Abstract
Importance
Plasma phosphorylated tau (p-tau) has proven to be an accurate biomarker for Alzheimer disease (AD) pathologic characteristics, offering a less expensive and less invasive alternative to cerebrospinal fluid (CSF) and positron emission tomography biomarkers for amyloid-β and tau. Alzheimer disease comorbid pathologic characteristics are common and are associated with more rapid cognitive decline in patients with dementia with Lewy bodies (DLB); therefore, it is anticipated that plasma p-tau concentrations may have utility in assessing cognitive impairment in individuals with this disorder.Objective
To measure the concentrations of plasma p-tau (p-tau181 and p-tau231) and evaluate their associations with cognitive decline in individuals with probable DLB.Design, setting, and participants
This multicenter longitudinal cohort study included participants from the European-DLB (E-DLB) Consortium cohort enrolled at 10 centers with harmonized diagnostic procedures from January 1, 2002, to December 31, 2020, with up to 5 years of follow-up. A total of 1122 participants with plasma samples were available. Participants with acute delirium or terminal illness and patients with other previous major psychiatric or neurologic disorders were excluded, leaving a cohort of 987 clinically diagnosed participants with probable DLB (n = 371), Parkinson disease (n = 204), AD (n = 207), as well as healthy controls (HCs) (n = 205).Main outcomes and measures
The main outcome was plasma p-tau181 and p-tau231 levels measured with in-house single molecule array assays. The Mini-Mental State Examination (MMSE) was used to measure cognition.Results
Among this cohort of 987 patients (512 men [51.9%]; mean [SD] age, 70.0 [8.8] years), patients with DLB did not differ significantly regarding age, sex, or years of education from those in the AD group, but the DLB group was older than the HC group and included more men than the AD and HC groups. Baseline concentrations of plasma p-tau181 and p-tau231 in patients with DLB were significantly higher than those in the HC group but lower than in the AD group and similar to the Parkinson disease group. Higher plasma concentrations of both p-tau markers were found in a subgroup of patients with DLB with abnormal CSF amyloid-β42 levels compared with those with normal levels (difference in the groups in p-tau181, -3.61 pg/mL; 95% CI, -5.43 to -1.79 pg/mL; P = .049; difference in the groups in p-tau231, -2.51 pg/mL; 95% CI, -3.63 to -1.39 pg/mL; P = .02). There was no difference between p-tau181 level and p-tau231 level across confirmed AD pathologic characteristcs based on reduced Aβ42 level in CSF in individuals with DLB. In DLB, a significant association was found between higher plasma p-tau181 and p-tau231 levels and lower MMSE scores at baseline (for p-tau181, -0.092 MMSE points; 95% CI, -0.12 to -0.06 MMSE points; P = .001; for p-tau231, -0.16 MMSE points; 95% CI, -0.21 to -0.12 MMSE points; P < .001), as well as more rapid MMSE decline over time. Plasma p-tau181 level was associated with a decrease of -0.094 MMSE points per year (95% CI, -0.144 to -0.052 MMSE points; P = .02), whereas plasma p-tau231 level was associated with an annual decrease of -0.130 MMSE points (95% CI, -0.201 to -0.071 MMSE points; P = .02), after adjusting for sex and age.Conclusions and relevance
This study suggests that plasma p-tau181 and p-tau231 levels may be used as cost-effective and accessible biomarkers to assess cognitive decline in individuals with DLB.Free full text

Association of Plasma p-tau181 and p-tau231 Concentrations With Cognitive Decline in Patients With Probable Dementia With Lewy Bodies
Key Points
Question
Are plasma p-tau concentrations associated with cognitive decline and Alzheimer disease (AD) pathologic characteristics in patients with probable dementia with Lewy bodies (DLB)?
Findings
In this cohort study of 987 participants from the European-DLB Consortium, concentrations of plasma p-tau181 and p-tau231 were significantly higher in individuals with DLB than in healthy controls and lower than in patients with AD. Plasma p-tau181 and p-tau231 levels were associated with cognitive impairment at baseline and more rapid cognitive decline during follow-up after adjusting for sex and age.
Meaning
Plasma p-tau concentrations have the potential to act as a cost-effective and accessible biomarker of AD pathologic characteristics and a prognostic marker of DLB.
Abstract
Importance
Plasma phosphorylated tau (p-tau) has proven to be an accurate biomarker for Alzheimer disease (AD) pathologic characteristics, offering a less expensive and less invasive alternative to cerebrospinal fluid (CSF) and positron emission tomography biomarkers for amyloid-β and tau. Alzheimer disease comorbid pathologic characteristics are common and are associated with more rapid cognitive decline in patients with dementia with Lewy bodies (DLB); therefore, it is anticipated that plasma p-tau concentrations may have utility in assessing cognitive impairment in individuals with this disorder.
Objective
To measure the concentrations of plasma p-tau (p-tau181 and p-tau231) and evaluate their associations with cognitive decline in individuals with probable DLB.
Design, Setting, and Participants
This multicenter longitudinal cohort study included participants from the European-DLB (E-DLB) Consortium cohort enrolled at 10 centers with harmonized diagnostic procedures from January 1, 2002, to December 31, 2020, with up to 5 years of follow-up. A total of 1122 participants with plasma samples were available. Participants with acute delirium or terminal illness and patients with other previous major psychiatric or neurologic disorders were excluded, leaving a cohort of 987 clinically diagnosed participants with probable DLB (n =
= 371), Parkinson disease (n
371), Parkinson disease (n =
= 204), AD (n
204), AD (n =
= 207), as well as healthy controls (HCs) (n
207), as well as healthy controls (HCs) (n =
= 205).
205).
Main Outcomes and Measures
The main outcome was plasma p-tau181 and p-tau231 levels measured with in-house single molecule array assays. The Mini-Mental State Examination (MMSE) was used to measure cognition.
Results
Among this cohort of 987 patients (512 men [51.9%]; mean [SD] age, 70.0 [8.8] years), patients with DLB did not differ significantly regarding age, sex, or years of education from those in the AD group, but the DLB group was older than the HC group and included more men than the AD and HC groups. Baseline concentrations of plasma p-tau181 and p-tau231 in patients with DLB were significantly higher than those in the HC group but lower than in the AD group and similar to the Parkinson disease group. Higher plasma concentrations of both p-tau markers were found in a subgroup of patients with DLB with abnormal CSF amyloid-β42 levels compared with those with normal levels (difference in the groups in p-tau181, −3.61 pg/mL; 95% CI, −5.43 to −1.79 pg/mL; P =
= .049; difference in the groups in p-tau231, −2.51 pg/mL; 95% CI, −3.63 to −1.39 pg/mL; P
.049; difference in the groups in p-tau231, −2.51 pg/mL; 95% CI, −3.63 to −1.39 pg/mL; P =
= .02). There was no difference between p-tau181 level and p-tau231 level across confirmed AD pathologic characteristcs based on reduced Aβ42 level in CSF in individuals with DLB. In DLB, a significant association was found between higher plasma p-tau181 and p-tau231 levels and lower MMSE scores at baseline (for p-tau181, −0.092 MMSE points; 95% CI, −0.12 to −0.06 MMSE points; P
.02). There was no difference between p-tau181 level and p-tau231 level across confirmed AD pathologic characteristcs based on reduced Aβ42 level in CSF in individuals with DLB. In DLB, a significant association was found between higher plasma p-tau181 and p-tau231 levels and lower MMSE scores at baseline (for p-tau181, −0.092 MMSE points; 95% CI, −0.12 to −0.06 MMSE points; P =
= .001; for p-tau231, −0.16 MMSE points; 95% CI, −0.21 to −0.12 MMSE points; P
.001; for p-tau231, −0.16 MMSE points; 95% CI, −0.21 to −0.12 MMSE points; P <
< .001), as well as more rapid MMSE decline over time. Plasma p-tau181 level was associated with a decrease of −0.094 MMSE points per year (95% CI, −0.144 to −0.052 MMSE points; P
.001), as well as more rapid MMSE decline over time. Plasma p-tau181 level was associated with a decrease of −0.094 MMSE points per year (95% CI, −0.144 to −0.052 MMSE points; P =
= .02), whereas plasma p-tau231 level was associated with an annual decrease of −0.130 MMSE points (95% CI, −0.201 to −0.071 MMSE points; P
.02), whereas plasma p-tau231 level was associated with an annual decrease of −0.130 MMSE points (95% CI, −0.201 to −0.071 MMSE points; P =
= .02), after adjusting for sex and age.
.02), after adjusting for sex and age.
Conclusions and Relevance
This study suggests that plasma p-tau181 and p-tau231 levels may be used as cost-effective and accessible biomarkers to assess cognitive decline in individuals with DLB.
Introduction
Dementia with Lewy bodies (DLB) is the second most frequent neurodegenerative dementia after Alzheimer disease (AD).1 The prognosis of DLB is usually poor,2 even compared with AD; however, a large variation in the rate of decline is observed.3
The neuropathologic characteristics of DLB are heterogeneous. In addition to the defining α-synucleinopathy in the brainstem, limbic system, and cortical areas, there is often coexisting AD neuropathologic characteristics.4 Cerebrospinal fluid (CSF) concentrations of the core AD biomarkers (amyloid-β42/40 [Aβ42/40], total tau, and phosphorylated tau [p-tau]) are associated with molecular imaging of Aβ plaques and tau tangles and are usually within normal ranges in individuals with non-AD dementia.5 In DLB, however, about 50% of individuals have low CSF Aβ42 concentrations, which is indicative of amyloid plaque pathologic characteristics (ie, the key feature of AD neurodegeneration).5 The degree, type, location, and spread of neurodegenerative changes in individuals with DLB are highly variable, which may explain the heterogeneous clinical presentation and rate of cognitive decline observed in clinical studies.6 Imaging and CSF biomarker studies support the concept that there is an association between the presence of AD pathologic characteristics and the rate of cognitive decline in DLB.7,8
There is now substantial evidence that blood biomarkers may be used to assess AD neuropathologic characteristics with high accuracy.9,10 In this regard, p-tau species, including p-tau181 and p-tau231, have emerged as leading candidates, and the diagnostic and prognostic markers first shown in CSF samples have been widely replicated in blood samples.11 Limited comparison data have suggested either a subtle difference or no difference between p-tau181 and p-tau231 levels in individuals with AD; however, these biomarkers have never been compared in individuals with DLB, to our knowledge.12 In this study, we sought to assess plasma p-tau181 and p-tau231 concentrations and their association with cognition in a large international, multicenter DLB cohort from the European-DLB (E-DLB) Consortium.
Methods
A total of 1122 participants with plasma samples (with 371 participants with probable DLB overall) were available for this study were enrolled at 10 participating E-DLB centers with harmonized diagnostic procedures from January 1, 2002, to December 31, 2020.13 Participants were referred from outpatient clinics, including memory, movement disorders, geriatric medicine, psychiatric, and neurology clinics. Participants with acute delirium or terminal illness and patients with other previous major psychiatric or neurologic disorders were excluded, leaving a cohort of 987 clinically diagnosed participants with probable DLB (n =
= 371), Parkinson disease (n
371), Parkinson disease (n =
= 204), or AD (n
204), or AD (n =
= 207) as well as healthy controls (HCs) (n
207) as well as healthy controls (HCs) (n =
= 205). Additional information on the diagnostic examination program, inclusion and exclusion criteria, p-tau blood measurements, and statistical procedures for this study can be found in the eMethods in Supplement 1. Patients with AD and Parkinson disease, as well as HCs, were included for comparison (eTable 1 in Supplement 1). A subgroup of patients with AD (113 of 207 [54.6%]) had CSF confirmation based on reduced Aβ42 concentrations. Likewise, 34.0% of the DLB group (126 of 371) had Aβ42 concentrations obtained from CSF samples according to local reference values (eTable 2 in Supplement 1). Global cognitive function was assessed using the Mini-Mental State Examination (MMSE). A subgroup of 182 participants with probable DLB (eFigure 1 in Supplement 1) had annual longitudinal cognitive assessments for up to 5 years. Details of the sample collection procedures in each center are available in eTable 2 in Supplement 1. The local ethics committee at each center (eTable 1 in Supplement 1) approved the incorporation of data in this study and the analysis of samples abroad. The participants gave their written consent to use the deidentified results of their clinical, instrumental, and laboratory data for research purposes.
205). Additional information on the diagnostic examination program, inclusion and exclusion criteria, p-tau blood measurements, and statistical procedures for this study can be found in the eMethods in Supplement 1. Patients with AD and Parkinson disease, as well as HCs, were included for comparison (eTable 1 in Supplement 1). A subgroup of patients with AD (113 of 207 [54.6%]) had CSF confirmation based on reduced Aβ42 concentrations. Likewise, 34.0% of the DLB group (126 of 371) had Aβ42 concentrations obtained from CSF samples according to local reference values (eTable 2 in Supplement 1). Global cognitive function was assessed using the Mini-Mental State Examination (MMSE). A subgroup of 182 participants with probable DLB (eFigure 1 in Supplement 1) had annual longitudinal cognitive assessments for up to 5 years. Details of the sample collection procedures in each center are available in eTable 2 in Supplement 1. The local ethics committee at each center (eTable 1 in Supplement 1) approved the incorporation of data in this study and the analysis of samples abroad. The participants gave their written consent to use the deidentified results of their clinical, instrumental, and laboratory data for research purposes.
Plasma p-tau181 and p-tau231 concentrations were measured using a clinically validated, in-house single molecule array (Simoa) at the Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden (eTable 3 in Supplement 1).
Statistical Analysis
We reported the baseline characteristics of the cohort as appropriate. After a significant 1-way analysis of variance, differences between groups at baseline were evaluated using a Bonferroni post hoc test. The receiver operating characteristic (ROC) curve explored the overall biomarker performance across confirmed AD pathologic characteristics based on reduced Aβ42 levels in CSF samples obtained from individuals with DLB, providing the area under the curve (AUC). We used the DeLong test14 to contrast the change in the AUC of both biomarkers. The MMSE score was transformed using the square root of 30 minus the MMSE score to achieve normal distribution. We fitted a linear regression with the MMSE score at baseline to evaluate the association of plasma p-tau181 and p-tau231 levels with cognition. We analyzed the longitudinal decrease in MMSE score by fitting a linear mixed-effect model with random intercept and slope for each participant, adjusted for potential confounders, such as age, sex, and years of education. All P values were from 1-sided tests and results were deemed statistically significant at P <
< .05.
.05.
Results
The baseline characteristics of the 987 participants (512 men [51.9%]; mean [SD] age, 70.0 [8.8] years) can be found in the Table. The DLB and AD groups did not differ significantly regarding age, sex, years of education, duration of symptoms, or MMSE baseline scores, but the DLB group was older than the HC group and included more men than did the AD and HC groups. In the DLB group with CSF analyses available, 63 of 126 patients (50.0%) had Aβ42 levels below the threshold.
Table.
| Characteristic | Mean (SD) value | P value | ||||
|---|---|---|---|---|---|---|
DLB (n = = 371 [37.6%]) 371 [37.6%]) | AD (n = = 207 [20.9%]) 207 [20.9%]) | PD (n = = 204 [20.7%]) 204 [20.7%]) | HC (n = = 205 [20.8%]) 205 [20.8%]) | Total (N = = 987) 987) | ||
| Sex, No. (%) | ||||||
| Female | 159 (42.9) | 121 (58.5) | 79 (38.7) | 116 (56.6) | 475 (48.1) | <.001 |
| Male | 212 (57.1) | 86 (41.5) | 125 (61.3) | 89 (43.4) | 512 (51.9) | |
| Age, y | 71.8 (8.0) | 71.7 (8.0) | 69.2 (9.9) | 65.1 (8.0) | 70.0 (8.8) | <.001a,b |
| Years of education | 9.2 (4.2) | 9.0 (4.0) | 9.9 (4.7) | 12.9 (3.6) | 9.79 (4.5) | <.001a |
| Duration of symptoms, moc | 25.2 (30.3) | 25.6 (26.6) | 71.5 (62.9) | 53.6 (61.1) | 41.2 (49.1) | <.001b |
| MMSE score | 21.7 (6.0) | 21.4 (5.3) | 26.9 (3.7) | 28.5 (1.7) | 23.2 (5.8) | <.001a,b |
| Plasma p-tau231 level, pg/mL | 12.8 (6.6) | 16.9 (12.1) | 12.3 (6.8) | 11.0 (6.9) | 13.2 (8.4) | <.001a,d |
| Plasma p-tau181 level, pg/mL | 18.2 (9.5) | 28.7 (28.4) | 17.0 (8.5) | 16.7 (18.5) | 18.1 (17.2) | <.001a,d |
| Abnormal CSF Aβ42 level, No. (%) | 63 (17.0) | 113 (54.6) | NA | NA | NA | NA |
Abbreviations: AD, Alzheimer disease; CSF, cerebrospinal fluid; DLB, dementia with Lewy bodies; HC, healthy control; MMSE, Mini-Mental State Examination; NA, not applicable; PD, Parkinson disease.
Associations of Plasma p-tau Concentrations With Diagnoses
The plasma p-tau181 and p-tau231 concentrations in the different groups are shown in Figure 1. Patients with DLB had increased plasma p-tau181 and p-tau231 concentrations compared with the HC group (p-tau181, 2.58 pg/mL; 95% CI, 1.79-3.37 pg/mL; P =
= .007; p-tau231, 1.96 pg/mL; 95% CI, 1.37-2.56 pg/mL; P
.007; p-tau231, 1.96 pg/mL; 95% CI, 1.37-2.56 pg/mL; P =
= .007) but lower p-tau181 and p-tau231 concentrations compared with the AD group (p-tau181, −3.53 pg/mL; 95% CI, −4.31 to −2.76 pg/mL; P
.007) but lower p-tau181 and p-tau231 concentrations compared with the AD group (p-tau181, −3.53 pg/mL; 95% CI, −4.31 to −2.76 pg/mL; P <
< .001; p-tau231, −3.30 pg/mL; 95% CI, −3.98 to −2.71 pg/mL; P
.001; p-tau231, −3.30 pg/mL; 95% CI, −3.98 to −2.71 pg/mL; P <
< .001). The numerical difference between DLB and AD increased when the analyses were performed using only the patients with AD (n
.001). The numerical difference between DLB and AD increased when the analyses were performed using only the patients with AD (n =
= 113) with confirmation of diagnosis based on low Aβ42 concentration in CSF samples. These results remained statistically significant after correcting for age and sex. When comparing patients with DLB and patients with AD with biomarker confirmation based on low Aβ42 concentrations in CSF samples, a greater effect size was seen for p-tau181 (Cohen d
113) with confirmation of diagnosis based on low Aβ42 concentration in CSF samples. These results remained statistically significant after correcting for age and sex. When comparing patients with DLB and patients with AD with biomarker confirmation based on low Aβ42 concentrations in CSF samples, a greater effect size was seen for p-tau181 (Cohen d =
= 0.53) compared with the full AD group (Cohen d
0.53) compared with the full AD group (Cohen d =
= 0.39). Effect sizes (Cohen d) for DLB compared with the HC group were similar for both plasma markers: 0.25 for p-tau231 and 0.23 for p-tau181. There were no statistically significant differences in either of the plasma p-tau concentrations between the Parkinson disease group and the DLB group. The DLB group with abnormal Aβ42 levels in CSF samples had higher plasma concentrations of both p-tau markers compared with those with normal levels in CSF samples (difference in p-tau181, −3.61 pg/mL; 95% CI, −5.43 to −1.79 pg/mL; P
0.39). Effect sizes (Cohen d) for DLB compared with the HC group were similar for both plasma markers: 0.25 for p-tau231 and 0.23 for p-tau181. There were no statistically significant differences in either of the plasma p-tau concentrations between the Parkinson disease group and the DLB group. The DLB group with abnormal Aβ42 levels in CSF samples had higher plasma concentrations of both p-tau markers compared with those with normal levels in CSF samples (difference in p-tau181, −3.61 pg/mL; 95% CI, −5.43 to −1.79 pg/mL; P =
= .049; difference in p-tau231, −2.51 pg/mL; 95% CI, −3.63 to −1.39 pg/mL; P
.049; difference in p-tau231, −2.51 pg/mL; 95% CI, −3.63 to −1.39 pg/mL; P =
= .02) (Figure 1). The AUC values of the ROC curves indicating the overall biomarker performance across AD pathologic confirmed cases based on reduced Aβ42 levels in CSF samples obtained from patients with DLB were larger for p-tau181 than for p-tau231 (0.62 vs 0.56), but the change in the AUC of both biomarkers was not statistically significant (eFigure 2 in Supplement 1).
.02) (Figure 1). The AUC values of the ROC curves indicating the overall biomarker performance across AD pathologic confirmed cases based on reduced Aβ42 levels in CSF samples obtained from patients with DLB were larger for p-tau181 than for p-tau231 (0.62 vs 0.56), but the change in the AUC of both biomarkers was not statistically significant (eFigure 2 in Supplement 1).
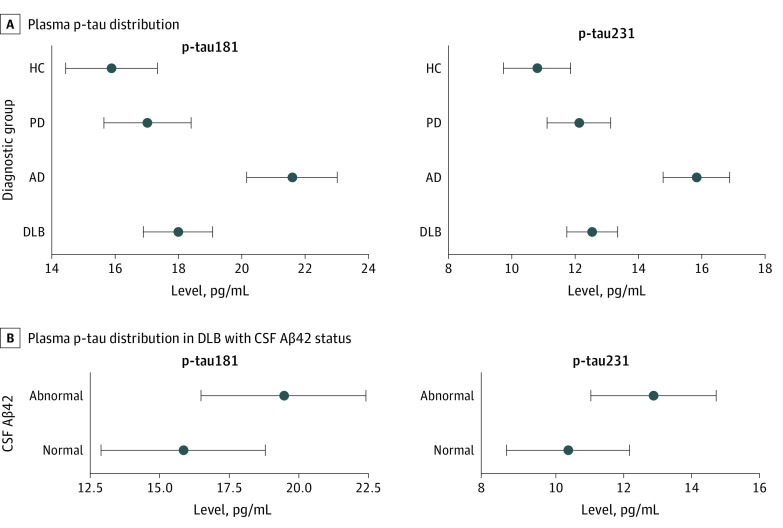
A, Distribution of plasma p-tau181 and p-tau231 concentrations across the different diagnostic groups. Dots indicate mean values, and the whiskers indicate their 95% CIs. Baseline concentrations of plasma p-tau181 and p-tau231 in patients with dementia with Lewy bodies (DLB) were significantly higher than those in the healthy control (HC) group but lower than those in the Alzheimer disease (AD) group. These differences between groups at baseline were evaluated using a Bonferroni post hoc test. B, Distribution of plasma p-tau181 and p-tau231 concentrations in patients with DLB with cerebrospinal fluid (CSF) amyloid-β42 (Aβ42) status. Baseline concentrations of plasma p-tau181 and p-tau231 in patients with DLB were significantly higher in the DLB group with abnormal CSF Aβ42 levels in both p-tau biomarkers compared with those with normal CSF Aβ42 levels. Patients were a mean (SD) age of 70.0 (8.8) years. PD indicates Parkinson disease.
Associations of Plasma p-tau Concentrations With Baseline and Longitudinal MMSE Score in Patients With DLB
We found that higher baseline plasma p-tau181 and p-tau231 concentrations were associated with a lower MMSE score at baseline (p-tau181, −0.092 MMSE points; 95% CI, −0.12 to −0.06 MMSE points; P =
= .001; p-tau231, −0.16 MMSE points; 95% CI, −0.21 to −0.12 MMSE points; P
.001; p-tau231, −0.16 MMSE points; 95% CI, −0.21 to −0.12 MMSE points; P <
< .001) after adjusting for age and sex. After adjustment of the model for years of education, the p-tau231 concentration, but not the p-tau 181 concentration, remained significantly associated with a decrease in MMSE score (p-tau231, −0.049 MMSE points; 95% CI, −0.15 to −0.04 MMSE points; P
.001) after adjusting for age and sex. After adjustment of the model for years of education, the p-tau231 concentration, but not the p-tau 181 concentration, remained significantly associated with a decrease in MMSE score (p-tau231, −0.049 MMSE points; 95% CI, −0.15 to −0.04 MMSE points; P =
= .049). The mean change estimated for the other diagnostic groups can be found in eTable 4 in Supplement 1. In the longitudinal model (DLB subgroup with longitudinal measurements of cognition [n
.049). The mean change estimated for the other diagnostic groups can be found in eTable 4 in Supplement 1. In the longitudinal model (DLB subgroup with longitudinal measurements of cognition [n =
= 182] with a mean [SD] follow-up of 3.5 [1.7] years), the plasma p-tau181 concentration was associated with a decrease of −0.094 MMSE points per year (95% CI, −0.144 to −0.052 MMSE points; P
182] with a mean [SD] follow-up of 3.5 [1.7] years), the plasma p-tau181 concentration was associated with a decrease of −0.094 MMSE points per year (95% CI, −0.144 to −0.052 MMSE points; P =
= .02), whereas the plasma p-tau231 concentration was associated with an annual decrease of −0.130 MMSE points (95% CI, −0.201 to −0.071 MMSE points; P
.02), whereas the plasma p-tau231 concentration was associated with an annual decrease of −0.130 MMSE points (95% CI, −0.201 to −0.071 MMSE points; P =
= .02) (Figure 2).
.02) (Figure 2).
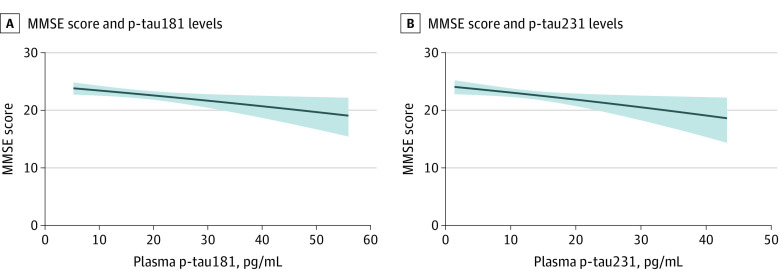
A, MMSE score and plasma levels of p-tau181. B, MMSE score and plasma levels of p-tau231. The solid line shows the estimated marginal model for the MMSE scores for a typical patient at a mean (SD) age of 70.0 (8.8) years and 1.5 years of follow-up. The gray area represents the 95% CIs around the mean values. Both graphs show the decrease in MMSE scores as a function of the increase in plasma concentrations of p-tau181 and p-tau231.
Discussion
Here we examined the concentrations of plasma p-tau181 and p-tau231 in 987 patients from the E-DLB Consortium. We found that the baseline concentrations of plasma p-tau181 and p-tau231 in the DLB group were significantly higher than those in the HC group but lower than in the AD group. In addition, we found that both biomarkers were associated with more cognitive impairment in the DLB group at baseline as well as with more pronounced cognitive worsening over time during follow-up. Significance was lost after adjusting for years of education, possibly because of the smaller sample size with longitudinal follow-up and educational data available. Overall, the plasma p-tau181 concentration had a greater effect size when comparing DLB and AD, although it should not be used in isolation to make a clinical diagnosis. There was no difference between the use of p-tau181 and p-tau231 concentrations across confirmed AD pathologic characteristics based on reduced Aβ42 levels in CSF samples obtained from individuals with DLB. Moreover, plasma p-tau231 concentration had a larger association than p-tau181 concentration with cognition. The association with the decrease in MMSE score, although statistically significant, was small, and the clinical relevance may be questionable. Previously, plasma p-tau concentration has been shown to be strongly associated with cognitive decline in individuals with AD15 and is associated with AD neuropathologic characteristics. However, for individuals with DLB, plasma p-tau concentration has been examined only in small cohorts, to our knowledge.9,16 Hall et al16 revealed that plasma p-tau concentration had a high correlation with abnormal tau and amyloid positron emission tomography scans from individuals with DLB, highlighting its usefulness as a marker for AD comorbid pathologic characteristics in DLB. Lantero Rodriguez et al,9 in neuropathologically confirmed cases, demonstrated increased plasma p-tau levels in those who had mixed AD and Lewy body pathologic characteristics a decade before death. In the small number of DLB cases without AD pathologic characteristics, the plasma p-tau levels were not elevated. In this study, we report that the levels of p-tau in individuals with DLB are intermediate between the HC and AD groups and significantly associated with cognitive decline in patients with DLB, suggesting that both p-tau181 and p-tau231 blood assays could be useful in assessing disease progression. Similar to Hall et al,16 we also demonstrate that plasma p-tau levels are elevated in DLB cases with AD comorbid pathologic findings. Further studies are needed to assess whether plasma p-tau levels can be used to assess the response of patients with DLB to treatments targeting AD pathophysiology.17
Limitations
This study has some limitations. For example, not all patients had a biomarker confirmation of the diagnosis. However, it has been shown that the clinical diagnosis of probable DLB has a very high specificity.18 To our knowledge, this is one of the largest DLB cohorts with biomarker data ever reported. However, owing to the retrospective multicenter design, there were missing data for some variables, including longitudinal data, CSF dementia markers, and years of education, which led to reduced statistical power for these analyses. Future studies should compare p-tau species with other candidate blood biomarkers of progression (eg, Aβ42/40, NfL [neurofilament light chain], or GFAP [glial fibrillary acidic protein]) and explore their ability to assess prodromal DLB.
Conclusions
Plasma concentrations of p-tau181 and p-tau231 are elevated in patients with DLB and further elevated in patients with DLB with confirmed AD comorbid pathologic characteristics. Plasma p-tau represents a promising biomarker to identify AD pathologic characteristics and a faster cognitive decline in individuals with this neurologic disorder.
Notes
Supplement 1.
eMethods.
eTable 1. Overview of Diagnostic Groups According to Centers
eTable 2. Overview of Plasma Sample Collection, Handling, and Storage and CSF Aβ42 Cutoff Values
eTable 3. Analytical Performance of the p-tau181 and p-tau231 Assays
eTable 4. MMSE Average Change per Group for Each Increased Unit (pg/mL) of Plasma p-tau at Baseline
eFigure 1. Flowchart of Number of DLB Patients Completing Annual Follow-up Assessments
eFigure 2. Plasma p-tau231 and p-tau181 Performance Across Confirmed AD Pathology Based on Reduced Aβ42 on CSF in DLB
eReferences.
References
Full text links
Read article at publisher's site: https://doi.org/10.1001/jamaneurol.2021.4222
Read article for free, from open access legal sources, via Unpaywall:
https://univoak.eu/islandora/object/islandora:146584/datastream/PDF/download/citation.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/117349703
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1001/jamaneurol.2021.4222
Article citations
Plasma NfL, GFAP, amyloid, and p-tau species as Prognostic biomarkers in Parkinson's disease.
J Neurol, 09 Sep 2024
Cited by: 0 articles | PMID: 39249107
Plasma biomarkers of amyloid, tau, axonal, and neuroinflammation pathologies in dementia with Lewy bodies.
Alzheimers Res Ther, 16(1):146, 03 Jul 2024
Cited by: 1 article | PMID: 38961441 | PMCID: PMC11221164
Nucleic acid aptamer-based electrochemical sensor for the detection of serum P-tau231 and the instant screening test of Alzheimer's disease.
Mikrochim Acta, 191(6):328, 14 May 2024
Cited by: 0 articles | PMID: 38743383
Plasma pTau181 Reveals a Pathological Signature that Predicts Cognitive Outcomes in Lewy Body Disease.
Ann Neurol, 96(3):526-538, 18 Jun 2024
Cited by: 1 article | PMID: 38888142
Neuroimaging and plasma evidence of early white matter loss in Parkinson's disease with poor outcomes.
Brain Commun, 6(3):fcae130, 16 Apr 2024
Cited by: 1 article | PMID: 38715714 | PMCID: PMC11073930
Go to all (28) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Association of Plasma Amyloid, P-Tau, GFAP, and NfL With CSF, Clinical, and Cognitive Features in Patients With Dementia With Lewy Bodies.
Neurology, 102(12):e209418, 03 Jun 2024
Cited by: 1 article | PMID: 38830138 | PMCID: PMC11244745
Comparison of Plasma Phosphorylated Tau Species With Amyloid and Tau Positron Emission Tomography, Neurodegeneration, Vascular Pathology, and Cognitive Outcomes.
JAMA Neurol, 78(9):1108-1117, 01 Sep 2021
Cited by: 104 articles | PMID: 34309632 | PMCID: PMC8314178
Association of Phosphorylated Tau Biomarkers With Amyloid Positron Emission Tomography vs Tau Positron Emission Tomography.
JAMA Neurol, 80(2):188-199, 01 Feb 2023
Cited by: 67 articles | PMID: 36508198 | PMCID: PMC9856704
Blood Biomarkers for the Diagnosis of Neurodegenerative Dementia: A Systematic Review.
J Geriatr Psychiatry Neurol, 36(4):267-281, 24 Nov 2022
Cited by: 2 articles | PMID: 36423207
Review
Funding
Funders who supported this work.
Medical Research Council (1)
Grant ID: UKDRI-1003
NIA NIH HHS (1)
Grant ID: R01 AG068398
 1
,
2
,
3
1
,
2
,
3




