Abstract
Free full text

BRCA 1/2 Germline Mutation Predicts the Treatment Response of FOLFIRINOX with Pancreatic Ductal Adenocarcinoma in Korean Patients
Abstract
Simple Summary
In pancreatic ductal adenocarcinoma, FOLFIRINOX and nab-paclitaxel are recommended as first-line chemotherapy regimens. However, there are limited data to predict the efficacy of the FOLFIRINOX regimen in patient outcomes. Platinum-based chemotherapy is tolerable and responsible in patients with DNA damage repair gene mutations. However, data are still limited, and no Asian data are available yet. Here, we sought to investigate the proportion of germline BRCA 1/2 mutations in patients with germline blood tests. Finally, we investigated the treatment response of FOLFIRINOX in patients with BRCA 1/2 mutations. We found that the presence of germline BRCA 1/2 mutations was associated with an improved overall response rate in pancreatic ductal adenocarcinoma patients treated with FOLFIRINOX. The high response rate in this analysis supports the preferential use of FOLFIRINOX therapy for patients harboring a BRCA germline mutation, and supports the need for early germline testing in order to select the best therapy.
Abstract
We evaluated the proportion of BRCA 1/2 germline mutations in Korean patients with sporadic pancreatic ductal adenocarcinoma (PDAC) and its effect on the chemotherapeutic response of FOLFIRINOX. This retrospective study included patients who were treated at two tertiary hospitals between 2012 and 2020, were pathologically confirmed to have PDAC, and had undergone targeted next-generation sequencing-based germline genetic testing. Sixty-six patients were included in the study (24 men; median age 57.5 years). In the germline test, BRCA 1/2 pathogenic mutations were found in nine patients (9/66, 13%, BRCA 1, n = 3; BRCA 2, n = 5; and BRCA 1/2, n = 1). There was no significant difference in the baseline characteristics according to BRCA mutation positivity. Among patients who underwent FOLFIRINOX chemotherapy, patients with a BRCA 1/2 mutation showed a higher overall response rate than those without a BRCA 1/2 mutation (71.4% vs. 13.9%, p = 0.004). Patients with a germline BRCA 1/2 mutation showed longer progression-free survival than those without a BRCA 1/2 mutation, without a significant time difference (18 months vs. 10 months, p = 0.297). Patients with a BRCA 1/2 mutation in the germline blood test had a higher response rate to FOLFIRINOX chemotherapy in PDAC. The high proportion of BRCA 1/2 germline mutations and response rate supports the need for germline testing in order to predict better treatment response.
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is expected to become the second leading cause of cancer-related deaths in the US before 2030 [1]. In the NCCN (National Comprehensive Cancer Network) guidelines, germline testing is recommended for patients with PDAC, using comprehensive gene panels for hereditary cancer syndromes [2]. The genes commonly associated with pathogenic germline alterations are BRCA 1/2, ATM, PALB2, MLH1, MSH2, MSH6, PMS2, CDKN2A, and TP53 [3]. Among them, the frequency of detected BRCA 1/2 (breast cancer susceptibility gene-1 and -2) is 4% to 7% [4,5]. The risk for pancreatic cancer is elevated two- to six-fold in these patients [6,7].
Recently, the POLO trial showed the benefit of poly ADP ribose polymerase (PARP) inhibitors in BRCA mutations. BRCA genes encode proteins involved in homologous recombination repair, and cells with mutations are sensitive to PARP inhibitors. However, there was no difference in overall survival between the PARP inhibitor and placebo groups (p = 0.68) [8]. Furthermore, in the real world, it is difficult for clinicians to change regimens in patients who are tolerant to FOLFIRINOX (oxaliplatin, irinotecan, folinic acid, and fluorouracil) chemotherapy.
In PDAC, FOLFIRINOX and nab-paclitaxel are recommended as first-line chemotherapy regimens. The guidelines recommend FOLFIRINOX in patients who are young and with better performance status (ECOG 0–1) [2]. However, there are limited data to predict the efficacy of the FOLFIRINOX regimen in patient outcomes [9]. Platinum-based chemotherapy is tolerable and responsible in patients with DNA damage repair gene mutations [10,11]. However, data are still limited, and no Asian data are available yet [12,13,14,15,16].
Here, we sought to investigate the proportion of germline BRCA 1/2 mutations in patients with germline blood tests. Finally, we investigated the treatment response of FOLFIRINOX in patients with a BRCA 1/2 mutation.
2. Materials and Methods
2.1. Study Population
This dual institutional retrospective analysis was performed on all patients diagnosed with PDAC who underwent a germline blood test between January 2012 and February 2020. We identified 66 patients who underwent a germline blood test. Of these, two patients were excluded from the study on account of insufficient clinical data (n = 2). One patient was diagnosed and treated at another hospital, and one patient died shortly after diagnosis due to deterioration of the condition. The remaining 64 patients were included in the analysis. This study was performed in accordance with the Declaration of Helsinki, as reflected by the institutional review board of Severance Hospital (approval number 4-2021-1151).
2.2. Variables
We evaluated patient characteristics, laboratory variables, tumor characteristics, progression-free survival (PFS), overall survival (OS), and overall response rate (ORR). Patient demographics and clinical characteristics, including age, sex, personal and family history of cancer, hypertension, diabetes mellitus, smoking history, body mass index (BMI), systemic chemotherapy, and response to treatment, were obtained from medical records and imaging studies. BMI, defined as body weight divided by the square of the height, was categorized following the guidelines of the World Health Organization (WHO 2000) (BMI < 18.5, underweight; 18.5–24.9, normal range; ≥25.0, overweight; and ≥30.0, obese). Tumor characteristics (location, extent, and number of metastatic organs) and laboratory characteristics (carbohydrate antigen [CA] 19-9) were also investigated.
The date of death and the date of the last follow-up were reviewed to estimate the OS and PFS. We observed both survival and follow-up data until 5 March 2021. OS was defined as the interval from the start of FOLFIRINOX until death. PFS was defined as the interval from the start of FOLFIRINOX to progressive disease (PD) or death. Patients who remained without death or PD were censored at the time of the last follow-up. Responses were determined using RECIST (response evaluation criteria in solid tumors) v1.1. ORR was defined as the percentage of patients who had a best response rating of complete response (CR) or partial response (PR) at any time point during treatment with chemotherapy. Patients without measurable disease at baseline were excluded from the ORR analysis.
2.3. DNA Extraction and Sequencing
Genomic DNA was extracted from peripheral blood using a QIAamp DNA Blood Mini Kit (Qiagen, Venlo, The Netherlands). The amount of input DNA was approximately 500 ng. DNA was fragmented into segments between 150 and 250 bp using the Bioruptor® Pico sonication system (Diagenode, Liege, Belgium), end-repaired, and ligated to Illumina adapters (Illumina, San Diego, CA, USA) and indices. Sequencing libraries were hybridized with capture probes (Celemic, Seoul, Korea). The enriched DNA was then amplified, and clusters were generated and sequenced on a NextSeq 550 instrument (Illumina) with 2 × 151 bp reads [17]. Pathogenicity interpretations of the variants were performed according to the 2015 American College of Medical Genetics and Genomics guidelines by professional medical geneticists, using evidence from variant type assessments, population allele frequency, prediction algorithm results, and searches within databases such as ClinVar.
2.4. Statistical Analysis
The baseline demographics and characteristics of the patients were analyzed using descriptive statistics. The differences in baseline characteristics and ORR between BRCA-positive and BRCA-negative groups were analyzed using the chi-square test for categorical variables and the Student’s t-test for continuous variables. We estimated the median OS and PFS according to BRCA mutations using Kaplan–Meier curves and compared them using the log-rank test. A time-dependent Cox regression analysis was applied to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) of pancreatic cancer mortality associated with BRCA mutations. Statistical significance was set at p < 0.05. All analyses were conducted using SPSS version 26.0 (SPSS, Chicago, IL, USA).
3. Results
3.1. Patients’ Characteristics and BRCA 1/2 Gene Mutations
A total of 66 PDAC patients underwent germline mutation analysis. Of all participants, three patients (4.5%) had a BRCA1 mutation, five (7.6%) had a BRCA2 mutation, and one patient (1.5%) had a BRCA 1/2 mutation. None had germline ATM or PALB2 mutations. Two patients had KRAS mutations, and one patient each had TP53, CDK2NA, SMAD4, and MUTYH mutations. Ten patients (15.2%) had BRCA variants of unknown significance (VUS). The specific BRCA mutations are listed in Supplementary Table S1. During the study period, somatic mutation tests were performed on 31 patients not included in this study. Two patients (2/31, 6.5%) had a BRCA1 mutation, and two patients (2/31, 6.5%) had a BRCA2 mutation. They were not included in this analysis. Of these 66 patients, 2 patients were excluded due to insufficient clinical data. Of these 64 patients, 7 patients had resectable PDAC, 20 patients had borderline resectable or locally advanced PDAC, and 37 patients had metastatic PDAC. Seven patients with resectable PDAC underwent curative intent resection (Figure 1). This study was performed in accordance with the Declaration of Helsinki, as reflected by the institutional review board of Severance Hospital (approval number 4-2021-1151).
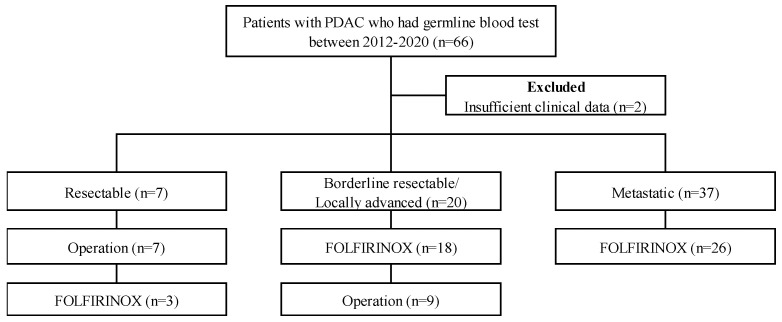
Selection of study population with PDAC, pancreatic ductal adenocarcinoma; FOLFIRINOX: oxaliplatin, irinotecan, folinic acid, and fluorouracil.
The patient demographics and clinical characteristics are summarized in Table 1. The median age of the patients was 57.5 years (interquartile range, 48.0–66.8 years), and 37.5% were men. Overall, 29.7% (19/64) of the patients in our study also had a personal history of malignancy, including breast cancer (6/64, 9.4%), thyroid cancer (2/64, 3.1%), and ovarian cancer (1/64, 1.6%). A higher percentage of patients in the BRCA-positive group had a prior history of malignancy (6/9, 66.7% vs. 13/55, 23.6%, p = 0.016) and breast cancer (4/9, 44.4% vs. 2/55, 3.64%). Of the 20 patients who had a family history of cancer, 10 (22.7%) were of pancreatic cancer, 3 (6.8%) were of breast cancer, and 12 (27.3%) were of other malignancies. It was not possible to identify whether the family history of cancer was from a first-degree relative or not. The proportion of family history of any malignancy and number of metastatic sites was greater in the BRCA-positive group than in the BRCA-negative group; however, the difference was not significant (all, p > 0.05). The other variables showed no significant differences between the two groups. In the Kaplan–Meier survival analysis, there was no significant difference seen in OS between the BRCA-positive and BRCA-negative groups (p = 0.888) (Supplementary Figure S1). The multivariable Cox regression model showed no significant improvement in OS in the presence of BRCA 1/2 mutations (HR, 0.128; 95% CI, 0.021–1.618) (Supplementary Table S2). The risk factors related to OS were tumor location (HR, 7.335; 95% CI, 2.030–26.503, p = 0.002), T stage (HR, 0.333; 95% CI, 0.115–0.963, p = 0.042), and M stage (HR, 7.661; 95% CI, 2.188–26.824, p = 0.001) in the multivariate analysis.
Table 1
Baseline characteristics of patients who had germline genetic blood tests.
| Variables | Total (n = 64) | BRCA Mutation (+) | BRCA Mutation (–) | p Value | ||
|---|---|---|---|---|---|---|
| (n = 9) | (n = 55) | |||||
| Age at diagnosis (year) | 57.5 (48.0–66.8) | 50.0 (47.0–60.0) | 59.0 (48.0–71.0) | 0.122 | ||
| Male | 24 (37.5%) | 2 (22.2%) | 22 (40.0%) | 0.464 | ||
| History of prior malignancy, n (%) | ||||||
| Yes | 19 (29.7%) | 6 (66.7%) | 13 (23.6%) | 0.016 | ||
| Breast | 6 (9.4%) | 4 (44.4%) | 2 (3.64%) | 0.014 | ||
| Family history of any malignancy, n (%) | ||||||
| Yes | 29 (45.3%) | 6 (66.7%) | 23 (41.8%) | 0.279 | ||
| Pancreas | 14 (21.9%) | 2 (22.2%) | 12 (21.8%) | 1.000 | ||
| Breast | 3 (4.7%) | 1 (11.1%) | 2 (3.6%) | 1.000 | ||
| Tobacco use (%) | ||||||
| Yes (past or current) | 16 (36.4%) | 3 (33.3%) | 19 (34.5%) | 1.000 | ||
| BMI (kg/m2) | ||||||
| ≥25.0 | 9 (14.1%) | 1 (11.1%) | 8 (14.5%) | 1.000 | ||
| Diabetes Mellitus | 18 (28.1%) | 1 (11.1%) | 17 (30.9%) | 0.425 | ||
| Hypertension | 21 (32.8%) | 2 (22.2%) | 19 (34.5%) | 0.706 | ||
| CA 19-9 (U/mL) | ||||||
| Elevated (>34.0U/mL) | 51 (79.7%) | 8 (88.9%) | 43 (78.2%) | 0.672 | ||
| Pathology | ||||||
| Well-differentiated | 2 (3.1%) | 0 (0.0%) | 2 (3.6%) | |||
| Moderately differentiated | 24 (37.5%) | 7 (77.8%) | 17 (30.9%) | |||
| Poorly differentiated | 7 (10.9%) | 2 (22.2%) | 5 (9.1%) | |||
| Clinical T stage | ||||||
| T1/2 | 25 (39.1%) | 3 (33.3%) | 22 (40.0%) | 1.000 | ||
| T3/4 | 39 (60.9%) | 6 (66.7%) | 33 (60.0%) | |||
| Clinical n stage | ||||||
| N0 | 26 (40.6%) | 5 (55.6%) | 21 (38.2%) | 0.467 | ||
| Location of primary tumor | ||||||
| Head | 32 (50.0%) | 4 (44.4%) | 28 (50.9%) | 1.000 | ||
| Metastasis site | ||||||
| Liver | 22 (34.4%) | 6 (66.7%) | 16 (29.1%) | 0.053 | ||
| Peritoneum | 13 (20.3%) | 0 (0.0%) | 13 (23.6%) | 0.185 | ||
| Distant LN | 9 (14.1%) | 1 (11.1%) | 8 (14.5%) | 1.000 | ||
| Number of metastasis site | ||||||
| 0 site | 33 (51.6%) | 3 (33.3%) | 30 (54.5%) | 0.296 | ||
| 1 or more sites | 31 (48.4%) | 6 (66.7%) | 25 (45.5%) |
Data are in n (%) or median (IQR). BRCA: breast cancer susceptibility gene; BMI: body mass index; CA: carbohydrate antigen; LN: lymph node; IQR: interquartile range.
3.2. FOLFIRINOX Treatment and Overall Response Rate
In total, 47 patients of the study participants received FOLFIRINOX chemotherapy. Of these 47 patients, 4 had no response evaluation and were hence excluded from the ORR analysis. The patient demographics and clinical characteristics are summarized in Table 2. Of these 43 patients, 7 (16.3%) had a BRCA mutation. The median age of the patients was 51.0 years (interquartile range, 46.0–65.0 years), and 41.9% were male. Of the BRCA-positive group, 57.1% (4/7) had a history of prior malignancy, compared with 16.7% (6/36) of the BRCA-negative group, and there was a significant difference observed between the groups (p = 0.040). A history of breast cancer was reported in 42.9% (3/7) and 2% (2/36) of the BRCA-positive and BRCA-negative groups, respectively (p = 0.024). A median of 12.0 FOLFIRINOX cycles were administered to the BRCA-positive patients, and 9.0 cycles were administered to the BRCA-negative patients. FOLFIRINOX therapy was mostly administered in the first-line setting: 93.0% (40/43) in the first-line setting and 7.0% (3/43) in the second-line setting. The ORR, as defined by RECIST v1.1, was significantly higher in BRCA-positive patients than in BRCA-negative patients (5/7, 71.4% vs. 5/36, 13.9%; p = 0.004) (Table 3). For BRCA-positive patients, partial response and stable disease were observed in 71.4% (5/7) and 28.6% (2/7) of patients, respectively. None of the BRCA-positive patients showed a complete response. Of the 43 patients, 7 (16.3%) had a BRCA mutation and 5 (11.6%) had a BRCA mutation of unknown significance. The ORR was significantly higher in BRCA-positive patients, including those with mutations of unknown significance, than in BRCA-negative patients (7/12, 58.3% vs. 3/31, 9.7%; p = 0.002) (Supplementary Table S3). A subset analysis was performed to test the effect of gemcitabine/nab-paclitaxel on the ORR. Patients with a BRCA 1/2 mutation did not show a significantly better response than those without a BRCA 1/2 mutation (1/3, 33.3% vs. 0/17, 0.0%, p = 0.154).
Table 2
Baseline characteristics of the patients who received FOLFIRINOX treatment and had response evaluation results.
| Variables | Total (n = 43) | BRCA Mutation (+) | BRCA Mutation (–) | p Value | ||
|---|---|---|---|---|---|---|
| (n = 7) | (n = 36) | |||||
| Age at diagnosis (year) | 51.0 (46.0–65.0) | 49.0 (46.0–56.0) | 51.5 (44.5–65.8) | 0.508 | ||
| Male | 18 (41.9%) | 2 (28.6%) | 16 (44.4%) | 0.680 | ||
| History of prior malignancy, n (%) | ||||||
| Yes | 10 (23.3%) | 4 (57.1%) | 6 (16.7%) | 0.040 | ||
| Breast | 5 (11.6%) | 3 (42.9%) | 2 (5.6%) | 0.024 | ||
| Family history of any malignancy, n (%) | ||||||
| Yes | 22 (51.2%) | 6 (85.7%) | 16 (44.4%) | 0.095 | ||
| Pancreas | 10 (23.3%) | 2 (28.6%) | 8 (22.2%) | 0.656 | ||
| Breast | 3 (7.0%) | 1 (14.3%) | 2 (5.6%) | 0.421 | ||
| Tobacco use (%) | ||||||
| Yes (past or current) | 15 (34.9%) | 3 (42.9%) | 12 (33.3%) | 0.680 | ||
| BMI (kg/m2) | ||||||
| ≥25.0 | 9 (20.9%) | 1 (14.3%) | 5 (13.9%) | 1.000 | ||
| Diabetes Mellitus | 9 (20.9%) | 0 (0.0%) | 9 (25.0%) | 0.314 | ||
| Hypertension | 11 (25.6%) | 2 (28.6%) | 9 (25.0%) | 1.000 | ||
| CA 19-9 (U/mL) | ||||||
| Elevated (>34.0 U/mL) | 36 (83.7%) | 6 (85.7%) | 30 (83.3%) | 1.000 | ||
| Pathology | ||||||
| Well-differentiated | 1 (2.3%) | 0 (0.0%) | 1 (2.8%) | |||
| Moderately differentiated | 16 (37.2%) | 5 (71.4%) | 11 (30.6%) | |||
| Poorly differentiated | 5 (11.6%) | 2 (28.6%) | 3 (8.3%) | |||
| Clinical T stage | ||||||
| T1/2 | 16 (37.2%) | 2 (28.6%) | 14 (38.9%) | 0.695 | ||
| T3/4 | 27 (62.8%) | 5 (71.4%) | 22 (61.1%) | |||
| Clinical n stage | ||||||
| N0 | 16 (37.2%) | 3 (42.9%) | 13 (36.1%) | 1.000 | ||
| Location of primary tumor | ||||||
| Head | 22 (51.2%) | 3 (42.9%) | 19 (52.8%) | 0.698 | ||
| Metastasis site | ||||||
| Liver | 17 (39.5%) | 5 (71.4%) | 12 (33.3%) | 0.093 | ||
| Peritoneum | 8 (18.6%) | 0 (0.0%) | 8 (22.2%) | 0.315 | ||
| Distant LN | 5 (11.6%) | 1 (14.3%) | 4 (11.1%) | 1.000 | ||
| Number of metastasis site | ||||||
| 0 site | 19 (44.2%) | 2 (28.6%) | 17 (47.2%) | 0.243 | ||
| 1 or more sites | 24 (55.8%) | 5 (71.4%) | 19 (52.8%) |
Data are in n (%) or median (IQR). BRCA: breast cancer susceptibility gene; BMI: body mass index; CA: carbohydrate antigen; LN: lymph node; IQR: interquartile range.
Table 3
Overall response rate to FOLFIRINOX in patients with a germline BRCA 1/2 mutation was significantly higher than patients without the mutation.
| Outcome | BRCA Mutation (+) | BRCA Mutation (-) | p Value | |
|---|---|---|---|---|
| (n = 7) | (n = 36) | |||
| Overall response rate, n (%) | 5 (71.4%) | 5 (13.9%) | 0.004 | |
| Complete response | 0 | 0 | ||
| Partial response | 5 (71.4%) | 5 (13.9%) | ||
| Stable disease | 2 (28.6%) | 26 (72.2%) | ||
| Progressive disease | 0 | 5 (13.9%) | ||
| Line of FOLFIRINOX therapy | ||||
| First | 6 (85.7%) | 34 (94.4%) | 0.421 | |
| Second | 1 (14.3%) | 2 (5.6%) |
Response criteria according to RECIST (response evaluation criteria in solid tumours) v1.1. FOLFIRINOX: oxaliplatin, irinotecan, folinic acid, and fluorouracil; BRCA: breast cancer susceptibility gene.
3.3. Progression-Free Survival and Overall Survival
In our study, 71.2% (47/66) of the patients received FOLFIRINOX treatment. Of the 47 patients, 32 patients with locally advanced, metastatic, or recurrent PDAC were treated with palliative first-line FOLFIRINOX. These cases were included in the survival analyses. As a clinically relevant surrogate of the durability of FOLFIRINOX responses, we utilized PFS, defined as the date of first FOLFIRINOX chemotherapy administration to the date of clinical treatment failure. BRCA-positive patients had longer PFS than BRCA-negative patients. However, this association was not significant (p = 0.423). The median PFS was 18.0 months for BRCA-positive patients and 10.0 months for BRCA-negative patients. The OS for BRCA-positive and BRCA-negative patients did not reach the median (Figure 2).
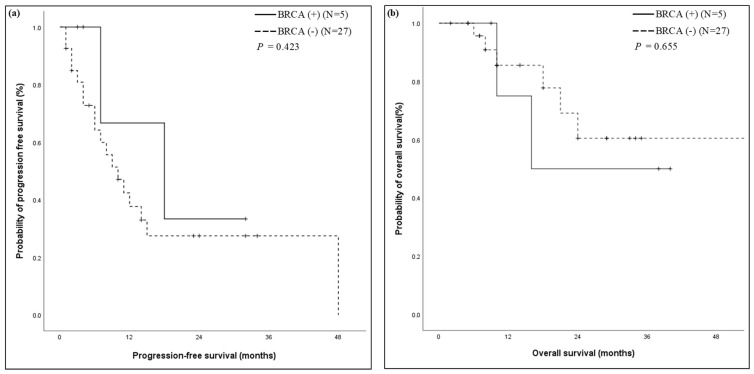
Kaplan–Meier curve in patients with first-line FOLFIRINOX stratified by the presence of a germline BRCA gene mutation. (a) The PFS was 18 months (95% confidence interval 0.4–35.6) in the BRCA-positive group, as compared to 10 months (95% confidence interval 5.5–14.5) in the BRCA-negative group (p = 0.423); (b) Kaplan–Meier survival curves for overall survival of the patients were not reached. FOLFIRINOX: oxaliplatin, irinotecan, folinic acid, and fluorouracil; BRCA: breast cancer susceptibility gene.
4. Discussion
In this study, BRCA 1/2 germline mutations predicted the treatment response of FOLFIRINOX in patients with PDAC. The PFS was longer in patients with a BRCA 1/2 mutation than those with a wild type, even though the difference was not statistically significant. In this study, the rate of BRCA 1/2 mutations was 13.6%. The data values were slightly higher than previous data (range 4–7%) in the general population [4,5]. The higher proportion of BRCA 1/2 mutations may be due to the change in detection method with the adoption of next-generation sequencing. In addition, considering that a high proportion of patients were previously diagnosed with breast cancer in this study, the results are similar to those of previous studies. The prevalence of BRCA 1/2 mutations in Asian patients with familial breast cancer and early-onset breast cancer was reported to be 2.8% to 31.8% [18]. Previous studies showed that BRCA gene mutations were associated with patients’ survival outcomes [12,13,19]. In this study, patients with BRCA gene mutations did not show different survival outcomes on account of the small number of patients.
The clinical significance and prognostic value of germline BRCA pathogenic mutations in tumors are well-known, but whether missense variants of uncertain significance (VUS) have clinical impact is not known. Variants in the gene were often classified as VUSs because of an insufficient understanding of the gene’s role. Variants can be reclassified from VUS to likely pathogenic, and further, to pathogenic. Phosphorylation of BRCA 1/2 mutations plays an important role in their function as regulators of DNA repair, transcription, and cell cycles in response to DNA damage. Tram et al. suggested that VUS have the potential to interfere with the phosphorylation process via abolishing or creating phosphorylation sites on BRCA 1/2 [20]. Hu et al. reported that germline VUS variant carriers had superior disease-free survival when compared with wild-type PDAC patients receiving adjuvant chemotherapy (16.5 months vs. 13.1 months, p = 0.007) [21]. Previous statistics indicate that between 10–20% of BRCA sequencing results are VUSs, and of these, more than 50% are missense mutations [22]. In this study, BRCA 1/2 missense mutations (VUSs) were detected in 15.2% of our cohort (Supplementary Table S1). The ORR was significantly higher in BRCA-positive patients, including missense mutations of VUS, than in BRCA-negative patients (7/12, 58.3% vs. 3/31, 9.7%, p = 0.002). With the further accumulation of data in the future, VUS can be reclassified as pathogenic.
Previously, several studies reported on the proportion of BRCA 1/2 mutations and their impact on patients with PDAC [9,12,13,16,19,23,24,25,26,27,28]. Golan et al. showed a difference in survival outcome for stage 3 or 4 PDAC patients with BRCA 1/2 mutations in platinum-based chemotherapy (22 months vs. 9 months, p = 0.039) [19]. Wattenberg et al. reported on the treatment response of platinum-based chemotherapy in PDAC patients with BRCA 1/2 mutations (58% vs. 21%, p = 0.002) [12]. In the present study, patients who received FOLFIRINOX chemotherapy showed a better treatment response in BRCA-positive patients compared to BRCA-negative patients. However, patients who received nab-paclitaxel chemotherapy did not show any difference in treatment response, irrespective of BRCA mutations.
Recently, several studies attempted to identify patients who benefit from palliative first-line FOLFIRINOX chemotherapy. Transcriptomic analysis showed that the basal type showed a better treatment response to FOLFIRINOX chemotherapy. The immunohistochemistry stained marker KRT81 may be a predictive marker to identify patients in the clinical field [29]. Circulating blood markers, such as ctDNA and exosomes, were also suggested as predictors for FOLFIRINOX response [30]. In other studies, protein markers, CES2 expression, and female gender predicted the response to FOLFIRINOX in PDAC [31,32]. The ideal predictor is a non-invasive clinically feasible tool during patient treatment. In this study, BRCA 1/2 was a predictor of the response to FOLFIRINOX. However, the proportion of BRCA cases was very low in patients with PDAC. Several clinical trials are currently ongoing to identify better blood germline biomarkers (ClinicalTrials.gov NCT04289961; NCT04143152).
Despite the efficacy of BRCA on treatment response in patients, the present study did not show survival benefits in patients who underwent FOLFIRINOX. Regardless of how good a prognostic or therapeutic predictive marker may be, it cannot outperform clinical parameters, such as cancer stage, age, sex, and metastasis, on their prognosis. Germline mutations can be used to predict FOLFIRINOX treatment response; however, they are still limited in predicting patient prognosis. A previous study by Sehdev et al. and Golan et al. also showed a significant difference in the prognosis of BRCA-positive patients who received platinum-based chemotherapy [16,24].
Our study has strengths. This is the first report of the ORR in numerous patients with BRCA 1/2 mutations following the use of FOLFIRINOX in Asia. In a previous study, less was known about the prevalence and treatment outcomes of FOLFIRINOX involving BRCA 1/2 mutations in Asia [14]. The high ORR of 71.4% with FOLFIRINOX therapy in BRCA-positive patients suggests that platinum therapy may be particularly desirable for this subset of patients in clinical scenarios marked by high disease burden and symptomatic disease, and for patients with PDAC. This study may help guide treatment decisions for patients with PDAC.
This study has several limitations. First, this is a retrospective study. Although we adjusted several factors via multivariate analysis, selection and/or information bias could remain. The lack of statistically significant differences in both OS and PFS in this study population may be attributed to the limited number of patients enrolled in the study: only 32 patients in our study were treated with palliative, first-line FOLFIRINOX. Second, although we found no significant difference in the proportion of males between groups, there were fewer males in the BRCA-positive group (2/9, 22.2% vs. 22/55, 40.0%, p = 0.464) [32]. In this study, relatively young patients were enrolled compared to previous studies (median 57.5 years) [12,19,33]. Both findings could plausibly skew bias toward the null hypothesis. Third, we were unable to control for mortality comorbidities that might have affected our results. However, since FOLFIRINOX is indicated for relatively healthier PDAC patients with good performance status, we do not think that the difference in comorbidities is the only explanation for our results.
5. Conclusions
We found that the presence of germline BRCA 1/2 mutations is associated with an improved ORR in PDAC patients treated with FOLFIRINOX. These results validate the association of germline BRCA 1/2 mutations with platinum sensitivity, as reported by other results in patients with PDAC. Notably, the high response rate in this analysis supports the preferential use of FOLFIRINOX therapy for patients with PDAC harboring a BRCA germline mutation, and supports the need for early germline testing in order to select the best therapy. Further prospective studies are needed to refine the treatment paradigms for this important subset of patients with PDAC.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14010236/s1, Figure S1: Kaplan–Meier curve for overall survival in patients stratified by presence of germline BRCA gene mutation, Table S1: Pathogenic mutations seen in 9 patients (ID) and variant of unknown significant mutations seen in 10 patients in BRCA genes of our cohort, Table S2: Univariate and multivariate analysis using the Cox regression analysis to identify the risk factors for overall survival, Table S3: Overall response rate to FOLFIRINOX in patients with a germline BRCA 1/2 mutation including variant of unknown significance was significantly higher than patients without the mutation.
Author Contributions
J.H.P. and H.S.L. had the initial idea and performed the research. J.H.P. and H.S.L. wrote the paper. J.H.J., S.I.J., M.J.C., J.Y.P., S.W.P., S.B., S.Y.S., H.S.L. and J.H.C. evaluated and suggested changes to the manuscript. H.S.L. and J.H.C. approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a faculty research grant of the Yonsei University College of Medicine (6-2018-0082).
Institutional Review Board Statement
The study was performed in accordance with the Declaration of Helsinki, as reflected by the institutional review board of Severance Hospital (approval number 4-2021-1151).
Informed Consent Statement
Informed consent was waived because of the retrospective nature of the study, and the analysis used anonymous clinical data.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Articles from Cancers are provided here courtesy of Multidisciplinary Digital Publishing Institute (MDPI)
Full text links
Read article at publisher's site: https://doi.org/10.3390/cancers14010236
Read article for free, from open access legal sources, via Unpaywall:
https://www.mdpi.com/2072-6694/14/1/236/pdf?version=1641287503
Citations & impact
Impact metrics
Citations of article over time
Article citations
Consensus, debate, and prospective on pancreatic cancer treatments.
J Hematol Oncol, 17(1):92, 10 Oct 2024
Cited by: 0 articles | PMID: 39390609 | PMCID: PMC11468220
Review Free full text in Europe PMC
Understanding the Genetic Landscape of Pancreatic Ductal Adenocarcinoma to Support Personalized Medicine: A Systematic Review.
Cancers (Basel), 16(1):56, 21 Dec 2023
Cited by: 2 articles | PMID: 38201484 | PMCID: PMC10778202
Review Free full text in Europe PMC
Therapeutic developments in pancreatic cancer.
Nat Rev Gastroenterol Hepatol, 21(1):7-24, 05 Oct 2023
Cited by: 28 articles | PMID: 37798442
Review
State-of-the-Art and Upcoming Innovations in Pancreatic Cancer Care: A Step Forward to Precision Medicine.
Cancers (Basel), 15(13):3423, 30 Jun 2023
Cited by: 7 articles | PMID: 37444534 | PMCID: PMC10341055
Review Free full text in Europe PMC
The Crucial Findings Derived from the Special Issue "Inside Cancer Genomics: From Structure to Therapy".
Cancers (Basel), 15(13):3488, 04 Jul 2023
Cited by: 0 articles | PMID: 37444598 | PMCID: PMC10341065
Go to all (7) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (1 citation) ClinicalTrials.gov - NCT04143152
- (1 citation) ClinicalTrials.gov - NCT04289961
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Association between BRCA Gene Variants and the Response to Modified FOLFIRINOX in Patients with Unresectable Pancreatic Cancer.
Acta Med Okayama, 77(5):517-525, 01 Oct 2023
Cited by: 0 articles | PMID: 37899263
Prognostic value of the <i>TP53</i> mutation in patients with pancreatic ductal adenocarcinoma receiving FOLFIRINOX.
Ther Adv Med Oncol, 16:17588359241290482, 23 Oct 2024
Cited by: 0 articles | PMID: 39449732 | PMCID: PMC11500227
Dramatic response of FOLFIRINOX regimen in a collision pancreatic adenocarcinoma patient with a germline BRCA2 mutation: a case report.
Jpn J Clin Oncol, 49(11):1049-1054, 01 Dec 2019
Cited by: 9 articles | PMID: 31612916
The role of PARP inhibitors in germline BRCA-associated pancreatic ductal adenocarcinoma.
Clin Adv Hematol Oncol, 18(3):168-179, 01 Mar 2020
Cited by: 6 articles | PMID: 32609666
Review
Funding
Funders who supported this work.
National Research Foundation of Korea (1)
Grant ID: NRF-2021R1A2C1008898
Yonsei University (2)
Grant ID: 6-2020-0079
Grant ID: 6-2018-0082




