Abstract
Purpose
Descemet stripping only is an emerging surgical technique used to remove central Descemet membrane and corneal endothelial cells in patients with corneal endothelial disease. Here, we describe a murine model of this procedure to help facilitate basic science investigation and evaluation of postoperative outcomes using this surgical technique.Methods
Slitlamp biomicroscopy, central corneal thickness assessment (by optical coherence tomography), and immunohistochemistry were used to assess the model through 7 weeks of follow-up.Results
Complete removal of the endothelium and Descemet membrane was confirmed by slitlamp biomicroscopy and by histology. Central corneal thickness peaked at day 1 postinjury and then declined over the course of 2 weeks to a stable level of persistent edema. Seven weeks postinjury, immunohistochemical staining for ZO-1 showed the area of Descemet stripping was fully covered by enlarged and dysmorphic corneal endothelial cell. No significant ocular complications were appreciated through the end of the follow-up.Conclusions
We demonstrate the feasibility of and provide detailed instructions for a murine model of Descemet stripping only. This model provides a potential in vivo platform to investigate the mechanisms and biology of this emerging surgical procedure.Free full text

Descemet Stripping Only (DSO) Technique for Corneal Endothelial Damage in Mice
Abstract
Purpose:
Descemet Stripping Only (DSO) is an emerging surgical technique used to remove central Descemet’s membrane and corneal endothelial cells (CEnC) in patients with corneal endothelial disease. Here we describe a murine model of this procedure to help facilitate basic science investigation and evaluation of postoperative outcomes using this surgical technique.
Methods:
Slit lamp biomicroscopy, central corneal thickness (CCT) assessment (by optical coherence tomography) and immunohistochemistry were used to assess the model through 7 weeks of follow-up.
Results:
Complete removal of the endothelium and Descemet’s membrane was confirmed by slit lamp biomicroscopy and by histology. CCT peaked at day 1 post-injury and then declined over the course of 2 weeks to a stable level of persistent edema. Seven weeks post-injury, immunohistochemical staining for ZO-1 showed the area of Descemet stripping was fully covered by enlarged and dysmorphic CEnC. No significant ocular complications were appreciated through the end of the follow up.
Conclusion:
We demonstrate the feasibility of and provide detailed instructions for a murine model of DSO. This model provides a potential in vivo platform to investigate the mechanisms and biology of this emerging surgical procedure.
INTRODUCTION
Descemet Stripping Only (DSO) (a.k.a. Descemetorhexis Without Endothelial Keratoplasty [DWEK]) is a corneal surgical technique consisting of removal of Descemet’s membrane (DM), without subsequent endothelial transplantation, that has proven to be efficacious in select patients with endothelial dysfunction, primarily in the setting of Fuchs’ Endothelial Corneal Dystrophy (FECD).1 FECD is a non-inflammatory dystrophy of the corneal endothelium that leads to corneal edema and subsequent impairment of visual acuity, potentially leading to corneal blindness.2 DSO reduces the need for corneal transplantation and is now a viable treatment option in select patients with FECD.3
Animal models of mechanical corneal injury resembling DSO have been described previously including in rats4, rabbits5 and non-human primates6. While larger animals are excellent models for reproducing human surgical techniques, cost and administrative restrictions in animal research can limit their use.7 This makes development of a model of DSO in smaller animals appealing, and a murine model would be invaluable thanks to the well-defined genetics of in-bred mouse strains and wide availability of reagents. A murine model of DSO would provide a foundation for basic scientific investigation into this surgical technique and the underlying mechanisms of corneal endothelial wound healing. Of note, the lower proliferation rate of corneal endothelial cells (CEnC) in adult mice relative to, i.e. rabbits, approximates the minimal proliferative capacity of CEnC in humans.7
Over the past three decades our laboratory has developed experimental murine models for a number of corneal procedures in order to gain a better understanding of corneal transplant failure, the host alloimmune response to corneal transplants, the role of angiogenesis in immunological responses and assessment of novel therapeutics. Here, we report a novel adaptation of the DSO technique, as commonly used in the clinical setting, to a murine model. A step-by-step description of the technique is provided, and we additionally describe follow-up with slit-lamp and optical coherence tomography (OCT) imaging as well as pathology sections to confirm the feasibility of this technique.
METHODS
Animals
Surgery was performed in six-to-eight-week-old male BALB/c mice. The BALB/c strain was chosen to maintain consistency with well-established murine models of corneal transplantation and endothelial keratoplasty.8–10 In addition, BALB/c mice have transparent irises, which facilitates intraocular structure visualization and helps to prevent perioperative injuries.
Descemet Stripping Only (DSO) Procedure
Animals were housed in the Schepens Eye Research Institute animal vivarium and treated according to the guidelines set forth by the Association for Research in Vision and Ophthalmology (ARVO). All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee. Figures 1 and and22 depict the DSO procedure and details are described below (Also see Supplementary Video 1). Required reagents and materials are listed in Supplementary Table 1.
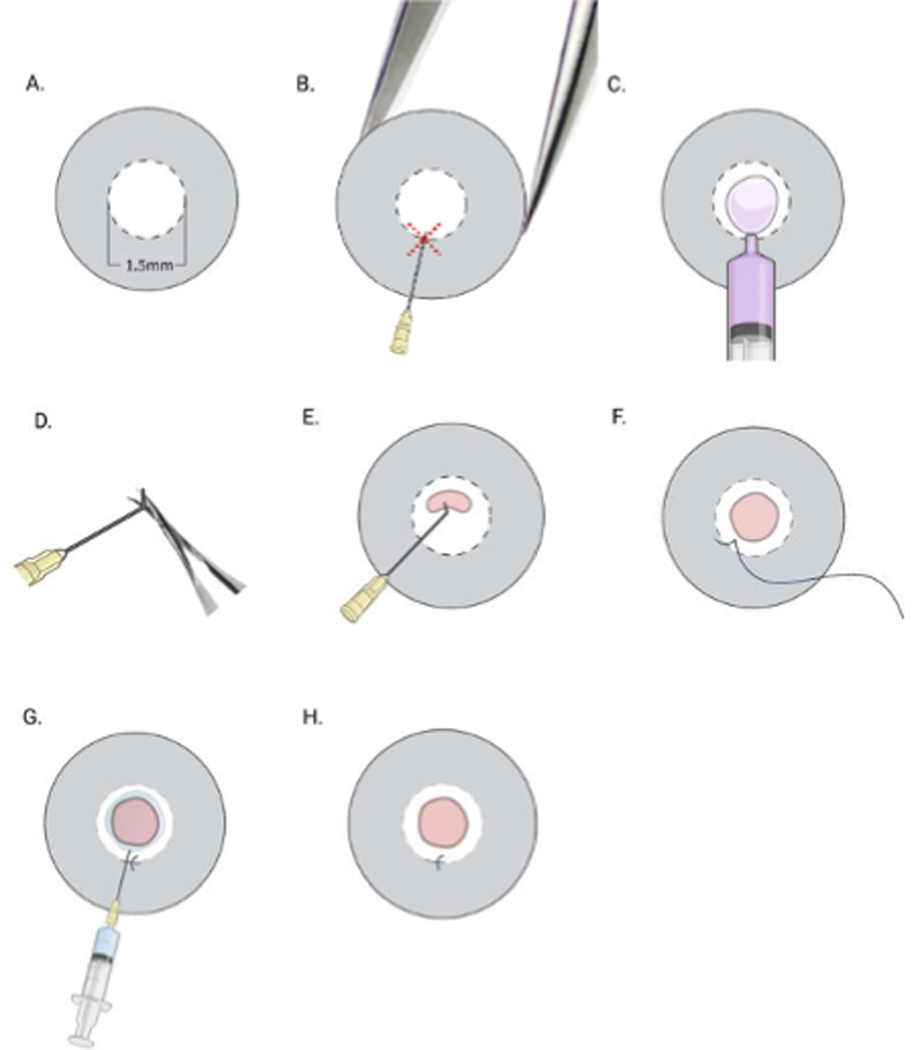
(A) Mark the central cornea with a 1.5-mm trephine; (B) support the globe using jeweler’s forceps, and make a tunnel incision into the cornea at ~6 to 9 o’clock along the border of the marked area (red dashed cross) using a 30G needle with the bevel pointed down; (C) use the proximal 1/4 of a 30G needle (by cutting the distal 3/4) to inject viscoelastic substance to fill the anterior chamber; (D) using a needle driver, bend the tip of a 30G needle; (E) introduce the bent 30G needle into the anterior chamber via the stromal tunnel and make a semicircular scratch (~120o) from 10 o’clock to 2 o’clock (120o) within the marked zone. Use the bent tip of the needle and scrape the endothelium gently to remove the endothelium in the marked area; (F) close the tunnel with an 11–0 nylon intrastromal suture; (G) irrigate the anterior chamber with PBS; (H) evaluate anterior chamber depth, iris integrity, pupil shape and suture placement. The red area depicts extent of scraped Descemet’s membrane.
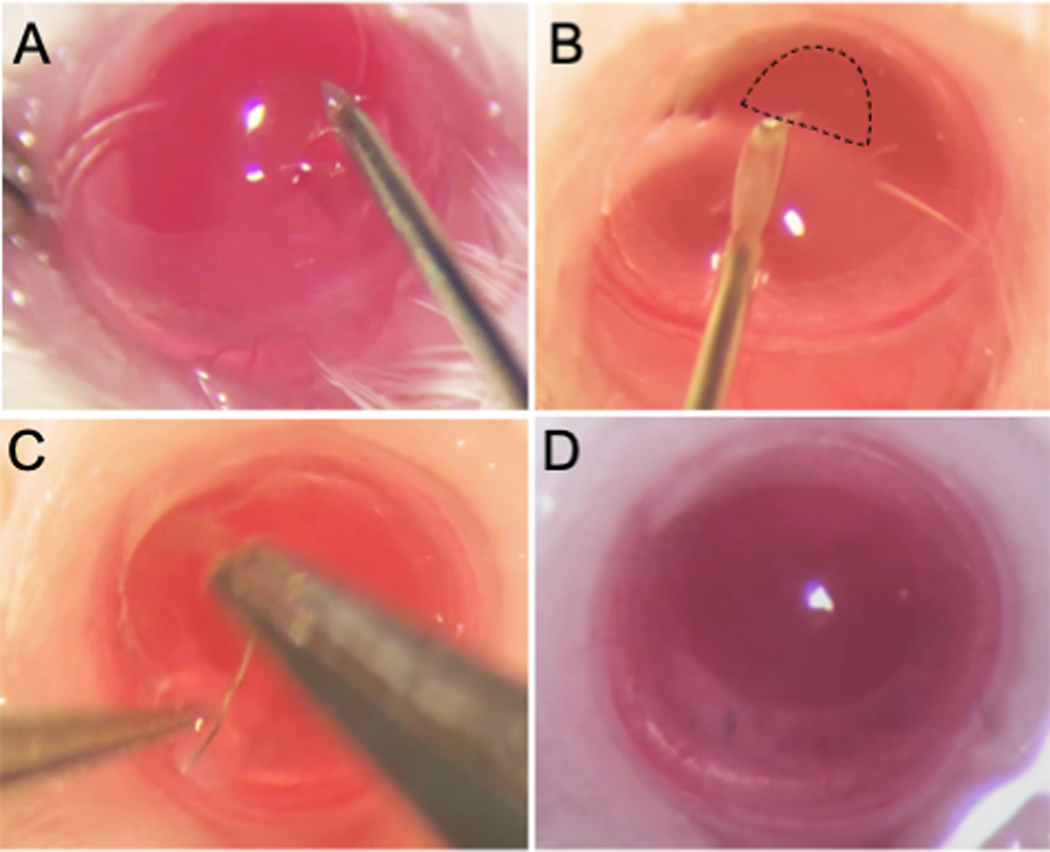
(A) Support the globe by holding the corneal limbus at the 6 and 12 o’clock positions with jeweler’s forceps. Mark the Descmetorhexis field using a 1.5-mm trephine. Make a tunnel incision in the cornea extending at least 0.3 mm in width between the 6 and 9 o’clock surgical positions using a 30G needle with the bevel pointing downwards. (B) Fill the anterior chamber with viscoelastic using a blunt 30G needle. Insert a bent 30G needle through the tunnel incision, with the bevel pointing downwards, and scratch the corneal endothelium from 10 o’clock to 2 o’clock (120o) in the previously marked area. Use the bent tip of the needle to contact the detached edge of Descemet’s membrane and scrape it gently in a circular fashion to extract the detached membrane. The scraped area appears relatively darker and hazier as compared to unscraped tissue when viewed through a microscope (outlined by dashed border). (C) Close the incision using an 11–0 nylon intrastromal suture. (D) Irrigate the anterior chamber with PBS through the sutured incision using the tip of a 30G needle (with bevel pointing away from the suture). Examine the depth of the anterior chamber, integrity of the iris, the pupil shape, and tightness of the suture.
Preparation: Six to eight week old male BALB/c mice were anesthetized by intraperitoneal injection of ketamine (120 mg/kg) and xylazine (20 mg/kg) using a 25G needle. A drop of 0.5% proparacaine was applied to the mouse ocular surface. After the animal was fully anesthetized, as confirmed by hind limb pinch, one drop each of 2.5% phenylephrine hydrochloride and 1% tropicamide was applied to achieve mydriasis during the procedure.
Corneal Incision: The mouse was placed in the lateral recumbent position ensuring the head is positioned so the eye can be visualized under the microscope. The ocular surface was irrigated with PBS and dried using eye spears. The central cornea was marked with a 1.5-mm diameter trephine to outline the area from which Descemet’s membrane is to be removed. Adequate pressure was applied to make a superficial partial thickness trephination without perforating the cornea (Fig. 1A). A paracentral corneal tunnel was made using a 30G needle to enter the anterior chamber and confirmed by positive Seidel sign and partial collapse of the anterior chamber (Fig. 1B). The distal 3/4 of the 30G needle was removed and the proximal 1/4 of the needle was used to enter the anterior chamber and deliver the ocular viscoelastic device (OVD). The depth of the anterior chamber was restored by injecting OVD and the corneal surface was dried carefully with eye spears. (Fig. 1C).
Scraping of the Endothelial Layer: The beveled tip of a 30G needle was bent using a needle-driver (Fig. 1D) and inserted into the anterior chamber through the prepared tunnel, with the bevel pointing downwards, and then it was rotated so its tip pointed upwards. Then corneal endothelium was gently contacted, and the bent sharp needle tip was used to engage endothelium and Descemet’s membrane and scrape them within the area previously demarcated with the trephine (Fig. 1E) until complete removal of Descemet’s membrane was achieved by pulling and externalizing scraped tissue. To ensure complete removal of the tissue, the scraped region was visualized under the microscope as a darker and slightly hazier area relative to the surrounding cornea with intact Descemet’s membrane. Thereafter, to maintain the anterior chamber depth, OVD was injected as needed and an 11–0 nylon suture was placed to close the corneal incision (Fig. 1F). OVD was removed by irrigating the anterior chamber with PBS (Fig. 1G).
Final Intraoperative Evaluation: The eye was examined to confirm a round pupil and normal anterior chamber depth (Fig. 1H). Antibiotic ointment was applied to the operated eye. To control post-operative pain, buprenorphine (0.05–0.1 mg/kg) was subcutaneously injected with a 25G needle immediately after the surgery and then every 12h for the next 48 hours.
Postoperative Clinical Assessment: Mice were followed post-operatively on a weekly basis for a total duration of 8 weeks. The corneal suture was removed after 1 week. At each follow up time point, the cornea and anterior chamber were examined by slit-lamp examination and corneal thickness was recorded using optical coherence tomography (OCT). Eyes were examined for development of any post-operative complications including hyphema, cataract, infection and collapsed anterior chamber.
RESULTS
The DSO technique was successfully developed in mice as shown in the schematic outline in Fig. 1. The critical steps of this procedure are depicted in Fig. 2 and include creating a stromal tunnel incision (Fig. 2A), scraping of the endothelium in the marked area (Fig. 2B), closure of the incision using an 11–0 nylon intrastromal suture (Fig. 2C), and examination of the depth of the anterior chamber, integrity of the iris, pupil shape, and tightness of the suture (Fig. 2D). Removal of Descemet’s membrane can be confirmed by direct visualization, as the scraped area appears relatively darker and hazier compared to the unscraped area (Fig. 2B, ,3A).3A). Following surgery, we were able to evaluate the cornea’s response to DSO by slit-lamp examination and by OCT (Fig. 3B, ,C).C). As expected, on postoperative day 1, the cornea was edematous and partially opaque (Fig. 3A). Pre-injury, the CCT of mice was measured to be 116±9 mm, and on post-operative day 1, the average corneal thickness was approximately 300 μm and gradually subsided over 2 weeks to eventually stabilize at approximately 128–140 μm between days 14 and 35, with a statistically significant difference compared to pre-injury corneal thickness (Fig. 3B–C). No complications were observed through 8-weeks of follow-up. The anterior chamber remained formed after the procedure and no synechiae were observed. We did not observe corneal neovascularization, including neovascularization to the placed suture. Periodic Acid–Schiff (PAS) staining was used to confirm successful removal of the corneal endothelium and its associated Descemet’s membrane (Fig. 4A–B).
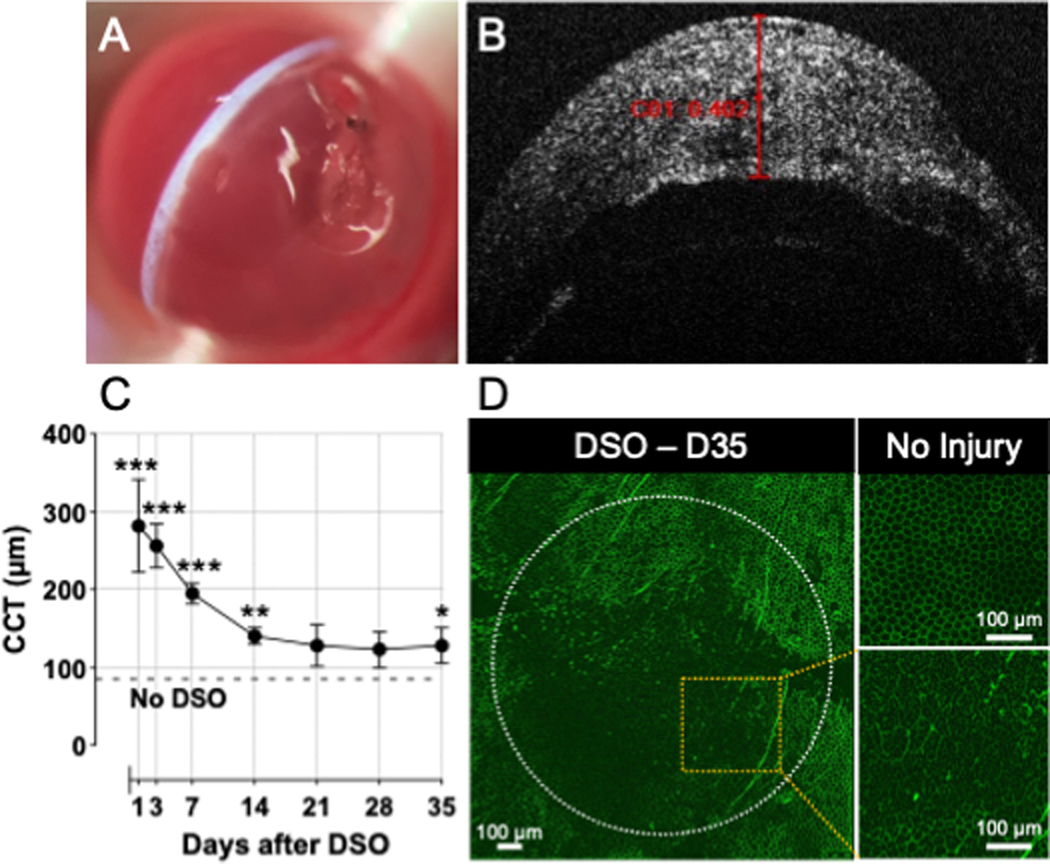
(A) Stripped corneal endothelium and increased corneal thickness can be visualized on slit-lamp exam using a narrow slit-lamp light beam. (B) AS-OCT examination shows corneal thickness greater than 400 μm at post-operative day 1. (C) Serial corneal thickness measurements via OCT examination show a decrease in corneal thickness through 35 days of follow-up. Data is presented as mean ± SEM; comparison was made by multiple paired t-test at each time point (DSO vs. No DSO [Pre-injury]) N=6/group; *, P<0.05; **, P<0.01, ***, P<0.001. CCT, Central Corneal Thickness. (D) Staining of corneal endothelium with ZO-1 35 days (7wks) after DSO showed impaired corneal wound healing, evidenced by enlarged and dysmorphic corneal endothelial cells as compared to uninjured corneas.
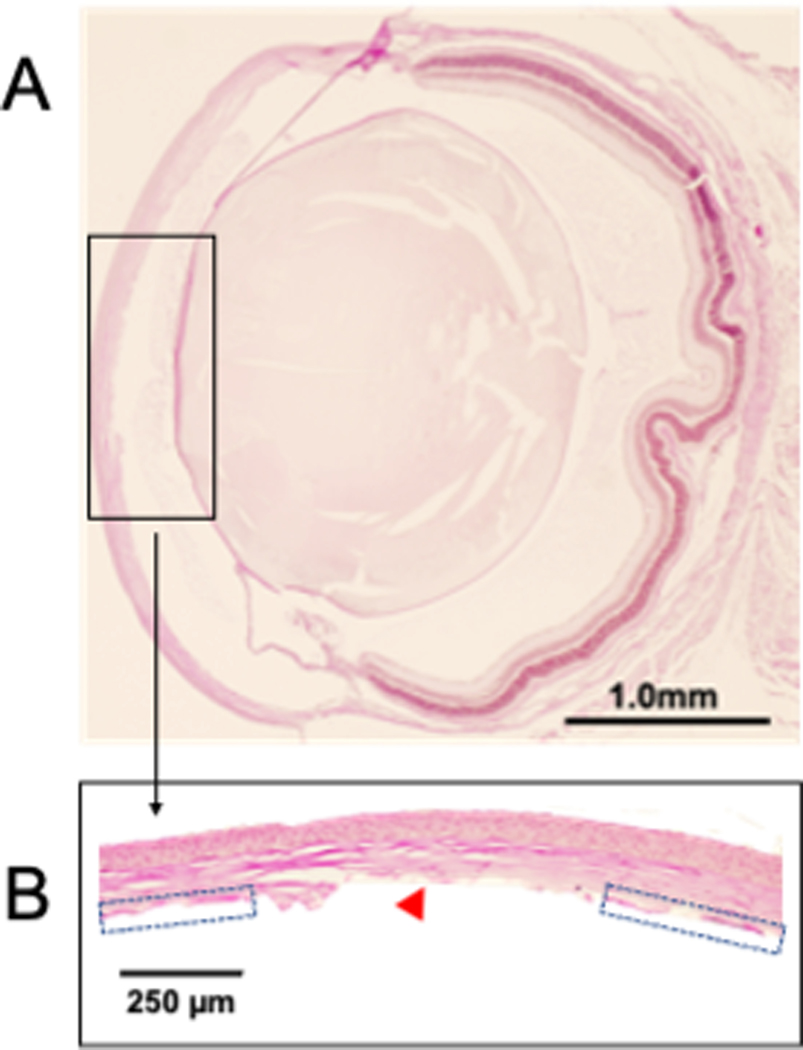
After finishing the procedure, corneas were collected and fixed in formalin (10%) and paraffin-embedded sections were stained with Periodic acid–Schiff (PAS) to visualize Descemet’s membrane. (A) Anterior chamber depth was preserved after the procedure and no synechiae were observed. (B) Removal of Descemet’s membrane is confirmed by the absence of membrane centrally (red arrowhead), which is in contrast to uninjured areas where Descemet’s membrane is visualized (blue arrow).
Of note, potential complications of the procedure include hyphema, which can result from trauma to the iris or conjunctival blood vessels and cataract. Cataract may result from contact with the lens capsule, and the chance of cataract formation can be minimized by using sufficient OVD in the anterior chamber, and potentially also by using older mice which have a relatively larger anterior chamber.11
DISCUSSION
DSO is an emerging surgical technique for the management of patients with diseases affecting the central corneal endothelium, including Fuchs’ Endothelial Dystrophy. To date, surgical interventions for endothelial disease consist of penetrating keratoplasty (PK) and endothelial keratoplasty (EK). The clinical importance of DSO lies in the fact that no donor tissue is needed, eliminating the possibility of graft rejection or failure. In addition, DSO is minimally invasive, induces little astigmatism and has fewer complications compared to PK and EK.12 Interestingly, one study has reported similar refractive results in DSO as compared to Descemet Stripping Automated Endothelial Keratoplasty (DSAEK).12 DSO may also be useful when surgery is performed in areas with limited access to donor tissue and in circumstances where patients decline donor tissue.13 In the present report, we describe a novel murine model of this procedure which may permit future basic science investigation into mechanisms of DSO success or failure, as well as corneal endothelial cell biology.
DSO has been shown to be an effective management strategy for patients affected by diseases affecting the central corneal endothelium. Corneal endothelial cells are responsible for maintaining appropriate corneal hydration and are thus critical to corneal clarity and vision. A prerequisite for DSO is the presence of healthy peripheral endothelial cells, as these cells are believed to migrate and cover the induced central defect in the weeks to months following surgery. When performing DSO, a “peeling” technique (i.e. removal of Descemet’s membrane in a smooth and continuous fashion) is preferred over a “scoring” technique so as to avoid stromal trauma and stromal tags which impede endothelial cell migration from the periphery towards the center.14 15 One main limitation of DSO is that it takes longer for corneal edema to clear after surgery as compared to endothelial keratoplasty;12 in humans, corneal clearance can take up to 1 to 3 months after the procedure. 12 14 Clinically, following DSO, the central cornea initially undergoes thickening before deturgescence. In this study, we used slit-lamp microscopy and OCT examination to assess corneal thickness through 8 weeks of follow-up and we observed a similar pattern in our murine model of DSO (Fig. 3C). It is important to note that CEC wound healing is more delayed with DM stripping as compared to simple CEC scraping; in this model we removed both CEC and DM together.
Compared to other DSO models, as the mouse cornea is smaller than that of other animals, we adjusted the size of the injury area to 1.5-mm diameter based on our previously established PK model in mice.9,16 Recovery after endothelial injury and DSO depends on the intrinsic proliferative capacity of CEnC, which seems to vary among species.7 Interestingly, in this model, we saw repopulation of the denuded area with CEnC, similar to DSO in humans, and CCT remained elevated compared to pre-injury through the follow up period. Although CCT has been reported to return to baseline levels in a feline and rat scraping model4,17, several other reports using scraping in cats18, as well as in rabbits19–21 and non-human primates6,19, showed persistent corneal edema. We believe the technique we describe in this report using mice is a feasible and reproducible model to study corneal endothelial regeneration while also reproducing clinical outcomes in humans.
An emerging area of research in endothelial cell biology is the role of Rho-associated kinase inhibitors in promoting the migration of endothelial cells. Treatment with Ripasudil, approved in Japan as an anti-glaucoma therapy, has been shown to decrease time to corneal clearance and increase final endothelial cell counts in DSO.22 A pilot study using Netarsudil has been shown to significantly reduce the time to corneal clearance after DSO,23 24 and an international, multi-center, placebo-controlled trial of Ripasudil after DSO is currently enrolling and should yield results this year. In the future, one potential role for the murine model of DSO described here could be to help investigate the effect of Rho-associated kinase inhibitors on endothelial cell migration and the underlying mechanisms.
In summary, in the present report we show that DSO is feasible and reproducible in a murine model, and that this model recapitulates the post-operative course seen clinically. This model may be a useful tool to aid the study of endothelial cell biology and to better understand the behavior of corneal endothelial cells after DSO in order to improve clinical outcomes in patients undergoing this procedure.
Supplementary Material
Supplemental Video File
Supplemental Video 1. Video of Descemet Stripping Only (DSO) Surgical Technique in Mice. Video depicting the step-by-step technique for this procedure and the appearance of the mouse cornea immediately following completion.
Supplemental Data File (.doc, .tif, pdf, etc.)
Supplemental Table 1. Equipment and Reagents for Descemet Stripping Only (DSO) Technique in Mice.
Cover Letter
ACKNOWLEDGEMENT
Funding:
This study was supported by the National Eye Institute/National Institutes of Health (R01 EY012963 to RD and K08 EY031759 to THD) and Department of Defense (W81XWH2110855 to RD)
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1097/ico.0000000000003223
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10117527
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/140597697
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Case Report: Role of Anterior Segment Optical Coherence Tomography for Managing Failed Endothelial Keratoplasty Graft.
Optom Vis Sci, 100(12):882-886, 25 Oct 2023
Cited by: 0 articles | PMID: 37890116
Where is endothelial keratoplasty going: from Descemet stripping (automated) endothelial keratoplasty to Descemet membrane endothelial keratoplasty to Descemet membrane endothelial transfer?
Can J Ophthalmol, 47(3):197-200, 01 Jun 2012
Cited by: 6 articles | PMID: 22687292
Long-term observation of graft thickness and shape in Descemet stripping and automated endothelial keratoplasty.
Eur J Ophthalmol, 30(1):155-161, 24 Jan 2019
Cited by: 0 articles | PMID: 30678489
Funding
Funders who supported this work.
NEI NIH HHS (3)
Grant ID: K08 EY031759
Grant ID: P30 EY003790
Grant ID: R01 EY012963



