Abstract
Study objectives
Obstructive sleep apnea (OSA) has been proposed as a risk factor for severe COVID-19. Confounding is an important consideration as OSA is associated with several known risk factors for severe COVID-19. Our aim was to assess the association of OSA with hospitalization due to COVID-19 using a population-based cohort with detailed information on OSA and comorbidities.Methods
Included were all community-dwelling Icelandic citizens 18 years of age and older diagnosed with SARS-CoV-2 infection in 2020. Data on demographics, comorbidities, and outcomes of COVID-19 was obtained from centralized national registries. Diagnosis of OSA was retrieved from the centralized Sleep Department Registry at Landspitali - The National University Hospital. Severe COVID-19 was defined as the composite outcome of hospitalization and death. The associations between OSA and the outcome were expressed as odds ratios (OR) with 95% confidence intervals (95% CI), calculated using logistic regression models and inverse probability weighting.Results
A total of 4,756 individuals diagnosed with SARS-CoV-2 infection in Iceland were included in the study (1.3% of the Icelandic population), of whom 185 had a diagnosis of OSA. In total, 238 were hospitalized or died, 38 of whom had OSA. Adjusted for age, sex, and BMI, OSA was associated with poor outcome (OR 2.2, 95% CI 1.4-3.5). This association was slightly attenuated (OR 2.0, 95% CI 2.0, 1.2-3.2) when adjusted for demographic characteristics and various comorbidities.Conclusions
OSA was associated with twofold increase in risk of severe COVID-19, and the association was not explained by obesity or other comorbidities.Free full text

Obstructive sleep apnea is an independent risk factor for severe COVID-19: a population-based study
Abstract
Study Objectives
Obstructive sleep apnea (OSA) has been proposed as a risk factor for severe COVID-19. Confounding is an important consideration as OSA is associated with several known risk factors for severe COVID-19. Our aim was to assess the association of OSA with hospitalization due to COVID-19 using a population-based cohort with detailed information on OSA and comorbidities.
Methods
Included were all community-dwelling Icelandic citizens 18 years of age and older diagnosed with SARS-CoV-2 infection in 2020. Data on demographics, comorbidities, and outcomes of COVID-19 was obtained from centralized national registries. Diagnosis of OSA was retrieved from the centralized Sleep Department Registry at Landspitali – The National University Hospital. Severe COVID-19 was defined as the composite outcome of hospitalization and death. The associations between OSA and the outcome were expressed as odds ratios (OR) with 95% confidence intervals (95% CI), calculated using logistic regression models and inverse probability weighting.
Results
A total of 4,756 individuals diagnosed with SARS-CoV-2 infection in Iceland were included in the study (1.3% of the Icelandic population), of whom 185 had a diagnosis of OSA. In total, 238 were hospitalized or died, 38 of whom had OSA. Adjusted for age, sex, and BMI, OSA was associated with poor outcome (OR 2.2, 95% CI 1.4–3.5). This association was slightly attenuated (OR 2.0, 95% CI 2.0, 1.2–3.2) when adjusted for demographic characteristics and various comorbidities.
Conclusions
OSA was associated with twofold increase in risk of severe COVID-19, and the association was not explained by obesity or other comorbidities.
Introduction
Most individuals with SARS-CoV-2 infection develop mild and self-limiting disease, but approximately one in every ten patients experiences severe symptoms [1]. Previous studies have identified several comorbid conditions that are associated with an increased risk of severe COVID-19 [2–4]. These include obesity, heart failure, chronic kidney disease (CKD), diabetes mellitus, chronic lung disease, chronic liver disease, and malignancies.
Obstructive sleep apnea (OSA) is among the most common chronic diseases in Western countries, although it is often underdiagnosed [5, 6]. OSA has been proposed as a risk factor for severe COVID-19, but clinical data reflecting the general population have been scarce [7]. Previous studies [8–17], have not sufficiently elucidated whether OSA is a true risk factor for severe COVID-19 (Table 1). Most studies [8–12] have suggested an association but have been limited by highly selective study populations and/or inability to adequately adjust for important confounding factors. Confounding is especially important as OSA is known to be strongly associated with male gender, obesity, diabetes mellitus, and heart failure [5, 18, 19]; all of which are well established risk factors for severe COVID-19 [2–4].
Table 1.
Previous studies on the association of obstructive sleep apnea and severe COVID-19
| Study | C-19 | OSA and C-19 | Findings* | Strengths | Limitations |
|---|---|---|---|---|---|
| Cade et al. 2020 [13] | 4,668 patients (PCR- confirmed) | 443 patients (diagnosis codes) | OR 1.2 (95% CI, 0.8–1.7) for death. OR 1.04 (0.8–1.3) for death, mechanical ventilation or ICU admission | Large cohort. PCR test required for inclusion. CPAP usage assessed | Not population-based. Strict inclusion criteria. Definitions of comorbidities were not detailed. No information on clinical sleep analysis |
| Cariou et al. 2020 [8] | 1,317 patients (some cases not PCR- confirmed) | 144 patients (“treated OSA”, medical records) | OR 1.2 (0.8–1.7) for tracheal intubation and or death. OR 2.7 (95% CI, 1.4–5.2) for death | One of the first studies | Cohort limited to patients with diabetes who were diagnosed with COVID-19. Not designed for assessing association with OSA specifically. Adjustment for confounding factors limited. No information on clinical sleep analysis |
| Chung et al. 2021 [14] | 622 patients (self-reported) | 40 patients (self-reported) | OR 2.1 (95% CI, 1.1–4.0) for hospitalization or ICU admission | Assessed the association of sleep-related symptoms with OSA | Not population-based. Low number of patients with OSA and COVID-19. Self-administered online survey. Diagnosis and prognosis limited by the self-reported nature of the data. OR only presented for patients at high risk of OSA (STOP criteria). No information on clinical sleep analysis |
| Goldstein et al. 2021 [10] | 572 patients (PCR- confirmed) | 113 patients (diagnosis codes) | OR 1.8 (95% CI, 0.9–3.8) for death. OR 1.5 (95% CI, 0.9–2.6) for mechanical ventilation | PCR test required for inclusion. Sleep study data included. Other sleep disorders simultaneously evaluated | Not population-based. Only adjusted for age, sex, BMI and race. Sleep study data was only available for 13% of patients |
| Gottlieb et al. 2020 [9] | 8,673 patients (PCR- confirmed) | 288 patients (from electronic health records) | OR 1.05 (95% CI, 0.8–1.5) for hospitalization and OR 1.6 (95% CI, 1.1–2.3) for ICU admission | PCR test required for inclusion | Not population-based. Not designed for assessing association with OSA specifically. OSA information only from diagnosis codes. No information on clinical sleep analysis |
| Ioannou et al. 2020 [15] | 10,131 patients (PCR- confirmed) | 2,720 patients (diagnosis codes) | OR 1.07 (95% CI, 0.99–1.2) for hospitalization and OR 1.11 (95% CI, 0.9–1.3) for mortality | PCR test required for inclusion Large study group | Not population-based. Not designed for assessing association with OSA specifically. Unusually high rate of OSA (26.8%). Only 9% of the sample was female. No information on clinical sleep analysis |
| Izquierdo et al. 2020 [16, 17] | 10,504 patients (some cases not PCR-confirmed) | 212 patients (machine learning and natural language processing from medical records) | No OR reported for OSA, but a meta-analysis[17] reported OR of 0.59 (95% CI, 0.08–4.26) for hospitalization based on the data | Data from a large portion of a single region in Spain | Not designed for assessing association with OSA specifically. Large portion of information gathered using machine learning. No information on clinical sleep analysis |
| Maas et al. 2020 [11] | 9,405 patients (some cases not PCR- confirmed) | Not provided in the report (approx. 699 patients according to percentages, used diagnosis codes for defining OSA) | OR 1.7 (95% CI, 1.4–2.0) for hospitalization | Large group of patients with OSA and COVID-19 | Only adjusted for diabetes, hypertension and BMI. U07 diagnosis code for COVID-19 includes all subcategories (U07.2 is “COVID-19, virus not identified”). No information on clinical sleep analysis. The report lacked detailed information on methodology and crude numbers. |
| Strausz et al. 2021 [12] | 445 patients (PCR- confirmed) | 38 patients (diagnosis codes) | OR 2.9 (95% CI, 1.0-8.4) for hospitalization | PCR test required for inclusion. Information on OSA treatment for 11/38 OSA patients with COVID-19 | Observational study using participants in a genetic study. Small sample of OSA patients with COVID-19 |
*From adjusted models.
Using data from centralized registries in Iceland, the opportunity to investigate the association of OSA with severe COVID-19 is unique. Early in the pandemic, Icelandic health authorities adopted a strategy of widespread testing of symptomatic individuals for SARS-CoV-2 and tracing contacts of confirmed infections and placing them in quarantine [1, 20]. Furthermore, all SARS-CoV-2-positive individuals in Iceland were enrolled in a telehealth monitoring service at the COVID-19 Outpatient Clinic of Landspitali – The National University Hospital of Iceland (LUH) at the time of diagnosis [20]. The Division of Respiratory Medicine and Sleep at LUH is the only referral center for OSA diagnosis in Iceland and the sole provider of positive airway pressure (PAP) treatment.
The aim of the study was to evaluate whether OSA is an independent risk factor for severe COVID-19 in a nationwide cohort. A secondary aim was to explore the risk of severe COVID-19 in the subgroup of OSA patients treated with a PAP device and if there are any indications that PAP treatment mitigates the risk.
Methods
Ethical approval
The study was approved by the National Bioethics Committee (VSN20-078) and the research adhered to the Declaration of Helsinki. No informed consent was obtained as this study only utilized observational data that was converted to a non-identifiable form.
Study population
Included were all persons 18 years of age and older in Iceland who were SARS-CoV-2-positive by a PCR test and were considered to have an active infection between February 28 and December 31, 2020 [1]. All SARS-CoV-2-positive persons were enrolled into a telehealth monitoring service provided by the COVID-19 Outpatient Clinic at LUH. During the telehealth enrollment interview, those individuals who were asymptomatic and had tested positive on a naso- and oropharyngeal sample obtained as part of entry screening at the border or random population screening were evaluated for infection status. In these cases, blood samples for SARS-CoV-2-antibodies and repeat upper respiratory samples for PCR testing were collected at the COVID-19 Outpatient Clinic within 24 h. Based on these data, determination of an active infection was made by a physician. Patients were followed from diagnosis until at least 14 days had passed from the initial positive PCR test and they had been free of symptoms for at least seven days. During monitoring, patients with concerning symptoms were evaluated in person at the COVID Outpatient Clinic. Those who required hospitalization were subsequently admitted to either LUH or Akureyri Hospital. The organization of the telehealth monitoring service has previously been described in detail [20]. Nursing home residents and individuals who were hospitalized or undergoing treatment in inpatient rehabilitation facilities at the time of SARS-CoV-2 infection were excluded from the analysis.
Data collection and definitions
Data on age, sex, body mass index (BMI), current smoking, and hospital admission were prospectively collected during telehealth monitoring [20]. Information on OSA diagnosis, PAP treatment, time on PAP, apnea–hypopnea index (AHI), time since AHI measurement, average nighttime saturation and time below 90% saturation was obtained from the centralized database at LUH Division of Respiratory Medicine and Sleep. Furthermore, BMI data that were missing in the records of the COVID-19 Outpatient Clinic were obtained from the Sleep database. OSA diagnosis was double-checked by assessing all prior clinical sleep studies performed at LUH in SARS-CoV-2-positive patients and OSA was defined as an AHI greater than or equal to five [21].
Severe COVID-19 was defined as hospitalization and/or death while receiving care at the COVID-19 Outpatient Clinic. Description of the definitions of covariates is provided in Supplementary Text and Supplementary Table S1.
Statistical analysis
Missing data were imputed using multiple imputation by chained equations (MICE); 100 imputations of the dataset were performed. Associations were estimated with logistic regression models and the results reported as odds ratios (OR) with 95% confidence intervals (95% CI). Age was modeled with a four-knot restricted cubic spline. The outcome was defined as a composite of hospital admission and death. Multiple imputation and regression modeling was carried out using the rms package in R [22].
Inverse probability weighting was performed using the MatchThem package in R for imputed data [23], and combined employing the “within” approach [24]. The propensity score was calculated using a priori defined variables: age, sex, BMI, hypertension, diabetes, heart failure, CKD, COPD, and current smoking status. Associations were estimated with logistic regression after pooling of the results using Rubin’s rules [24]. Average treatment effect was estimated using propensity score weighting.
A sensitivity analysis was performed and included only individuals with complete information, that is, without imputation.
Results
Baseline characteristics
A total of 6,126 individuals were SARS-CoV-2-positive by PCR testing during the study period, of whom 4,756 adult persons were included (Figure 1). In total, 185 (3.9%) had SARS-CoV-2 and OSA (Table 2). Compared to patients without OSA, those with OSA were older (median age 59 vs. 39 years, p < 0.001), had higher BMI (median of 32 vs. 26, p < 0.001), more likely to be male (72% vs. 51%, p < 0.001), and more likely to have hypertension (44% vs. 11%, p < 0.001), diabetes mellitus (18% vs. 3%, p < 0.001), CKD (9% vs. 2%, p < 0.001), COPD (6% vs. 1%, p < 0.001), and heart failure (5% vs. 1%, p < 0.001) (Table 2). No significant difference was observed between patients with and without OSA with respect to current smoking (7% vs. 9%, p = 0.36). In total, 38 (21%) patients with OSA experienced the composite outcome of hospital admission or death, compared with 200 (4%) of those without a diagnosis of OSA.
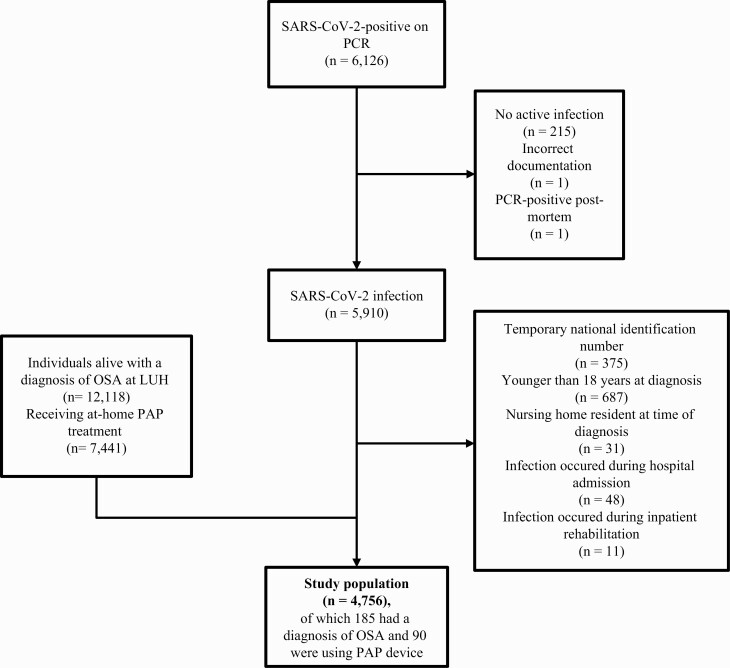
Flowchart illustrating the study sample. The total population of Iceland on January 1, 2021 was 368,792. The final study population comprised 4,756 persons (1.3% of the total Icelandic population). At the time of the study, 12,118 persons (3.3% of the Icelandic population) carried a diagnosis of OSA.
Table 2.
Baseline characteristics of persons with SARS-CoV-2 infection, stratified by presence of obstructive sleep apnea (OSA)
| Patients with OSA | Patients without OSA | |
|---|---|---|
| Number of patients | 185 (3.9%) | 4,571 (96.1%) |
| Median age* (IQR) | 59 (50-67) | 39 (28-53) |
| Female sex* | 52 (28%) | 2,249 (49%) |
| Median BMI* (IQR) [missing values] | 32 (29–36) [4, 2%] | 26 (23–29) [1376, 30%] |
| Median AHI (IQR) [missing values] | 20 (11–33) [9, 5%] | – |
| Hypertension* | 82 (44%) | 522 (11%) |
| Diabetes mellitus* | 34 (18%) | 146 (3%) |
| Heart failure* | 10 (5%) | 38 (1%) |
| Chronic kidney disease* | 17 (9%) | 98 (2%) |
| Chronic obstructive lung disease* | 11 (6%) | 56 (1%) |
| Current smoking [missing values] | 11 (7%) [22, 12%] | 384 (9%) [382, 8%] |
| PAP use: | ||
| Application for PAP device never submitted | 55 (30%) | – |
| On waiting list for PAP device | 15 (8%) | – |
| Currently using PAP | 90 (49%) | – |
| PAP device returned | 24 (13%) | – |
| Hospital admission or death* | 38 (21%) | 200 (4%) |
*p < 0.001.
AHI: apnea–hypopnea index; BMI: body mass index; IQR: interquartile range.
Of the 185 patients with OSA, 90 (49%) had been prescribed and were currently using PAP treatment, 24 (13%) had returned their PAP device and 15 (8%) were on a waiting list for a PAP device. Among the remaining 55 (30%) patients with OSA, PAP treatment had not been prescribed.
Obstructive sleep apnea and severity of COVID-19
Univariate analysis revealed that OSA was associated with an increased risk of severe COVID-19 (OR 5.6, 95% CI 3.8–8.3; Table 3). This association was attenuated following adjustment for age, sex, and BMI (OR 2.2, 95% CI 1.4–3.5). In a logistic regression model adjusting for demographic factors and comorbidities the OR was 2.0 (95% CI 1.2–3.2) and was 2.0 (95% CI 1.1–3.6) when assessed by the inverse probability weighted model.
Table 3.
The odds of severe COVID-19 in patients with obstructive sleep apnea
| Adjusted for | Odds ratio (95% CI) |
|---|---|
| No adjustment | 5.6 (3.8–8.3) |
| Age and sex | 2.9 (1.9–4.4) |
| Age, sex, and BMI | 2.2 (1.4–3.5) |
| Age, sex, BMI, and comorbidities* | 2.0 (1.2–3.2) |
| Inverse probability weighting** | 2.0 (1.1–3.6) |
BMI: body mass index.
* Hypertension, diabetes mellitus, heart failure, chronic kidney disease, chronic obstructive lung disease (COPD), and smoking.
**Weights calculated using age, sex, BMI, hypertension, diabetes mellitus, heart failure, chronic kidney disease, COPD, smoking status, and BMI.
Positive airway pressure treatment and outcomes of patients with SARS-CoV-2 infection
In an analysis to explore the association between PAP treatment and severe COVID-19 among OSA patients, the risk was significantly increased in a model adjusting for age, sex, and AHI (2.5, 95% CI 1.0–6.3), while the association was no longer present (OR 1.9. 95% CI 0.6–6.0) when comorbidities had been added to the model (Table 4). In another analysis of the whole cohort (thus without adjustment for AHI), OSA patients who were treated with PAP, had a significantly increased risk of severe COVID-19 (OR 2.4, 95% CI 1.3–4.5; Supplementary Table S3) compared with those without OSA, adjusted for baseline characteristics and comorbidities. Patients who did not have an indication for PAP treatment had a trend towards a higher risk (OR 2.0, 95% CI 0.9–4.7; Supplementary Table S3) compared with those who were on the waiting list for PAP treatment or had returned the PAP device (OR 1.3, 95% CI 0.5–3.5).
Table 4.
The risk of severe COVID-19 among patients with obstructive sleep apnea treated with a positive airway pressure (PAP) device compared with those who were not
| Adjusted for | Odds ratio (95% CI) |
|---|---|
| No adjustment | 2.1 (1.0–4.4) |
| Age, sex, and AHI | 2.5 (1.0–6.3) |
| Age, sex, AHI, and BMI | 2.3 (0.9–5.8) |
| Age, sex, AHI, BMI, and comorbidities* | 1.9 (0.6–6.0) |
BMI: body mass index.
*Hypertension, diabetes mellitus, heart failure, chronic kidney disease, chronic obstructive lung disease (COPD), and smoking.
In a fully adjusted model, patients treated with PAP for more than 4 years had an OR of 1.4 (95% CI 0.4–5.5) and patients treated with PAP for less than 4 years had an OR of 4.1 (0.9–18.6) for severe COVID-19, when compared with OSA patients without PAP treatment (Supplementary Table S4).
Sensitivity analysis
The effect sizes obtained from complete case analyses were comparable to the results of the main analysis (Supplementary Table S5).
Discussion
In this population-based observational study, OSA was independently associated with an increased risk of developing severe COVID-19. This association persisted after adjustment for several known confounding factors and was not modified by PAP treatment.
Obstructive sleep apnea as a risk factor for severe COVID-19
We observed a twofold increase in the risk of severe COVID-19 among OSA patients in variously adjusted models for important confounders, including a propensity score weighting. Most of the previous studies outlined in Table 1, have suggested that OSA may be a risk factor for severe COVID-19, although prior studies have suffered from methodologic limitations inherent in their design and available data. Nevertheless, these studies together with the results of the current population-based study strongly suggest that OSA is an independent contributor to the risk of more severe COVID-19 disease [8–11, 13].
The link between OSA and COVID-19 is biologically plausible. First, the chronic low-grade inflammation associated with OSA [25, 26] may hypothetically predispose to a more severe immune response to COVID-19. Also, the hypoxemia observed in COVID-19 may possibly be aggravated further by the intermittent nocturnal desaturation associated with OSA [25, 26], leading to more pronounced hypoxemia, oxidative stress and hypoxia-related manifestations. This notion is strengthened by a dose-response trend when OSA severity was defined by nighttime oxygen saturation levels (Supplementary Tables S7 and S8).
Taken together, our results, in addition to previous studies, indicate that OSA may be a risk factor for severe COVID-19. The results of our nationwide study on an unselected cohort of persons SARS-CoV-2 infected with detailed information on OSA and comorbid conditions show that OSA is likely to be an independent risk factor for severe COVID-19 in the general population. This finding should be taken into account during clinical evaluation of risk profiles for COVID-19.
PAP treatment and severe COVID-19
Several OSA-associated pathophysiological alterations could explain the increased risk of severe COVID-19, such as cardiovascular disorders and systemic inflammation and oxidative stress due to intermittent hypoxia [26–30]. Some of these pathophysiological changes are attenuated by PAP therapy, which has been proposed to mitigate the risk for severe COVID-19 among OSA patients [31]. On the other hand, PAP treatment could theoretically propel viral particles located in the upper respiratory tract further down the respiratory tract and thus increase the risk of viral pneumonia.
Two previous studies have observed a trend towards increased risk of pneumonia in patients on PAP treatment, compared with other OSA patients [32, 33]. In the current study, there was a trend towards increased risk for severe COVID-19 among OSA patients treated with PAP, to greater extent than in OSA patients who had returned the PAP device or had been prescribed but not yet received PAP treatment. These findings are difficult to interpret, particularly as the OSA groups that did not receive PAP treatment were relatively small and thereby limit the strength of this analysis. Furthermore, the results of our study provide some indications that long-term treatment with PAP alleviates the high risk of severe COVID-19. Therefore, our data are insufficient for providing specific clinical recommendations on the use of PAP treatment in COVID-19 patients. Further studies are needed to explore this association.
Strengths and weaknesses
The main strength of the current study is the nationwide design and a cohort of unselected patients with SARS-CoV-2 infection and complete information on PAP treatment. A comprehensive SARS-CoV-2 screening and contact tracing in Iceland [1, 20] further enhance the generalizability of the data. Finally, extensive information from medical records and structured clinical evaluation of all patients with SARS-CoV-2 infection allowed for meticulous adjustments for confounders. Although BMI values were missing for 30% of the control group, this is quite low considering a population-based cohort of persons with SARS-CoV-2 infection consisting largely of healthy individuals with few prior hospital visits. Notably, a BMI value was only missing for 2% of the OSA cohort as it is routinely obtained during OSA screening. Importantly, the results from sensitivity analysis and propensity score weighting indicate that the missing values are unlikely to have had a significant systematic effect on the results. An important limitation to our study is the lack of sleep studies in the control group, which is not surprising as OSA is known to be underdiagnosed [34]. Indeed, a study on 415 Icelanders, aged 40–65 years, found the prevalence of OSA, defined as AHI ≥15, was 15.4%, even though many of the participants had minimal symptoms [34]. Moreover, undiagnosed OSA in the control group would be expected to lead to underestimation of the risk of severe COVID-19, rather than overestimation. Another notable limitation is the low statistical power of the secondary analysis of the modifying effect of PAP treatment due to the small sample size, thus limiting the conclusions that can be drawn from this analysis. Furthermore, we did not have detailed information on PAP compliance which would have added depth to the PAP treatment analysis. Finally, infections caused by the SARS-CoV-2 variants of recent concern (Alpha, Beta and Delta variants) were not prevalent in Iceland during the study period, and the vaccination program had not been initiated [35]. Thus, future studies will have to establish whether the results are generalizable to other SARS-CoV-2 variants (including the Delta variant) and vaccinated populations.
Conclusion
The results from this nationwide study suggest that OSA is an independent risk factor for severe COVID-19, defined as hospitalization or death. These results are likely to be representative of the general population due to the nationwide study design and the unselected cohort of persons with SARS-CoV-2 infection. Larger studies are needed to further evaluate different phenotypes of OSA, examine possible pathophysiological mechanisms, and investigate the effect of PAP treatment on outcomes of COVID-19.
Supplementary Material
zsab272_suppl_Supplementary_Material
Acknowledgments
We would like to thank Ingibjörg Richter for her help with retrieving data from the Landspitali – The National University Hospital electronic medical record system.
Funding
This work was supported University of Iceland Research Fund and Landspitali University Hospital Scientific Fund (A-2021-018, A-2020-017).
Data Availability
The data used for the analyses presented in this article cannot be shared publicly according to the ethical approval.
References
Full text links
Read article at publisher's site: https://doi.org/10.1093/sleep/zsab272
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/sleep/article-pdf/45/3/zsab272/45287079/zsab272.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/117471359
Article citations
Superspreading of SARS-CoV-2 at a choir rehearsal in Finland-A computational fluid dynamics view on aerosol transmission and patient interviews.
PLoS One, 19(9):e0302250, 12 Sep 2024
Cited by: 0 articles | PMID: 39264883 | PMCID: PMC11392323
Exploring the relationship between HCMV serostatus and outcomes in COVID-19 sepsis.
Front Immunol, 15:1386586, 08 May 2024
Cited by: 1 article | PMID: 38779663 | PMCID: PMC11109369
Obstructive Sleep Apnea and Acute Lower Respiratory Tract Infections: A Narrative Literature Review.
Antibiotics (Basel), 13(6):532, 06 Jun 2024
Cited by: 0 articles | PMID: 38927198
Review
Identification of biomarkers and pathways for the SARS-CoV-2 infections in obstructive sleep apnea patients based on machine learning and proteomic analysis.
BMC Pulm Med, 24(1):112, 05 Mar 2024
Cited by: 2 articles | PMID: 38443855
Sleep and critical illness: a review.
Front Med (Lausanne), 10:1199685, 19 Sep 2023
Cited by: 1 article | PMID: 37828946 | PMCID: PMC10566646
Review Free full text in Europe PMC
Go to all (18) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Risk of post-acute sequelae of SARS-CoV-2 infection associated with pre-coronavirus disease obstructive sleep apnea diagnoses: an electronic health record-based analysis from the RECOVER initiative.
Sleep, 46(9):zsad126, 01 Sep 2023
Cited by: 10 articles | PMID: 37166330 | PMCID: PMC10485569
Sleep apnoea is a risk factor for severe COVID-19.
BMJ Open Respir Res, 8(1):e000845, 01 Jan 2021
Cited by: 68 articles | PMID: 33436406 | PMCID: PMC7804843
The association between high risk of sleep apnea, comorbidities, and risk of COVID-19: a population-based international harmonized study.
Sleep Breath, 25(2):849-860, 28 Apr 2021
Cited by: 24 articles | PMID: 33907966 | PMCID: PMC8079162
[Novel coronavirus disease (COVID-19) and obstructive sleep apnea: age aspects of comorbidity].
Zh Nevrol Psikhiatr Im S S Korsakova, 121(4. vyp. 2):110-115, 01 Jan 2021
Cited by: 0 articles | PMID: 34078869
Review
Funding
Funders who supported this work.
Landspitali University Hospital Scientific Fund (2)
Grant ID: A-2020-017
Grant ID: A-2021-018




