress and disease conditions.28,29,67, 68, 69, 70 For example, in gastrointestinal epithelial cells, a breakdown of the MC1R-signaling pathway led to a more severe course of colitis.29 Similarly, MC1R activation was directly neuroprotective in a mouse model of neuroinflammatory disease28 and was critical for proper cartilage development and strength in an osteoarthritis mice model.32
There are several studies focusing on the intracellular pathways on how α-MSH/MC1R activation suppresses cell death in various stress conditions. In the current in vitro experiments, α-MSH suppressed IFN-γ–mediated CEnC apoptosis, a critical mechanism for CEnC death and graft edema (failure) after transplantation.65,71 Paired high-quality human donor corneas were recently used to demonstrate that α-MSH treatment also reduced CEnC death during hypothermic storage under cytokine and oxidative-induced stress.36 However, further studies need to be conducted to better clarify the exact intracellular molecular pathways underlying α-MSH's cytoprotective effect on increasing CEnCs resilience to stress. Although the exact intracellular signaling pathways by which α-MSH exerts its cytoprotective effect on CEnCs survival are still under investigation, previous studies in several other cell types have shown that several molecular pathways can be involved. For example, retinal pigment epithelial cells were protected from hydrogen peroxide-induced apoptosis by α-MSH–activated Akt/mammalian target of rapamycin activation and Erk1/2 signaling.31 In another study, Zhang et al35 suggested that the cytoprotective effect in retinal vascular endothelial cells of early diabetic rats is mediated through α-MSH–mediated inhibition of Foxo4 up-regulation. Moreover, α-MSH also increases levels of enzymes essential for base excision repair, independently of melanin synthesis, enhancing the repair of DNA photoproducts in skin melanocytes.30 Activation of MC1R by α-MSH contributes to P53 activation, a sensor of DNA damage, which is mediated by the cAMP/PKA pathway and by the activation of phosphoinositide 3-kinase ATR and DNA protein kinase.30 α-MSH also increases nuclear factor Nrf2 and Nrf-dependent genes, which are important regulators of cellular resistance to oxidants reduced after UVB irradiation, highlighting another cytoprotective and antioxidative mechanism.54 Given the high exposure of CEnCs to UV radiation through life and the UV radiation–induced formation of reactive oxygen species, one can only speculate that a molecular mechanism essential for DNA repair might also be involved in preventing CEnCs apoptosis in human corneas.72
In summary, this study demonstrates, for the first time, that in addition to its well-known immunomodulatory effect, α-MSH has a pivotal cytoprotective effect on CEnC survival. These data, in conjunction with previous work,27,36 highlight the function of α-MSH and its native receptor MC1R, in preventing CEnC loss after transplantation.
Footnotes
Supported by NIH grants R21 EY029387 and R01 EY012963 (R.D.), NIH grant 1K08EY031340 (J.Y.), and an Eye Bank Association of America Richard Lindstrom Research grant (Z.L.M.).
Disclosures: R.D. is a consultant for Aramis Biosciences, Claris Biotherapeutics, GelMEDIX, Kala, Novartis, and GSK, and has equity in Claris Biotherapeutics, GelMEDIX and Aramis Biosciences; J.Y. is a consultant for Claris Biotherapeutics; S.K.C. is a consultant and equity holder in Claris Biotherapeutics and Aramis Biosciences; U.V.J. is a consultant for Claris Biotherapeutics; J.Y. and R.D. have financial interest in Kera Therapeutics, a company focused on developing alpha-melanocyte stimulating hormone for management of corneal edema; this interest has been reviewed and managed by Massachusetts Eye and Ear and Mass General Brigham under their conflict-of-interest policies.
Author Contributions
R.D. and J.Y. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; Z.L.M., Z.S., S.K.C., A.W.T., U.V.J., J.Y., and R.D. conceived and designed the study; Z.L.M., T.B., Z.S., H.A., G.O., H.N., J.Y., and R.D. acquired, analyzed, or interpreted data; Z.L.M., T.B., Z.S., J.Y., and R.D. drafted the manuscript; S.K.C., A.W.T., U.V.J., J.Y., and R.D. critically revised the manuscript; Z.L.M., Z.S., and J.Y. performed statistical analysis; Z.L.M., U.V.J., J.Y., and R.D. obtained funding; T.B., Z.S., H.N., H.A., and A.W.T. provided administrative, technical, or material support; U.V.J., J.Y., and R.D. supervised the study.
References
Articles from The American Journal of Pathology are provided here courtesy of American Society for Investigative Pathology
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.ajpath.2021.10.016
Read article for free, from open access legal sources, via Unpaywall:
http://ajp.amjpathol.org/article/S0002944021004776/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/117050223
Article citations
Neuropeptide alpha-Melanocyte stimulating hormone preserves corneal endothelial morphology in a murine model of Fuchs dystrophy.
Sci Rep, 14(1):18842, 14 Aug 2024
Cited by: 0 articles | PMID: 39138334 | PMCID: PMC11322312
Myeloid-derived suppressor cells promote allograft survival by suppressing regulatory T cell dysfunction in high-risk corneal transplantation.
Am J Transplant, 24(9):1597-1609, 19 Mar 2024
Cited by: 0 articles | PMID: 38514014
Therapeutic Effects of Stimulating the Melanocortin Pathway in Regulating Ocular Inflammation and Cell Death.
Biomolecules, 14(2):169, 31 Jan 2024
Cited by: 4 articles | PMID: 38397406 | PMCID: PMC10886905
Review Free full text in Europe PMC
The Neuropeptide α-Melanocyte-Stimulating Hormone Prevents Persistent Corneal Edema following Injury.
Am J Pathol, 194(1):150-164, 11 Oct 2023
Cited by: 5 articles | PMID: 37827217
Descemet Stripping Only Technique for Corneal Endothelial Damage in Mice.
Cornea, 42(4):470-475, 22 Dec 2022
Cited by: 0 articles | PMID: 36728991 | PMCID: PMC10117527
Go to all (7) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Association of α-Melanocyte-Stimulating Hormone With Corneal Endothelial Cell Survival During Oxidative Stress and Inflammation-Induced Cell Loss in Donor Tissue.
JAMA Ophthalmol, 138(11):1192-1195, 01 Nov 2020
Cited by: 8 articles | PMID: 32940642 | PMCID: PMC7499243
The Neuropeptide α-Melanocyte-Stimulating Hormone Prevents Persistent Corneal Edema following Injury.
Am J Pathol, 194(1):150-164, 11 Oct 2023
Cited by: 5 articles | PMID: 37827217
Regulatory T cells promote corneal endothelial cell survival following transplantation via interleukin-10.
Am J Transplant, 20(2):389-398, 30 Oct 2019
Cited by: 10 articles | PMID: 31587452 | PMCID: PMC6984989
Alpha-melanocyte stimulating hormone (α-MSH): biology, clinical relevance and implication in melanoma.
J Transl Med, 21(1):562, 22 Aug 2023
Cited by: 5 articles | PMID: 37608347 | PMCID: PMC10463388
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Eye Bank Association of America
NEI NIH HHS (3)
Grant ID: R01 EY012963
Grant ID: K08 EY031340
Grant ID: R21 EY029387
National Institutes of Health (3)
Grant ID: R01 EY012963
Grant ID: R21 EY029387
Grant ID: 1K08EY031340




