Abstract
Free full text

Consensus, debate, and prospective on pancreatic cancer treatments
Associated Data
Abstract
Pancreatic cancer remains one of the most aggressive solid tumors. As a systemic disease, despite the improvement of multi-modality treatment strategies, the prognosis of pancreatic cancer was not improved dramatically. For resectable or borderline resectable patients, the surgical strategy centered on improving R0 resection rate is consensus; however, the role of neoadjuvant therapy in resectable patients and the optimal neoadjuvant therapy of chemotherapy with or without radiotherapy in borderline resectable patients were debated. Postoperative adjuvant chemotherapy of gemcitabine/capecitabine or mFOLFIRINOX is recommended regardless of the margin status. Chemotherapy as the first-line treatment strategy for advanced or metastatic patients included FOLFIRINOX, gemcitabine/nab-paclitaxel, or NALIRIFOX regimens whereas 5-FU plus liposomal irinotecan was the only standard of care second-line therapy. Immunotherapy is an innovative therapy although anti-PD-1 antibody is currently the only agent approved by for MSI-H, dMMR, or TMB-high solid tumors, which represent a very small subset of pancreatic cancers. Combination strategies to increase the immunogenicity and to overcome the immunosuppressive tumor microenvironment may sensitize pancreatic cancer to immunotherapy. Targeted therapies represented by PARP and KRAS inhibitors are also under investigation, showing benefits in improving progression-free survival and objective response rate. This review discusses the current treatment modalities and highlights innovative therapies for pancreatic cancer.
Introduction
Pancreatic cancer, specifically, pancreatic ductal adenocarcinoma (PDAC), has become the third leading cause of cancer-related death only behind lung cancer and colorectal cancer in the United States, and is predicted to rise to the second by 2030 [1]. In 2023, an estimated 64,050 new diagnoses and 50,550 deaths from pancreatic cancer occurred in the United States [1]. The prognosis of pancreatic cancer remains dismal, 5-year survival increased from 4% in 1997 to 12% in 2018 in pancreatic cancer patients of all stages [1]. This small incremental improvement is attributed to the development of multidisciplinary care and the improvement of multimodality therapies, including surgical resection, radiation, chemotherapy, immunotherapy, and targeted therapy. This article provides a comprehensive review of current treatment modalities for pancreatic cancer, with a particular focus on recent clinical advancements in the multi-modality treatments of resectable, borderline resectable, local advanced, and metastatic pancreatic cancer. We conducted a comprehensive literature search using databases such as PubMed, MEDLINE, and clinical trial registries, employing keywords such as “pancreatic cancer,” “treatment,” “neoadjuvant therapy,” and “targeted therapy.” Studies were selected based on relevance, recent publication date, and the quality of evidence presented. In addition, we discuss the latest research and development of innovative therapies for pancreatic cancer.
Multi-modality treatments for pancreatic cancer
The management of pancreatic cancer requires a comprehensive approach that integrates various treatment modalities, including surgery, radiotherapy, chemotherapy, and emerging therapies such as immunotherapy and targeted therapy. Given the aggressive nature of pancreatic cancer and the complexity of its treatment, a multi-modality strategy is essential to address the disease at different stages and to improve patient outcomes. This section will discuss the current standard of care treatments based on resectability status, emphasizing the evolving role of neoadjuvant and adjuvant therapies in conjunction with surgical intervention.
Surgical treatment
Pancreatectomy offers the only chance of cure for pancreatic cancer. Major pancreatectomy such as pancreaticoduodenectomy (also called the Whipple procedure) is safe when performed at high-volume centers with reported perioperative 30-day mortality rates of less than 3% [2, 3]. Based on the severity of the blood vessel involvement, localized pancreatic cancers are categorized into resectable pancreatic cancer, borderline resectable pancreatic cancer (BRPC), and locally advanced unresectable pancreatic cancer (LAPC) (Table 1) [4].
Table 1
NCCN criteria to assess and classify PDAC resectability status
| Resectability status | Resectable | Borderline resectable | Locally advanced |
|---|---|---|---|
| SMV/PV | No contact or contact ≤ ≤ 180° without vein contour irregularity 180° without vein contour irregularity | Contact > > 180°, or 180°, or ≤ ≤ 180° with contour irregularity or thrombosis 180° with contour irregularity or thrombosis | Unreconstructable |
| SMA | No contact | Contact ≤ ≤ 180° 180° | Contact > > 180° 180° |
| CHA | No contact | Contact without extension to celiac trunk or hepatic artery bifurcation | Unreconstructable |
| Coeliac trunk | No contact | Contact ≤ ≤ 180° 180° | Contact > > 180° 180° |
SMV superior mesenteric vein, PV portal vein, SMA,superior mesenteric artery, CHA common hepatic artery
Surgery for resectable pancreatic cancer
Although upfront surgical resection followed by adjuvant chemotherapy is the standard of care for patients with resectable pancreatic cancer, the role of neoadjuvant therapy in resectable pancreatic cancer remains controversial. A retrospective study favored neoadjuvant chemotherapy (n =
= 46), which showed superiority over upfront resection (n
46), which showed superiority over upfront resection (n =
= 113) in resectable pancreatic cancer, with a higher R0 resection rate (83% vs 53%), lower recurrence rate (31% vs 71%), and better overall survival (OS) (not reached vs 25.9 months) [5]. Two larger retrospective studies which included 13,674 and 5216 patients with resectable pancreatic cancer, also showed that neoadjuvant therapy with chemoradiotherapy or chemotherapy followed by surgery may improve OS compared to upfront surgery [6, 7]. A meta-analysis which included 6 randomized clinical trials (RCTs) with 469 resectable pancreatic cancer showed that, compared to upfront surgery, neoadjuvant chemotherapy with or without radiotherapy significantly improved OS, disease-free survival (DFS), and R0 resection rate [8]. Similar results were further corroborated in a more recent meta-analysis which included 50 studies with resectable pancreatic cancer and BRPC [9]. Many prospective clinical trials of neoadjuvant therapy for resectable pancreatic cancer as described below in detail, however, did not provide evidence to support neoadjuvant chemotherapy for this patient population. Two ongoing phase III RCTs including one Alliance trial conducted in the United States and the PREOPANC-3 trial conducted in Europe, both with larger sample sizes, are anticipated to provide more definitive answers to the questions about the role of multi-agent neoadjuvant therapy in resectable pancreatic cancer [10, 11]
113) in resectable pancreatic cancer, with a higher R0 resection rate (83% vs 53%), lower recurrence rate (31% vs 71%), and better overall survival (OS) (not reached vs 25.9 months) [5]. Two larger retrospective studies which included 13,674 and 5216 patients with resectable pancreatic cancer, also showed that neoadjuvant therapy with chemoradiotherapy or chemotherapy followed by surgery may improve OS compared to upfront surgery [6, 7]. A meta-analysis which included 6 randomized clinical trials (RCTs) with 469 resectable pancreatic cancer showed that, compared to upfront surgery, neoadjuvant chemotherapy with or without radiotherapy significantly improved OS, disease-free survival (DFS), and R0 resection rate [8]. Similar results were further corroborated in a more recent meta-analysis which included 50 studies with resectable pancreatic cancer and BRPC [9]. Many prospective clinical trials of neoadjuvant therapy for resectable pancreatic cancer as described below in detail, however, did not provide evidence to support neoadjuvant chemotherapy for this patient population. Two ongoing phase III RCTs including one Alliance trial conducted in the United States and the PREOPANC-3 trial conducted in Europe, both with larger sample sizes, are anticipated to provide more definitive answers to the questions about the role of multi-agent neoadjuvant therapy in resectable pancreatic cancer [10, 11]
Surgery for BRPC and LAPC
BRPCs portend relatively lower R0 resection rates, and a margin-negative resection for LAPCs is not achievable. Therefore, preoperative chemotherapy with or without radiation has become a standard approach for patients with BRPC. Evidence supporting a multidisciplinary management of BRPC, including chemotherapy, radiotherapy, and surgery, is described below in detail.
Compared with BRPCs, LAPCs have a lower tumor resection rate, even after neoadjuvant therapy. A recent meta-analysis which included 125 studies of either prospective trials or high-quality retrospective analyses found that the resection rates of BRPCs and LAPCs after neoadjuvant therapy were 60.6% and 22.2%, respectively; and surgical resection is associated with improved survival (BRPCs, 32.3 vs 13.9 months; LAPCs, 30.0 vs 14.6 months) for these localized pancreatic cancers [12]. Large, retrospective studies recently showed that conversion surgery for LAPCs after FOLFIRINOX chemotherapy was associated with improved survival [13, 14]. The largest single-center, comparative cohort study of portal vein resection in pancreatic cancer surgery showed concomitant portal vein resection (n =
= 694) significantly increased the 90-day mortality rate (6.3% vs 2.6%) compared to that without portal vein resection (n
694) significantly increased the 90-day mortality rate (6.3% vs 2.6%) compared to that without portal vein resection (n =
= 1571) [15]. Data on arterial resection and reconstruction are relatively few and varied by the resected arteries and the technical approaches. The reported mortality and morbidity rates for arterial resection in pancreatectomy were 5.7% and 41.5%, respectively [16]. The reported mortality and morbidity rates for celiac axis resection in pancreatectomy were 1.7% and 39.0%, respectively [17]. Thus, celiac axis involvement is not considered to be a strict contraindication for surgery in LAPCs. In addition, data (2015–2019) in a retrospective study revealed that arterial divestment has a significantly reduced mortality compared to arterial resection (2.3% vs 7.0%) in pancreatic cancer surgeries [18]. Notably, these aggressive operations should be performed only when long-term survival is expected.
1571) [15]. Data on arterial resection and reconstruction are relatively few and varied by the resected arteries and the technical approaches. The reported mortality and morbidity rates for arterial resection in pancreatectomy were 5.7% and 41.5%, respectively [16]. The reported mortality and morbidity rates for celiac axis resection in pancreatectomy were 1.7% and 39.0%, respectively [17]. Thus, celiac axis involvement is not considered to be a strict contraindication for surgery in LAPCs. In addition, data (2015–2019) in a retrospective study revealed that arterial divestment has a significantly reduced mortality compared to arterial resection (2.3% vs 7.0%) in pancreatic cancer surgeries [18]. Notably, these aggressive operations should be performed only when long-term survival is expected.
Surgery for metastatic pancreatic cancer
Metastatic pancreatic cancer has been traditionally regarded as a contraindication for surgical resection. However, with the use of potent multiagent chemotherapy, an increasing number of studies investigated the oncologic outcomes of surgical resection in metastatic or oligometastatic pancreatic cancer. A recent meta-analysis showed that for oligometastatic pancreatic cancer to the liver, surgical resection after initial chemotherapy achieved increased median OS compared to chemotherapy only (23.3–56.0 vs 11.0–16.4 months) [19]. A review included 6 studies for lung metastases from pancreatic cancer and showed that the median OS after lung resection ranged from 18.6 to 38.3 months [20]. In a multicenter phase II study that included 33 patients with peritoneal metastases, 8 patients underwent conversion surgery after paclitaxel/S-1 chemotherapy and achieved a median OS of 27.8 months, which was significantly higher than 14.2 months in nonsurgical patients [21]. Moreover, in one of the largest retrospective studies that included 93 metastatic pancreatic cancer following the resection of the primary tumor and metastatic sites after chemotherapy, 45 patients (48.4%) achieved complete pathological response in their metastases. This study also found that only patients with complete pathological responses in metastasis could obtain survival benefits from surgical resection [22]. In spite of these results, surgery for metastatic pancreatic cancer has not been widely accepted due to lack of high-quality clinical trials. More prospective studies are ongoing (NCT04617457, NCT03398291) [23, 24].
Minimally invasive surgery
Minimally invasive surgery for pancreatic cancer, including laparoscopic and robotic approaches, is technically challenging but is gradually being adopted by surgeons due to its potential value in improving the quality of life. A multicenter RCT compared the benefit and safety of laparoscopic pancreatoduodenectomy (LPD, n =
= 297) with open pancreatoduodenectomy (OPD, n
297) with open pancreatoduodenectomy (OPD, n =
= 297) in pancreatic or periampullary tumors and showed that LPD was associated with a shorter hospital stay (15.0 vs 16.0 days) and similar short-term morbidity (29% vs 23%) and mortality (2% vs 2%) rates [25]. A subsequent meta-analysis which included 3 RCTs reached similar conclusions [26]. Moreover, a propensity-matched analysis showed that robotic pancreatoduodenectomy (RPD, n
297) in pancreatic or periampullary tumors and showed that LPD was associated with a shorter hospital stay (15.0 vs 16.0 days) and similar short-term morbidity (29% vs 23%) and mortality (2% vs 2%) rates [25]. A subsequent meta-analysis which included 3 RCTs reached similar conclusions [26]. Moreover, a propensity-matched analysis showed that robotic pancreatoduodenectomy (RPD, n =
= 626) and LPD (n
626) and LPD (n =
= 2716) for pancreatic cancer achieve similar surgical and oncologic outcomes whereas RPD compared to LPD showed a lower rate of conversion to open (14.7% vs 20.2%) [27]. Accumulated studies have supported the advantages of RPD or LPD although these studies also included patients with benign pancreatic diseases [28]– [30]. Especially, a recent RCT compared the short-term postoperative outcomes of RPD (n
2716) for pancreatic cancer achieve similar surgical and oncologic outcomes whereas RPD compared to LPD showed a lower rate of conversion to open (14.7% vs 20.2%) [27]. Accumulated studies have supported the advantages of RPD or LPD although these studies also included patients with benign pancreatic diseases [28]– [30]. Especially, a recent RCT compared the short-term postoperative outcomes of RPD (n =
= 82) with those of OPD (n
82) with those of OPD (n =
= 82) and showed that RPD led to a shorter postoperative length of hospital stay (11.0 vs 13.5 days) and similar rates of perioperative complications and postoperative 90-day mortality [31].
82) and showed that RPD led to a shorter postoperative length of hospital stay (11.0 vs 13.5 days) and similar rates of perioperative complications and postoperative 90-day mortality [31].
For distal pancreatectomy, a recent meta-analysis included 5 matched studies and showed that laparoscopic distal pancreatectomy (n =
= 1180) is superior to open distal pancreatectomy (n
1180) is superior to open distal pancreatectomy (n =
= 1250) including higher R0 resection rates (84.3% vs 77.6%) and shorter time to adjuvant therapy (45.0 vs 51.0 days) [32]. Most recently, an international randomized trial (DIPLOMA) that included 114 minimally invasive distal pancreatectomy (either laparoscopic or robotic) and 110 open distal pancreatectomy for resectable pancreatic cancers showed the noninferiority of minimally invasive distal pancreatectomy (R0 resection rate, 73% vs 69%) and comparable postoperative outcomes including lymph node yield (22 vs 23), intraperitoneal recurrence rate (41% vs 38%), and survival rate (2-year, 46% vs 48%), compared to open surgery [33]. The first benchmark study from 16 international expert centers also demonstrates that, compared to laparoscopic approach, robotic distal pancreatectomy has a lower conversion rate and fewer overall complications. Additionally, compared to the open approach, robotic distal pancreatectomy is associated with reduced blood loss and a shorter hospital stay [34]. Taken together, the above studies support the applicability of minimally invasive surgery for pancreatic cancer. The 2022 European Guidelines for Minimally Invasive Pancreatic Surgery meeting in Brescia published the evidence-based guidelines for minimally invasive pancreatic surgery [35].
1250) including higher R0 resection rates (84.3% vs 77.6%) and shorter time to adjuvant therapy (45.0 vs 51.0 days) [32]. Most recently, an international randomized trial (DIPLOMA) that included 114 minimally invasive distal pancreatectomy (either laparoscopic or robotic) and 110 open distal pancreatectomy for resectable pancreatic cancers showed the noninferiority of minimally invasive distal pancreatectomy (R0 resection rate, 73% vs 69%) and comparable postoperative outcomes including lymph node yield (22 vs 23), intraperitoneal recurrence rate (41% vs 38%), and survival rate (2-year, 46% vs 48%), compared to open surgery [33]. The first benchmark study from 16 international expert centers also demonstrates that, compared to laparoscopic approach, robotic distal pancreatectomy has a lower conversion rate and fewer overall complications. Additionally, compared to the open approach, robotic distal pancreatectomy is associated with reduced blood loss and a shorter hospital stay [34]. Taken together, the above studies support the applicability of minimally invasive surgery for pancreatic cancer. The 2022 European Guidelines for Minimally Invasive Pancreatic Surgery meeting in Brescia published the evidence-based guidelines for minimally invasive pancreatic surgery [35].
Radiotherapy
Radiotherapy is one of the most commonly used local therapy approaches. Radiotherapy can be used as a neoadjuvant or adjuvant therapy to improve tumor resection rate or to reduce recurrence rate or as a definitive therapy in the localized, unresectable setting to improve local control. Of note, the exact indications for radiation across disease stages remain controversial. In part, this is reflective of variation in outcomes across historical studies that used techniques which are no longer applicable. However, recent data using modern techniques have shown increasing signals for the benefit that radiation therapy may offer across the neoadjuvant, adjuvant, and definitive settings.
Adjuvant radiotherapy
The role of radiation for adjuvant therapy following complete macroscopic resection of pancreatic adenocarcinoma has been historically controversial with unclear indications for its use. However, evidence supporting the use of adjuvant radiotherapy after pancreatectomy, regardless of margin status, is still lacking according to prospective, randomized controlled studies. Early data from randomized studies provided mixed results. In the 1970s, the Gastrointestinal Tumor Study Group (GITSG) conducted a study in which 43 patients with resected pancreatic cancer were randomized to either observation or 5-Fluorouracil (5-FU)-based chemoradiation [36, 37]. The study used a split-course radiation regimen, where patients received two courses of 2 Gy ×
× 10 delivered over two weeks, with a two-week break in between. A rudimentary anterior–posterior beam arrangement was utilized with optional field shaping. 5-FU was administered for three consecutive days at a dose of 500 mg/m2 during both courses of radiation, and continued once weekly as a maintenance regimen for up to two years or until recurrence. The median OS and 2-year OS in the chemoradiation arm were 20 months and 42%, respectively, while in the observation arm, these figures were significantly lower at 11 months and 15%, respectively. In contrast, two subsequent European RCTs did not show a benefit to adjuvant chemoradiation. In the European Organization for Research and Treatment of Cancer (EORTC) 40,891 study, 218 patients with resected pancreatic cancer were randomized to 5-FU-based chemoradiation versus observation alone [38]. The radiation regimen was similar to the split-course used in the GITSG study, although no maintenance chemotherapy was offered. Unlike the GITSG study, survival analysis yielded no significant difference between the treatment arms, with median OS values of 24.5 months and 19.0 months in the chemoradiation and observation arms, respectively (p
10 delivered over two weeks, with a two-week break in between. A rudimentary anterior–posterior beam arrangement was utilized with optional field shaping. 5-FU was administered for three consecutive days at a dose of 500 mg/m2 during both courses of radiation, and continued once weekly as a maintenance regimen for up to two years or until recurrence. The median OS and 2-year OS in the chemoradiation arm were 20 months and 42%, respectively, while in the observation arm, these figures were significantly lower at 11 months and 15%, respectively. In contrast, two subsequent European RCTs did not show a benefit to adjuvant chemoradiation. In the European Organization for Research and Treatment of Cancer (EORTC) 40,891 study, 218 patients with resected pancreatic cancer were randomized to 5-FU-based chemoradiation versus observation alone [38]. The radiation regimen was similar to the split-course used in the GITSG study, although no maintenance chemotherapy was offered. Unlike the GITSG study, survival analysis yielded no significant difference between the treatment arms, with median OS values of 24.5 months and 19.0 months in the chemoradiation and observation arms, respectively (p =
= 0.21). Similarly, the multicenter randomized trial (ESPAC-1) in 2004 observed a negative survival outcome from adjuvant chemoradiation (n
0.21). Similarly, the multicenter randomized trial (ESPAC-1) in 2004 observed a negative survival outcome from adjuvant chemoradiation (n =
= 145) compared to no adjuvant chemoradiation following surgery (n
145) compared to no adjuvant chemoradiation following surgery (n =
= 144) (median OS, 15.9 vs 17.9 months, respectively). In the same study, a significant survival benefit was observed from adjuvant chemotherapy (n
144) (median OS, 15.9 vs 17.9 months, respectively). In the same study, a significant survival benefit was observed from adjuvant chemotherapy (n =
= 147) with 5-FU, compared to no adjuvant chemotherapy following surgery (n
147) with 5-FU, compared to no adjuvant chemotherapy following surgery (n =
= 142) (median OS, 20.1 vs 15.5 months, respectively) [39]. The results of this study hindered the further application of adjuvant radiotherapy in Europe.
142) (median OS, 20.1 vs 15.5 months, respectively) [39]. The results of this study hindered the further application of adjuvant radiotherapy in Europe.
In 2010, a randomized phase II study (EORTC-40013–22012/FFCD-9203/GERCOR) found that adjuvant gemcitabine alone (n =
= 45) and gemcitabine-based chemoradiation (n
45) and gemcitabine-based chemoradiation (n =
= 45) after curative resection for pancreatic cancer (2004–2007) showed comparable median DFS (11 vs 12 months) and OS (24 vs 24 months); however, the chemoradiation group had a lower rate of first local recurrence compared to the chemotherapy alone group(11% vs 24%) [40]. In 2022, another randomized trial (NCT02461836) of stage II pancreatic cancer with negative margins (2015–2018) demonstrated neither a survival benefit (median recurrence-free survival, 5.3 vs 9.7 months; median OS, 15.0 vs 28.0 months) nor improved local tumor control with adjuvant gemcitabine following stereotactic body radiation (SBRT) (n
45) after curative resection for pancreatic cancer (2004–2007) showed comparable median DFS (11 vs 12 months) and OS (24 vs 24 months); however, the chemoradiation group had a lower rate of first local recurrence compared to the chemotherapy alone group(11% vs 24%) [40]. In 2022, another randomized trial (NCT02461836) of stage II pancreatic cancer with negative margins (2015–2018) demonstrated neither a survival benefit (median recurrence-free survival, 5.3 vs 9.7 months; median OS, 15.0 vs 28.0 months) nor improved local tumor control with adjuvant gemcitabine following stereotactic body radiation (SBRT) (n =
= 18) compared to adjuvant gemcitabine alone (n
18) compared to adjuvant gemcitabine alone (n =
= 20) [41].
20) [41].
Despite the mixed study results in the prospective, randomized setting above, there are still considerable data that argue for consideration of adjuvant radiation, particularly in settings associated with increased local recurrence. A number of patterns of failure studies have highlighted that while systemic failure certainly predominates over local failure, local failure rates remain generally high after complete resection of pancreatic adenocarcinoma [42]– [45]. Indeed, even in the PRODIGE24/CCTG PA6 study, 38% of first failures continue to have a local component of failure, including 20% with local failure alone. Moreover, retrospective data from high-volume institutions also have provided signals for the value of adjuvant radiation. As an example, data from Johns Hopkins on patterns of failure after pancreatic cancer resection from 2000 through 2013 showed that positive margins were the strongest risk factor for local recurrence, while the administration of the radiation therapy was the strongest predictor of local control [46]. Furthermore, data from Radiation Therapy Oncology Group (RTOG) 9704, which was a randomized study exploring an adjuvant chemotherapy question in which all patients received adjuvant chemoradiation in a “sandwich” schedule, showed that receipt of radiation per protocol was associated with improved outcomes, suggesting value to the delivery of quality radiation therapy [47]. Even more, patients on RTOG 9704 who received radiation per protocol had outcomes that far exceeded similarly treated patients who did not have radiation therapy on CONKO-001 [48, 49].
Taken together, the role of radiation therapy in the adjuvant setting remains undefined. Importantly, RTOG 0804 was designed to ask this question in a more modern fashion in which patients with resected pancreatic cancer were randomized to adjuvant chemotherapy alone versus adjuvant chemotherapy followed by consolidative chemoradiation [50]. Radiation therapy in this study was delivered using modern intensity modulated radiation therapy (IMRT). While we await the final publication of these results, early presentation suggests significant benefits in the node negative patient population [51]. These findings may be due to far higher systemic therapy failure risk in the setting of node positive disease that washes out the benefit of local recurrence risk reduction. In fact, it likely is that local recurrence is not driven by nodal positivity but instead by extra-pancreatic perineural invasion, with many Asian studies supporting this notion [52]– [56]. Indeed, the Japanese have intricately characterized the anatomy of extra-pancreatic neural tracts that are at risk for microscopic disease which may not be fully dissected at the time of surgery, which may not be fully sterilized by adjuvant chemotherapy, and which may therefore drive local failure. Moreover, data from a phase II study demonstrated that nodal involvement was associated with systemic failure, while extrapancreatic perineural involvement was the most important predictor of local failure [57]. As such, investigators are now actively exploring whether designing the radiation fields to target these neural tracts may improve outcomes for pancreatic cancer. While such exploration has been primarily in the neoadjuvant or definitive settings, it may also apply to the adjuvant setting.
Neoadjuvant radiotherapy
It is now widely accepted that the main purposes of neoadjuvant radiotherapy are to enhance the radical resection rate and to reduce the risk of local recurrence. Two early single-arm phase II trials demonstrated that neoadjuvant fluorouracil plus cisplatin [58] (n =
= 41) or gemcitabine [59] (n
41) or gemcitabine [59] (n =
= 41) with concurrent radiotherapy were tolerated and feasible in potentially resectable or nonmetastatic pancreatic cancers. However, the evidence on the efficacy of neoadjuvant radiotherapy for pancreatic cancer has been inconsistent. A subsequent meta-analysis found that not only did adjuvant chemoradiotherapy have no significant effect on OS and DFS (17 studies, n
41) with concurrent radiotherapy were tolerated and feasible in potentially resectable or nonmetastatic pancreatic cancers. However, the evidence on the efficacy of neoadjuvant radiotherapy for pancreatic cancer has been inconsistent. A subsequent meta-analysis found that not only did adjuvant chemoradiotherapy have no significant effect on OS and DFS (17 studies, n =
= 3088) in resectable pancreatic cancer, but there was no significant difference between neoadjuvant chemoradiotherapy and adjuvant chemoradiotherapy (3 studies, n
3088) in resectable pancreatic cancer, but there was no significant difference between neoadjuvant chemoradiotherapy and adjuvant chemoradiotherapy (3 studies, n =
= 189) [60]. Nevertheless, a single-arm phase II clinical trial found that neoadjuvant FOLFIRINOX followed by individualized chemoradiation in BRPCs (n
189) [60]. Nevertheless, a single-arm phase II clinical trial found that neoadjuvant FOLFIRINOX followed by individualized chemoradiation in BRPCs (n =
= 48) results in a high R0 resection rate in resected patients (31/32, 97%) and prolonged survival (median DFS, 14.7 months; median OS, 37.7 months), which supported further investigations [61]. A recent meta-analysis that included 15 studies (n
48) results in a high R0 resection rate in resected patients (31/32, 97%) and prolonged survival (median DFS, 14.7 months; median OS, 37.7 months), which supported further investigations [61]. A recent meta-analysis that included 15 studies (n =
= 512) also investigated the added value of radiotherapy following neoadjuvant FOLFIRINOX chemotherapy in resectable pancreatic cancer or BRPC demonstrated that radiotherapy following neoadjuvant FOLFIRINOX (n
512) also investigated the added value of radiotherapy following neoadjuvant FOLFIRINOX chemotherapy in resectable pancreatic cancer or BRPC demonstrated that radiotherapy following neoadjuvant FOLFIRINOX (n =
= 161) improved the R0 resection rate (97.6% vs 88.0%) compared to FOLFIRINOX alone (n
161) improved the R0 resection rate (97.6% vs 88.0%) compared to FOLFIRINOX alone (n =
= 351) although survival benefits (22.4 vs 21.6 months) were not observed [62]. The randomized multicenter phase III trial (CONKO-007) investigated induction chemotherapy followed by chemoradiation or chemotherapy alone in 525 nonresectable LAPCs (2013–2021) [63]. After induction chemotherapy of FOLFIRINOX or gemcitabine, 190 patients with tumor progression or toxicity were excluded; and the remaining 335 were randomized to chemotherapy (n
351) although survival benefits (22.4 vs 21.6 months) were not observed [62]. The randomized multicenter phase III trial (CONKO-007) investigated induction chemotherapy followed by chemoradiation or chemotherapy alone in 525 nonresectable LAPCs (2013–2021) [63]. After induction chemotherapy of FOLFIRINOX or gemcitabine, 190 patients with tumor progression or toxicity were excluded; and the remaining 335 were randomized to chemotherapy (n =
= 167) or gemcitabine-based chemoradiation (50.4 Gy, n
167) or gemcitabine-based chemoradiation (50.4 Gy, n =
= 168). The circumferential resection margin-negative resection rate (19.6% vs 9.0%) and complete pathological complete response (6.0% vs 0%) were significantly higher in the chemoradiotherapy arm compared to the chemotherapy arm; however, the 2-year OS rate (34.8% vs 32.5%) and the general R0-resection rate (25.0% vs 18.0%) did not differ significantly between these two arms. A retrospective study of 2019 pancreatic cancer cases after pancreatoduodenectomy (2014–2020) showed that preoperative chemoradiotherapy but not chemotherapy could reduce the postoperative pancreatic fistula rate (2.0% vs 4.2%) [64].
168). The circumferential resection margin-negative resection rate (19.6% vs 9.0%) and complete pathological complete response (6.0% vs 0%) were significantly higher in the chemoradiotherapy arm compared to the chemotherapy arm; however, the 2-year OS rate (34.8% vs 32.5%) and the general R0-resection rate (25.0% vs 18.0%) did not differ significantly between these two arms. A retrospective study of 2019 pancreatic cancer cases after pancreatoduodenectomy (2014–2020) showed that preoperative chemoradiotherapy but not chemotherapy could reduce the postoperative pancreatic fistula rate (2.0% vs 4.2%) [64].
While the added value of neoadjuvant radiotherapy remains to be established, some studies raised concerns on adding radiation to chemotherapy. A recent retrospective study (2014–2019) that investigated the neoadjuvant chemotherapy and radiotherapy outcomes in BRPCs and LAPCs (n =
= 52) found that patients who were candidates for surgery after receiving neoadjuvant chemotherapy of gemcitabine-based chemotherapy or FOLFIRINOX without radiotherapy had a higher R0 resection (35.0% vs 7.6% vs 7.6%) and a prolonged prognosis (median OS, 26.2 vs 14.9 vs 7.3 months) than chemotherapy followed by radiation or concurrent chemoradiotherapy [65]. Moreover, a two-arms phase II RCT (A021501) found that neoadjuvant modified FOLFIRINOX (mFOLFIRINOX) alone (n
52) found that patients who were candidates for surgery after receiving neoadjuvant chemotherapy of gemcitabine-based chemotherapy or FOLFIRINOX without radiotherapy had a higher R0 resection (35.0% vs 7.6% vs 7.6%) and a prolonged prognosis (median OS, 26.2 vs 14.9 vs 7.3 months) than chemotherapy followed by radiation or concurrent chemoradiotherapy [65]. Moreover, a two-arms phase II RCT (A021501) found that neoadjuvant modified FOLFIRINOX (mFOLFIRINOX) alone (n =
= 70) was associated with a favorable median OS (29.8 vs 17.1 months) in patients with BRPC compared to mFOLFIRINOX plus hypofractionated radiotherapy (n
70) was associated with a favorable median OS (29.8 vs 17.1 months) in patients with BRPC compared to mFOLFIRINOX plus hypofractionated radiotherapy (n =
= 56) [66]. It should be noted that the surgeries in this study were conducted in multiple centers with heterogenous experience in performing pancreatectomy following neoadjuvant radiation. Indeed, the outcomes from A021501 conflict with data from high volume centers regarding what should be achieved in the borderline resectable setting. As an example, investigators from Johns Hopkins recently reported their outcomes in borderline resectable patients treated with pre-operative SBRT over a similar time frame as the A021501 study, specifically 2016–2019 [67]. Over that time, 64 patients with BRPC were treated with pre-operative SBRT, which translated into 58 (91%) patients being surgically explored, 50 (78%) patients undergoing resection, and 48 (75%) patients undergoing resection with negative margins. This was dramatically different from what was achieved in Alliance, in which of the 40 patients who underwent SBRT, only 28 (70%) were explored, only 19 (48%) were resected, and only 14 (35%) were resected with negative margins. These raise serious questions regarding the validity of A021501 and its applicability to outcomes at high-volume centers. Moreover, they are in striking contrast to what was achieved in the PREOPANC-1 study, in which patients with resectable or borderline resectable pancreatic cancer were randomized to gemcitabine-based chemoradiation versus upfront surgery [68, 69]. Long-term follow-up of this study showed that OS was improved in the pre-operative chemoradiation arm, with a difference in 5-year OS of 20.5% vs 6.5%. Moreover, unplanned subset analysis showed a clear benefit in borderline resectable patients and even a striking trend towards improvement in resectable patients. Furthermore, the improved outcomes were driven by improvements in local control, not systemic failure, highlighting how a decrease in isolated local failure can translate into improvements in OS.
56) [66]. It should be noted that the surgeries in this study were conducted in multiple centers with heterogenous experience in performing pancreatectomy following neoadjuvant radiation. Indeed, the outcomes from A021501 conflict with data from high volume centers regarding what should be achieved in the borderline resectable setting. As an example, investigators from Johns Hopkins recently reported their outcomes in borderline resectable patients treated with pre-operative SBRT over a similar time frame as the A021501 study, specifically 2016–2019 [67]. Over that time, 64 patients with BRPC were treated with pre-operative SBRT, which translated into 58 (91%) patients being surgically explored, 50 (78%) patients undergoing resection, and 48 (75%) patients undergoing resection with negative margins. This was dramatically different from what was achieved in Alliance, in which of the 40 patients who underwent SBRT, only 28 (70%) were explored, only 19 (48%) were resected, and only 14 (35%) were resected with negative margins. These raise serious questions regarding the validity of A021501 and its applicability to outcomes at high-volume centers. Moreover, they are in striking contrast to what was achieved in the PREOPANC-1 study, in which patients with resectable or borderline resectable pancreatic cancer were randomized to gemcitabine-based chemoradiation versus upfront surgery [68, 69]. Long-term follow-up of this study showed that OS was improved in the pre-operative chemoradiation arm, with a difference in 5-year OS of 20.5% vs 6.5%. Moreover, unplanned subset analysis showed a clear benefit in borderline resectable patients and even a striking trend towards improvement in resectable patients. Furthermore, the improved outcomes were driven by improvements in local control, not systemic failure, highlighting how a decrease in isolated local failure can translate into improvements in OS.
Of note, a critical question is the optimal target volume design for pancreatic cancer. Historically, the approach was to target gross disease as well as involved vasculature in order to improve margin negative resection rates. This was the approach that was taken on A021501, for example. However, more recently, there has been interest in considering elective volume targeting. Indeed, investigators from Johns Hopkins demonstrated that while targeting of gross disease and involved vasculature led to high rates of margin negative resection in the aforementioned cohort from 2016 through 2019, local recurrence rates remained elevated with 1- and 2-year local progression-free survival (PFS) rates of 70.9% and 54.2%, respectively [67]. Importantly, when the locations of the local failures were mapped, they nearly universally mapped the aforementioned “Triangle volume,” which contains the extra-pancreatic neural tracts that have been identified to be at risk of harboring microscopic residual disease following resection, namely the pancreatic head plexus I, the pancreatic head plexus II, the celiac plexus, the superior mesenteric artery plexus, and the common hepatic artery plexus [70]. While more extended surgical dissection of the Triangle volume has been advocated for and is being explored, it also stands that the Triangle volume could serve as the basis for radiation field design [71]. Indeed, following realization that the Triangle volume mediated local failure, the Johns Hopkins investigators modified their target volume to include not only gross disease and involved vasculature but also the full Triangle volume. Recent analysis suggests that making this change in field design has dramatically decreased the local failure rate [72]. Furthermore, exploration into the role of dose-escalation through technologies such as intraoperative radiation is also being explored [73]– [75]. Ultimately, more prospective data is needed with respect to the role of modern field design and dose-escalation in the pre-operative setting.
Therefore, the role of radiotherapy as part of neoadjuvant therapy for BRPCs and LAPCs is still not conclusive. Several more studies are underway, including a randomized, multicenter phase II trial (NCT05083247) assessing the efficacy of adding isotoxic high-dose stereotactic body radiation (iHD-SBRT) to neoadjuvant mFOLFIRINOX or gemcitabine/nab-paclitaxel in BRPCs [76] and a randomized, phase II, clinical trial (NCT03704662) investigating the neoadjuvant chemoradiation with fractionated radiation therapy versus SBRT in combination with chemotherapy for resectable pancreatic cancers, BRPCs or LAPCs [77].
Radiotherapy for locally advanced disease
For unresectable LAPCs, radiotherapy is used as the primary modality for local control. The rationale for its use is based on the significant morbidity and even mortality that uncontrolled local progression can drive, which has been characterized in both autopsy series, which have demonstrated the not insignificant rate of patients dying from local disease complications, as well as additional data highlighting the frequency of hospital admissions related to complications from local progression [78, 79]. However, the prospective, randomized data has been mixed. A trial comparing gemcitabine plus radiotherapy (n =
= 34) to gemcitabine alone (n
34) to gemcitabine alone (n =
= 37) for LAPCs demonstrated an improved OS (11.1 vs 9.2 months) in the gemcitabine plus radiotherapy arm with acceptable toxicity [80]. However, the phase III RCT LAP07 found that no significant difference in OS (15.2 vs 16.5 months) between capecitabine-based chemoradiation of 54 Gy followed by 4 months of maintenance therapy of gemcitabine (n
37) for LAPCs demonstrated an improved OS (11.1 vs 9.2 months) in the gemcitabine plus radiotherapy arm with acceptable toxicity [80]. However, the phase III RCT LAP07 found that no significant difference in OS (15.2 vs 16.5 months) between capecitabine-based chemoradiation of 54 Gy followed by 4 months of maintenance therapy of gemcitabine (n =
= 109) and gemcitabine alone (n
109) and gemcitabine alone (n =
= 112) for LAPCs [81]. Nevertheless, chemoradiation decreased the local tumor progression (32% vs 46%). More recently, modern technologies have been developed to deliver higher “ablative” doses to those portions of the tumor that are spatially situated away from dose-limiting gastrointestinal luminal organs. Single-arm retrospective and prospective studies have demonstrated further improvements in local control rates with such dose-escalated radiation, although its benefit compared to chemotherapy alone in the prospective setting still needs to be demonstrated [82]– [90].
112) for LAPCs [81]. Nevertheless, chemoradiation decreased the local tumor progression (32% vs 46%). More recently, modern technologies have been developed to deliver higher “ablative” doses to those portions of the tumor that are spatially situated away from dose-limiting gastrointestinal luminal organs. Single-arm retrospective and prospective studies have demonstrated further improvements in local control rates with such dose-escalated radiation, although its benefit compared to chemotherapy alone in the prospective setting still needs to be demonstrated [82]– [90].
Notably, while LAPC used to be synonymous with unresectable, this is clearly not the case anymore, as series from high-volume institutions have shown the ability to achieve margin negative resection in a high proportion of patients [13, 91]. It should be noted that the vast majority of patients in these series were treated with pre-operative radiation such that the ability to achieve such high margin negative rates in the LAPC setting with chemotherapy alone is unclear. The value that pre-operative radiation may have in LAPC patients undergoing exploration is being explored on the CONKO-007 study [63]. While we await publication of results and longer-term follow-up, initial presentation has suggested improvement in margin negative resection rate with pre-operative radiation, with some signal that long term 5-year OS may also be higher in the chemoradiation arm (10.1% vs 3.8%, not formally statistically compared), which would mirror results from the PREOPANC-1 study referenced above. Ultimately, more data is needed in this regard.
Chemotherapy
First-line chemotherapy for advanced and metastatic pancreatic cancer
For patients with advanced or metastatic disease, chemotherapy is the only systemic therapy that offers a meaningful benefit. Before the registration of gemcitabine, 5-FU was the only chemotherapeutic option for treating pancreatic cancer for two decades. Then, gemcitabine has been approved as a first-line treatment for pancreatic cancer since 1997 (Fig. 1), when a randomized trial showed that gemcitabine (n =
= 63) is more effective by having a higher clinical response (23.8% vs 4.8%) and providing a moderately better survival advantage (median OS, 5.65 vs 4.41 months) than 5-FU (n
63) is more effective by having a higher clinical response (23.8% vs 4.8%) and providing a moderately better survival advantage (median OS, 5.65 vs 4.41 months) than 5-FU (n =
= 63) in advanced pancreatic cancer [92]. Subsequently, gemcitabine has been investigated in combination with other agents or compared with other combination chemotherapy; however, most of these studies did not achieve their primary endpoint, including 5-FU [93], irinotecan [94], cisplatin [95, 96], oxaliplatin [97], capecitabine [98]– [100], FOLFIRI.3 (irinotecan, leucovorin and 5-FU) [101], tipifarnib [102], cetuximab [103], bevacizumab [104], and axitinib [105]. Nevertheless, in a phase III study, gemcitabine plus erlotinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, emerged as a regimen conferring a statistically significant survival advantage over gemcitabine plus placebo (Fig. 1) [106], however, the prolongation of median OS by 10 days is not considered clinically meaningful.
63) in advanced pancreatic cancer [92]. Subsequently, gemcitabine has been investigated in combination with other agents or compared with other combination chemotherapy; however, most of these studies did not achieve their primary endpoint, including 5-FU [93], irinotecan [94], cisplatin [95, 96], oxaliplatin [97], capecitabine [98]– [100], FOLFIRI.3 (irinotecan, leucovorin and 5-FU) [101], tipifarnib [102], cetuximab [103], bevacizumab [104], and axitinib [105]. Nevertheless, in a phase III study, gemcitabine plus erlotinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, emerged as a regimen conferring a statistically significant survival advantage over gemcitabine plus placebo (Fig. 1) [106], however, the prolongation of median OS by 10 days is not considered clinically meaningful.
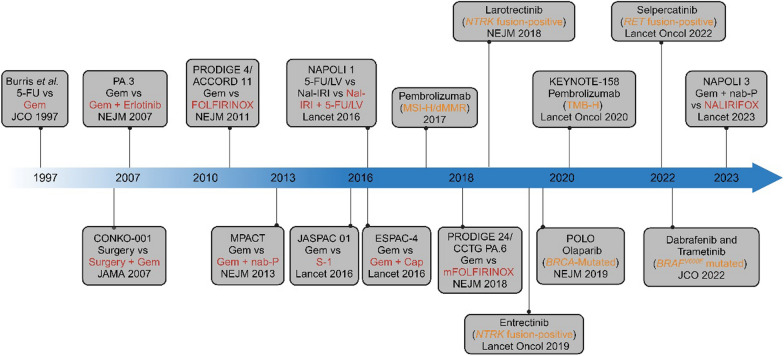
Timeline for pancreatic cancer treatment progression. Initial research demonstrated the efficacy of gemcitabine (Gem) as both adjuvant therapy for resectable pancreatic cancer and systemic treatment for advanced disease. Over the past decade, significant advancements have been made in chemotherapy options for pancreatic cancer, including the introduction of FOLFIRINOX (oxaliplatin, irinotecan, leucovorin, and fluorouracil), gemcitabine plus nab-paclitaxel (Gem +
+ nab-P), S-1, liposomal irinotecan (Nal-IRI), gemcitabine plus capecitabine (Gem
nab-P), S-1, liposomal irinotecan (Nal-IRI), gemcitabine plus capecitabine (Gem +
+ Cap), modified FOLFIRINOX (mFOLFIRINOX), and NALIRIFOX (liposomal irinotecan, oxaliplatin, leucovorin, and fluorouracil). Immunotherapy has also made strides, with pembrolizumab, which targets the PD-1/PD-L1 immune checkpoint pathway, showing improved outcomes in MSI-H/dMMR and TMB-H solid tumors. Erlotinib, an EGFR-targeting agent, showed slight improvement when combined with Gem compared to Gem alone. Recent clinical investigations have highlighted the efficacy of PARP inhibitors such as olaparib in significantly prolonging survival for patients with germline BRCA-mutated pancreatic cancer, leading to FDA approval for maintenance treatment in cases with non-progressing disease following at least 16 weeks of first-line platinum-based chemotherapy. Moreover, targeted therapies such as larotrectinib and entrectinib for NTRK fusion-positive solid tumors, dabrafenib and trametinib for solid tumors with BRAFV600E mutations, and selpercatinib for RET fusion-positive solid tumors have received FDA approval. In the figure, red font denotes treatment strategies that demonstrated superior outcomes in corresponding randomized controlled trials, while orange font highlights the specific genetic alterations or subtypes targeted by the therapies
Cap), modified FOLFIRINOX (mFOLFIRINOX), and NALIRIFOX (liposomal irinotecan, oxaliplatin, leucovorin, and fluorouracil). Immunotherapy has also made strides, with pembrolizumab, which targets the PD-1/PD-L1 immune checkpoint pathway, showing improved outcomes in MSI-H/dMMR and TMB-H solid tumors. Erlotinib, an EGFR-targeting agent, showed slight improvement when combined with Gem compared to Gem alone. Recent clinical investigations have highlighted the efficacy of PARP inhibitors such as olaparib in significantly prolonging survival for patients with germline BRCA-mutated pancreatic cancer, leading to FDA approval for maintenance treatment in cases with non-progressing disease following at least 16 weeks of first-line platinum-based chemotherapy. Moreover, targeted therapies such as larotrectinib and entrectinib for NTRK fusion-positive solid tumors, dabrafenib and trametinib for solid tumors with BRAFV600E mutations, and selpercatinib for RET fusion-positive solid tumors have received FDA approval. In the figure, red font denotes treatment strategies that demonstrated superior outcomes in corresponding randomized controlled trials, while orange font highlights the specific genetic alterations or subtypes targeted by the therapies
Combination chemotherapy for advanced and metastatic pancreatic cancer has made significant progress in the last two decades (Table 2). In 2003, Conroy and colleagues reported the results of an open-label phase I study demonstrating the safety and feasibility of a novel multi-agent chemotherapy regimen, the combination of oxaliplatin, irinotecan, and leucovorin/5-FU, designated FOLFIRINOX, for treating metastatic solid tumors [107]. In 2011, the PRODIGE 4/ACCORD 11 trial subsequently showed that FOLFIRINOX was associated with a better median OS (11.1 vs 6.8 months) and PFS (6.4 vs 3.3 months) in patients with metastatic pancreatic cancer, but increased toxicity including grade 3–4 neutropenia, febrile neutropenia, thrombocytopenia, diarrhea, and sensory neuropathy, compared to gemcitabine (Fig. 1) [108]. However, no treatment-related deaths occurred in the FOLFIRINOX arm while fewer patients in this arm experienced deterioration in quality of life at the 6-month timepoint compared to gemcitabine alone (31% vs 66%). This trial thus established FOLFIRINOX as a new standard for advanced or metastatic pancreatic cancer. Indeed, a dedicated quality of life analysis subsequently showed that FOLFIRINOX maintained or conferred even an improvement of quality of life compared to gemcitabine alone in patients with metastatic pancreatic cancer [109]. Subsequently, a multicenter phase II study accessed mFOLFIRINOX in locally advanced (n =
= 31) and metastatic (n
31) and metastatic (n =
= 44) pancreatic cancer and concluded that mFOLFIRINOX offers equivalent efficacy, but lower grade 3/4 adverse events including neutropenia (12.2% vs 45.7%), vomiting (2.7% vs 14.5%) and fatigue (12.2% vs 23.6%), compared to the original FOLFIRINOX in the PRODIGE 4/ACCORD 11 study [110]. Another randomized phase II trial (PANOPTIMOX-PRODIGE 35) evaluated an oxaliplatin stop-and-go strategy and a 5-FU maintenance strategy [111]. In this trial, patients were assigned to receive either 6 months of FOLFIRINOX (Arm A), 4 months of FOLFIRINOX followed by leucovorin plus 5-FU maintenance treatment (Arm B), or alternate between gemcitabine and FOLFIRI (the combination of 5-FU, leucovorin, and irinotecan) every 2 months as maintenance therapy (Arm C). Although this study did not reach the primary endpoint, median survival without deterioration in quality-of-life scores was the highest in Arm B with a maintenance strategy (11.4 months) compared to Arms A and C (7.2 and 7.5 months, respectively).
44) pancreatic cancer and concluded that mFOLFIRINOX offers equivalent efficacy, but lower grade 3/4 adverse events including neutropenia (12.2% vs 45.7%), vomiting (2.7% vs 14.5%) and fatigue (12.2% vs 23.6%), compared to the original FOLFIRINOX in the PRODIGE 4/ACCORD 11 study [110]. Another randomized phase II trial (PANOPTIMOX-PRODIGE 35) evaluated an oxaliplatin stop-and-go strategy and a 5-FU maintenance strategy [111]. In this trial, patients were assigned to receive either 6 months of FOLFIRINOX (Arm A), 4 months of FOLFIRINOX followed by leucovorin plus 5-FU maintenance treatment (Arm B), or alternate between gemcitabine and FOLFIRI (the combination of 5-FU, leucovorin, and irinotecan) every 2 months as maintenance therapy (Arm C). Although this study did not reach the primary endpoint, median survival without deterioration in quality-of-life scores was the highest in Arm B with a maintenance strategy (11.4 months) compared to Arms A and C (7.2 and 7.5 months, respectively).
Table 2
Chemotherapy based systemic treatment for locally advanced/metastatic pancreatic cancer
| Category (recruitment period) | Phase of trial | Number of patients | Disease stage | Candidate drug and combination regimen | Primary outcome | Survival | Clinical trial identifier and reference | |
|---|---|---|---|---|---|---|---|---|
| Months | HR (95% CI) | |||||||
| PRODIGE 4/ACCORD 11 (2005–2009) | III | 342 | Metastatic | FOLFIRINOX | OS | 11.1 | 0.57 (0.45–0.73) | NCT00112658 [108] |
| Gemcitabine | 6.8 | |||||||
MPACT (2009–2012) | III | 861 | Metastatic | GnP | OS | 8.5 | 0.72 (0.62–0.83) | NCT00844649 [112] |
| Gemcitabine | 6.7 | |||||||
| American multicenter (2011–2014) | II | 75 | Locally advanced | mFOLFIRINOX | OS | 26.6 | / | NCT01523457 [110] |
| Metastatic | 10.2 | |||||||
| PANOPTIMOX-PRODIGE 35 (2015–2016) | II | 276 | Metastatic | FOLFIRINOX | PFS at 6 months | 47.1% | – | NCT02352337 [111] |
| FOLFIRINOX followed by 5-FU/LV | 42.9% | |||||||
| Gemcitabine plus FOLFIRI | 34.1% | |||||||
| NAPOLI 3 (2020–2021) | III | 770 | Metastatic | NALIRIFOX | OS | 11.1 | 0.83 (0.70–0.99) | NCT04083235 [116] |
| GnP | 9.2 | |||||||
PASS-01 (2020–2024) | II | 140 | Metastatic | mFOLFIRINOX | PFS | 4.0 | – | NCT04469556 [117] |
| GnP | 5.1 | |||||||
| CONKO-003 (2004–2007) | III | 160 | Advanced; previously treated with gemcitabine-based therapy | FF | OS | 3.3 | 0.66 (0.48–0.91) | NCT00786058 [121] |
| OFF | 5.9 | |||||||
| PANCREOX (2010–2012) | III | 108 | Advanced; previously treated with gemcitabine-based therapy | mFOLFOX6 | PFS | 3.1 | 1.00 (0.66–1.53) | NCT01121848 [122] |
| 5-FU/LV | 2.9 | |||||||
NAPOLI-1 (2012–2013) | III | 417 | Metastatic; previously treated with gemcitabine-based therapy | Nanoliposomal irinotecan | OS | 4.9 | 0.67 (0.49–0.92) ** | NCT01494506 [123] |
| 5-FU/LV | 4.2 | |||||||
| Nanoliposomal irinotecan plus 5-FU/LV | 6.1 | |||||||
LAPACT (2015–2018) | II | 106 | Locally advanced | GnP | time to treatment failure | 9.0 | – | NCT02301143 [118] |
| NEOLAP-AIOPAK-0113 (2014–2018) | II | 130 | Locally advanced | GnP | Surgical conversion rate | 35.9% | 0.72*** (0.35–1.45) | NCT02125136 [119] |
| GnP followed by FOLFIRINOX | 43.9% | |||||||
PRODIGE 29/NEOPAN (2015–2022) | III | 171 | Locally advanced | FOLFIRINOX | PFS | 9.8 | 0.57 (0.3–1.08) | NCT02539537 [120] |
| Gemcitabine | 7.5 | |||||||
FOLFIRINOX: oxaliplatin, irinotecan, fluorouracil, and leucovorin; GnP: gemcitabine/nab-paclitaxel; mFOLFIRINOX: modified FOLFIRINOX; 5-FU/LV: fluorouracil and leucovorin; FOLFIRI: fluorouracil, leucovorin, and irinotecan; NALIRIFOX: liposomal irinotecan, oxaliplatin, fluorouracil and leucovorin; FF: folinic acid and fluorouracil; OFF: oxaliplatin, folinic acid and fluorouracil. mFOLFOX6: oxaliplatin, fluorouracil and leucovorin. *97.5% CI; ** 6.1 vs 4.2 months; *** OR: odds ratio
Albumin-bound paclitaxel (nab-paclitaxel) is a nanoparticle form of paclitaxel. In 2013, the large, open-label, international, randomized, phase III MPACT trial enrolled 861 patients with metastatic pancreatic cancer and no prior chemotherapy and randomized them to receive gemcitabine plus nab-paclitaxel or gemcitabine alone (Fig. 1) [112]. Improved survival was observed in the gemcitabine plus nab-paclitaxel arm compared to gemcitabine alone (median OS, 8.5 vs 6.7 months; median DFS, 5.5 vs 3.7 months; response rate, 23% vs 7%, respectively). Updated results at the 42-month landmark revealed that, while no patients remained alive in the gemcitabine alone arm, a small yet impactful proportion of 3% in the gemcitabine plus nab-paclitaxel arm still survived [113].
Nano-liposomal irinotecan represents an innovative drug delivery system in which the active chemotherapeutic agent, irinotecan sucrosofate salt, is encapsulated within diminutive pegylated liposomal particles [114]. In a phase I/II study, the NALIRIFOX regimen, which is based on mFOLFIRINOX with irinotecan replaced by liposomal irinotecan, demonstrated a median PFS of 9.2 months and median OS of 12.6 months as the first-line treatment in locally advanced/metastatic PDACs [115]. In 2023, the phase III trial (NAPOLI 3) showed that NALIRIFOX (n =
= 383) as the first-line treatment had a significantly better median OS (11.1 vs 9.2 months) than gemcitabine/nab-paclitaxel (n
383) as the first-line treatment had a significantly better median OS (11.1 vs 9.2 months) than gemcitabine/nab-paclitaxel (n =
= 387) (Fig. 1) [116]. Grade 3 or higher treatment-emergent adverse events and treatment-related deaths were comparable between the two arms [116]. These results led the Food and Drug Administration (FDA) to approve liposomal irinotecan for the first-line treatment of pancreatic cancer in 2024.
387) (Fig. 1) [116]. Grade 3 or higher treatment-emergent adverse events and treatment-related deaths were comparable between the two arms [116]. These results led the Food and Drug Administration (FDA) to approve liposomal irinotecan for the first-line treatment of pancreatic cancer in 2024.
By far, no formal comparison was made between FOLFIRINOX and gemcitabine/nab-paclitaxel. PASS-01 is a multicenter, randomized phase II trial evaluating the benefit of first-line mFOLFIRINOX vs gemcitabine/nab-paclitaxel in untreated metastatic pancreatic cancer patients whose baseline tumor biopsies were obtained for whole genome/transcriptional sequencing and for establishing patient-derived organoids [117]. Preliminary analysis showed an over 80% success rate in obtaining whole genomes and a 50% success rate in establishing patient-derived organoids for drug sensitivity tests. Interestingly, median PFS was 5.1 months in the gemcitabine/nab-paclitaxel arm (n =
= 69) and 4.0 months in the mFOLFIRINOX arm (n
69) and 4.0 months in the mFOLFIRINOX arm (n =
= 71) although PFS in both arms appears to be poorer than historical controls.
71) although PFS in both arms appears to be poorer than historical controls.
Several studies have investigated the efficacy of gemcitabine/nab-paclitaxel and FOLFIRINOX in LAPCs. In a phase II study (LAPACT) investigating gemcitabine/nab-paclitaxel for treating 106 patients, 62 patients (58%) completed induction therapy and 17 (16%) underwent surgery (7 had R0 resection, 9 had R1), with a median time to treatment failure of 9.0 months [118]. Another randomized phase II study (NEOLAP-AIO-PAK-0113) of 64 patients yielded a higher surgical conversion rate and median OS with gemcitabine/nab-paclitaxel (n =
= 64) at 35.9% and 18.5 months, respectively and with gemcitabine/nab-paclitaxel followed by FOLFIRINOX (n
64) at 35.9% and 18.5 months, respectively and with gemcitabine/nab-paclitaxel followed by FOLFIRINOX (n =
= 66) at 43.9% and 20.7 months, respectively [119]. A phase III study (PRODIGE 29/NEOPAN) also showed that FOLFIRINOX yielded a significantly longer PFS (9.8 vs 7.5 months) compared to gemcitabine with similar grade
66) at 43.9% and 20.7 months, respectively [119]. A phase III study (PRODIGE 29/NEOPAN) also showed that FOLFIRINOX yielded a significantly longer PFS (9.8 vs 7.5 months) compared to gemcitabine with similar grade ≥
≥ 3 adverse events (41% vs 38%) [120]. These findings thus support using FOLFIRINOX or gemcitabine/nab-paclitaxel in the induction chemotherapy for LAPCs.
3 adverse events (41% vs 38%) [120]. These findings thus support using FOLFIRINOX or gemcitabine/nab-paclitaxel in the induction chemotherapy for LAPCs.
Second-line chemotherapy for advanced and metastatic pancreatic cancer
Second-line regimens after gemcitabine-based chemotherapy for advanced pancreatic cancer have been studied in several trials (Table 2). The CONKO-003 randomized phase III trial demonstrated that second-line treatment with oxaliplatin plus folinic acid and fluorouracil (OFF) significantly extended survival compared to folinic acid and fluorouracil (FF) alone (median OS, 5.9 vs 3.3 months) [121]. However, results from the phase III PANCREOX trial showed that the addition of oxaliplatin to 5-FU/leucovorin (mFOLFOX6) as second-line treatment may be detrimental compared to 5-FU/leucovorin in patients with advanced pancreatic cancer who progressed on gemcitabine-based treatment, including worse median OS (6.1 vs 9.9 months) and increased grade 3/4 adverse events (63% vs 11%) [122]. Later, the randomized phase III trial NAPOLI-1 supported liposomal irinotecan with 5-FU/leucovorin (5-FU/liposomal irinotecan) as a standard of care second-line therapy in metastatic pancreatic cancer after previous gemcitabine-based therapy by showing a significantly longer median OS with 5-FU/liposomal irinotecan than that with 5-FU/leucovorin (6.1 vs 4.2 months) (Fig. 1) [123]. 5-FU/liposomal irinotecan became the only standard of care second-line therapy after gemcitabine-based therapy for metastatic pancreatic cancer. Nevertheless, it would be appropriate to use mFOLFIRINOX as second-line therapy for selected patients with a good performance status after gemcitabine/nab-paclitaxel.
As a second-line systemic treatment after progression with FOLFIRINOX has not been standardized, the current clinical practice is to switch to gemcitabine/nab-paclitaxel or gemcitabine-based regimen. On another hand, FOLFIRINOX is also a choice of second-line treatment if the patient progresses through a gemcitabine-based first-line treatment. Multiple retrospective studies supported the use of gemcitabine/nab-paclitaxel as a second-line regimen for patients who cannot tolerate or progress with FOLFIRINOX [124]– [126].
Adjuvant chemotherapy
Adjuvant systemic treatment is universally recommended for all eligible patients undergoing resection for PDAC. Gemcitabine monotherapy has been a cornerstone of adjuvant chemotherapy for pancreatic cancer for decades. However, compelling evidence supporting gemcitabine as the standard of care for adjuvant treatment was not established until 2007 by the randomized controlled trial CONKO-001 (Table 3) [127]. Preliminary results of this trial showed that postoperative adjuvant gemcitabine therapy significantly delayed the development of recurrent disease after complete resection of pancreatic cancer compared to observation alone (median DFS, 13.4 vs 6.9 months; DFS at 5-year, 16.5% vs 5.5%). Long-term outcomes from this trial also showed improvements in 5-year OS (20.7% vs 10.4%) and 10-year OS (12.2% vs 7.7%) [128]. In 2010, the RCT ESPAC-3 investigated adjuvant chemotherapy with 5-FU plus folinic acid regimen (n =
= 551) versus gemcitabine (n
551) versus gemcitabine (n =
= 537) following pancreatic cancer resection. The study reported no statistically significant difference between the two arms in terms of median OS (23.0 vs 23.6 months), PFS (14.1 vs 14.3 months), or quality-of-life outcomes [129]. In 2016, a phase III, randomized, non-inferiority trial conducted in Japan (JASPAC 01) reported that adjuvant chemotherapy with S-1 was non-inferior, but offered a superior survival (5-year OS, 44.1% vs 24.4%; median OS, 46.5 vs 25.5 months) compared to gemcitabine (Fig. 1) [130]. Consequently, S-1 became the standard of care for adjuvant chemotherapy in East Asia. However, S-1 has not been widely adopted in North America and Europe, where gemcitabine remains the standard of care. This is primarily due to the lower maximum tolerated dose of S-1 in Caucasians compared to East Asians, largely because of increased gastrointestinal toxicity, particularly diarrhea [131]. As a result, it has been difficult to replicate the favorable outcomes seen in Asian clinical trials in Western populations, leading to the continued preference for gemcitabine in these regions.
537) following pancreatic cancer resection. The study reported no statistically significant difference between the two arms in terms of median OS (23.0 vs 23.6 months), PFS (14.1 vs 14.3 months), or quality-of-life outcomes [129]. In 2016, a phase III, randomized, non-inferiority trial conducted in Japan (JASPAC 01) reported that adjuvant chemotherapy with S-1 was non-inferior, but offered a superior survival (5-year OS, 44.1% vs 24.4%; median OS, 46.5 vs 25.5 months) compared to gemcitabine (Fig. 1) [130]. Consequently, S-1 became the standard of care for adjuvant chemotherapy in East Asia. However, S-1 has not been widely adopted in North America and Europe, where gemcitabine remains the standard of care. This is primarily due to the lower maximum tolerated dose of S-1 in Caucasians compared to East Asians, largely because of increased gastrointestinal toxicity, particularly diarrhea [131]. As a result, it has been difficult to replicate the favorable outcomes seen in Asian clinical trials in Western populations, leading to the continued preference for gemcitabine in these regions.
Table 3
Adjuvant systemic treatment for resected PDAC
| Category/Target (recruitment period) | Phase of trial | Number of patients | Disease stage | Candidate drug and combination regimen | Primary outcome | Survival | Clinical trial identifier and reference | |
|---|---|---|---|---|---|---|---|---|
| Months | HR (95% CI) | |||||||
| CONKO-001 (1998–2004) | III | 354 | Resectable | Gemcitabine | DFS | 13.4 | – | ISRCTN34802808 [127] |
| Observation | 6.7 | |||||||
ESPAC-3 (2000–2007) | III | 1088 | Resectable | Folinic acid and fluorouracil | OS | 23.0 | 0.94 (0.81–1.08) | NCT00058201 [129] |
| Gemcitabine | 23.6 | |||||||
| JASPAC 01 (2007–2010) | III | 385 | Resectable | S-1 | OS | 46.5 | 0.57 (0.44–0.72) | UMIN000000655 [130] |
| Gemcitabine | 25.5 | |||||||
ESPAC-4 (2008–2014) | III | 730 | Resectable | Gemcitabine plus capecitabine | OS | 28.0 | 0.82 (0.68–0.98) | ISRCTN96397434 [132] |
| Gemcitabine | 25.5 | |||||||
| CONKO-005 (2008–2013) | III | 436 | Resectable (R0 resection) | Gemcitabine plus erlotinib | DFS | 11.4 | 0.94 (0.76–1.15) | [133] |
| Gemcitabine | 11.4 | |||||||
| RTOG 0848 (2009–2018) | II/III | 354 | Resectable | Gemcitabine based chemotherapy | OS | 31 | – | NCT01013649 [51] |
Gemcitabine based chemotherapy + + 5FU/capecitabine/RT 5FU/capecitabine/RT | 27 | |||||||
| PRODIGE 24/CCTG PA6 (2012–2016) | III | 493 | Resectable | mFOLFIRINOX | DFS | 21.6 | 0.58 (0.46–0.73) | NCT01526135 [135] |
| Gemcitabine | 12.8 | |||||||
APACT (2014–2016) | III | 597 | Resectable | GnP | DFS | 19.4 | 0.88 (0.73–1.06) | NCT01964430 [134] |
| Gemcitabine | 18.8 | |||||||
In 2017, the multicenter, randomized phase III trial ESPAC-4 compared adjuvant therapy with gemcitabine and capecitabine versus gemcitabine monotherapy. The trial demonstrated that the median OS in the gemcitabine plus capecitabine arm (n =
= 364) was significantly improved (28.0 vs 25.5 months) compared to the gemcitabine monotherapy arm (n
364) was significantly improved (28.0 vs 25.5 months) compared to the gemcitabine monotherapy arm (n =
= 366) [132]. This finding supports the use of gemcitabine in combination with capecitabine as a standard-of-care adjuvant therapy for pancreatic cancer.
366) [132]. This finding supports the use of gemcitabine in combination with capecitabine as a standard-of-care adjuvant therapy for pancreatic cancer.
In contrast, the CONKO-005 trial, which investigated the addition of erlotinib to gemcitabine, did not show any benefit from the addition of erlotinib as an adjuvant therapy [133]. The phase II/III RTOG 0848 trial (NCT01013649) demonstrated that while adding adjuvant radiation to chemotherapy did not improve OS across the entire study population, it did improve DFS, with both OS and DFS showing enhancement in the node-negative subgroup patients [51]. In the randomized phase III trial (APACT), adjuvant gemcitabine/nab-paclitaxel (n =
= 432) offered improved survival (41.8 vs 37.7 months) compared to gemcitabine alone (n
432) offered improved survival (41.8 vs 37.7 months) compared to gemcitabine alone (n =
= 434); however, the primary endpoint of DFS was not achieved (19.4 vs 18.8 months) [134]. Finally, in 2018, the PRODIGE-24/CCTG PA6 trial reported its meeting of the primary endpoint by showing adjuvant therapy with mFOLFIRINOX significantly improved median DFS (21.6 vs 12.8 months) and OS (54.4 vs 35.0 months) compared to gemcitabine (Fig. 1) [135]. Since then, mFOLFIRINOX has become a standard-of-care adjuvant chemotherapy option, alongside the combination of gemcitabine and capecitabine.
434); however, the primary endpoint of DFS was not achieved (19.4 vs 18.8 months) [134]. Finally, in 2018, the PRODIGE-24/CCTG PA6 trial reported its meeting of the primary endpoint by showing adjuvant therapy with mFOLFIRINOX significantly improved median DFS (21.6 vs 12.8 months) and OS (54.4 vs 35.0 months) compared to gemcitabine (Fig. 1) [135]. Since then, mFOLFIRINOX has become a standard-of-care adjuvant chemotherapy option, alongside the combination of gemcitabine and capecitabine.
Neoadjuvant chemotherapy as a part of multidisciplinary management
Pancreatic cancer frequently presents with micrometastatic disease even at early stages, underscoring the necessity of a systemic treatment paradigm [136]. Moreover, approximately one-third of patients are unable to complete planned adjuvant chemotherapy regimens following pancreatic resection due to postoperative complications [137]. Neoadjuvant therapy aims to optimize patient tolerance and the delivery of full-dose chemotherapy regimens, mitigating the risk of inadequate management of subclinical metastatic deposits that often drive mortality. Neoadjuvant therapy also provides opportunities to evaluate in vivo tumor responses to chemotherapeutics and potentially identify patients most likely to benefit from surgical resection. Additionally, neoadjuvant therapy may increase the rate of R0 margin-negative resections by downsizing primary and nodal tumor burdens before surgery. Recent clinical trials investigating neoadjuvant therapy for resectable pancreatic cancer and BRPC are summarized in Table 4.
Table 4
Chemotherapy or chemoradiation therapy based neoadjuvant systemic treatment for borderline resectable and resectable PDAC
| Category/Target (Recruitment period) | Phase of trial | Number of patients | Disease stage | Candidate drug and combination regimen | Primary outcome/endpoint | Months/rates | HR (95% CI) | Clinical trial identifier and reference |
|---|---|---|---|---|---|---|---|---|
PACT-15 (2010–2015) | II/III | 88 | Resectable | Surgery > > 6 cycles gemcitabine 6 cycles gemcitabine | Event-free at 1 year | 23% | – | NCT01150630 [139] |
Surgery > > 6 cycles PEXG 6 cycles PEXG | 50% | |||||||
3 cycles PEXG > > Surgery Surgery > > 3 cycles PEXG 3 cycles PEXG | 66% | |||||||
Prep-02/JSAP-05 (2013–2016) | II/III | 364 | Resectable | 2 cycles gemcitabine/S-1 > > surgery surgery > > 6-month S-1 6-month S-1 | OS | 36.7 | 0.72 (0.55–0.94) | UMIN000009634 [140] |
Surgery > > 6-month S-1 6-month S-1 | 26.6 | |||||||
| PREOPANC-1 (2013–2017) | III | 246 | Resectable/ borderline resectable | 3 cycles gemcitabine based chemoradiotherapy > > surgery surgery > > 4 cycles gemcitabine 4 cycles gemcitabine | OS | 15.7 | 0.73 (0.56–0.96) | EudraCT 2012–003181-40 [69] |
Upfront surgery > > six cycles of adjuvant gemcitabine six cycles of adjuvant gemcitabine | 14.3 | |||||||
SWOG S1505 (2015–2018) | II | 102 | Resectable | 3 cycles mFOLFIRINOX > > surgery surgery > > 3 cycles mFOLFIRINOX 3 cycles mFOLFIRINOX | 2-year OS | 41.6% | – | NCT02562716 [145] |
3 cycles GnP > > surgery surgery > > 3 cycles GnP 3 cycles GnP | 48.8% | |||||||
NEONAX (2015–2019) | II | 118 | Resectable | 2 cycles GnP > > surgery surgery > > 4 cycles GnP 4 cycles GnP | DFS at 18 months | 33.3% | – | NCT02047513 [142] |
Surgery > > 6 cycles GnP 6 cycles GnP | 41.4% | |||||||
PANACHE01-PRODIGE48 (2017–2020) | II | 146 | Resectable | 4 cycles mFOLFIRINOX > > surgery surgery > > chemotherapy chemotherapy | 1-year OS | 84.1% | – | NCT02959879 [143] |
4 cycles FOLFOX > > surgery surgery > > chemotherapy chemotherapy | 71.8% | |||||||
surgery > > chemotherapy chemotherapy | 80.8% | |||||||
NORPACT-1 (2017–2021) | II | 140 | Resectable | 4 cycles FOLFIRINOX > > surgery surgery > > 8 cycles mFOLFIRINOX 8 cycles mFOLFIRINOX | OS at 18 months | 60% | – | NCT02919787 [144] |
Surgery > > 12 cycles mFOLFIRINOX 12 cycles mFOLFIRINOX | 73% | |||||||
PREOPANC-2 (2018–2021) | III | 375 | Resectable/ borderline resectable | 8 cycles mFOLFIRINOX > > surgery surgery | OS | 21.9 | 0.87 (0.68–1.12) | EudraCT 2017–002036-17 [146] |
3 cycles gemcitabine with hypofractionated radiotherapy > > surgery surgery > > 4 cycles gemcitabine 4 cycles gemcitabine | 21.3 | |||||||
nITRO (2018–2022) | II | 107 | Resectable | 3 cycles NALIRIFOX > > surgery surgery > > 3 cycles NALIRIFOX 3 cycles NALIRIFOX | R0 resection rate | 65.3% | – | NCT03528785 [147] |
NEO-Nal-IRI (2019–2023) | II | 45 | Resectable/ borderline resectable | 8 cycles NALIRIFOX > > surgery surgery | Composite 30 day postoperative major complication rate | 10% | – | NCT03483038 [148] |
PREOPANC-3 Ongoing | III | 378 (Estimated) | Resectable | 8 cycles mFOLFIRINOX > > surgery surgery > > 4 cycles mFOLFIRINOX 4 cycles mFOLFIRINOX | OS | – | – | NCT04927780 [11] |
Surgery > > 12 cycles mFOLFIRINOX 12 cycles mFOLFIRINOX | ||||||||
Alliance A021806 Ongoing | III | 352 (Estimated) | Resectable | 8 cycles mFOLFIRINOX > > surgery surgery > > 4 cycles mFOLFIRINOX 4 cycles mFOLFIRINOX | OS | – | – | NCT04340141 [10] |
Surgery > > 12 cycles mFOLFIRINOX 12 cycles mFOLFIRINOX | ||||||||
NeoFOL-R Ongoing | III | 609 (Estimated) | Resectable | 6 cycles of mFOLFIRINOX > > surgery surgery > > 6 cycles of mFOLFIRINOX 6 cycles of mFOLFIRINOX | 2-year survival rate | – | – | NCT05529940 |
Surgery > > 12 cycles mFOLFIRINOX 12 cycles mFOLFIRINOX | ||||||||
Alliance A021101 (2013–2014) | I | 22 | Borderline resectable | 4 cycles of mFOLFIRINOX > > 5.5 weeks of capecitabine-based chemoradiation 5.5 weeks of capecitabine-based chemoradiation > > surgery surgery | Pancreatectomy rate | 68% | – | NCT01821612 [149] |
| America single center (2012–2016) | II | 48 | Borderline resectable | 8 cycles of FOLFIRINOX > > capecitabine based chemoradiotherapy capecitabine based chemoradiotherapy > > surgery surgery | R0 resection rate | 65% | – | NCT01591733 [61] |
ESPAC-5 (2014–2018) | II | 90 | Borderline Resectable | Surgery > > adjuvant therapy adjuvant therapy | Recruitment rate; R0 resection | 68%; 14% | – | ISRCTN, 89500674 [150] |
Gemcitabine/capecitabine, FOLFIRINOX or capecitabine-based chemoradiotherapy > > surgery surgery > > adjuvant therapy adjuvant therapy | 55%; 23% | |||||||
| Alliance A021501 (2017–2019) | II | 126 | Borderline resectable | 8 cycles of mFOLFIRINOX > > surgery surgery > > 4 cycles of FOLFOX6 4 cycles of FOLFOX6 | 18-month OS rate | 66.7% | – | NCT02839343 [66] |
7 cycles of mFOLFIRINOX > > radiotherapy radiotherapy > > surgery surgery > > 4 cycles of FOLFOX6 4 cycles of FOLFOX6 | 47.3% | |||||||
GABARNANCE (2017–2022) | II/III | 112 | Borderline resectable | GnP > > surgery surgery > > S-1 S-1 | OS | 23.1 | 0.76 (0.47–1.22) | UMIN-CTR 000026858 [130] |
S-1 + + chemoradiotherapy chemoradiotherapy > > surgery surgery > > S-1 S-1 | 31.5 | |||||||
| AGICC (2016–2022) | II | 49 | Resectable/ borderline resectable | 3 cycles of GnP + + SBRT SBRT > > surgery surgery > > 3 cycles of GnP 3 cycles of GnP | R0 resection rate | 72.7% | – | NCT02723331 [132] |
PANDAS-PRODIGE 44 Ongoing | II | 130 | Borderline resectable | mFOLFIRINOX + + capecitabine-based chemoradiotherapy capecitabine-based chemoradiotherapy > > surgery surgery > > chemotherapy chemotherapy | R0 resection margin rate | – | – | NCT02676349 |
mFOLFIRINOX > > surgery surgery > > chemotherapy chemotherapy |
PEXG: cisplatin, epirubicin, gemcitabine, and capecitabine; FOLFOX6: oxaliplatin, leucovorin, fluorouracil
Neoadjuvant therapy for resectable pancreatic cancer
One of the primary objectives of neoadjuvant chemotherapy for resectable pancreatic cancer is to enhance the likelihood that patients will benefit from a multi-modal treatment approach that combines systemic therapy with surgical intervention. A propensity-matched observational analysis of over 15,000 resected patients demonstrated significantly improved OS among those receiving neoadjuvant therapy compared to those without neoadjuvant therapy (median OS, 26 vs 21 months) [138]. Preliminary data from the randomized phase II/III PACT-15 trial showed that neoadjuvant chemotherapy with the PEXG regimen (cisplatin, epirubicin, capecitabine, and gemcitabine) improved the R0 resection rate and OS (63%, 38.2 months) compared with adjuvant gemcitabine (27%, 20.4 months) and adjuvant PEXG (37%, 26.4 months) for resectable pancreatic cancer [139]. However, with the change in standard of care for adjuvant therapy, the phase III component of PACT-15 was discontinued, leaving the trial inconclusive. Another phase II/III study (Prep-02/JSAP-05) randomized predominantly resectable cases to receive neoadjuvant gemcitabine plus S-1 followed by surgery or undergo upfront surgery followed by six months of adjuvant S-1 [140]. Although the neoadjuvant arm demonstrated improved median OS (36.7 vs 26.6 months), the difference may have been influenced by the unequal chemotherapy regimens between arms. While essentially all the patients in the neoadjuvant arm underwent surgical resection, the trial did not replicate the results of the pivotal phase III JASPAC 01 trial, which reported a median OS of 46.5 months with adjuvant S-1 therapy [130].
Conversely, several RCTs have not supported neoadjuvant therapy. A randomized phase II trial comparing neoadjuvant chemoradiation therapy with gemcitabine/cisplatin to upfront surgery was terminated early due to slow patient recruitment [141]. With only 66 analyzable patients, no significant differences were observed between the two arms in terms of R0 resection rates (52% vs 48%), pathologic node negativity rates (39% vs 30%), or OS (25.0 vs 18.9 months). The NEONAX trial compared perioperative gemcitabine plus nab-paclitaxel to adjuvant gemcitabine plus nab-paclitaxel [142]. The primary endpoint was to achieve an 18-month DFS rate of 55%, based on a 38% 18-month DFS seen with gemcitabine alone in the CONKO-001 trial; however, neither arm met this endpoint, with rates of 33.3% in the perioperative arm and 41.4% in the adjuvant arm [143].
Several phase II studies have evaluated the feasibility and efficacy of neoadjuvant mFOLFIRINOX compared to upfront surgery. The prospective, multicenter, non-comparative phase II PANACHE01-PRODIGE48 trial randomly assigned 146 patients to neoadjuvant mFOLFIRINOX, FOLFOX or upfront surgery arms [144]. The FOLFOX arm was discontinued for lack of efficacy, and the mFOLFIRINOX arm showed no significant improvement in the 1-year OS rate (84.1% vs 80.8%) compared to upfront surgery. The recent multicenter, randomized phase II NORPACT-1 trial included 77 patients receiving neoadjuvant FOLFIRINOX and 63 patients undergoing upfront surgery for resectable pancreatic head cancer [145]. Surprisingly, the median OS in the neoadjuvant FOLFIRINOX arm was 13.4 months shorter than that in the adjuvant FOLFIRINOX arm (25.1 vs 38.5 months), as well as lower resection rate (82% vs 89%). However, this study had several limitations. The phase II design limited the conclusiveness of the results. The use of full-dose neoadjuvant FOLFIRINOX instead of a modified regimen may have contributed to increased toxicity and reduced compliance. Additionally, histological confirmation of PDAC was not mandatory before randomization, leading to the inclusion of 11 patients (8%) with non-PDAC diagnoses, which may have influenced the outcomes although these conditions would be anticipated to have a more favorable outcome. The phase II SWOG S1505 trial evaluated the efficacy of neoadjuvant mFOLFIRINOX versus nab-paclitaxel/gemcitabine for resectable patients. Median OS was 22.4 months for mFOLFIRINOX and 23.6 months for gemcitabine/nab-paclitaxel, failing to show a clinically meaningful improvement over upfront surgery [146]. The multicenter, randomized phase III PREOPANC-2 trial enrolled patients with both BRPC and resectable pancreatic cancer patients across 19 Dutch centers [147]. Patients were randomized to receive either neoadjuvant FOLFIRINOX followed by surgery without adjuvant treatment or neoadjuvant gemcitabine-based chemoradiotherapy followed by adjuvant gemcitabine. This trial found no differences in OS (21.9 vs 21.3 months) or resection rate (77% vs 75%) between the two arms. However, the gemcitabine chemoradiotherapy group achieved significantly higher lymph node-negative (N0) status post-operatively compared to the FOLFIRINOX group (58% vs 47%) [147]. As described above, the field is hoping that the Alliance A021806 trial and PREOPANC-3 trial which are evaluating the efficacy of perioperative mFOLFIRINOX versus adjuvant mFOLFIRINOX will provide a more definitive answer on the role of neoadjuvant chemotherapy in resectable pancreatic cancer [10, 11].
Notably, given the promising role of NALIRIFOX in systemic treatment, this regimen is currently being evaluated in the neoadjuvant setting. For example, in the phase II nITRO trial, NALIRIFOX demonstrated promising outcomes with an R0 resection rate of 65.3% among 76 resected patients, and a median DFS and OS of 31.3 and 44.9 months, respectively [148]. Similarly, in the phase II NEO-Nal-IRI trial, NALIRIFOX as neoadjuvant therapy achieved an R0 resection rate of 89% in 29 patients (14 resectable and 15 borderline resectable) [149]. While these preliminary results suggest potential efficacy of NALIRIFOX as a neoadjuvant therapy, further studies are needed to confirm its benefits. Moreover, the development of a biomarker assay to guide individualized selection of neoadjuvant chemotherapy regimens is crucial. Such an assay could also be applicable for selecting adjuvant chemotherapy regimens. The key challenge remains in identifying biomarkers that can accurately predict which patients are most likely to benefit from neoadjuvant chemotherapy.
The indiscriminate administration of neoadjuvant chemotherapy to all surgically resectable pancreatic cancer patients without appropriate selection could potentially cause unintended harm, as tumor progression or chemotherapy toxicity may render patients ineligible for surgery, which remains the only curative treatment option. Without a biomarker for patient selection, current considerations include selecting individuals with high-risk features associated with an increased likelihood of local or distant recurrence post-resection, such as elevated preoperative carbohydrate antigen 19–9 (CA19-9) levels, radiological evidence of suspicious lymphadenopathy or metastases, or the presence of circulating tumor cells or cell-free DNA. Further validation is needed to determine whether using these criteria to select patients for neoadjuvant therapy would enhance its benefits.
Neoadjuvant therapy for BRPC
There is growing consensus on the benefits of neoadjuvant therapy for BRPC. A key advantage of neoadjuvant chemotherapy in BRPC is the potential increase in the likelihood of achieving R0 resection status. Single-arm studies, such as the Alliance A021101 trial, have provided preliminary evidence supporting the safety and efficacy of neoadjuvant therapy with mFOLFIRINOX followed by capecitabine-based chemoradiation for BRPCs. Of the 15 participants who completed the preoperative protocols (68%), all but one achieved negative margins on pancreatectomy. The median OS for the full cohort reached 21.7 months [150]. The phase III PREOPANC-1 trial randomized 246 patients with resectable or borderline resectable disease to neoadjuvant chemoradiation or upfront surgery (approximately 50% in each group). The neoadjuvant chemoradiotherapy regimen consisted of three cycles of gemcitabine combined with 36 Gy radiotherapy in 15 fractions during the second cycle, followed by four cycles of adjuvant gemcitabine. In contrast, patients in the upfront surgery arm received six cycles of adjuvant gemcitabine. Neoadjuvant therapy improved the R0 resection rate (72% vs 43%) and decreased tumor size (25 vs 33 mm) as well as the percentage of node-positive status (35% vs 82%), perineural invasion (45% vs 85%), and vascular invasion (36% vs 65%). However, the median OS was only slightly prolonged by 1.4 months, from 14.3 to 15.7 months [69]. The ESPAC-5 phase II study randomized 90 BRPCs to short-course neoadjuvant multi-agent chemotherapy, capecitabine-based chemoradiation or upfront surgery alone and showed that neoadjuvant chemotherapy with either FOLFIRINOX or gemcitabine/capecitabine improved 1-year OS compared to upfront surgery (84% and 78%, respectively vs 39%) whereas neoadjuvant 50.4 Gy capecitabine-based chemoradiation did not (60% vs 39%), thus supporting short-course neoadjuvant chemotherapy for BRPCs. Although R0 resection rates did not statistically differ between arms, all arms had low R0 resection rates likely due to the differences among centers in the definition of BRPCs resulting in the inclusion of LAPCs [151].
Immunotherapy
Immunotherapy has achieved significant clinical breakthroughs in various solid tumors, such as non-small cell lung cancer (NSCLC) and melanoma [152, 153]. Targeting immune checkpoint molecules, such as programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), leads to the reinvigoration of the anti-tumor immune response in cancers, resulting in improved clinical outcomes [154]– [156]. Unfortunately, pancreatic cancer responds poorly to immunotherapy, particularly immune checkpoint inhibitors (ICIs), due to its poor immunogenic properties, low number of neoantigens, and immunosuppressive tumor microenvironment (TME) [157, 158]. Single-agent ICIs have thus far proven clinically ineffective, but multi-modal therapies targeting mechanisms of ICI resistance still hold promise, as described in detail below. Strategies seeking to combine chemotherapy with ICIs have not proven effective in pancreatic cancer [159, 160].
Notably, a rare subtype of pancreatic cancer, accounting for approximately 0–1.3% of all pancreatic cancers, is characterized by microsatellite instability-high (MSI-H) or mismatch repair deficiency (dMMR), which confers high immunogenicity [161]. In 2017, pembrolizumab was approved for the treatment of patients with unresectable or metastatic MSI-H/dMMR solid tumors, including pancreatic cancer, that had progressed on prior treatment with no satisfactory alternative treatment options (Fig. 1) [162]. In the initial study demonstrating the benefits of pembrolizumab in dMMR solid tumors, among 8 patients with pancreatic cancer, 5 (62.5%) achieved complete or partial responses [163]. In the subsequent phase II KEYNOTE-158 study of pembrolizumab in patients with previously treated, advanced cancer, which included 233 MSI-H/dMMR non-colorectal patients with 27 tumor types, the objective response rate (ORR) was 34.3%, and the median DFS and OS were 4.1 and 23.5 months, respectively [164]. Among 22 patients with pancreatic cancer in this study, the ORR was 18.2%, and the median PFS and OS were 2.1 and 4.0 months, respectively.
Another rare subtype of MMR-proficient (MMR-p) pancreatic cancer is characterized by a high tumor mutation burden (TMB). In 2020, pembrolizumab was approved for treating patients with TMB-high (≥ 10 mutations/megabase) metastatic or unresectable solid tumors (Fig. 1). This approval was based on the KEYNOTE-158 study descripted above, in which the TMB-high group (n
10 mutations/megabase) metastatic or unresectable solid tumors (Fig. 1). This approval was based on the KEYNOTE-158 study descripted above, in which the TMB-high group (n =
= 102) achieved significantly higher ORR (29% vs 6%) compared to the TMB-low group (n
102) achieved significantly higher ORR (29% vs 6%) compared to the TMB-low group (n =
= 688) [165]. Additionally, another study compared clinical and genomic data from 1,662 patients with advanced cancer who were treated with ICIs to 5,371 patients without ICI treatments, finding that TMB-high was associated with improved survival in patients receiving ICI treatment across a wide variety of cancer types [166]. Therefore, for pancreatic cancer with MSI-H, dMMR, or TMB-high, ICIs are a recommended, standard-of-care treatment option [167].
688) [165]. Additionally, another study compared clinical and genomic data from 1,662 patients with advanced cancer who were treated with ICIs to 5,371 patients without ICI treatments, finding that TMB-high was associated with improved survival in patients receiving ICI treatment across a wide variety of cancer types [166]. Therefore, for pancreatic cancer with MSI-H, dMMR, or TMB-high, ICIs are a recommended, standard-of-care treatment option [167].
Targeted therapy
Over the past decade, the development of molecular targeted therapeutics for pancreatic cancer has undergone a significant transformation. Advances in next-generation sequencing technology and bioinformatics have facilitated the discovery of driver mutations and dysregulated pathways in pancreatic cancer, leading to the identification of novel therapeutic targets [168, 169]. Consequently, innovative targeted therapies, derived from genomic data, hold promise for enhancing survival rates and quality of life for pancreatic cancer patients. Targeted therapy underscores the association between tumor characteristics and individualized treatment responses, with biomarkers and genomic mutations serving as potential therapeutic targets or prognostic indicators based on their expression. Broadly, targeted therapies encompass three principal strategies: inhibiting the aberrant activation of oncogenes, interfering with the inactivation of tumor suppressor genes, and exploiting biological functional defects in specific genes [170]. Oncogenic mutations in the KRAS gene and loss of the tumor suppressor genes like TP53, CDKN2A, and SMAD4 are frequently observed in pancreatic cancer, and were deemed as driver mutations [171]. Besides, homologous recombination deficiency (HRD) in pancreatic cancer, loss of a critical DNA repair pathway, will increase sensitivity to certain DNA damaging agents, including platinum-based chemotherapy and poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitors. Targeted therapies for pancreatic cancer have made new progress mainly in KRAS and PARP inhibitors.
EGFR signaling is required for KRAS oncogene-driven PDAC [172]. In 2005, the FDA approved gemcitabine in combination with EGFR inhibitor erlotinib as the first-line treatment of locally advanced, unresectable, or metastatic PDAC, marking erlotinib as the first targeted drug approved for pancreatic cancer (Fig. 1). This approval was based on a phase III trial that demonstrated a statistically significant, though not clinically meaningful, improvement in median OS with the combination of erlotinib and gemcitabine compared to gemcitabine alone (6.2 vs 5.9 months) [106]. Consequently, erlotinib was eventually abandoned in clinical practice for pancreatic cancer treatment.
Cells employ multiple DNA repair mechanisms, including base excision, nucleotide excision, mismatch repair, homologous recombination, and non-homologous end joining, to maintain genomic integrity in response to DNA damage [173]. Mutations in these DNA repair pathways can lead to genomic instability and an increased risk of tumorigenesis. In PDAC, approximately 19% of patients had HRD, that is germline or somatic homologous recombination gene mutations such as BRCA1, BRCA2, PALB2, ATM, BAP1 [174]. A meta-analysis involving 21,842 PDAC patients revealed that whole genome or whole exome sequencing can detect a higher proportion of patients with HRD (24–44%) compared to gene-level hotspot/targeted next-generation sequencing (14.5–16.5%). Specific prevalence rates for germline and somatic HRD mutations in pancreatic cancer include BRCA1: 0.9%, BRCA2: 3.5%, PALB2: 0.2%, ATM: 2.2%, and CHEK2: 0.3% [175]. Detection of HRD pathway deficiencies, often referred to as “BRCAness”, is crucial for PDAC treatment, as patients with these deficiencies not only respond to platinum-based therapies like cisplatin but also benefit from PARP inhibitors such as Olaparib [176]. These agents act by interfering with DNA repair mechanisms, causing DNA damage, and subsequently inducing tumor apoptosis [177, 178]. A randomized phase II trial assessed cisplatin-gemcitabine with and without the PARP inhibitor veliparib in 50 patients with advanced germline BRCA-mutated and PALB2-mutated pancreatic cancer, showing ORRs of 74.1% and 65.2% (P =
= 0.55), respectively [179]. Retrospective analyses have shown higher ORR in germline BRCA-mutated and BRCA2-mutated PDAC patients treated with FOLFIRINOX (71.4% vs 13.9%; P
0.55), respectively [179]. Retrospective analyses have shown higher ORR in germline BRCA-mutated and BRCA2-mutated PDAC patients treated with FOLFIRINOX (71.4% vs 13.9%; P =
= 0.004) and in patients with germline BRCA-mutated and PALB2-mutated advanced pancreatic cancer treated with platinum-based therapies (58% vs 21%; P
0.004) and in patients with germline BRCA-mutated and PALB2-mutated advanced pancreatic cancer treated with platinum-based therapies (58% vs 21%; P =
= 0.002) [180, 181]. Another retrospective study also showed BRCA-mutated BRPCs showed a significantly higher rate of complete pathologic response (44.4% vs 10%, P
0.002) [180, 181]. Another retrospective study also showed BRCA-mutated BRPCs showed a significantly higher rate of complete pathologic response (44.4% vs 10%, P =
= 0.009) after neoadjuvant FOLFIRINOX [182]. PARP inhibitors are being explored in both maintenance and adjuvant settings for pancreatic cancer patients with BRCA1 and BRCA2 mutations [183]. Olaparib, a PARP inhibitor, has demonstrated efficacy in tumors with germline BRCA1 or BRCA2 mutations. In 2019, a randomized, double-blind, phase III trial (POLO) reported on the primary endpoint of PFS. The trial showed that among patients with germline BRCA mutation and metastatic pancreatic cancer who had not progressed during platinum-based chemotherapy, maintenance olaparib (n
0.009) after neoadjuvant FOLFIRINOX [182]. PARP inhibitors are being explored in both maintenance and adjuvant settings for pancreatic cancer patients with BRCA1 and BRCA2 mutations [183]. Olaparib, a PARP inhibitor, has demonstrated efficacy in tumors with germline BRCA1 or BRCA2 mutations. In 2019, a randomized, double-blind, phase III trial (POLO) reported on the primary endpoint of PFS. The trial showed that among patients with germline BRCA mutation and metastatic pancreatic cancer who had not progressed during platinum-based chemotherapy, maintenance olaparib (n =
= 92) achieved a longer PFS (7.4 vs 3.8 months) compared with placebo (n
92) achieved a longer PFS (7.4 vs 3.8 months) compared with placebo (n =
= 62) [184]. The ORR was 23% in the olaparib group and 12% in the placebo group. Based on these results, the FDA approved olaparib for the treatment of germline BRCA-mutated metastatic pancreatic adenocarcinoma at the end of 2019 (Fig. 1). Although no statistically significant OS benefit was observed between the olaparib and placebo groups (19.0 vs 19.2 months), the Kaplan–Meier OS curves began to separate at approximately 24 months, with survival rates of 33.9% vs 17.8% at the greatest point of separation (36 months), further supporting the clinically meaningful benefits of maintenance olaparib [185].
62) [184]. The ORR was 23% in the olaparib group and 12% in the placebo group. Based on these results, the FDA approved olaparib for the treatment of germline BRCA-mutated metastatic pancreatic adenocarcinoma at the end of 2019 (Fig. 1). Although no statistically significant OS benefit was observed between the olaparib and placebo groups (19.0 vs 19.2 months), the Kaplan–Meier OS curves began to separate at approximately 24 months, with survival rates of 33.9% vs 17.8% at the greatest point of separation (36 months), further supporting the clinically meaningful benefits of maintenance olaparib [185].
In addition to the HRD pathway, gene fusions can also drive tumorigenesis and serve as potential therapeutic targets, such as neurotrophic tyrosine receptor kinase (NTRK) and neuregulin 1 (NRG1) [186]. These gene fusions typically detected in KRAS wild-type pancreatic cancers. The frequencies of NTRK and NRG1 fusions are 0.3% and 0.5%, respectively [187]. In 2018, the FDA approved the use of the highly selective TRK inhibitor larotrectinib for adults and children with solid tumors harboring an NTRK gene fusion without a known acquired resistance mutation (Fig. 1). This approval was based on three multicenter clinical trials (NCT02122913, NCT02637687, and NCT02576431) that demonstrated marked and durable antitumor activity of larotrectinib in 55 patients with 17 TRK fusion-positive cancers, including pancreatic cancer [188]. The ORR was 75%, and 86% of the patients who responded were either continuing treatment or had undergone surgery at a median follow-up of 9.4 months. Notably, this study included only one patient with pancreatic cancer. In 2019, the FDA also approved another TRK inhibitor, entrectinib, for solid tumors with NTRK gene fusions. This approval was supported by an analysis of three phase I-II trials (ALKA-372-001, STARTRK-1, and STARTRK-2), which found that entrectinib induced durable and clinically meaningful responses in patients with NTRK fusion-positive solid tumors (Fig. 1) [189]. Among the 54 patients enrolled, the ORR was 57%, and 7% achieved complete responses. This study included three patients with pancreatic cancer. Additionally, in 2022, the FDA approved dabrafenib and trametinib for solid tumors with BRAFV600E mutations, based on the results of the NCI-MATCH Trial (NCT02465060) Subprotocol H, which reported an ORR of 38% and a PFS of 11.4 months, with responses observed across seven different tumor types, including pancreatic cancer (Fig. 1) [190]. In the same year, selpercatinib received FDA approval for rearranged during transfection (RET) fusion-positive solid tumors, based on the results of the LIBRETTO-001 basket trial (NCT03157128), which achieved an ORR of 54.5% in pancreatic cancer patients (Fig. 1) [191].
Novel therapeutic development for pancreatic Cancer
Emerging immunotherapeutic strategies beyond current standards
As described above, pancreatic cancer is characterized by low immunogenicity and immunosuppressive TME. Although immunotherapy still holds a promise as a potential breakthrough for pancreatic cancer, novel approaches for immunotherapy must be taken to overcome both low immunogenicity and immunosuppressive TME in pancreatic cancer (Fig. 2) [158, 192]– [194].
Immunomodulatory strategies for reprogramming the tumor microenvironment to enhance antitumor immunity. The pancreatic tumor microenvironment (TME) is characterized by an abundance of immunosuppressive cells, such as tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), myeloid-derived suppressor cells (MDSCs), and cancer-associated fibroblasts (CAFs), all embedded within a dense fibrotic stroma. Targeting these immunosuppressive myeloid cells involves inhibiting pathways such as CSF-1R, CCL2/CCR2, CXCR1/2, CXCL8, and CXCL12/CXCR4, while activating CD40 and CD11b to prevent their migration and reduce immunosuppression. Innate immune responses are stimulated through small molecule innate agonists or radiation to create a proinflammatory microenvironment and boost antitumor immunity. Cancer vaccines—including whole-cell, antigen-specific, and neoantigen-based vaccines—activate dendritic cells (DCs) to present tumor antigens, promoting targeted immune responses. Cell therapies, including TILs, CAR-T, CAR-NK, and TCR-engineered T-cell therapies, are employed to enhance cytotoxic activity against tumor cells by targeting specific antigens such as HER2, CEA, EGFR, mesothelin, and mutant KRAS. Immune checkpoint inhibitors targeting PD-1/PD-L1, CTLA-4, and other emerging checkpoints like LAG-3, TIM-3, and TIGIT are utilized to unleash T cell-mediated responses, overcoming immune evasion within the TME. Additionally, stroma modulation is achieved through the use of MMP inhibitors, TGF-β inhibitors, FAP-targeting agents, and FAK inhibitors, which disrupt the tumor-supportive environment. These strategies collectively aim to shift the TME from an immunosuppressive to an immunostimulatory state, augmenting therapeutic outcomes in pancreatic cancer, particularly when combined with chemotherapy and/or targeted therapy
Cancer vaccines
Whole-cell vaccines
Due to a lack of knowledge on specific pancreatic tumor-associated antigens (TAAs), whole-cell vaccines were developed for treating pancreatic cancer, particularly the GVAX vaccine [195]. The pancreatic cancer GVAX developed by Johns Hopkins University is comprised of two, irradiated, allogenic human PDAC cell lines transfected with granulocyte–macrophage colony-stimulating factor (GM-CSF) and administered intradermally. In its phase I trial, 14 pancreatic cancer patients receiving multiple vaccinations after pancreaticoduodenectomy demonstrated its safety and tolerability, with three patients remaining disease-free for over 10 years [195]. A subsequent phase II study with 60 resected PDAC patients reported median DFS of 17.3 months and OS of 24.8 months [196]. Through these studies, mesothelin was identified as pancreatic cancer-associated T cell antigens and demonstrated that mesothelin-specific CD8+ T cell responses correlated with longer survival [197]. Further investigation demonstrated that combining GVAX with low-dose cyclophosphamide (Cy) to deplete regulatory T cells (Tregs) resulted in higher avidity mesothelin-specific T cell responses and longer survival with minimal toxicity in patients with metastatic PDAC compared to GVAX alone [198].
Another allogenic whole-cell vaccine that had shown clinical promise in pancreatic cancer is algenpantucel-L which leverages the concept of hyperacute rejection by using PDAC cell lines engineered to express α-galactose epitopes on membrane glycoproteins and glycolipids to induce immune-mediated tumor cell destruction [199]. The phase II trial evaluating algenpantucel-L plus gemcitabine or 5-FU-based chemoradiotherapy in patients with resected pancreatic cancer showed that algenpantucel-L improved survival to a 12-month DFS at 62% and OS at 86% [200]. However, the subsequent phase III trial failed to support the efficacy of algenpantucel-L [201].
Vaccine therapy to prime TME
As vaccine therapy by itself has a limited efficacy, the institution focused on evaluating changes in the TME of pancreatic cancer following GVAX treatment. A window-of-opportunity neoadjuvant clinical trial for resectable pancreatic cancer was developed and demonstrated that GVAX treatment induces intratumoral tertiary lymphoid aggregates, indicating the conversion of pancreatic cancer from a non-immunogenic tumor into an immunogenic one [202, 203]. The study also raised the hypothesis that vaccine therapy primes the TME and thus sensitize pancreatic cancer for ICI treatment. This hypothesis was tested in a followup clinical trial with the same neoadjuvant therapy platform design (NCT02451982). The study showed that, in response to anti-PD-1 nivolumab treatment, GVAX-induced tertiary lymphoid aggregates became immune-regulatory sites. The study also found that higher densities of tumor-associated neutrophils (TANs) following the combination of GVAX and nivolumab portend poorer OS, leading to the development of a new arm in this platform trial to test the combination of nivolumab and anti-interleukin 8 (IL-8) antibody that blocks TANs [204]. The clinical trial added another arm to test anti-CD137 agonist antibody urelumab in combination of GAVX and nivolumab because the study found that CD8+ T cell expression of CD137 was required for optimal T cell activation and that increased T cells expressing CD137 correlated with increased OS [202, 204, 205]. This new arm showed that the triple combination of GVAX, nivolumab, and urelumab meets the primary endpoint by significantly increasing intratumoral CD137+CD8+ T cells compared to the double combination of GVAX and Nivolumab and resulted in a numerically improved DFS to 33.51 months and OS to 35.55 months although the improvement is not statistically significant due to small sample size [206].
Antigen-specific vaccines
The development of antigen-specific vaccines has not been successful in pancreatic cancer treatment by stimulating the immune system to target tumor-specific antigens. Several peptide-based vaccines are currently under investigation. GV1001, derived from the telomerase reverse-transcriptase portion, showed significant immune responses and improved median survival in a phase I/II trial of advanced pancreatic cancer patients [207]. However, a subsequent phase III trial combining GV1001 with chemotherapy did not significantly improve OS, suggesting that new strategies are required to enhance the immune response to telomerase vaccines during chemotherapy [208]. Another vaccine, KIF20A-66, demonstrated safety and modest efficacy in a phase I/II trial [209]. Interestingly, a phase I trial combining KIF20A-derived peptide with gemcitabine in advanced pancreatic cancer patients showed no serious adverse effects and induced interferon (IFN)-γ-producing cells in four out of nine patients [210, 211]. Additional phase II trials confirmed these results, indicating promise for this combination therapy in advanced pancreatic cancer [212, 213]. Mucin-1 (MUC-1), a transmembrane protein overexpressed in pancreatic cancer, has also been targeted with peptide vaccines. Initial clinical trials showed that MUC-1-specific vaccines could elicit T cell responses and potentially improve OS [214]. Additionally, dendritic cell (DC)-based vaccines loaded with peptides like Wilms tumor 1 (WT1) and MUC-1 combined with standard chemotherapy in patients with advanced or relapsed PDAC have shown enhanced tumor-specific immunity and favorable outcomes, with the median PFS and OS as 8.1 months and 15.1 months, respectively [215]. These results suggest that peptide-based and DC vaccines, especially when combined with conventional therapies, hold potential for improving outcomes in pancreatic cancer, particularly in patients with recurrent or refractory disease [216].
Mesothelin-specific immune responses observed in patients with increased DFS after receiving GVAX have positioned mesothelin as a promising candidate for antigen-specific vaccines [197]. CRS-207, a recombinant live-attenuated Listeria monocytogenes engineered to secrete tumor antigens, was developed to stimulate both innate and adaptive immunity. In a phase II trial, combining Cy/GVAX with CRS-207 in metastatic pancreatic cancer patients resulted in a median OS of 6.1 months, compared to 3.9 months for Cy/GVAX alone, and 9.7 months versus 4.6 months for those receiving at least three doses of the vaccine [217]. However, a later phase IIb trial found no survival advantage for the combination of Cy/GVAX and CRS-207 over single-agent chemotherapy in previously treated metastatic pancreatic cancer patients [218]. Despite the initial promising results, including immunological activation and mesothelin-specific T-cell responses, further studies are needed to determine the efficacy of CRS-207 in PDAC. A recent trial also evaluated the combination of Cy/GVAX and CRS-207 with or without nivolumab (NCT02243371), showing non-durable objective responses in a small percentage of patients, but significant immunological changes in the TME [219]. This result suggests the need for further ongoing research to optimize combined treatment strategies with antigen-specific cancer vaccines such as CRS-207 (NCT03006302, NCT05014776).
Neoantigen-based vaccines
Neoantigen-based vaccines are an emerging approach in cancer immunotherapy, leveraging the immunogenicity of tumor-specific mutations. Neoepitopes, which arise from these mutations, can be recognized by T cells, offering a targeted immune response against the tumor [220]. Although pancreatic cancers have a lower mutation rate compared to melanoma or NSCLC, they still express neoepitopes that can serve as potent targets for vaccination. The rationale is that these neoepitopes are less likely to evade the immune system, making neoantigen-based vaccines more immunogenic [221].
Oncogenic mutations in KRAS are present in up to 90% of PDAC, making vaccination against mutant KRAS (mKRAS) a promising immunotherapeutic approach. Johns Hopkins University has developed an mKRAS peptide vaccine targeting six common KRAS mutations (G12V, G12A, G12C, G12R, G12D, G13D) (NCT04117087). In an early trial, the vaccine, combined with ipilimumab and nivolumab, showed that 8 out of 11 patients with resected PDAC developed a significant mKRAS-specific T cell response, characterized by an increase in IFNγ-producing T cells. Cytometry by time-of-flight analysis revealed the expansion of polyfunctional mKRAS-specific CD4 and CD8 T cells, with CD4 T cells being more prominently induced. Single-cell analysis further identified a novel CD4+ T cell receptor (TCR) that recognizes KRASG12V in the context of human leukocyte antigens (HLA)-DRB1*07:01, underscoring the vaccine's potential to induce high-quality T cells. Ongoing studies are focused on further characterizing TCR diversity and clonality to identify biomarkers for predicting response to mKRAS-targeted immunotherapy [222]. Additionally, a phase II trial (NCT06411691) is evaluating the efficacy and immune response of this mKRAS vaccine combined with Balstilimab (an anti-PD-1 antibody) and Botensilimab (an anti-CTLA-4 antibody) in patients with unresectable or MMR-p colorectal cancer or PDAC, following first-line FOLFIRINOX/FOLFOXIRI treatment, aiming to expand upon these initial findings in a broader patient population.
Despite their promise, peptide vaccines face several challenges, including HLA-type restriction, which limits their use to patients with matching HLA types, and the frequent occurrence of immune evasion when the vaccine's anti-tumor activity relies on a response to a single epitope. Additionally, peptide- or protein-based vaccines often require combination with adequate immune adjuvants or vectors to elicit a strong immune response [223]. To address these issues, researchers are developing immune-dominant antigens that can trigger responses to multiple epitopes and creating new vaccine vector systems to enhance efficacy. A promising development in overcoming these challenges is the ELI-002 2P vaccine, which targets KRAS mutations (G12D and G12R) with modified long peptides and a Toll-like receptor (TLR) 9 agonist, found in a significant portion of solid tumors. In a first-in-human phase I trial, ELI-002 2P demonstrated robust immunogenicity in 84% of patients with minimal residual disease relapse after locoregional treatment. Importantly, this vaccine does not require HLA-type matching, effectively overcoming one of the major limitations of traditional peptide vaccines. The induced T cell responses correlated with significant reductions in tumor biomarkers and improved relapse-free survival, highlighting the potential of ELI-002 2P as a promising therapeutic option for patients with KRAS-mutated tumors [224].
One promising example is autogene cevumeran, an mRNA vaccine designed similarly to COVID-19 vaccines, formulated with uridine mRNA-lipoplex nanoparticles encoding up to 20 neoantigens per patient. In a phase I trial (NCT04161755), patients underwent tumor resection, followed by a single dose of anti-PD-L1 antibody atezolizumab six weeks post-surgery, eight doses of autogene cevumeran, standard adjuvant mFOLFIRINOX, and a ninth vaccine booster dose upon completion of mFOLFIRINOX [225]. Initial results showed that this combination was safe and feasible, generating substantial neoantigen-specific T cell responses in 50% of unselected resectable PDAC patients. The vaccine-induced T cells, which could comprise up to 10% of the total circulating T cells, demonstrated significant clonal expansion and targeted pancreatic cancer neoantigens. Responders to the vaccine had significantly prolonged recurrence-free survival compared to non-responders (median not reached versus 13.7 months) after a median follow-up of 18 months [225]. The eight patients with vaccine-induced T-cell responses continued to have significantly longer median recurrence-free survival compared to those without an immune response after a median follow-up of 3 years [226]. These encouraging results have led to the initiation of a randomized phase II trial (IMCODE003, NCT05968326) to further evaluate the efficacy and safety of autogene cevumeran in combination with atezolizumab and mFOLFIRINOX as adjuvant therapy, compared to standard-of-care mFOLFIRINOX, in patients with resectable pancreatic cancer (NCT05968326).
Despite the potential of neoantigen-based immunotherapy, it faces significant challenges in pancreatic cancer. High intertumoral heterogeneity and the scarcity of shared mutations complicate the development of personalized treatments. Additionally, the specialized TME in PDAC limits the effectiveness of immunotherapy, making neoantigen-based strategies more viable as complementary treatments rather than standalone options. These challenges have driven ongoing research to overcome these barriers and enhance the effectiveness of these therapies (Table 5) [221].
Table 5
Selected ongoing trials of cancer vaccines in pancreatic cancer therapy
| Drug Category | Phase of trial | Number of patients | Disease stage | Candidate drug and combination regimen | Primary outcome | Survival/Rate (Month) | Clinical trial identifier |
|---|---|---|---|---|---|---|---|
| GVAX | II | 76 | Resectable | GVAX/Cyclophosphamide (Cy) | IL17A expression; Intratumoral CD8+CD137+cells; Intratumoral granzyme B+PD-1+CD137+ cells; Pathologic Response | 23.59 | NCT02451982 [206] |
| GVAX/Cy, Nivolumab | 27.01 | ||||||
| GVAX/Cy, Nivolumab, Urelumab | 35.55 | ||||||
| Nivolumab and BMS-986253 (anti-IL-8 antibody) | N/A | ||||||
| I/II | 30 | Locally advanced | SBRT, Nivolumab, BMS-813160 (CCR2/CCR5 inhibitor) | Drug-related toxicities; Immune response rate | N/A | NCT03767582 [320] | |
| SBRT, Nivolumab, BMS-813160, GVAX | |||||||
| II | 41 | Metastatic | Epacadostat, Pembrolizumab, GVAX/Cy, CRS-207 | Maximum Tolerated Dose of Epacadostat; 6-month OS | N/A | NCT03006302 | |
| Epacadostat, Pembrolizumab, CRS-207 | |||||||
| WT1-targeted vaccine | I/II | 10 | Refractory or advanced solid tumors | IL15-transpresenting WT1-targeted Dendritic Cell Vaccine | Feasibilty and safety | N/A | NCT05964361 |
| CRS-207 | II | 17 | Metastatic | Tadalafil, Pembrolizumab, Ipilimumab, CRS-207 | ORR using immune Response Evaluation Criteria for Solid Tumors | N/A | NCT05014776 |
| OSE2101 | II | 106 | Locally advanced or metastatic | FOLFIRI | OS | N/A | NCT03806309 |
| FOLFIRI, OSE2101 | |||||||
| Personalized synthetic peptide vaccine | I | 150 | advanced or metastatic PDAC | Imiquimod (personalized vaccine) | Treatment-related AEs | N/A | NCT02600949 |
| Imiquimod, pembrolizumab | |||||||
| Imiquimod, pembrolizumab, sotigalimab | |||||||
| Neoantigen Vaccines | I | 30 | Resectable or borderline resectable | poly-ICLC, surgery, vaccine (adjuvant) | Safety | N/A | NCT05111353 |
| poly-ICLC, vaccine, surgery (neoadjuvant) | |||||||
| ELI-002 | I | 25 | KRAS Mutated PDAC | ELI-002 2P | Maximum Tolerated Dose; Safety | N/A | NCT04853017 |
| Long Peptide Vaccine | I | 30 | Resectable | KRAS peptide vaccine, nivolumab, ipilimumab | Safety; T cell response | N/A | NCT04117087 |
| Long Peptide Vaccine | I | 37 | High risk of developing pancreatic cancer | KRAS peptide vaccine with poly-ICLC | Drug-related toxicities; T cell response | N/A | NCT05013216 |
| Personalized tumor vaccines | I | 29 | Resectable | Atezolizumab, personalized cancer vaccine RO7198457, mFOLFIRINOX | Drug related toxicity | N/A | NCT04161755 [225] |
| Personalized tumor vaccines | II | 260 | Resectable | Autogene cevumeran, atezolizumab, mFOLFIRINOX | DFS | N/A | NCT05968326 |
| mFOLFIRINOX | |||||||
| KRAS-Targeted Vaccine | II | 50 | Stage IV MMR-p PDAC | KRAS Vaccine with Poly-ICLC adjuvant, balstilimab, botensilimab | PFS; ORR; Drug related toxicity | N/A | NCT06411691 |
Gem/nP gemcitabine plus nab-paclitaxel, TRAE Treatment Related Adverse Events, N/A not applicable, PR partial response, SD stable disease, AEs Adverse Events, PFS Progression-free survival, DLT Dose limiting toxicities, DFS disease-free survival, OS overall survival, RFS recurrence free survival, PRR pathologic response rate
Immune checkpoint inhibitors
PD-1/PD-L1 axis and CTLA-4
Following the success of anti-PD-1/PD-L1 therapies in melanoma, researchers began exploring the role of the PD-1/PD-L1 axis in PDAC. The interaction between PD-1 and PD-L1 leads to T cell exhaustion by blocking T cell activation, while CTLA-4 inhibition can modulate Tregs and enhance T cell priming [227]. However, these ICIs have not yielded significant clinical benefits in pancreatic cancer, which is often characterized by a lack of pre-existing T cell immunity [228, 229]. For instance, the KEYNOTE-028 trial found that pancreatic cancer was the only tumor type among 475 PD-L1-positive advanced solid tumors that did not respond to pembrolizumab therapy [230]. Even in the small subset of PDAC patients with MSI-H or high TMB profiles, responses to PD-1/PD-L1 inhibitors are less robust compared to other MSI-H tumors [164, 163, 231]. Similarly, clinical trials investigating CTLA-4 inhibitors in PDAC have been disappointing, with minimal efficacy and challenging side effect profiles [232]. Additionally, unlike in other solid tumors, combining anti-PD-1/PD-L1 and anti-CTLA-4 therapies has not substantially improved outcomes in pancreatic cancer. For instance, a phase II trial evaluating durvalumab with or without tremelimumab in previously treated metastatic PDAC patients reported an ORR of 3.1% for the combination therapy and 0% for monotherapy, with no significant improvement in PFS (1.5 vs 1.5 months) or OS (3.1 vs 3.6 months) [233].
Given these challenges, ongoing clinical trials are focusing on combining ICIs with chemotherapy and/or radiotherapy to enhance antitumor responses in pancreatic cancer. For instance, the CISPD-4 randomized phase II trial (NCT03983057) is investigating mFOLFIRINOX with or without anti-PD-1 antibody as neoadjuvant therapy in BRPC and LAPC. Preliminary data suggest similar resection rates between the two groups in BRPC (51.7% vs 47.4%), with a higher resection rate observed in the PD-1 group for LAPC (48.0% vs 37.1%) [234]. However, combining anti-CTLA-4 therapy with chemotherapy has not demonstrated superiority over PD-1 combinations. For instance, a phase Ib study combining ipilimumab with gemcitabine in advanced PDAC reported an ORR of 14% and a median OS of 6.9 months, indicating that while the combination is safe, it does not offer significant efficacy advantages over gemcitabine alone [159]. Similarly, the CCTG PA.7 phase II trial (NCT02879318) found that adding durvalumab and tremelimumab to gemcitabine/nab-paclitaxel did not significantly improve median OS (9.8 vs 8.8 months) in metastatic PDAC, though it slightly increased ORR (30.3% vs 23.0%) [235]. Overall, these findings suggest that while combinations of PD-1/PD-L1 and/or CTLA-4 inhibitors with chemotherapy or other treatment methods are being actively investigated, further research is needed to identify the subsets of PDAC patients who may benefit most from these regimens (Table 6).
Table 6
Selected ongoing trials of ICIs in pancreatic cancer therapy
| ICI Target | Phase of trial | Number of patients | Disease stage | Candidate drug and combination regimen | Primary outcome | Survival/Rate (Month) | Clinical trial identifier |
|---|---|---|---|---|---|---|---|
| PD-1/PD-L1 axis and CTLA-4 | II | 80 | Metastatic | Nivolumab, Ipilimumab, Radiation Therapy | Disease Control Rate | 20% | NCT03104439 |
| II | 30 | Metastatic | Nivolumab, Ipilimumab, Radiation Therapy | Overall response rate | 3% | NCT04361162 | |
| II | 180 | Metastatic | Gemcitabine, Nab-paclitaxel | Progression Free Survival; Objective Response Rate | 8.8, 30.3% | NCT02879318 [235] | |
| Gemcitabine, Nab-paclitaxel, Durvalumab, Tremelimumab | 9.8, 23.0% | ||||||
| I | 45 | Metastatic | Nivolumab, Ipilimumab, nP/gem | Incidence and severity of adverse events | Grade 3–4 60% TRAEs | NCT04787991 [331] | |
| Hydroxychloroquine, Ipilimumab, nP/gem | Grade 3–4 53% TRAEs | ||||||
| NG-350A (anti-CD40 antibody), Ipilimumab, nP/gem | N/A | ||||||
| II | 36 | Advanced | Pembrolizumab, Azacitidine | Progression-Free Survival | 1.51 | NCT03264404 | |
| I/II | 36 | Borderline resectable and locally advanced | Durvalumab, Stereotactic Ablative Body Radiotherapy | Dose limiting toxicities; Progression Free Survival; Proportion of downstaging rates | Median PFS 8.7 m, median OS 16 m | NCT03245541 | |
| II | 168 | Borderline resectable and locally advanced | FOLFIRINOX, SBRT, Surgery | R0 resection tare | N/A | NCT03563248 | |
| FOLFIRINOX, Losartan, SBRT, Surgery | |||||||
| FOLFIRINOX, Losartan, SBRT, Nivolumab, Surgery | |||||||
| FOLFIRINOX, SBRT, Nivolumab, Surgery | |||||||
| III | 146 | Borderline resectable and locally advanced | modified-FOLFIRINOX | Progression-free survival | NCT03983057 [234] | ||
| modified-FOLFIRINOX, Anti-PD-1 antibody | |||||||
| TIGIT | I/II | 340 | Metastatic | nP/gem | Objective response rates; Safety | atezolizumab plus PEGPH20 (n ORRs 6.1% vs. 2.4%; grade 3/4 AEs 65.2% vs. 61.9%; grade 5 AEs 4.5% vs. 2.4% | NCT03193190 |
| Atezolizumab, nP/gem, Selicrelumab | |||||||
| Atezolizumab, nP/gem, Bevacizumab | |||||||
| Atezolizumab, nP/gem, AB928 | |||||||
| Atezolizumab, nP/gem, Tiragolumab | |||||||
| Atezolizumab, Cobimetinib | |||||||
| Atezolizumab, PEGPH20 | |||||||
| Atezolizumab, BL-8040 | |||||||
| Atezolizumab, RO6874281 | |||||||
| nP/gem or mFOLFOX | |||||||
| Atezolizumab, nP/gem, Tocilizumab | |||||||
| TIM-3 | I | 447 | Advanced Solid Tumors | TSR-022 | Dose limiting toxicity; Safety; Overall response rate | N/A | NCT02817633 |
| TSR-022, Nivolumab | |||||||
| TSR-022, TSR-042, TSR-033 | |||||||
| TSR-022, TSR-042 | |||||||
| TSR-022, TSR-042, Docetaxel | |||||||
| TSR-022, TSR-042, Pemetrexed, Cisplatin | |||||||
| TSR-022, TSR-042, Pemetrexed, Carboplatin |
nP/gem nab-paclitaxel plus gemcitabine, TRAE Treatment Related Adverse Events, AEs Adverse Events; ORR Objective response rates
Others
Despite significant advancements, the efficacy of antibodies targeting CTLA-4 or PD-1/PD-L1 has not been satisfactory in all cases. This has prompted researchers to explore new immune checkpoints as potential targets for ICIs. Three promising candidates currently under clinical investigation are lymphocyte activation gene-3 (LAG-3), T-cell immunoglobulin and mucin-domain containing-3 (TIM3), and T cell immunoreceptor with Ig and ITIM domains (TIGIT) [236].
LAG-3, also known as CD223, is expressed on various cell types, including CD4+ and CD8+ T cells, as well as Tregs, and plays a crucial role in T cell regulation and homeostasis [237]. In cancer, persistent antigen stimulation leads to chronic LAG-3 expression, contributing to T cell exhaustion [238]. Several neutralizing antibodies targeting LAG-3, including relatlimab, fianlimab, and favezelimab, are currently in phase III clinical trials for various cancers, although none have been tested specifically in PDAC [239]. Interestingly, unlike PD-1 and CTLA-4, which inhibit CD28-mediated co-stimulation, LAG-3 impedes the TCR signal, suggesting that combined targeting of these pathways may be beneficial [240, 241]. Preclinical studies in murine models of melanoma, colon adenocarcinoma, and ovarian cancer have demonstrated that co-blockade of LAG-3 and PD-1, expressed on both CD4+ and CD8+ tumor-infiltrating lymphocytes (TILs), enhances anti-tumor responses [241, 242]. Relatlimab, the first FDA-approved LAG-3 inhibitor, in combination with nivolumab (marketed as Opdualag), has been shown to double median PFS (10.1 vs 4.6 months) in patients with unresectable or metastatic melanoma compared to nivolumab alone, highlighting the potential efficacy of targeting LAG-3 in cancer therapies [243]. In PDAC, elevated LAG-3 levels in tumor-infiltrating T cells have been associated with poorer DFS [244]. A phase I/II trial involving ieramilimab (anti-LAG-3) with or without anti-PD-1 antibody spartalizumab in advanced malignancies, including pancreatic cancer (NCT02460224), reported an ORR of 0% with single-agent treatment and 10.7% with combination therapy, suggesting that dual targeting of LAG-3 and PD-1 may also hold promise in PDAC by enhancing T cell priming [245]. In a previously mentioned clinical study combining GVAX vaccine and nivolumab as neoadjuvant therapy for resectable PDAC (NCT02451982), it was observed that higher densities of TANs in vaccine-induced tertiary lymphoid aggregates within the TME were correlated with increased densities of LAG-3+ tumor-infiltrating T cells, but not with TIM-3+ or EOMES+ T cells [204]. This finding suggests that modulating TANs in PDAC may facilitate the recovery of exhausted T cell populations through LAG-3 targeting.
TIM-3 regulates type 1 immune responses and is expressed on IFNγ-secreting CD4+ and CD8+ T lymphocytes, natural killer (NK) cells, myeloid cells, mast cells, and a subset of B lymphocytes [246, 247]. TIM-3 has been identified as a marker of terminally dysfunctional CD8+ T cells more effectively than PD-1 in both cancer models and human samples, making it a promising target for immunotherapy [248, 249]. Moreover, TIM-3 is highly expressed on Foxp3+ Tregs, adding complexity to its role in immune regulation [250]. Since 2016, several anti-TIM-3 antibodies and PD-1/TIM-3 bispecific antibodies, such as TSR-022 (Tesaro), have entered clinical trials. However, these therapies have not yet been tested in pancreatic cancers.
TIGIT is a co-inhibitory receptor induced by TCR stimulation, expressed on NK cells, Tregs, Th1 cells, follicular Th cells, and dysfunctional CD8+ T cells [251]– [254]. TIGIT’s ligands, CD155 and CD112, are shared with DNAM-1 and are found on antigen-presenting cells and tumor cells [255]. High TIGIT expression in PDAC-infiltrating T cells correlates with anti-inflammatory and exhausted phenotypes, highlighting its potential as a therapeutic target [256]. Ongoing trials such as NCT03193190 are testing the efficacy of combining tiragolumab, a TIGIT monoclonal antibody, with other therapies in metastatic PDAC (Table 6).
Agents targeted tumor microenvironment
Activation of innate immunity
The innate immune system, primarily composed of myeloid/macrophages, NK cells, and DCs, represents the first line of defense against invading microbial pathogens. This system utilizes pattern recognition receptors (PRRs) to identify conserved structures on pathogens, known as pathogen-associated molecular patterns [257]. However, research has shown that innate immune activation can also occur in the absence of pathogen infection, a process termed sterile inflammation. Sterile inflammation is often associated with radiation-induced innate immune responses, where PRRs detect damage-associated molecular patterns (DAMPs) originating from stressed or damaged cells [258]. The ligation of PRRs, such as TLRs and the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) signaling pathway, has been investigated to enhance proinflammatory DC phenotypes and bolster antitumor immunity in PDAC. Radiation can induce DAMPs, further enhancing PRR-mediated activation of the immune system, which complements the effects of immunotherapy [259].
TLR3 agonist Toll-like receptor 3 (TLR3) is expressed in various cell types, including DCs, macrophages, epithelial cells, and fibroblasts [260, 261]. In myeloid lineage cells, TLR3 functions as a cell surface receptor recognizing viral double-stranded RNA motifs, contributing to innate antiviral responses [262]. In tumors, TLR3 activation triggers interferon regulatory factor 3, leading to IFN-β production, which inhibits tumor growth and angiogenesis. It also enhances the activation and proliferation of tumor-specific T cells while inducing caspase-dependent apoptosis [263]. A TLR3 agonist polyinosinic-polycytidylic acid (poly-IC) has been shown to enhance C-X-C chemokine receptor type 7 (CCR7) expression on DCs and increase the frequency of mature DCs in draining lymph nodes [264]. The CCR7-CCL19/CCL21 axis facilitates the migration of mature DCs bearing tumor antigens to draining lymph nodes, where they prime antigen-specific T cell responses [265, 266]. Researchers have developed an enhanced form of poly-IC, known as poly-ICLC, to boost antitumor immunity, which has been evaluated in several clinical trials for its safety and efficacy [267]. In a clinical study involving patients with locally advanced and metastatic pancreatic cancer, the combination of a poly-(ICLC)-activated autologous DC vaccine with peptides induced tumor-specific T-cell responses, prolonged survival, and was well-tolerated [268]. In addition, a single-center study evaluated a restricted TLR3 agonist Rintatolimod as maintenance therapy in patients with locally advanced or metastatic PDAC previously treated with FOLFIRINOX chemotherapy, demonstrating improved median OS (19.0 vs 12.5 months) compared to matched control [269]. Post-intervention blood analysis revealed increased frequencies of BDCA-3+ CD141+ conventional DCs and higher expression of costimulatory molecules CD80 and CD86 [270]. Current clinical trials are investigating the combination of Rintatolimod with FOLFIRINOX (NCT05494697) or anti-PD-L1 antibody durvalumab (NCT05927142) in locally advanced or metastatic PDAC [271].
TLR7/8 agonist TLR7 and TLR8 share structural motifs and are both localized within intracellular endosomal compartments [272]. Natural agonists, such as single-stranded RNA or deoxyribonucleotides with cytosine-phosphate-guanine motifs, activate innate immune cells through these TLRs [273]. TLR7 is primarily expressed by plasmacytoid DCs and B cells, while TLR8 is expressed by myeloid lineage cells, including monocytes, macrophages, and myeloid DCs [272]. A study investigating TLR expression in pancreatic cancer patients receiving neoadjuvant therapy found that TLR7 and TLR9 were both associated with favorable postoperative outcomes [274]. At present, the AGADIR trial (NCT03915678), a multicenter, phase II study is evaluating the novel TLR7/8 agonist BDB001 in combination with atezolizumab and SBRT in patients with advanced pancreatic adenocarcinoma. This study met its first endpoint for disease control rate (DCR) at 38.0%, and of the 21 patients enrolled, 9.5% achieved a partial response, 28.5% had stable disease, and 62.0% experienced progressive disease [275].
TLR9 agonist TLR9 is a PRR expressed intracellularly in various immune effector cells, including DCs, macrophages, and NK cells [276]. TLR9 agonism has been shown to enhance immune responses, circumvent tumor immune evasion, and optimize immunotherapy outcomes [277, 278]. SD-101 is a synthetic oligonucleotide containing cytidine-phospho-guanosine (CpG) motifs that stimulates plasmacytoid DCs via TLR9, leading to IFN-α release and maturation into antigen-presenting cells, thereby enhancing innate and adaptive immune responses [279]. A pilot study combining intratumoral SD-101 with nivolumab and radiotherapy for chemotherapy-refractory metastatic pancreatic adenocarcinoma has been completed, with results pending (NCT04050085) [280]. Additionally, the PERIO-03 pilot study is currently evaluating the intratumoral administration of SD-101 via pressure-enabled intrapancreatic infusion alongside nivolumab in patients with LAPC, demonstrating favorable tolerability and potentially beneficial immune alterations, such as decreases in myeloid-derived suppressor cells (MDSCs)-associated gene expression in both peripheral blood mononuclear cells and tumors (NCT05607953) [281].
STING agonist STING is a transmembrane protein localized to the endoplasmic reticulum that, upon binding with cyclic guanosine monophosphate–adenosine monophosphate, induces the production of class I interferons in DCs [282, 283]. STING agonists, which activate innate immune responses and counteract tumor-induced immunosuppression, are emerging as promising agents in cancer immunotherapy [284]. While first-generation synthetic cyclic dinucleotide based STING agonists showed encouraging preclinical results, challenges such as technical difficulties with intratumoral delivery, systemic toxicity, and tumor resistance have limited their clinical application [285]. This has led to the development of new generation STING agonists suitable for systemic administration, such as BMS-986301 (NCT03956680), TAK-676 (NCT04420884), and SNX281 (NCT04609579) [286, 287]. BMS-986301, for example, has demonstrated similar efficacy with intramuscular injection compared to traditional intratumoral injection in preclinical studies, and it attenuates T cell exhaustion and immunosuppressive signals while upregulating CTLA-4 checkpoint signals in tumor-infiltrating T cells [288]. This led to the initiation of a clinical trial combining BMS-986301 with nivolumab and ipilimumab in advanced solid cancers (NCT03956680). Another innovative, systemically administered innate agonist is Decoy20, a detoxified, inactivated bacterial product that retains agonistic activity for multiple innate immune pathways, including endogenous TLR1,2,6,8,9, nucleotide oligomerization domain containing 2, and STING. Early results from a phase I trial in advanced solid malignancies showed systemic immune activation and preliminary evidence of stable disease after only one infusion (NCT05651022) [289, 290].
Radiation as an innate immune agonist Emerging evidence suggests that radiotherapy can initiate an innate immune response by inducing immunogenic cell death and subsequently activating adaptive immunity, functioning as an "in situ vaccination" within the TME [259, 291, 292]. Preclinical studies support combining radiotherapy with ICIs in pancreatic cancer [293, 294], with clinical trials showing modest benefits and well-tolerated safety in metastatic PDAC when radiotherapy is combined with ICIs like durvalumab, tremelimumab, ipilimumab, and nivolumab [295, 296]. Notably, the randomized phase II CheckPAC study (NCT02866383) demonstrated that combining SBRT with nivolumab and ipilimumab significantly improved antitumor activity in chemotherapy-refractory metastatic PDAC patients compared to SBRT with nivolumab alone, showing higher clinical benefit rate (37.2% vs 17.1%) and ORR (14.0% vs 2.4%) [297]. However, results from the phase II TRIPLE-R study (NCT04258150) of ipilimumab, nivolumab, IL-6 inhibitor tocilizumab combined with SBRT indicated limited efficacy, with no observed responses and a median OS of only 5.3 months, potentially due to the complex role of IL-6 in the PDAC microenvironment [298]. Overall, while combining radiotherapy with immunotherapy has shown potential, the integration of precision radiotherapy with more personalized and diverse immunotherapy regimens is crucial in PDAC treatment [299]. For instance, combining GVAX, PD-1 blockade pembrolizumab, and SBRT in LAPC patients (NCT02648282) increased antitumor immune responses but also elevated immunosuppressive M2-like tumor-associated macrophages (TAMs), highlighting the need for further studies targeting TAMs in radioimmunotherapy [300, 301].
Targeting myeloid cells
Myeloid cells play a critical role in shaping the TME of PDAC, significantly contributing to tumor progression and immune evasion. These cells, which include macrophages, neutrophils, DCs, and MDSCs, often adopt phenotypes that support tumor growth and suppress anti-tumor immune responses [158, 302, 303].
CSF-1R inhibitor The recruitment and survival of TAMs in PDAC are regulated by the colony-stimulating factor 1 (CSF-1)/CSF-1 receptor (CSF-1R) axis and the CCL2/CCR2 signaling pathway [304, 305]. Preclinical models have demonstrated that blocking CSF-1R can reprogram TAMs to enhance antigen presentation and prime anti-tumor T cell responses [306]. Early-phase clinical investigations with inhibitors targeting CCR2 and CSF-1R showed promise in advanced PDAC patients [307, 308]. Recent research utilizing data from the TCGA PanCancer Atlas categorized PDAC patients into CSF-1R high and CSF-1R low groups, revealing that higher CSF-1R expression correlates with increased immune infiltration. This suggests potential for targeting CSF-1R in combination immunotherapy strategies for PDAC [309]. A clinical trial explored the safety and immunologic effects of combining GVAX with Cy, pembrolizumab, and IMC-CS4 (a CSF-1R inhibitor) in PDAC patients (NCT03153410) [310]. The study reported a median DFS of 12.6 months and OS of 20.4 months, with 78% achieving major pathological response post-surgery. Notably, while the primary immunologic endpoint was met, showing an increase in CD8+ T cells and CD8+Granzyme B+ T cells, no significant change in myeloid cell density was observed, suggesting that the treatment reprogrammed rather than depleted the macrophages [310]. However, a recent phase II study (NCT03336216) that combined the CSF-1R inhibitor cabiralizumab with nivolumab and chemotherapy did not significantly improve median PFS compared to chemotherapy alone in advanced PDAC patients (3.68 months, 3.22 months, and 3.25 months, respectively) [311]. The failure of this cabiralizumab-based regimen may be attributed to ineffective targeting of myeloid cells, lack of T cell priming agents, or inadequate combinatorial effects from chemotherapy. Pexidartinib, a more potent CSF-1R inhibitor, was evaluated in a small phase I study (NCT02777710) involving pancreatic and colorectal cancer patients (n =
= 19). The combination of pexidartinib and the anti-PD-L1 antibody durvalumab showed a response rate of 21% among the 19 enrolled patients [312]. Further analysis suggested that pexidartinib impacts Fms-like tyrosine kinase 3-dependent DC differentiation and may antagonize the effect of durvalumab, indicating that inhibition of Fms-like tyrosine kinase 3 should be considered when combining CSF-1R inhibitors with PD-L1 inhibitors [313].
19). The combination of pexidartinib and the anti-PD-L1 antibody durvalumab showed a response rate of 21% among the 19 enrolled patients [312]. Further analysis suggested that pexidartinib impacts Fms-like tyrosine kinase 3-dependent DC differentiation and may antagonize the effect of durvalumab, indicating that inhibition of Fms-like tyrosine kinase 3 should be considered when combining CSF-1R inhibitors with PD-L1 inhibitors [313].
CCL2/CCR2 antagonist CCR2 and its ligand CCL2 are crucial in recruiting immunosuppressive cells, such as M2-like TAMs and MDSCs, into tumors [314]. In PDAC, elevated CCL2 expression alongside low CD8+ T cell infiltration is associated with significantly poorer patient survival [315]. Preclinical studies have shown that disrupting the CCL2–CCR2 axis can enhance chemotherapy efficacy and bolster antitumor T cell responses [305, 316]. A phase Ib study (NCT01413022) exploring the combination of the CCR2 antagonist PF-04136309 with FOLFIRINOX in patients with BRPC and LAPC reported a promising ORR of 49% in 33 evaluable patients, with no dose-limiting toxicities observed [307]. However, a subsequent trial combining PF-04136309 with gemcitabine/nab-paclitaxel revealed a high incidence of pulmonary toxicity (24%) and did not demonstrate an efficacy advantage over chemotherapy alone [317]. CCR5, another chemokine receptor, plays a role in TAM and Treg infiltration into tumors [318]. Preclinical research indicated that a dual CCR2/CCR5 antagonist BMS-687681 could counteract radiotherapy-induced suppressive signals in myeloid cells and upregulate effector T cell pathways, thus supporting an ongoing clinical trial combining radiotherapy, a CCR2/CCR5 dual antagonist BMS-813160, and nivolumab for LAPC treatment(NCT03767582) [319, 320]. Additionally, a phase Ib/II trial (NCT03184870) is currently assessing BMS-813160 as monotherapy or in combination with chemotherapy or nivolumab in patients with advanced pancreatic or colorectal cancer. The trial has completed enrollment, and its results are awaited [321, 322].
CD40 agonist CD40, a member of the tumor necrosis factor receptor superfamily, is predominantly expressed on DCs, macrophages, and B cells [323]. Its ligand, CD40L, is primarily found on activated T cells and interacts with CD40 on antigen-presenting cells, promoting the upregulation of IL-12, MHC-II, costimulatory, and adhesion molecules. CD40 agonist immunotherapy leverages this interaction to stimulate endogenous effector T cells via host conventional DCs, providing a robust therapeutic benefit [324]. There are several agonistic anti-CD40 antibodies, such as SGN-40, SEA-CD40, selicrelumab, APX005M, CDX-1140, and ADC1013, applicable in clinical trials [325]. In a phase I study evaluating the CD40 agonist selicrelumab as neoadjuvant therapy in resectable PDAC (NCT02588443), treated patients exhibited more mature DCs, more active and proliferative T cells, fewer M2-like TAMs, and reduced fibrosis compared to treatment-naïve or chemotherapy-only patients [326]. When combined with chemotherapeutic agents like gemcitabine/nab-paclitaxel, which induce PDAC cellular apoptosis and expose TAAs, CD40 engagement has been shown to induce DC-dependent cellular immune responses [327]. In a prior phase I trial, combining CD40 agonism CP-870893 with gemcitabine in advanced PDAC resulted in an ORR of 19%, with a median PFS of 5.2 months and OS of 8.4 months [328]. A phase Ib trial combining gemcitabine/nab-paclitaxel, and the CD40 agonist APX005M (sotigalimab), with or without the anti-PD-1 inhibitor nivolumab for metastatic PDAC (NCT03214250), reported an ORR of 58% among the 24 evaluated subjects, though most participants experienced grade 3 or 4 treatment-related adverse events, including lymphopenia, anemia, and neutropenia [329]. Despite these promising results, the subsequent phase II PRINCE trial (NCT03214250) for first-line treatment of metastatic PDAC revealed that the primary endpoint of 1-year OS was only met for the nivolumab plus chemotherapy group (57.7% compared to a historical 1-year OS of 35%), but not for the sotigalimab plus chemotherapy (48.1%) or sotigalimab, nivolumab plus chemotherapy groups (41.3%) [330]. These findings suggest that CD40 agonist regimens may not be optimal for unselected PDAC, although several immune signatures identified are being further evaluated in the ongoing REVOLUTION platform trial (NCT04787991) [331]. Additionally, combination therapy using a DC vaccine loaded with tumor antigens and CD40 agonism has shown promise in enhancing tumor-specific T cell responses. The phase Ib/II OPTIMIZE-1 study (NCT04888312), which evaluated mitazalimab, a human CD40 agonistic IgG1 antibody combined with mFOLFIRINOX in previously untreated metastatic PDAC, demonstrated encouraging anti-tumor activity with an ORR of 40% among the 57 evaluated patients [332]. An ongoing phase I REACTIVE-2 trial (NCT05650918) is currently assessing the safety and tumor-specific immunologic endpoints of a DC vaccine loaded with tumor lysates in combination with CD40 agonism [333].
CD11b agonist Integrin αMβ2 (CD11b/CD18), expressed on macrophages, monocytes, neutrophils, and some DC subsets, plays a crucial role in leukocyte adhesion to vasculature and transmigration under inflammatory conditions by binding fibrinogen and endothelial ICAM-1 [334, 335]. Preclinical evidence suggests that the small molecule CD11b agonist ADH-503 may suppress myeloid infiltration into inflamed sites by enhancing CD11b-dependent adhesion to endothelial ICAM-1 [336]. Partial CD11b activation by ADH-503 led to TAM repolarization, reduced immunosuppressive infiltrates, and enhanced DC responses in PDAC models, thereby improving antitumor T cell immunity and the efficacy of checkpoint inhibitors [337]. However, a phase I trial of the CD11b modulator GB1275 as monotherapy or in combination with pembrolizumab in advanced solid tumors, including PDAC, was terminated due to lack of observed benefit (NCT04060342) [338]. Rational combination approaches incorporating CD11b agonism with other therapeutic modalities, such as chemotherapy, may unlock clinical efficacy not seen with single-agent approaches.
Neutrophil modulation Neutrophils are considered one of the main immune cells in the PDAC TME. Increasing research over recent years has revealed the critical roles neutrophils play in PDAC tumorigenesis, progression, and metastasis, prompting significant attention to their study in PDAC [303]. Recent studies have highlighted the importance of polymorphonuclear MDSCs, a subpopulation of neutrophils with immunosuppressive functions, in mediating resistance to therapies such as CSF-1R blockade in PDAC [339]– [341]. Targeting the CXCL8-CXCR1/2 axis has shown promise in preclinical PDAC models for neutrophil-targeted therapy [342]. As described before, an anti-IL-8 antibody (BMS-986253) is currently being tested in the neoadjuvant platform clinical trial for resectable PDAC (NCT02451982). Further, SX-682, an orally available allosteric inhibitor of CXCR1 and CXCR2, is currently being tested in combination with nivolumab (NCT04477343) and tislelizumab (NCT05604560) in unresectable and resectable PDAC, respectively [343]. Another agent, AZD5069, a selective CXCR2 antagonist, is under investigation in combination with durvalumab for metastatic PDAC, though results are yet to be reported (NCT02583477).
AMD3100, a CXCL12/CXCR4 antagonist, has demonstrated promising results in preclinical and clinical studies. A completed dose-escalation study (NCT02179970) in patients with advanced pancreatic cancer showed that AMD3100 treatment significantly reduced circulating tumor DNA and CXCL8 levels by impairing CXCR4-mediated chemokine receptor function, which is crucial for intratumoral immune cell accumulation [344]. A recently completed phase II clinical trial evaluating the safety and clinical activity of AMD3100 in combination with cemiplimab (PD-1 blockade) in patients with metastatic pancreatic cancer (NCT04177810) indicated that the combination therapy significantly mobilized myeloid cells and increased their infiltration into the hepatic metastatic microenvironment of PDAC, suggesting a potential resistance mechanism to CXCR4-targeted therapy [345]. BL-8040 is a small synthetic peptide that binds to CXCR4 with higher affinity and longer receptor occupancy than AMD3100, and has also shown potential in clinical studies [346]– [348]. A phase IIa, open-label, two-cohort study (NCT02826486) assessed the safety, efficacy, and immunobiological effects of BL-8040 combined with pembrolizumab and chemotherapy in metastatic PDAC. The results demonstrated that BL-8040 can increase CD8+ effector T cell tumor infiltration, decrease MDSCs, and reduce circulating Tregs, suggesting that co-inhibition of CXCR4 and PD-1 may enhance chemotherapy outcomes in PDAC patients [349].
Targeting stroma
Stromal elements play crucial roles in determining the biology of PDAC and their response to chemotherapy and/or immunotherapy. Therefore, designing therapeutic strategies targeting the tumor stroma in PDAC is also of paramount importance [158]. Each PDAC, due to the physicochemical properties of stromal fibrosis, possesses multiple therapeutic obstacles that prevent proper vascularization, thereby limiting chemotherapy exposure and resulting in poor immune cell infiltration [350, 351]. Neuzillet et al. demonstrated through transcriptomic analysis that cancer-associated fibroblasts (CAFs) derived from human PDAC exhibit a high level of intertumor and intratumor heterogeneity, with at least four subtypes identified [352]. Independent research from our institution corroborated these findings, indicating inter- and intratumoral heterogeneity in stromal signaling, revealing potential mechanisms for CAF heterogeneity at the transcriptomic level [353]. Approaches to deconstruct the stroma generally involve the use of matrix metalloproteinase (MMP) inhibitors, hyaluronidase, Sonic Hedgehog (SHH) inhibitors, fibroblast activation protein (FAP) targeting agents, and focal adhesion kinase (FAK) inhibitor.
Despite preclinical success in other cancers such as melanoma and overall tolerability in patients, MMP inhibitors like marimastat and tanomastat failed to demonstrate significant clinical activity in advanced pancreatic cancer patients [354, 355]. This suggests that nonspecific targeting of the extracellular matrixc (ECM) alone is ineffective against pancreatic cancer. A more specific approach to disrupting the ECM's hardened barrier is targeting hyaluronic acid. A randomized phase II trial showed that adding PEGPH20, a pegylated recombinant human hyaluronidase, to gemcitabine/nab-paclitaxel improved PFS (6.0 vs 5.3 months) in patients with untreated metastatic PDAC [356]. However, another phase Ib/II trial indicated that adding PEGPH20 reduced OS (7.7 vs 14.4 months) in patients with metastatic PDAC receiving FOLFIRINOX [357]. Additionally, a subsequent phase III trial showed that combining PEGPH20 with gemcitabine/nab-paclitaxel did not improve OS (11.2 vs 11.5 months) compared to chemotherapy alone [358]. The inability of PEGPH20 to enhance chemotherapy efficacy does not necessarily exclude ECM-targeting drugs from future anti-cancer treatments but suggests that targeting this fibrotic barrier component alone is insufficient to explain chemotherapy's ineffectiveness in PDAC. There is evidence that SHH contributes to both intrinsic cellular carcinogenesis and the profibrotic process [359]. Hence, inhibiting SHH has been explored as a therapeutic strategy for PDAC. However, clinical trials with SHH inhibitors such as saridegib, vismodegib, and vismodegib have been largely disappointing [158].
Elevated activation of FAK signaling potently regulates the formation of profibrotic stromal matrix deposition and immunosuppressive TME properties [360]. Complementing this understanding, preclinical evidence has provided mechanistic insight demonstrating synergistic recruitment of anti-tumor effector memory T lymphocyte populations following combined targeting of prominent oncogenic pathways governing the dense desmoplasia. Namely, concurring disruption of hyaluronic acid-rich stroma and inhibition of CXCR4 receptor signaling coupled with interdiction of FAK activation within CAFs successfully reprograms the hostile immune environment [361]. The current randomized phase II trial (NCT03727880) evaluates the use of pembrolizumab with or without Defactinib, a FAK inhibitor, as sequential neoadjuvant and adjuvant therapy in patients with high-risk (CA19-9 >
> 200) resectable PDAC (NCT03727880) [362]. The preliminary findings showed that pembrolizumab combined with defactinib was associated with lower fibroblast infiltration, higher anti-tumor M1 macrophage expression and increased CD8+ T-cell infiltration into the TME, versus pembrolizumab alone. The increased expression of CXCR4 across both treatment arms may represent a resistance mechanism and support CXCR4 as an additional TME target. Furthermore, preclinical research has indicated that FAK inhibition can sensitize PDAC to radiotherapy-induced antitumor immunity. The combination of FAK inhibitors with radiotherapy has shown potential in sustaining checkpoint immunotherapy efficacy, leading to prolonged tumor control and potential eradication [363]. Based on these findings, a clinical trial (NCT04331041) is currently testing the efficacy of combining FAK inhibition (defactinib) with SBRT in patients with LAPC, although further data are still pending.
200) resectable PDAC (NCT03727880) [362]. The preliminary findings showed that pembrolizumab combined with defactinib was associated with lower fibroblast infiltration, higher anti-tumor M1 macrophage expression and increased CD8+ T-cell infiltration into the TME, versus pembrolizumab alone. The increased expression of CXCR4 across both treatment arms may represent a resistance mechanism and support CXCR4 as an additional TME target. Furthermore, preclinical research has indicated that FAK inhibition can sensitize PDAC to radiotherapy-induced antitumor immunity. The combination of FAK inhibitors with radiotherapy has shown potential in sustaining checkpoint immunotherapy efficacy, leading to prolonged tumor control and potential eradication [363]. Based on these findings, a clinical trial (NCT04331041) is currently testing the efficacy of combining FAK inhibition (defactinib) with SBRT in patients with LAPC, although further data are still pending.
In addition, reprogramming the ECM also involves targeting the cells that deposit ECM components. CAFs are the major components of the PDAC stroma and are heterogeneous, including myofibroblastic, inflammatory, and antigen-presenting subtypes [364]. However, the heterogeneity of CAFs makes direct targeting complex and may lead to unexpected biological outcomes, contributing to the failure of FAP inhibitors [365].
Cell therapy
Tumor-infiltrating lymphocytes therapy
TILs represent a polyclonal population with diverse TCRs capable of recognizing a wide array of TAAs, which may offer advantages over genetically engineered immune cells for treating solid tumors [366]. In the immunosuppressive environment of PDAC, endogenous TILs often lack the necessary quality and quantity to mount a robust antitumor response. However, ex vivo expansion of TILs from surgically resected pancreatic tumors has demonstrated that these cells can maintain functionality and effectively target HLA-matched pancreatic tumor cells [367]. Reinfusing this polyclonal TIL product may help overcome immunosuppressive barriers and induce potent tumor regression, avoiding the selective pressures associated with single-antigen targeting therapies. The landmark C-144–01 trial showed that the autologous TIL preparation lifileucel achieved an ORR of 31.4%, including 8 complete responses and 40 partial responses in advanced melanoma patients following the failure of anti-checkpoint and targeted therapies [368]. Additionally, the phase II C-144–01 study results indicated that lifileucel had clinically meaningful and durable effects in 15 patients with the refractory mucosal melanoma subtype (ORR: 50%), leading to lifileucel's recognition as the first FDA-approved TIL therapy [369]. In PDAC, research has shown that expanded TILs can recognize pancreatic cancer-associated antigens [370]. Current studies are evaluating TIL therapy in advanced PDAC (NCT05098197, NCT03935893, NCT03610490, and NCT01174121), with results yet to be published (Table 7) [371]– [373].
Table 7
Ongoing clinical trials in pancreatic cancer investigating the effectiveness of cell therapy
| Drug Category | Phase of trial | Number of patients | Disease stage | Candidate drug and combination regimen | Primary outcome | Survival/Rate (Month) | Clinical trial identifier |
|---|---|---|---|---|---|---|---|
| Tumor-infiltrating lymphocytes (TILs) | II | 332 | Advanced or metastatic cancer including PDAC | Young TIL, Aldesleukin, Cyclophosphamide, Fludarabine | ORR | N/A | NCT01174121 [371] |
| Young TIL, Aldesleukin, Cyclophosphamide, Fludarabine, Pembrolizumab | |||||||
| I | 50 | Advanced hepatobiliary-pancreatic cancers | autologous TILs | AEs, ORR, DCR, DOR, PFS, OS | N/A | NCT05098197 | |
| II | 240 | Advanced solid cancers including PDAC | TIL, Fludarabine, Cyclophosphamide | ORR | N/A | NCT03935893 | |
| II | 60 | Recurrent or refractory cancers including PDAC | MDA-TIL, Cyclophosphamide, Fludarabine, Interleukin-2 | ORR | N/A | NCT03610490 [373] | |
| CAR-T | I/II | 10 | HER2-positive advanced cancers | Anti-HER2 CAR-T cells | Safety | 2 patients with SD | NCT01935843 |
| I | 5 | Metastatic | Anti-CEA CAR-T cells | Safety | N/A | NCT02850536 | |
| I/II | 60 | Metastatic | Anti-EGFR CAR-T cells | TRAEs | N/A | NCT01869166 | |
| I | 18 | Locally advanced and metastatic | Anti-mesothelin CAR-T cells | TRAEs | N/A | NCT03323944 | |
| N/A | 10 | Locally advanced and metastatic | Anti-mesothelin CAR-T cells | TRAEs | N/A | NCT03638193 | |
| I | 134 | Refractory Metastatic | Anti-claudin18.2 CAR-T cells (CT041) | Safety | N/A | NCT03874897 | |
| CT041, Toripalimab | |||||||
| CT041, Chemotherapy | |||||||
| I/II | 192 | Advanced pancreatic cancer | Anti-claudin18.2 CAR-T cells (CT041) | TRAEs; Maximum Tolerated Dose; PFS | N/A | NCT04581473 | |
| I/II | 110 | Advanced or metastatic cancers | Anti-claudin18.2 CAR-T cells | ORR | N/A | NCT04404595 | |
| I | 56 | Advanced pancreatic cancer | Anti-claudin18.2 CAR-T cells | Recommended dose for expansion; Recommended Phase 2 dose | N/A | NCT05539430 | |
| CAR-NK | I | 20 | Locally Advanced | CAR-NK | Maximum tolerated dose; DLTs | N/A | NCT06478459 |
| I | 30 | Advanced gastric and pancreatic cancer | CAR-NK Cells targeting Claudin18.2 | Maximum tolerated dose; DLTs | N/A | NCT06464965 | |
| TCR-engineered T-cell therapy | I/II | 110 | Advanced or metastatic cancers including PDAC | Cyclophosphamide, Fludarabine, Anti-KRAS G12V mTCR PBL, Aldesleukin | ORR; TRAEs | N/A | NCT03190941 |
| I/II | 30 | Advanced or metastatic cancers including PDAC | Cyclophosphamide, Fludarabine, Mutant KRAS G12V-specific TCR transduced autologous T cells, Anti-PD-1 antibody | ORR; TRAEs | N/A | NCT04146298 | |
| I/II | 70 | Advanced or metastatic cancers including PDAC | Cyclophosphamide, Fludarabine, anti-KRAS G12D mTCR PBL, Aldesleukin | ORR; TRAEs | N/A | NCT03745326 | |
| I | 24 | Advanced or metastatic cancers including PDAC | NT-112: Autologous, engineered T Cells targeting KRAS G12D | Maximum Tolerated Dose (MTD) and Recommended Phase 2 Dose (RP2D(s)); Treatment-related AEs | N/A | NCT06218914 | |
| I | 15 | Metastatic | Autologous Mesothelin-specific TCR-T Cells, Cyclophosphamide, Fludarabine, Bendamustine | Treatment-related AEs, DLT | N/A | NCT04809766 [405] | |
| Autologous Mesothelin-specific TCR-T Cells, Cyclophosphamide, Fludarabine |
TRAE Treatment Related Adverse Events, AEs Adverse Events, ORR Objective Response Rates, DCR Disease Control Rate, DOR Duration of Response, PFS Progression-Free Survival, OS Overall Survival
Chimeric antigen receptor (CAR) cell therapy
CAR-T cell therapy involves isolating and genetically modifying patient-derived T lymphocytes ex vivo to express engineered CAR constructs on their surface. Antigen selection remains a significant challenge for CAR-T strategies targeting PDAC. Most efforts have focused on TAAs, which often exhibit variable or heterogeneous expression among tumor cells, posing a high risk of on-target, off-tumor toxicity. Antigens currently under clinical investigation for CAR-T therapy in PDAC include prostate stem cell antigen, carcinoembryonic antigen (CEA), MUC-1, human epidermal growth factor receptor 2 (HER2), EGFR, CD133, epithelial cell adhesion molecule, and Claudin 18.2 (CLDN18.2) [374, 375]. Early-phase clinical trials have demonstrated preliminary therapeutic potential, but serious adverse events, particularly with HER2 and CEA, have limited their evaluation as CAR-T targets in PDAC [376]– [379]. Mesothelin, a glycosylphosphatidylinositol-anchored glycoprotein, is overexpressed in 80–85% of PDAC cases and has low expression in normal tissues, making it a promising target antigen [380]. In a phase I clinical trial, six patients with treatment-refractory metastatic PDAC received autologous CAR-T cells targeting mesothelin. The treatment was well-tolerated, with no cytokine release syndrome or neurological adverse events observed, and two patients achieved stable disease with PFS of 3.8 and 5.4 months, respectively [381]. CLDN18.2, a tight junction protein, is ectopically expressed in PDAC and associated precancerous lesions, indicating early expression in PDAC development [382]. CT041, an autologous T cell therapy genetically modified to express a CAR targeting CLDN18.2, was evaluated in a single-arm, open-label, first-in-human phase I pilot study (NCT03159819) to investigate its safety and efficacy. The study demonstrated the safety of CT041 and showed potential therapeutic benefits [383]. In another study, two patients with metastatic PDAC who received CT041 treatment after standard treatment failure both achieved a partial response, further supporting the translational value of CAR-CLDN18.2 [384]. Further data from the same study, which included an analysis of 24 patients, demonstrated an ORR of 16.7% and a DCR of 70.8%, with a median OS of 10.0 months, highlighting the potential efficacy of CT041 in heavily pretreated pancreatic cancer patients [385]. Currently, another ongoing clinical trial is evaluating CLDN18.2-targeted CAR-T therapies (LB1908) in locally advanced or metastatic PDAC (NCT05539430) (Table 7).
Unlike T cells, NK cells lack clonotypic TCR-CD3 complexes for signal transduction. Their potent cytotoxic function is mediated by the release of lytic granules and cytokines upon forming an immunological synapse with targets, and they also exhibit memory-like functions [386, 387]. Recent single-cell analyses have identified potentially dysfunctional states in NK cells across various cancers, similar to T cell exhaustion [388]. In PDAC, circulating tumor cells may evade NK surveillance via the HLA-E:CD94-NKG2A checkpoint [389]. Adoptive NK cell therapies genetically engineer CAR expression for tumor-specific targeting [390]. As HLA-unrestricted cytotoxic effectors, NK cells offer a universal immunotherapeutic approach without the risk of graft-versus-host disease [391]. However, CAR-NK cells also face challenges, such as short-lived responses that may require repeated administrations for sustained efficacy, difficulties in proper antigen selection, antigen heterogeneity, and donor selection [392]. Importantly, NK cells express several inhibitory killer cell immunoglobulin-like receptors that interact with their HLA molecule ligands. The widespread expression of HLA molecules on nucleated cells can inhibit CAR-NK cell function [393]. Preclinical studies have evaluated the anti-tumor efficacy of CAR-NK cells targeting prostate stem cell antigen and mesothelin in PDAC [394, 395]. A first-of-its-kind, off-the-shelf iPSC-derived NK cell therapy, FT500, is currently under evaluation in a phase I clinical trial for advanced solid tumors, including pancreatic cancer, both as a monotherapy and in combination with checkpoint inhibitors (nivolumab, pembrolizumab, atezolizumab), IL-2, Cy, and fludarabine (NCT03841110) [396].In addition, several ongoing phase I trials are assessing the clinical application of CAR-NK cells in advanced pancreatic cancer, including NKG2D CAR-NK (NCT06478459) and CLDN18.2 CAR-NK (NCT06464965) (Table 7) [397, 398].
TCR-engineered T-cell therapy
TCRs engineered to target neoantigen epitopes resulting from somatic mutations present a promising approach in cancer therapy. These TCRs can bypass central tolerance mechanisms due to the evasion of negative selection in the thymus, allowing for the identification of high-avidity TCRs with enhanced anti-tumor efficacy and reduced off-target toxicity [399, 400]. In PDAC, the high prevalence and conserved mutational profile of mKRAS provide a unique opportunity for developing neoantigen-directed TCR-T therapies. Specifically, HLA-C*08:02-restricted TCRs that recognize the KRASG12D mutation have been isolated from TILs of colorectal cancer patients [401]. A case report detailed a patient with advanced PDAC harboring a KRAS c.35G >
> A (p.G12D) mutation who received autologous T cells engineered to express two allogeneic HLA-C*08:02-restricted TCRs targeting mutant KRASG12D, leading to a partial response with over 72% regression of visceral metastases maintained for six months. Engineered T cells constituted over 2% of circulating T cells thereafter, suggesting the potential for sustained therapeutic efficacy [402]. This therapy could benefit any patient with this specific HLA allele and tumor-expressing KRASG12D mutation, particularly when combined with treatments addressing immune resistance mechanisms. However, the therapy's applicability is limited to patients with the HLA-C*08:02 allele, restricting its potential patient population [403]. Ongoing clinical investigations are evaluating mutant KRASG12D- or KRASG12V-specific TCR-transduced T cell therapies for advanced PDAC (NCT03190941, NCT03745326, NCT04146298, NCT06218914) (Table 7) [404]. Additionally, a phase I trial is exploring the safety and efficacy of autologous mesothelin-specific TCR T cells in metastatic PDAC (NCT04809766), representing another avenue for TCR-engineered T-cell therapy in this challenging cancer type [405].
A (p.G12D) mutation who received autologous T cells engineered to express two allogeneic HLA-C*08:02-restricted TCRs targeting mutant KRASG12D, leading to a partial response with over 72% regression of visceral metastases maintained for six months. Engineered T cells constituted over 2% of circulating T cells thereafter, suggesting the potential for sustained therapeutic efficacy [402]. This therapy could benefit any patient with this specific HLA allele and tumor-expressing KRASG12D mutation, particularly when combined with treatments addressing immune resistance mechanisms. However, the therapy's applicability is limited to patients with the HLA-C*08:02 allele, restricting its potential patient population [403]. Ongoing clinical investigations are evaluating mutant KRASG12D- or KRASG12V-specific TCR-transduced T cell therapies for advanced PDAC (NCT03190941, NCT03745326, NCT04146298, NCT06218914) (Table 7) [404]. Additionally, a phase I trial is exploring the safety and efficacy of autologous mesothelin-specific TCR T cells in metastatic PDAC (NCT04809766), representing another avenue for TCR-engineered T-cell therapy in this challenging cancer type [405].
Experimental targeted therapies on the horizon
PARP inhibitors
As previously described, based on the results of the POLO trial, the FDA has approved olaparib for the treatment of germline BRCA-mutated metastatic PDAC. Currently, olaparib is being tested in the APOLLO trial, a randomized phase II study investigating its efficacy compared to placebo in patients with resected pancreatic cancer harboring pathogenic BRCA1, BRCA2, or PALB2 mutations [406]. However, despite promising results in the POLO trial, approximately 25% of patients experienced disease progression within 2 months of initiating PARP inhibitor treatment, suggesting the development of resistance to PARP inhibitors [185]. The mechanisms behind this resistance in pancreatic cancer remain largely unclear, highlighting the importance of strategies to predict and manage resistance [183]. Researchers are exploring various approaches to enhance PARP inhibitor efficacy. These include developing new PARP inhibitors and combining them with other agents to achieve synergistic anti-tumor effects. For instance, a phase II single-arm study evaluated another PARP inhibitor rucaparib as maintenance therapy in 42 patients with advanced pancreatic cancer with germline or somatic pathogenic variants in BRCA1, BRCA2, or PALB2. This study reported a 6-month PFS rate of 59.5% and an ORR of 41.7%, with median PFS and OS of 13.1 and 23.5 months, respectively [407]. Other next-generation PARP inhibitors, such as fuzuloparib, have been tested in ovarian cancer (NCT04517357); however, their application in pancreatic cancer has not yet been explored.
PARP inhibitors have been shown to increase the TMB in pancreatic cancer, augment cytoplasmic DNA, and potentially activate immunostimulatory pathways through the release of DAMPs. This has led to the investigation of combining PARP inhibitors with ICIs as a therapeutic strategy [408, 409]. A multi-cohort phase II trial (NCT04666740) is evaluating the combination of pembrolizumab with olaparib (POLAR) as maintenance therapy for patients with HRD and platinum-sensitive metastatic PDAC. Preliminary results show that in Cohort B (patients with platinum-sensitive PDAC with non-core homologous recombination gene mutations, such as ATM, BAP1, etc.) and Cohort C (platinum-sensitive patients without known HRD), the combined median PFS is approximately 4 months, while median OS has increased to 14 months [410]. Building on the POLO trial results, the SWOG2001 trial (NCT04548752) is assessing the combination of olaparib and pembrolizumab versus olaparib alone as maintenance therapy in metastatic PDAC, with the primary objective of increasing median PFS from approximately 7 months to 11.7 months [411]. Another phase II study (NCT04493060) is evaluating the PD-1 inhibitor dostarlimab with the PARP inhibitor niraparib in patients with metastatic PDAC harboring somatic or germline mutations in homologous recombination genes (including BRCA1 and BRCA2) following platinum-based chemotherapy [412]. The randomized phase Ib/II PARPVAX trial is investigating the antitumor activity of niraparib combined with either nivolumab (n =
= 46) or ipilimumab (n
46) or ipilimumab (n =
= 45) in patients with advanced pancreatic cancer who have not progressed after over 16 weeks of platinum-based therapy. This trial achieved 44% and 59.6% 6-month PFS rates, respectively [413]. Additional trials investigating PARP inhibitor and immunotherapy combinations in PDAC are ongoing or awaiting results, such as NCT05093231, NCT04753879, NCT03851614, NCT04493060, and NCT04673448 (Table 8). Clinical trials are also examining PARP inhibitors combined with FOLFIRI chemotherapy (NCT02890355) and the anti-angiogenic agent cediranib (NCT02498613). Moreover, PARP inhibitors are also being combined with novel therapeutic approaches, such as bromodomain and extraterminal (BET) inhibitors. The BET protein family plays a critical role in gene transcription, making it an attractive target for cancer therapy [414]. NUV-868 is a novel, highly selective BD2-specific BET inhibitor that, when used in combination with olaparib or androgen receptor antagonist enzalutamide, has been shown to inhibit tumor xenograft growth [415]. The ongoing phase I trial (NCT05252390) aims to evaluate NUV-868 as a monotherapy or in combination with olaparib or enzalutamide in patients with advanced solid tumors, including PDAC.
45) in patients with advanced pancreatic cancer who have not progressed after over 16 weeks of platinum-based therapy. This trial achieved 44% and 59.6% 6-month PFS rates, respectively [413]. Additional trials investigating PARP inhibitor and immunotherapy combinations in PDAC are ongoing or awaiting results, such as NCT05093231, NCT04753879, NCT03851614, NCT04493060, and NCT04673448 (Table 8). Clinical trials are also examining PARP inhibitors combined with FOLFIRI chemotherapy (NCT02890355) and the anti-angiogenic agent cediranib (NCT02498613). Moreover, PARP inhibitors are also being combined with novel therapeutic approaches, such as bromodomain and extraterminal (BET) inhibitors. The BET protein family plays a critical role in gene transcription, making it an attractive target for cancer therapy [414]. NUV-868 is a novel, highly selective BD2-specific BET inhibitor that, when used in combination with olaparib or androgen receptor antagonist enzalutamide, has been shown to inhibit tumor xenograft growth [415]. The ongoing phase I trial (NCT05252390) aims to evaluate NUV-868 as a monotherapy or in combination with olaparib or enzalutamide in patients with advanced solid tumors, including PDAC.
Table 8
Selected ongoing trials of PARP inhibitors in pancreatic cancer therapy
| Category/Target | Phase of trial | (Estimated) Enrollment | Disease stage | Combination regimen | Primary outcome/end point | Survival Months/rates | Clinical trial identifier and reference |
|---|---|---|---|---|---|---|---|
| Olaparib | II | 152 | Resected | N/A | RFS | N/A | NCT04858334 [406] |
| II | 63 | Metastatic | Pembrolizumab | PFS | N/A | NCT04666740 [410] | |
| II | 88 | Metastatic | Pembrolizumab | PFS | N/A | NCT04548752 [411] | |
| II | 20 | Metastatic | Pembrolizumab | ORR | N/A | NCT05093231 | |
| II | 38 | Metastatic | Low dose gemcitabine, nab-paclitaxel, capecitabine, cisplatin, and irinotecan; Pembrolizumab | PFS after 6 months | N/A | NCT04753879 | |
| II | 90 | Advanced solid tumors including PDAC | Durvalumab | Changes in genomic and immune biomarkers | N/A | NCT03851614 | |
| II | 122 | Advanced solid tumors including PDAC | Cediranib | ORR | N/A | NCT02498613 | |
| I/II | 657 | Advanced solid tumors including PDAC | NUV-868 (BD2 inhibitor), enzalutamide | DLTs, ORR | N/A | NCT05252390 | |
| Niraparib | II | 22 | Metastatic | Dostarlimab | DCR at 12 weeks | N/A | NCT04493060 [412] |
| I | 18 | Locally advanced or metastatic cancer including PDAC | Dostarlimab | Best objective response | N/A | NCT04673448 | |
| Veliparib | II | 123 | Metastatic | (m)FOLFIRI | OS | N/A | NCT02890355 |
| II | 107 | Locally advanced or metastatic | Gemcitabine Hydrochloride and Cisplatin | ORR | N/A | NCT01585805 |
DCR, disease control rate; DLTs, dose-limiting toxicities; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; RFS, relapse-free survival
ATM/ATR inhibitors
Ataxia-telangiectasia mutated (ATM) and ATM/Rad3-related (ATR) protein kinases are also key regulators of the homologous recombination repair, involved in the activation and regulation of a large number of highly interconnected proteins [416]. They are emerging as promising targets for anti-cancer drug development [417, 418]. Conventional cancer treatments, such as radiotherapy and chemotherapy, often face resistance due to enhanced DDR mechanisms. Therefore, DDR inhibitors are being used in conjunction with these therapies to overcome such resistance [418]. ATM inhibitors currently in clinical trials include XRD-0394, M4076, AZD1390, and AZD0156. XRD-0394, a novel dual ATM/DNA-dependent protein kinase inhibitor, is being evaluated for safety and tolerability in combination with radiotherapy in advanced solid tumor patients in a phase I trial (NCT05002140) [419]. M4076, when combined with radiotherapy, has shown enhanced anti-tumor activity and complete tumor regression in immunodeficient mice with human tumor xenografts [420]. M4076 is now being tested in a phase I clinical trial for advanced solid tumors (NCT04882917), where preliminary results have established the maximum tolerated dose and confirmed safety [421]. AZD0156 is also under investigation in a phase I trial, either as a monotherapy or in combination with chemotherapies and olaparib for advanced cancer patients (NCT02588105).
Several ATR inhibitors are currently under evaluation in clinical trials. A phase I trial (NCT02723864) evaluated ATR inhibitor M6620 in combination with PARP inhibitor veliparib and cisplatin in patients with advanced solid tumors. This trial, which included 23 patients, demonstrated that this triple therapy was both safe and effective in tumors with homologous recombination deficiencies [422]. Another phase I study (NCT02487095) assessed M6620 combined with the chemotherapy topotecan, a selective Topo1 inhibitor, in 21 patients (including 2 with pancreatic cancer). This combination proved to be tolerable and effective [423]. Additionally, a phase I trial (NCT02157792) evaluated M6620 either alone or in combination with carboplatin in 40 patients with advanced solid tumors, including 1 pancreatic cancer, showing good tolerability and antitumor responses [424]. A phase I trial (NCT04170153) is ongoing to assess another ATR inhibitor M1774 either as a monotherapy or in combination with the PARP inhibitor niraparib in patients with advanced solid tumors. Preliminary results indicate that M1774 is well-tolerated [425, 426]. Another phase I/II trial (NCT04497116) is optimizing the regimen for ATR inhibitor camonsertib (RP-3500) as monotherapy in advanced solid tumors, including 14 pancreatic cancer patients [427]. ATR inhibitors under investigation include AZD6738 (NCT02264678), BAY1895344 (NCT04267939, NCT03188965), RP-3500 (NCT04972110, NCT04497116), M4344 (NCT04149145), and ATRN-119 (NCT04905914). These trials are exploring the use of these inhibitors as monotherapy or in combination with chemotherapy and/or radiotherapy in advanced solid tumors, including pancreatic cancer [418, 428].
Targeting oncogenic KRAS signaling
RAS is a subfamily of small GTPases, including KRAS, HRAS and NRAS isoforms [429], which act as molecular switches to regulate intracellular signal transduction. RAS proteins are activated when bound to guanosine triphosphate (GTP), and inactivated when bound to guanosine diphosphate (GDP). When activated, these proteins can “switch on” downstream pathways and regulate cell survival, proliferation, and differentiation. Their genes have almost the same structure, but in cancer, they mutate at different frequencies. KRAS, a major oncogenic driver gene mutated in over 90% of PDAC cases, has revealed new insights into pancreatic carcinogenesis and opened opportunities for targeted therapies [430]– [432]. The mutations, particularly at codons G12, G13, and Q61, result in the constitutive activation of KRAS, driving tumorigenesis through persistent activation of downstream signaling pathways such as RAF/MEK/ERK and PI3K/Akt/mTOR (Fig. 3, Table 9) [172, 433, 434].

Therapeutic strategies targeting KRAS mutations in pancreatic cancer. KRAS, a GTPase, transitions between an inactive GDP-bound state and an active GTP-bound state, driving downstream signaling pathways that promote cell proliferation and survival, such as the PI3K/AKT/mTOR and RAF/MEK/ERK pathways. Therapeutic strategies targeting KRAS aim to prevent its activation, disrupt its signaling, or indirectly inhibit the KRAS pathway upstream. KRAS inhibitors can directly bind to either the GDP-bound or GTP-bound state of KRAS, disrupting further signaling. Specific inhibitors for KRASG12C (e.g., Sotorasib, Adagrasib) and KRASG12D (e.g., MRTX1133, ASP3082) mutations target specific isoforms of mutated KRAS, while pan-RAS inhibitors (e.g., RMC-6236) offer a broader approach by targeting multiple RAS isoforms. Indirect inhibition of the KRAS pathway is being explored through upstream inhibitors, such as SHP2 and SOS1 inhibitors. Downstream inhibitors disrupt key signaling pathways activated by KRAS, with examples including PI3K/AKT inhibitors (Rigosertib, Inavolisib), mTOR inhibitors (Everolimus), and RAF/MEK inhibitors (Avutometinib). Additionally, novel KRAS-directed delivery routes, including vaccines targeting specific KRAS mutations (e.g., ELI-002), CAR-T cell therapies, and exosomes loaded with siRNA targeting the KRASG12D mutation, are also under investigation
Table 9
Selected ongoing trials for KRAS-targeted therapy
| Category/Target | Phase of trial | (Estimated) Enrollment | Disease stage | Combination regimen | Primary outcome/end point | Survival Months/rates | Clinical trial identifier and reference | |
|---|---|---|---|---|---|---|---|---|
| KRAS G12C inhibitor | Sotorasib (AMG 510) | I/II | 713 | Advanced solid tumors including PDAC | N/A | AEs, DLTs, ORR | 21% in 38 PDAC patients | NCT03600883 [442] |
| Adagrasib (MRTX849) | I/II | 822 | Advanced solid tumors including PDAC | Pembrolizumab; Cetuximab; Afatinib | AEs, ORR | 33.3% in 21 PDAC patients | NCT03785249 [443] | |
| Glecirasib (JAB-21822) | I/II | 311 | Advanced solid tumors including PDAC | N/A | AEs, DLTs, ORR | 46.4% in 28 PDAC patients | NCT05009329 NCT05002270 [449] | |
Olomorasib (LY3537982) | I | 550 | Advanced solid tumors including PDAC | Pembrolizumab; Cetuximab; Pemetrexed; Cisplatin; Carboplatin | AEs, DLTs, antitumor activity | N/A | NCT04956640 | |
| Divarasib (GDC-6036) | I | 498 | Advanced solid tumors including PDAC | Atezolizumab; Cetuximab; Bevacizumab; Erlotinib; GDC-1971 (SHP2 inhibitor); Inavolisib | AEs, DLTs | N/A | NCT04449874 [451] | |
| RMC-6291 | I | 222 | Advanced solid tumors including PDAC | N/A | AEs, DLTs | N/A | NCT05462717 | |
| JDQ443 | I/II | 475 | Advanced solid tumors including PDAC | TNO155(SHP2 inhibitor), tislelizumab | AEs, DLTs, ORR | N/A | NCT04699188 | |
| D-1553 | I/II | 180 | Advanced solid tumors including PDAC | Pembrolizumab, cetuximab | AEs, DLTs | N/A | NCT04585035 | |
| FMC-376 | I/II | 403 | Advanced solid tumors including PDAC | N/A | AEs, DLTs | N/A | NCT06244771 | |
| BI-1823911 | I | 30 | Advanced solid tumors including PDAC | BI 1701963 (SOS1 inhibitor) | DLTs, ORR | N/A | NCT04973163 | |
| KRAS G12D inhibitor | MRTX1133 | I/II | 386 | Advanced solid tumors including PDAC | N/A | DLTs, AEs, ORR, PFS, OS | N/A | NCT05737706 |
| ASP3082 | I | 541 | Advanced solid tumors including PDAC | Cetuximab, chemotherapy | AEs, DLTs | N/A | NCT05382559 [457] | |
| RMC-9805 | I | 290 | Advanced solid tumors including PDAC | N/A | AEs, DLTs | N/A | NCT06040541 | |
| HRS-4642 | I | 108 | Advanced solid tumors including PDAC | N/A | AEs, DLTs | N/A | NCT05533463 [458] | |
| pan-RAS inhibitor | RMC-6236 | I | 474 | Advanced solid tumors including PDAC | N/A | AEs, DLTs | N/A | NCT05379985 [462] |
| RMC-6236 | I | 210 | Advanced solid tumors including PDAC | RMC-6291 | AEs, DLTs | N/A | NCT06128551 | |
| Upstream | BI1701963 (SOS1 inhibitor) | I | 71 | Advanced solid tumors including PDAC | Trametinib | DLTs, ORR | N/A | NCT04111458 [ [471] |
| MRTX0902 (SOS1 inhibitor) | I/II | 228 | Advanced solid tumors including PDAC | Adagrasib | DLTs, AEs, ORR, PFS, OS | N/A | NCT05578092 | |
| TNO155 (SHP2 inhibitor) | I | 227 | Advanced solid tumors including PDAC | Nazartinib | AEs, DLTs | N/A | NCT03114319 | |
| TNO155 (SHP2 inhibitor) | I | 89 | Advanced solid tumors including PDAC | Adagrasib | AEs | N/A | NCT04330664 [477] | |
| RMC-4630 (SHP2 inhibitor) | I | 133 | Relapsed/Refractory solid tumors including PDAC | N/A | AEs, DLTs | N/A | NCT03634982 | |
| Downstream | Binimetinib (MEK inhibitor) | I | 39 | Metastatic | Hydroxychloroquine | MTD | N/A | NCT04132505 |
| Binimetinib (MEK inhibitor) | II | 199 | Metastatic | Palbociclib | ORR | N/A | NCT05554367 | |
| Trametinib (MEK inhibitor) | I | 39 | Locally advanced or metastatic | Hydroxychloroquine | DLTs | N/A | NCT03825289 | |
| MEK inhibitor (Trametinib, cobimetinib or binimetinib) | II | 20 | Refractory malignancies including PDAC | Hydroxychloroquine, bevacizumab | ORR | N/A | NCT06229340 | |
| IMM-1–104 (MEK inhibitor) | I/II | 210 | Advanced solid tumors including PDAC | Gemcitabine/nab-Paclitaxel, mFOLFIRINOX | AEs, DLTs, ORR | N/A | NCT05585320 | |
| Avutometinib (VS-6766, RAF/MEK clamp) | Ib/II | 40 | Metastatic | Defactinib, gemcitabine and nab-paclitaxel | DLTs, ORR | N/A | NCT05669482 [484] | |
| Nab-sirolimus (mTOR inhibitor) | I | 79 | Advanced solid tumors including PDAC | Adagrasib | AEs, MTD, ORR | N/A | NCT05840510 | |
AEs Adverse events, DFS disease free survival, DLTs Dose limiting toxicities, MTD Maximum tolerated dose, ORR Objective response rate, OS overall survival
Agents directly targeting KRAS mutation
For decades, cancer drug development has focused on directly targeting RAS function with small molecules and peptides. However, due to the complex protein structure, high affinity for GTP, and multiple alternative signaling pathways, the KRAS protein has proven to be an “undruggable” target [435]. Groundbreaking research in 2013 identified small molecules capable of covalently binding to KRASG12C-GDP, making KRAS more likely to bind to GDP and thus become inactivate [436]. These inhibitors specifically bind to the KRASG12C mutant protein, with minimal binding affinity for the wild-type KRAS protein. Subsequently, a series of KRASG12C inhibitors were developed, including ARS853 [437], ARS-1260 [438], AMG 510 [439], MRTX849 [440], and AMG 510 being the first KRASG12C inhibitor to enter clinical development. In 2021, the FDA approved the first KRAS-targeted drug, sotorasib (AMG 510), for patients with previously treated NSCLC with KRASG12C mutations [441]. The approval was based on results from the phase II CodeBreaK 100 trial (NCT03600883), which demonstrated an 80.6% DCR, a median PFS of 6.8 months, and a median OS of 12.5 months in patients with KRASG12C-mutated advanced NSCLC who had been previously treated with standard therapies [442]. In 2022, the FDA approved the second KRASG12C inhibitor, adagrasib (MRTX849), an oral small molecule, for the treatment of KRASG12C-mutated locally advanced or metastatic NSCLC. This approval was based on the results of the phase II KRYSTAL-1 trial (NCT03785249), in which adagrasib achieved an ORR of 42.9%, a DCR of 79.5%, a median PFS of 6.5 months, and a median OS of 12.6 months in patients with advanced or metastatic NSCLC harboring a KRASG12C mutation [443]. Furthermore, the phase III CodeBreaK 300 trial (NCT05198934) demonstrated a PFS benefit with two different doses of the KRASG12C inhibitor sotorasib plus the EGFR inhibitor panitumumab in patients with refractory colorectal cancer harboring KRASG12C mutations (5.6 months, 3.9 months, respectively, compared to 2.2 months in the standard-care group), supporting sotorasib 960 mg plus panitumumab as a potential standard of care in KRASG12C mutated metastatic colorectal cancer [444, 445]. This combination therapy is currently under review by the FDA.
KRASG12C inhibitors have also shown breakthroughs in treating pancreatic cancer, in which the KRASG12C mutation is present in approximately 3% of patients [446]. CodeBreaK100 (NCT03600883), an international, single arm, phase I/II trial evaluating the efficacy and safety of sotorasib in patients with KRASG12C-mutated advanced solid tumors, including pancreatic cancer, enrolled 38 patients with PDAC as of November 2021, achieving a 21.1% ORR, an 84.2% DCR, a median PFS of 3.98 months, and a median OS of 6.87 months, with good tolerability [447]. The KRYSTAL-1 trial (NCT03785249) is a multicohort phase I/II study evaluating the KRASG12C inhibitor adagrasib in patients with advanced solid tumors harboring a KRASG12C mutation [448]. As of October 2022, 21 patients with PDAC were enrolled, showing a 33.3% ORR, an 81.0% DCR, a median PFS of 5.4 months, and a median OS of 8.0 months, demonstrating promising clinical activity in PDAC. Glecirasib (JAB-21822), another highly selective KRASG12C inhibitor, has demonstrated promising clinical activity in NSCLC and colorectal cancer, and is now being evaluated in PDAC with manageable side effects. Two phase I/II trials (NCT05009329 in China and NCT05002270 in US) pooled data from 48 patients, including 28 with PDAC. Among these PDAC patients, 13 achieved a partial response (46.4% ORR), with a DCR of 96.4%, a median duration of response of 4.1 months, and a median PFS of 5.5 months [449]. Olomorasib (LY3537982), a potent and highly selective second-generation inhibitor of GDP-bound KRASG12C, demonstrated promising efficacy and safety in a phase I trial (NCT04956640). Among 24 pancreatic cancer patients treated, preliminary results showed encouraging antitumor activity and a favorable safety profile, with no dose-limiting toxicities observed; diarrhea was the most common side effect [450]. Divarasib (GDC-6036) is another covalent KRASG12C inhibitor whose safety was evaluated in a phase I study (NCT04449874) [451], enrolling 137 patients with a KRASG12C mutations, including those with NSCLC, colorectal cancer, and other solid tumors. Divarasib showed durable clinical responses with mostly low-grade adverse events. Among the 7 patients with PDAC enrolled, partial responses were observed in 3 patients (42.9%), and stable disease in 4 patients (57.1%). Additional KRASG12C inhibitors currently in clinical trials include RMC-6291 (NCT05462717), JDQ443 (NCT04699188), D-1553 (NCT04585035), FMC-376 (NCT06244771), and BI-1823911 (NCT04973163) (Table 9) [452].
The major KRAS mutant isoforms found in PDAC are G12D, G12V, and G12R. The success of KRASG12C inhibitors has inspired the development of KRASG12D inhibitors. The KRASG12D mutation, found in approximately one-third of PDAC patients, has been targeted with MRTX1133, a non-covalent selective inhibitor developed through structure-based drug design [453, 454]. MRTX1133 induced deep tumor regression in preclinical KRASG12D mouse tumor models and altered the TME, including reduction of MDSCs, increase of M1-like macrophages, and increase of tumor-infiltrating cytotoxic T cells [455]. Given these strong preclinical data, a phase I trial (NCT05737706) is currently enrolling patients to investigate its use in KRASG12D advanced solid tumors. However, MRTX1133 has pharmacokinetic issues, and a new formulation is being developed [456]. Other KRASG12D targeted therapies are in earlier phases of drug development, such as direct inhibitor HRS-4642 (NCT05533463), KRASG12D targeted degrader ASP3082 (NCT05382559), and molecular glue inhibitor RMC-9805 (NCT06040541) (Table 9) [457, 458].
Given the heterogeneity of RAS mutations among patients, dual or pan-RAS inhibition has emerged as a promising therapeutic strategy [459]. RMC-6236 is an oral small molecule inhibitor designed to target cancers driven by diverse RAS mutations [460]. Preclinical study on cell line-derived and patient-derived xenograft models of KRAS mutant PDAC demonstrated impressive antitumor activity [461]. Another study in mouse xenograft models with KRASG12X demonstrated that RMC-6236 achieved tumor regressions across multiple tumor types, including NSCLC and PDAC [462]. An ongoing phase I/II clinical trial (NCT05379985) is evaluating the effectiveness of RMC-6236 in patients with specific RAS mutant advanced solid tumors [462]. As of April 2023, 22 PDAC patients with KRASG12X were enrolled. Among 10 PDAC patients with at least 8 weeks of RMC-6236 treatment, objective response was observed in 2 patients (ORR, 20%), and DCR was 80%, exhibiting promising anti-tumor activity with well tolerance [463]. Notably, to balance the benefit and toxicity of pan-RAS/KRAS drugs, a phase I study combining KRASG12C inhibitor RMC-6291 with RMC-6236 (pan-RAS) is now underway in solid tumors (NCT06128551). BI-2865 is a novel pan-KRAS inhibitor that affects a wide spectrum of mutated KRAS and has demonstrated in vivo tumor reduction capability without detrimental effects on the animals [464, 465]. A phase I trial of BI-3706674, a similar compound to BI-2865 that inhibits multiple KRAS variants, is now under investigation in patients with advanced stomach and esophagus cancer(NCT06056024) [466]. Additionally, RMC-7977, another promising pan-RAS inhibitor targeting the active state of both mutant and wild-type KRAS, NRAS, and HRAS, has exhibited efficacy against RAS-dependent tumors with diverse RAS genotypes in preclinical models, particularly those harboring the KRASG12X mutation [467].
Although the development of allele-specific inhibitors has transformed KRAS into a targetable protein, responses occur in only about 20–30% of patients, and these responses are often partial and not durable [468]. Thus, extensive efforts are underway to overcome intrinsic and acquired drug resistance. Research has shown that drug resistance emerged with MRTX1133 treatment in the KRASG12D mouse tumor model, characterized by amplifications of KRAS, Yap1, Myc, and Cdk6/Abcb1a/b, and co-evolution of drug-resistant transcriptional programs [469]. Combining MRTX1133 with chemotherapy or co-targeting of EGFR may result in better antitumor effects than MRTX1133 alone in PDAC mouse models [469, 470].
Targeting upstream SOS1, SHP2
Inhibiting upstream effectors that support KRAS function could target all mKRAS alleles indiscriminately, and thus they are attractive targets for combination therapies with KRAS inhibitors. Son of sevenless 1 (SOS1) triggers GTP loading of KRAS through its nucleotide exchange activity, and Src homology region 2-containing protein tyrosine phosphatase 2 (SHP2) directly activates SOS1 activity. Therefore, inhibition of SOS1 or SHP2 could maintain GDP-bound KRAS in an inactive form. A phase I clinical trial (NCT04111458) is ongoing in patients with KRAS mutated advanced or metastatic solid tumors to evaluate the safety and efficacy of the first SOS1 inhibitor BI-1701963 alone and in combination with the MEK inhibitor trametinib [471]. The rationale for adding the MEK inhibitor is to eliminate the negative feedback in the MEK/ERK signaling pathway caused by SOS1 inhibition. The phase I trial KRYSTAL-14 (NCT04975256) evaluated BI-1701963 in combination with KRASG12C inhibitor adagrasib in patients with advanced KRASG12C mutated solid tumors. Another SOS1 inhibitor MRTX0902 also showed enhanced antitumor activity in combination with KRASG12C inhibitor MRTX849 in mouse xenograft tumor model [472]. A phase I/II trial (NCT05578092) is ongoing in patients with advanced solid tumor malignancy harboring mutations in the KRAS-MAPK pathways to evaluate the safety and efficacy of MRTX0902 alone and in combination with adagrasib. BI-3406, a selective SOS1-KRAS interaction inhibitor, reduced formation of GTP-loaded RAS and thus limited cellular proliferation of KRAS-driven tumors [473]. BI-3406 also attenuated feedback reactivation induced by MEK inhibitors, and is a suitable candidate in combination with MEK inhibitors in KRAS-driven tumors. Other SOS1 inhibitors under development include RM-0331, RMC-5845, GH52, and ERAS-9 [474]. SHP2, a tyrosine phosphatase activated by receptor tyrosine kinases, is essential for RAS activation [475]. SHP2 inhibitor TNO155 has demonstrated synergy with KRASG12C inhibitor and greatly enhanced efficacy against KRASG12C tumor cells [476]. The phase I trial (NCT03114319) is ongoing to evaluate the safety and tolerability of TNO155 alone and in combination with EGFR inhibitor EGF816 (nazartinib) in patients with advanced solid tumors. And another phase I/II trial (NCT04330664) is ongoing to evaluate TNO155 alone and in combination with adagrasib in patients with KRASG12C mutated advanced solid tumors [477]. Trials are also underway testing other SHP2 inhibitors combinations in patients with KRAS-mutated tumors such as RMC-4630 (NCT03634982) (Table 9).
Targeting downstream RAS signaling pathways
Before the development of direct RAS inhibitors, efforts were primarily focused on targeting downstream RAS signaling pathways, such as RAF/MEK/ERK and PI3K/PDK1/AKT/mTOR. Given the emergence of primary and acquired resistance to KRAS inhibitors, these pathways have garnered renewed attention. The MEK inhibitor selumetinib was previously compared with gemcitabine as a second-line treatment for pancreatic cancer but did not show a difference in OS [478]. Similarly, another MEK inhibitor, trametinib, failed to improve survival when used in combination with gemcitabine in previously untreated PDAC [479]. However, combining MEK inhibitors with other agents has shown promise. In vitro studies and patient-derived xenograft models have demonstrated that MEK inhibition affects a key regulatory axis of autophagy, and that combined inhibition of MEK and autophagy could synergistically suppress PDAC cell proliferation [480, 481]. Hydroxychloroquine, known for its autophagy inhibitory properties [482], is currently being evaluated in combination with various ERK inhibitors, such as LY3214996 (NCT04386057), binimetinib (NCT04132505), ulixertinib (NCT04145297), and trametinib (NCT03825289) in pancreatic cancer (Table 9). Paradoxically, MEK inhibition can induce RAF-MEK complex formation in KRAS mutant models through feedback pathways, and disrupting this loop through multi-node inhibition represents a promising therapeutic strategy [483]. Avutometinib (VS-6766), a novel RAF/MEK clamp, is in phase I trials in combination with adagrasib (NCT05375994) and sotorasib (NCT05074810) for patients with progression on prior KRAS-directed treatment, and with chemotherapy and the FAK inhibitor defactinib in PDAC (NCT05669482), showing notable preliminary efficacy [484]. ERK1/2 inhibitors have shown limited monotherapy efficacy. In the HERKULES-1 I/IIb trial, 10 pancreatic cancer patients received the ERK1/2 inhibitor ERAS-007, but of the seven evaluable patients, six discontinued treatment within two months due to disease progression [485]. Interestingly, MEK inhibitors are more effective in inhibiting ERK signaling in BRAFV600E than in KRAS mutant tumors [483]. An ongoing phase II trial (NCT04390243) is investigating the combination of the BRAF inhibitor encorafenib and the MEK inhibitor binimetinib in pancreatic cancer patients with a somatic BRAFV600E mutation.
Rigosertib is a multi-kinase inhibitor that induces apoptosis by inhibiting the PI3K/Akt pathway. In a phase II/III trial, adding rigosertib to gemcitabine for previously untreated PDAC did not demonstrate clinical benefit compared to gemcitabine alone [486]. The ongoing phase I trial (NCT04449874) is evaluating the KRASG12C inhibitor divarasib, both as a monotherapy and in combination with other anti-cancer therapies, including the PI3K inhibitor inavolisib, in advanced or metastatic KRASG12C-mutated solid tumors. Everolimus, an oral mTOR inhibitor, showed moderate activity when combined with capecitabine in patients with advanced pancreatic cancer, achieving a median OS of 8.9 months [487]. The ongoing KRYSTAL-19 trial (NCT05840510) is exploring the combination of adagrasib with nab-sirolimus, a novel albumin-bound mTOR inhibitor, in patients with advanced solid tumors and NSCLC with KRASG12C mutations.
Other molecular therapeutic targets
NRG1 fusions
NRG1 fusions occur in approximately 0.5% of pancreatic cancers, often presenting in younger patients [187]. The most common NRG1 fusion involves ERBB3/HER3, leading to the overactivation of ERBB3/HER3, which drives tumor growth and cancer cell survival. Seribantumab, a fully humanized anti-HER3 IgG2 monoclonal antibody, has shown the ability to inhibit tumor growth in preclinical models driven by NRG1 fusions [488]. The phase II CRESTONE trial (NCT04383210) is currently evaluating seribantumab in patients with locally advanced or metastatic solid tumors harboring NRG1 fusions. Preliminary data from this trial suggest that seribantumab induces durable responses with a favorable safety profile [489]. Additionally, zenocutuzumab, a bispecific antibody targeting NRG1 fusion signaling, has demonstrated promising results in a phase I-II clinical trial (NCT02912949), with an ORR of 40% and a DCR of 90% in 10 pancreatic cancer patients with NRG1 fusions [490].
Claudin18.2
CLDN18.2 is a tight junction protein involved in the formation of tight junction complexes, maintaining the barrier function of epithelial cells [382]. It is stably and highly expressed in gastric cancer tissues and ectopically expressed in several other tumor types, including pancreatic, esophageal, and ovarian cancers, making it a novel target for anti-tumor therapy [491]. Current therapies targeting CLDN18.2 include monoclonal antibodies, bispecific antibodies, antibody–drug conjugates (ADCs), CAR-T cells, and CAR-NK cells. Zolbetuximab (IMAB362), an anti-CLDN18.2 monoclonal antibody, has been evaluated in two randomized phase III trials (SPOTLIGHT and GLOW) to assess its efficacy and safety in combination with standard chemotherapy for HER2-negative, CLDN18.2-positive locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma (GC/GEJA) patients. These trials demonstrated significantly prolonged OS and PFS with zolbetuximab treatment (NCT03504397, NCT03653507) [492, 493]. In March 2024, zolbetuximab received its first approval in Japan for treating HER2-negative, CLDN18.2-positive unresectable advanced or recurrent gastric cancer [494]. Notably, CLDN18.2 positivity is reported in nearly 60% of pancreatic cancer patients [495]. A phase II trial (NCT03816163) is currently evaluating zolbetuximab in combination with gemcitabine/nab-paclitaxel as a first-line therapy for CLDN18.2-positive metastatic pancreatic cancer patients [496]. Another multicenter phase I trial (NCT04400383) assessed AB011, a recombinant humanized anti- CLDN18.2 monoclonal antibody, as monotherapy or in combination with capecitabine and oxaliplatin in patients with advanced solid tumors, including GC/GEJA and pancreatic cancer, showing an ORR of 65.2% with a favorable safety profile [497].
ADC have become an important strategy for targeted therapies. CMG901, an CLDN18.2-ADC, showed an ORR of 75.0% and a DCR of 100% in a phase Ia trial (NCT04805307) for patients with resistant/refractory solid tumors, including pancreatic cancer [498]. Another ongoing phase II trial (NCT06219941) is evaluating CMG901 in patients with CLDN18.2-expressing advanced solid tumors, including GC/GEJA and PDAC [499]. IBI343, another ADC targeting CLDN18.2, demonstrated an ORR of 28.0% and a DCR of 80.0% in a phase I trial (NCT05458219) involving patients with advanced PDAC or biliary tract cancer, with manageable safety profiles [500].
CLDN18.2 is also being targeted by bispecific T cell or macrophage engagers. IBI389, an anti-CLDN18.2 bispecific antibody, showed an ORR of 30.4% and a DCR of 69.6% in a phase I trial (NCT05164458) with pancreatic cancer patients [501]. LB4330, a bi-functional peptide targeting CLDN18.2 with IL-10 expression, is anticipated to activate tumor antigen-specific CD8+ T cells in the TME and is being evaluated in a phase Ib/II trial (TRIGGERCD8, NCT06468358) in combination with LB1410, a TIM-3/PD-1 bispecific antibody, in patients with advanced or metastatic solid tumors. Additionally, PM1032, a CLDN18.2:4-1BB bispecific antibody, is under evaluation in a phase I/II trial (NCT05839106) in patients with advanced solid tumors [502]. PT886, a CLDN18.2 bispecific antibody that blocks the "do-not-eat-me" signal on macrophages, is also being tested in a phase I/II clinical trial for pancreatic cancer (NCT05482893) [503]. Furthermore, as previously described, CLDN18.2-specific CAR-T cells or CAR-NK cells are also being explored as potential therapies for pancreatic cancers.
Molecular biomarkers
With the advancement of molecular profiling technologies, several studies have categorized PDAC into distinct subtypes based on tumor-specific gene expression profiles. In 2011, Collisson et al. delineated three PDAC subtypes, classical, quasi-mesenchymal, and exocrine-like, through an analysis of transcriptional profiles from PDAC samples and cell lines, demonstrating distinct differences in clinical outcomes and therapeutic responses among these subtypes [504]. In 2015, Moffitt et al. further refined this classification by identifying two primary tumor subgroups, namely basal-like and classical, noting that the basal-like subtype is associated with significantly poorer survival outcomes but exhibits a better response to adjuvant therapy [505]. Building on this, more granular classifications based on Moffitt’s subtypes have been proposed, including “basal-like A”, “basal-like B”, “hybrid”, “classical A”, and “classical B” subtypes, to better distinguish gene expression patterns at various disease stages [506]. Despite variations in specific genes and classifiers across studies, the basal-like and classical subtypes are widely supported by most researchers.
In the COMPASS trial, whole-genome sequencing and RNA sequencing were performed on 195 patients with advanced PDAC treated with mFOLFIRINOX, categorizing 39 as basal-like and 156 as classical. The basal-like subtype was associated with resistance to mFOLFIRINOX, reflected in a lower ORR (10% vs 33%) and shorter median OS (6.5 vs 10.6 months). GATA6 expression emerged as a prognostic biomarker capable of distinguishing basal-like from classical PDAC [507]. The PASS-01 trial is further investigating GATA6 and other biomarkers of first-line chemotherapy response as secondary endpoints, with results pending [508]. Molecular characterization of long-term (over 2 years) versus short-term (less than 3 months) survivors of advanced PDAC revealed that short-term survivors were more frequently classified as the basal-like subtype (30% vs 3%) [509]. Additionally, researchers have developed the GemPred transcriptomic signature to predict adjuvant gemcitabine sensitivity in PDAC patients [510]. In the PRODIGE 24-ACCORD/CCTG PA 6 trial, GemPred-positive patients treated with resected PDAC had significantly longer median DFS (27 vs 10 months) and OS (68 vs 29 months) compared to GemPred-negative patients. However, GemPred did not show predictive value in the mFOLFIRINOX treatment arm [511]. Rashid et al. developed the Purity Independent Subtyping of Tumors (PurIST) classifier, which accurately differentiates tumor samples into classical and basal-like subtypes, and observed that basal-like PDACs are less responsive to FOLFIRINOX treatment [512]. The ongoing phase II PANCREAS trial (NCT04683315) is evaluating an adaptive neoadjuvant chemotherapy approach guided by PurIST classification for patients with resectable pancreatic cancer and BRPC, with classical subtype patients receiving mFOLFIRINOX and basal-like subtype patients receiving gemcitabine/nab-paclitaxel. These studies aim to enhance our understanding of the prognostic and predictive implications of current therapeutic strategies in relation to distinct PDAC molecular subtypes. Other biomarkers, such as circulating tumor DNA, are also being explored for early detection, surveillance, and monitoring in high-risk PDAC patients (NCT03334708, NCT03568630).
Challenges and future directions
Over the past decade, extensive preclinical and clinical research has underscored the pivotal roles of both adaptive and innate immune systems in PDAC immunotherapy. While ICIs have revolutionized cancer treatment, their efficacy in pancreatic cancer remains constrained by the immunosuppressive and inaccessible TME. The clinical benefits of ICIs in pancreatic cancer have been largely restricted to a small subset of patients characterized by dMMR, MSI-H, and elevated TMB. The underlying reasons for the lack of response in the majority of PDAC patients to immunotherapy remain elusive. Current consensus suggests that single-mechanism immunotherapies are insufficient to combat pancreatic cancer. Optimizing existing treatment strategies targeting immunosuppressive TME through rational combinations is believed to offer greater benefits.
Recent biotechnological advances have led to the emergence of promising new molecules that directly or indirectly target KRAS. KRAS-directed therapies, including small molecular inhibitors, TCR-engineered T cells targeting KRAS mutations and personalized mRNA vaccines, have shown encouraging results. However, the emergence of KRAS-driven resistance mechanisms poses a significant challenge. Tumors frequently acquire secondary mutations or activate alternative signaling pathways to evade KRAS inhibition, leading to treatment failure [432]. Future clinical research must prioritize strategies to overcome both primary and acquired resistance to KRAS-targeted therapies, such as combining KRAS inhibitors with other targeted therapies or immunomodulators to achieve more durable and effective responses.
Moreover, with advancements in sequencing technologies and multi-omics analyses, there is now an opportunity to explore the diverse cell lineages within pancreatic cancer. Despite the promising research, there remains a significant challenge in identifying reliable biomarkers to predict therapeutic response and toxicity. Biomarkers can stratify patients based on their likelihood of responding to specific therapies, enabling personalized treatment approaches. While high-evidence biomarkers, such as MSI-H, high TMB, and DDR deficiencies, have shown promise in predicting ICI response, their applicability remains limited. Researchers have proposed tumor-derived CAFs, microbiomes, and exosomes as potential biomarkers for tracking pancreatic cancer immunotherapy response [513]. Additionally, recent studies have suggested that a lower neutrophil-to-lymphocyte ratio may predict a better response to PD-1 inhibitors in pancreatic cancer patients [514]. Advances in multi-omics analysis and liquid biopsy technologies have also facilitated the identification of potential biomarkers, such as circulating tumor DNA, immune cell profiles, and specific protein expressions, which can predict treatment response, monitor disease progression, and detect early signs of resistance [515, 516]. Integrating biomarker-driven strategies into clinical practice is crucial for optimizing therapeutic efficacy and improving patient prognosis in PDAC.
In conclusion, the evolving therapeutic landscape is fundamentally reshaping our approach to pancreatic cancer treatment. Innovative strategies are being clinically applied across various solid tumors, and as more clinical data become available, the use of advanced tools such as machine learning and artificial intelligence will further enhance our understanding of pancreatic cancer biology, ultimately accelerating the development of more promising new drugs.
Abbreviations
| 5-FU | 5-Fluorouracil |
| ADC | Antibody–drug conjugate |
| ATM | Ataxia-telangiectasia mutated |
| ATR | ATM/Rad3-related |
| BET | Bromodomain and extraterminal |
| BRPC | Borderline resectable pancreatic cancer |
| CA19-9 | Carbohydrate antigen 19–9 |
| CAFs | Cancer-associated fibroblasts |
| CAR | Chimeric antigen receptor |
| CCR7 | C-X-C chemokine receptor type 7 |
| cGAS | Cyclic GMP-AMP synthase |
| CLDN18.2 | Claudin18.2 |
| CpG | Cytidine-phospho-guanosine |
| CSF-1 | Colony-stimulating factor 1 |
| CSF-1R | CSF-1 receptor |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| Cy | Cyclophosphamide |
| DAMPs | Damage-associated molecular patterns |
| DC | Dendritic cell |
| DCR | Disease control rate |
| DDR | DNA damage response |
| DFS | Disease-free survival |
| dMMR | Mismatch repair deficiency |
| EGFR | Epidermal growth factor receptor |
| EORTC | European Organization for Research and Treatment of Cancer |
| FAK | Focal adhesion kinase |
| FAP | Fibroblast activation protein |
| FF | Folinic acid and fluorouracil |
| gBRCAm | Germline BRCA-mutated |
| GC/GEJA | Gastric or gastroesophageal junction adenocarcinoma |
| GITSG | Gastrointestinal Tumor Study Group |
| GDP | Guanosine diphosphate |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor |
| GTP | Guanosine triphosphate |
| HER2 | Human epidermal growth factor receptor 2 |
| HRR | Homologous recombination repair |
| ICIs | Immune checkpoint inhibitors |
| IFN | Interferon |
| iHD-SBRT | Isotoxic high-dose stereotactic body radiation |
| IL | Interleukin |
| IMRT | Intensity modulated radiation therapy |
| LAG-3 | Lymphocyte activation gene-3 |
| LAPC | Locally advanced unresectable pancreatic cancer |
| LPD | Laparoscopic pancreatoduodenectomy |
| MDSCs | Myeloid-derived suppressor cells |
| mKRAS | Mutant KRAS |
| MMP | Matrix metalloproteinase |
| MMR-p | MMR-proficient |
| MSI-H | Microsatellite instability-high |
| MUC-1 | Mucin-1 |
| NSCLC | Non-small cell lung cancer |
| OFF | Oxaliplatin plus folinic acid and fluorouracil |
| OPD | Open pancreatoduodenectomy |
| ORR | Objective response rate |
| OS | Overall survival |
| PARP | Poly (adenosine diphosphate-ribose) polymerase |
| PD-1 | Programmed death-1 |
| PDAC | Pancreatic ductal adenocarcinoma |
| PD-L1 | Programmed death-ligand 1 |
| PFS | Progression-free survival |
| poly-IC | Polyinosinic-polycytidylic acid |
| PRRs | Pattern recognition receptors |
| RCTs | Randomized clinical trials |
| RPD | Robotic pancreatoduodenectomy |
| RTOG | Radiation therapy oncology group |
| SBRT | Stereotactic body radiation |
| SHH | Sonic Hedgehog |
| SHP2 | Src homology region 2-containing protein tyrosine phosphatase 2 |
| SOS1 | Son of sevenless 1 |
| STING | Stimulator of interferon genes |
| TAAs | Tumor-associated antigens |
| TAMs | Tumor-associated macrophages |
| TANs | Tumor-associated neutrophils |
| TCR | T cell receptor |
| TIGIT | T cell immunoreceptor with Ig and ITIM domains |
| TILs | Tumor-infiltrating lymphocytes |
| TIM-3 | T-cell immunoglobulin and mucin-domain containing-3 |
| TLR | Toll-like receptor |
| TMB | Tumor mutation burden |
| TME | Tumor microenvironment |
| Treg | Regulatory T cell |
| WT1 | Wilms tumor 1 |
Author contributions
Junke Wang, and Jie Yang contributed equally to this project. Concept was conceived by K.L. and L.Z. Literatures were collected by J.Y., J.W., and K.L. Original draft manuscript was written by J.W., J.Y., and K.L. Manuscript was reviewed and revised was by K.L., A.N., J.H., C.W., and L.Z. Supervision was made by L.Z. The project administrator is K.L. and L.Z.
Funding
L.Z. is supported by an NIH Grant R01 CA169702, an NIH Grant R01 CA197296, an NIH Grant P01 CA247886, an NIH SPORE Grant P50 CA062924, and an NIH Cancer Center Support Grant P30 CA006973. K.L. is supported by a National Natural Science Foundation of China 82303740, a Key Research and Development Project of Science and Technology Department of Sichuan Province 2023YFS0167, a China Postdoctoral Science Foundation 2023T160451, and a Postdoctor Research Fund of West China Hospital, Sichuan University 2023HXBH053. J.W. is supported by a National Natural Science Foundation of China for Young Scientists Fund 82403318, a Sichuan Natural Science Foundation Youth Foundation Project 2024NSFSC1949, and a Postdoctor Research Fund of West China Hospital, Sichuan University 2024HXBH134.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Not applicable.
L.Z. receives grant support from Bristol-Meyer Squibb, Merck, AstraZeneca, iTeos, Amgen, NovaRock, Inxmed, Halozyme and Abmeta. L.Z. is a paid consultant/Advisory Board Member at Biosion, Alphamab, NovaRock, Ambrx, Akrevia/Xilio, QED, Novagenesis, Snow Lake Capitals, Amberstone, Pfizer, Tavotek, and Mingruizhiyao. L.Z. holds shares at Alphamab, Amberstone, Mingruizhiyao, and Cellaration. LZ is an editorial board member of Journal of Hematology and Oncology.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Junke Wang and Jie Yang contributed equally to this work and are co–first authors.
References
Articles from Journal of Hematology & Oncology are provided here courtesy of BMC
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/169251038
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
Clinical Trials (Showing 167 of 167)
- (3 citations) ClinicalTrials.gov - NCT05968326
- (3 citations) ClinicalTrials.gov - NCT03785249
- (3 citations) ClinicalTrials.gov - NCT02451982
- (2 citations) ClinicalTrials.gov - NCT03634982
- (2 citations) ClinicalTrials.gov - NCT06411691
- (2 citations) ClinicalTrials.gov - NCT04753879
- (2 citations) ClinicalTrials.gov - NCT04699188
- (2 citations) ClinicalTrials.gov - NCT04161755
- (2 citations) ClinicalTrials.gov - NCT04956640
- (2 citations) ClinicalTrials.gov - NCT05462717
- (2 citations) ClinicalTrials.gov - NCT02879318
- (2 citations) ClinicalTrials.gov - NCT04809766
- (2 citations) ClinicalTrials.gov - NCT05539430
- (2 citations) ClinicalTrials.gov - NCT04973163
- (2 citations) ClinicalTrials.gov - NCT05737706
- (2 citations) ClinicalTrials.gov - NCT04493060
- (2 citations) ClinicalTrials.gov - NCT03193190
- (2 citations) ClinicalTrials.gov - NCT04585035
- (2 citations) ClinicalTrials.gov - NCT05379985
- (2 citations) ClinicalTrials.gov - NCT06478459
- (2 citations) ClinicalTrials.gov - NCT05093231
- (2 citations) ClinicalTrials.gov - NCT01013649
- (2 citations) ClinicalTrials.gov - NCT02890355
- (2 citations) ClinicalTrials.gov - NCT04787991
- (2 citations) ClinicalTrials.gov - NCT05002270
- (2 citations) ClinicalTrials.gov - NCT05009329
- (2 citations) ClinicalTrials.gov - NCT04548752
- (2 citations) ClinicalTrials.gov - NCT03983057
- (2 citations) ClinicalTrials.gov - NCT03851614
- (2 citations) ClinicalTrials.gov - NCT04666740
- (2 citations) ClinicalTrials.gov - NCT04330664
- (2 citations) ClinicalTrials.gov - NCT05840510
- (2 citations) ClinicalTrials.gov - NCT04673448
- (2 citations) ClinicalTrials.gov - NCT06464965
- (2 citations) ClinicalTrials.gov - NCT05252390
- (2 citations) ClinicalTrials.gov - NCT06244771
- (2 citations) ClinicalTrials.gov - NCT03727880
- (2 citations) ClinicalTrials.gov - NCT03114319
- (2 citations) ClinicalTrials.gov - NCT03214250
- (2 citations) ClinicalTrials.gov - NCT04111458
- (2 citations) ClinicalTrials.gov - NCT02498613
- (2 citations) ClinicalTrials.gov - NCT03767582
- (2 citations) ClinicalTrials.gov - NCT05578092
- (2 citations) ClinicalTrials.gov - NCT03600883
- (2 citations) ClinicalTrials.gov - NCT05669482
- (2 citations) ClinicalTrials.gov - NCT04449874
- (1 citation) ClinicalTrials.gov - NCT01964430
- (1 citation) ClinicalTrials.gov - NCT02562716
- (1 citation) ClinicalTrials.gov - NCT03874897
- (1 citation) ClinicalTrials.gov - NCT05651022
- (1 citation) ClinicalTrials.gov - NCT05964361
- (1 citation) ClinicalTrials.gov - NCT02122913
- (1 citation) ClinicalTrials.gov - NCT06040541
- (1 citation) ClinicalTrials.gov - NCT03704662
- (1 citation) ClinicalTrials.gov - NCT01526135
- (1 citation) ClinicalTrials.gov - NCT01121848
- (1 citation) ClinicalTrials.gov - NCT03323944
- (1 citation) ClinicalTrials.gov - NCT05554367
- (1 citation) ClinicalTrials.gov - NCT02352337
- (1 citation) ClinicalTrials.gov - NCT01869166
- (1 citation) ClinicalTrials.gov - NCT03264404
- (1 citation) ClinicalTrials.gov - NCT04083235
- (1 citation) ClinicalTrials.gov - NCT06128551
- (1 citation) ClinicalTrials.gov - NCT04683315
- (1 citation) ClinicalTrials.gov - NCT04146298
- (1 citation) ClinicalTrials.gov - NCT06218914
- (1 citation) ClinicalTrials.gov - NCT01935843
- (1 citation) ClinicalTrials.gov - NCT02047513
- (1 citation) ClinicalTrials.gov - NCT04340141
- (1 citation) ClinicalTrials.gov - NCT04390243
- (1 citation) ClinicalTrials.gov - NCT04400383
- (1 citation) ClinicalTrials.gov - NCT04132505
- (1 citation) ClinicalTrials.gov - NCT05013216
- (1 citation) ClinicalTrials.gov - NCT03610490
- (1 citation) ClinicalTrials.gov - NCT03190941
- (1 citation) ClinicalTrials.gov - NCT02539537
- (1 citation) ClinicalTrials.gov - NCT04117087
- (1 citation) ClinicalTrials.gov - NCT03184870
- (1 citation) ClinicalTrials.gov - NCT04331041
- (1 citation) ClinicalTrials.gov - NCT03806309
- (1 citation) ClinicalTrials.gov - NCT04177810
- (1 citation) ClinicalTrials.gov - NCT01174121
- (1 citation) ClinicalTrials.gov - NCT05111353
- (1 citation) ClinicalTrials.gov - NCT06229340
- (1 citation) ClinicalTrials.gov - NCT03104439
- (1 citation) ClinicalTrials.gov - NCT01494506
- (1 citation) ClinicalTrials.gov - NCT02460224
- (1 citation) ClinicalTrials.gov - NCT02839343
- (1 citation) ClinicalTrials.gov - NCT04581473
- (1 citation) ClinicalTrials.gov - NCT03483038
- (1 citation) ClinicalTrials.gov - NCT05014776
- (1 citation) ClinicalTrials.gov - NCT02461836
- (1 citation) ClinicalTrials.gov - NCT04060342
- (1 citation) ClinicalTrials.gov - NCT02817633
- (1 citation) ClinicalTrials.gov - NCT00112658
- (1 citation) ClinicalTrials.gov - NCT06219941
- (1 citation) ClinicalTrials.gov - NCT02125136
- (1 citation) ClinicalTrials.gov - NCT03504397
- (1 citation) ClinicalTrials.gov - NCT04383210
- (1 citation) ClinicalTrials.gov - NCT03956680
- (1 citation) ClinicalTrials.gov - NCT04361162
- (1 citation) ClinicalTrials.gov - NCT02301143
- (1 citation) ClinicalTrials.gov - NCT03153410
- (1 citation) ClinicalTrials.gov - NCT01591733
- (1 citation) ClinicalTrials.gov - NCT02850536
- (1 citation) ClinicalTrials.gov - NCT02912949
- (1 citation) ClinicalTrials.gov - NCT05098197
- (1 citation) ClinicalTrials.gov - NCT03745326
- (1 citation) ClinicalTrials.gov - NCT03825289
- (1 citation) ClinicalTrials.gov - NCT00844649
- (1 citation) ClinicalTrials.gov - NCT02465060
- (1 citation) ClinicalTrials.gov - NCT03935893
- (1 citation) ClinicalTrials.gov - NCT02637687
- (1 citation) ClinicalTrials.gov - NCT02588105
- (1 citation) ClinicalTrials.gov - NCT01821612
- (1 citation) ClinicalTrials.gov - NCT01523457
- (1 citation) ClinicalTrials.gov - NCT03638193
- (1 citation) ClinicalTrials.gov - NCT00058201
- (1 citation) ClinicalTrials.gov - NCT05533463
- (1 citation) ClinicalTrials.gov - NCT04497116
- (1 citation) ClinicalTrials.gov - NCT03006302
- (1 citation) ClinicalTrials.gov - NCT05494697
- (1 citation) ClinicalTrials.gov - NCT05002140
- (1 citation) ClinicalTrials.gov - NCT05164458
- (1 citation) ClinicalTrials.gov - NCT04469556
- (1 citation) ClinicalTrials.gov - NCT02600949
- (1 citation) ClinicalTrials.gov - NCT05927142
- (1 citation) ClinicalTrials.gov - NCT05839106
- (1 citation) ClinicalTrials.gov - NCT04858334
- (1 citation) ClinicalTrials.gov - NCT03653507
- (1 citation) ClinicalTrials.gov - NCT03563248
- (1 citation) ClinicalTrials.gov - NCT05529940
- (1 citation) ClinicalTrials.gov - NCT00786058
- (1 citation) ClinicalTrials.gov - NCT02723864
- (1 citation) ClinicalTrials.gov - NCT04927780
- (1 citation) ClinicalTrials.gov - NCT05375994
- (1 citation) ClinicalTrials.gov - NCT01150630
- (1 citation) ClinicalTrials.gov - NCT04805307
- (1 citation) ClinicalTrials.gov - NCT03841110
- (1 citation) ClinicalTrials.gov - NCT02959879
- (1 citation) ClinicalTrials.gov - NCT04404595
- (1 citation) ClinicalTrials.gov - NCT05482893
- (1 citation) ClinicalTrials.gov - NCT01585805
- (1 citation) ClinicalTrials.gov - NCT02676349
- (1 citation) ClinicalTrials.gov - NCT03157128
- (1 citation) ClinicalTrials.gov - NCT05650918
- (1 citation) ClinicalTrials.gov - NCT06468358
- (1 citation) ClinicalTrials.gov - NCT05198934
- (1 citation) ClinicalTrials.gov - NCT06056024
- (1 citation) ClinicalTrials.gov - NCT03245541
- (1 citation) ClinicalTrials.gov - NCT05458219
- (1 citation) ClinicalTrials.gov - NCT04170153
- (1 citation) ClinicalTrials.gov - NCT03915678
- (1 citation) ClinicalTrials.gov - NCT05585320
- (1 citation) ClinicalTrials.gov - NCT05382559
- (1 citation) ClinicalTrials.gov - NCT02576431
- (1 citation) ClinicalTrials.gov - NCT04975256
- (1 citation) ClinicalTrials.gov - NCT02723331
- (1 citation) ClinicalTrials.gov - NCT04882917
- (1 citation) ClinicalTrials.gov - NCT03528785
- (1 citation) ClinicalTrials.gov - NCT05074810
- (1 citation) ClinicalTrials.gov - NCT04853017
- (1 citation) ClinicalTrials.gov - NCT02919787
- (1 citation) ClinicalTrials.gov - NCT02243371
- (1 citation) ClinicalTrials.gov - NCT02157792
- (1 citation) ClinicalTrials.gov - NCT05083247
- (1 citation) ClinicalTrials.gov - NCT03816163
Show less
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Neoadjuvant plus adjuvant or only adjuvant nab-paclitaxel plus gemcitabine for resectable pancreatic cancer - the NEONAX trial (AIO-PAK-0313), a prospective, randomized, controlled, phase II study of the AIO pancreatic cancer group.
BMC Cancer, 18(1):1298, 29 Dec 2018
Cited by: 25 articles | PMID: 30594153 | PMCID: PMC6311014
AGITG MASTERPLAN: a randomised phase II study of modified FOLFIRINOX alone or in combination with stereotactic body radiotherapy for patients with high-risk and locally advanced pancreatic cancer.
BMC Cancer, 21(1):936, 19 Aug 2021
Cited by: 6 articles | PMID: 34412605 | PMCID: PMC8377976
Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial.
Lancet Gastroenterol Hepatol, 8(2):157-168, 12 Dec 2022
Cited by: 90 articles | PMID: 36521500
Update on management of pancreatic cancer: a literature review.
Chin Clin Oncol, 13(3):41, 17 May 2024
Cited by: 0 articles | PMID: 38769794
Review
Funding
Funders who supported this work.
China Postdoctoral Science Foundation (1)
Grant ID: 2023T160451
NCI NIH HHS (1)
Grant ID: P30 CA006973
NIH HHS (5)
Grant ID: P01 CA247886
Grant ID: P50 CA062924
Grant ID: R01 CA169702
Grant ID: P30 CA006973.
Grant ID: R01 CA197296
National Institutes of Health (5)
Grant ID: P01 CA247886
Grant ID: R01 CA197296
Grant ID: P50 CA062924
Grant ID: R01 CA169702
Grant ID: P30 CA006973.
National Natural Science Foundation of China (2)
Grant ID: 82403318
Grant ID: 82303740
Postdoctor Research Fund of West China Hospital, Sichuan University (2)
Grant ID: 2024HXBH134
Grant ID: 2023HXBH053
Science and Technology Department of Sichuan Province (2)
Grant ID: 2024NSFSC1949
Grant ID: 2023YFS0167

 2,4,5 and
2,4,5 and 

