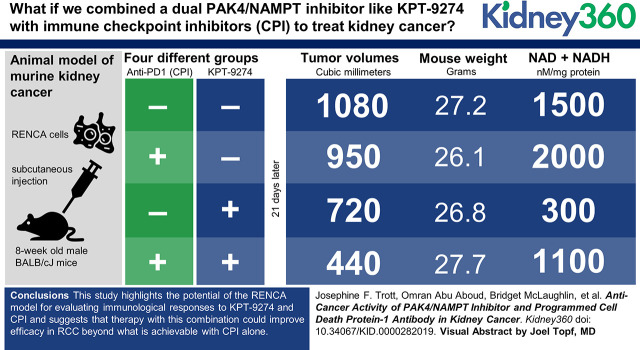
Abstract
Background
Kidney cancer (or renal cell carcinoma, RCC) is the sixth most common malignancy in the United States and is increasing in incidence. Despite new therapies, including targeted therapies and immunotherapies, most RCCs are resistant to treatment. Thus, several laboratories have been evaluating new approaches to therapy, both with single agents as well as combinations. Although we have previously shown efficacy of the dual PAK4/nicotinamide phosphoribosyltransferase (NAMPT) inhibitor KPT-9274, and the immune checkpoint inhibitors (CPI) have shown utility in the clinic, there has been no evaluation of this combination either clinically or in an immunocompetent animal model of kidney cancer.Methods
In this study, we use the renal cell adenocarcinoma (RENCA) model of spontaneous murine kidney cancer. Male BALB/cJ mice were injected subcutaneously with RENCA cells and, after tumors were palpable, they were treated with KPT-9274 and/or anti-programmed cell death 1 (PDCD1; PD1) antibody for 21 days. Tumors were measured and then removed at animal euthanasia for subsequent studies.Results
We demonstrate a significant decrease in allograft growth with the combination treatment of KPT-9274 and anti-PD1 antibody without significant weight loss by the animals. This is associated with decreased (MOUSE) Naprt expression, indicating dependence of these tumors on NAMPT in parallel to what we have observed in human RCC. Histology of the tumors showed substantial necrosis regardless of treatment condition, and flow cytometry of antibody-stained tumor cells revealed that the enhanced therapeutic effect of KPT-9274 and anti-PD1 antibody was not driven by infiltration of T cells into tumors.Conclusions
This study highlights the potential of the RENCA model for evaluating immunologic responses to KPT-9274 and checkpoint inhibitor (CPI) and suggests that therapy with this combination could improve efficacy in RCC beyond what is achievable with CPI alone.Free full text

Anti-Cancer Activity of PAK4/NAMPT Inhibitor and Programmed Cell Death Protein-1 Antibody in Kidney Cancer
Visual Abstract
Abstract
Background
Kidney cancer (or renal cell carcinoma, RCC) is the sixth most common malignancy in the United States and is increasing in incidence. Despite new therapies, including targeted therapies and immunotherapies, most RCCs are resistant to treatment. Thus, several laboratories have been evaluating new approaches to therapy, both with single agents as well as combinations. Although we have previously shown efficacy of the dual PAK4/nicotinamide phosphoribosyltransferase (NAMPT) inhibitor KPT-9274, and the immune checkpoint inhibitors (CPI) have shown utility in the clinic, there has been no evaluation of this combination either clinically or in an immunocompetent animal model of kidney cancer.
Methods
In this study, we use the renal cell adenocarcinoma (RENCA) model of spontaneous murine kidney cancer. Male BALB/cJ mice were injected subcutaneously with RENCA cells and, after tumors were palpable, they were treated with KPT-9274 and/or anti–programmed cell death 1 (PDCD1; PD1) antibody for 21 days. Tumors were measured and then removed at animal euthanasia for subsequent studies.
Results
We demonstrate a significant decrease in allograft growth with the combination treatment of KPT-9274 and anti-PD1 antibody without significant weight loss by the animals. This is associated with decreased (MOUSE) Naprt expression, indicating dependence of these tumors on NAMPT in parallel to what we have observed in human RCC. Histology of the tumors showed substantial necrosis regardless of treatment condition, and flow cytometry of antibody-stained tumor cells revealed that the enhanced therapeutic effect of KPT-9274 and anti-PD1 antibody was not driven by infiltration of T cells into tumors.
Conclusions
This study highlights the potential of the RENCA model for evaluating immunologic responses to KPT-9274 and checkpoint inhibitor (CPI) and suggests that therapy with this combination could improve efficacy in RCC beyond what is achievable with CPI alone.
Introduction
Recent advances in immunotherapy have revolutionized the field of cancer treatment. Using antibodies against programmed cell death 1 (PDCD1; PD1) and/or PD1 ligand 1 (CD274; PD-L1) to block the inhibition on immune recognition of tumor cells (i.e., immune checkpoint) results in clearance of a wide range of tumor types by T cells (1). Specifically, immune checkpoint inhibitors (CPIs) have shown promise in multiple clinical trials of patients with renal cell carcinoma (RCC) with objective response rates of 12%–31% (2). Unfortunately, in patients with RCC, complete responses to a single PD1/PD-L1 antibody in the absence of an accompanying conventional or targeted therapeutic are rare (2). For this reason, many clinical researchers are investigating the treatment of RCC using combinations of CPI with angiogenesis inhibitors (2) or with targeted agents.
KPT-9274 is an orally bioavailable, small molecule that modulates the activities of both p21 (RAC1) activated kinase 4 (PAK4) and nicotinamide phosphoribosyltransferase (NAMPT) (3). KPT-9274 reduces the steady-state level of PAK4 in breast cancer cells (4) and kidney cancer cell lines (3). KPT-9274 has also been shown to have potent antitumor activity in murine xenograft models, including RCC (3–,10), and in trials of companion dogs with lymphoma (11). KPT-9274 is currently in a phase 1 study (NCT02702492) to assess the safety, tolerability, and preliminary efficacy in patients with advanced solid malignancies or non-Hodgkin lymphoma (NCT02702492) (12).
NAMPT catalyzes the rate-limiting step in the main salvage pathway used to produce oxidized beta-nicotinamide adenine dinucleotide (NAD+), an essential coenzyme in energy-producing catabolic reactions (13). In vertebrates, NAD+ can be synthesized de novo from tryptophan or salvaged from nicotinamide (through NAMPT), nicotinic acid (through nicotinate phosphoribosyltransferase, NAPRT), or nicotinamide riboside (through nicotinamide riboside kinase) (4,13). Cancer cells preferentially generate NAD through nicotinamide and NAMPT, most likely because de novo NAD+ synthesis occurs predominately in the liver and NAPRT is often epigenetically downregulated in cancer cells through hypermethylation of the NAPRT promoter (found in 5%–65% of samples tested, depending on tumor type). This correlates with low NAPRT expression in these lines and tumor samples (14). We have previously shown that two human RCC cell lines have very low levels of NAPRT expression, and treatment with KPT-9274 reduces proliferation and induces apoptosis in these cells (3). Additionally, a selective inhibitor of NAMPT (FK866/APO866) was found to have antitumorigenic, antimetastatic, and antiangiogenic activity in a syngeneic mouse model of renal cell adenocarcinoma (RENCA) (15).
The signaling molecule PAK4 is involved in multiple pathways, including WNT/β-catenin signaling (16), whose target genes include CCND1 and MYC (17), both of which have key roles in cell proliferation (18). PAK4 regulates the activity of CDKN1A (p21) and thereby regulates normal progression of the cell cycle (19). PAK4 has also been implicated in the oncogenic transformation of cells (20,21). In a recent publication, the epithelial-to-mesenchymal transition (EMT) of gastric cancer cell lines was correlated to the loss of NAPRT expression (22). The authors suggested that NAPRT expression destabilizes β-catenin and acts as a tumor suppressor protein prior to the cells undergoing the EMT phenotypic change. However, when NAPRT is lost through the dynamic process of epigenetic silencing (possibly through selective pressure on cancer cells), β-catenin is stabilized, resulting in the activation of an EMT program in these cell lines. At the same time, the EMT cells become sensitive to NAMPT inhibition (22). PAK4 also affects β-catenin stability by inhibiting degradation of β-catenin (16,23).
Recently, a correlation was reported between activation of the PAK4 and WNT/β-catenin signaling pathway and T cell exclusion in samples of patients with melanoma (24,25). The low immune cell infiltration is also associated with resistance to PD-L1/CTLA4 antibody therapy (24). This correlation has been further validated in other cancer types including breast, colorectal, non-small cell lung, and RCC (26), and these data suggested that combination therapies in which activated β-catenin is inhibited simultaneously with targeting PD1, as we have investigated in this study, may be particularly effective. Thus, PAK4/WNT/β-catenin, NAMPT, and the immune system seem to converge, making KPT-9274 and immune CPI combination a viable therapy for patients with RCC.
In this study, we show that PD1 inhibition enhances the antitumor effect of the novel RCC therapeutic KPT-9274, which specifically targets tumors they havedue to metabolic reprogramming of the NAD+ biosynthesis pathway. Thus, combination immunotherapies using dual PAK4/NAMPT inhibition as well as CPI antibodies are now primed to be studied in the oncology clinic.
Materials and Methods
Tissues and Cells
Mouse RENCA-luciferase (RENCA-luc) cells were derived from a spontaneous kidney adenocarcinoma in BALB/cCr mice (27–,29), and were authenticated by short tandem repeat and species analysis (IDEXX BioAnalytics, Colombia, MO). They were injected into mice at passage 4–6. RCC cell lines (786-O, Caki-1, and ACHN) were purchased from and authenticated by ATCC and passaged <20 times. All cells were confirmed mycoplasma-free and were handled one at a time in the cell culture hood, each with their own media and trypsin bottle. Tissues from human clear cell RCC (ccRCC) tumors and normal human kidney tissues (adjacent to a tumor) were archived following Institutional Review Board approval at the Department of Pathology, University of California, Davis (UC Davis).
In Vivo Experiments
Animal experiments were performed in accordance with guidelines set forth by the Institutional Animal Care and Use Committee at UC Davis. Male BALB/cJ mice (Jackson Laboratories, Bar Harbor, ME) that were 6 weeks old had ad libitum access to standard laboratory mouse chow and water. RENCA-luc cells (2.5×105) were injected in 100 μl of OptiMEM with 33% BD Matrigel Matrix (Corning, Tewksbury, MA) subcutaneously in the right flank of male mice when they reached 8 weeks of age. Male mice were used because RCC is more severe and twice as common in men as in women (30). Treatments were started 10 days after injection. Four separate batches of mice were injected with RENCA-luc cells and then treated. Treatments were assigned randomly to mice within each batch. Anti-mouse PD1 or isotype control antibody (250 µg/dose) was injected intraperitoneally twice a week. KPT-9274 (200 mg/kg) or vehicle was administered orally twice a day, 5 days per week. Mice were weighed at the start of the treatment period and once a week thereafter. Subcutaneous tumors were measured (length and width) in situ every 2–3 days using calipers, and tumor volume was calculated using the equation 1/2×length×width2. RENCA tumors were dissected from BALB/cJ mice after 21 days of treatments. The length, width, and depth of tumors were measured using a caliper, and tumor volume was calculated using the equation 4/3×3.142× (length/2) × (width/2) × (depth/2). Tumors were dissected and a small piece was frozen, a second small piece was fixed in 10% formalin for histology and immunohistochemistry, and the remainder was processed for flow cytometry. Spleens were also harvested, either for flow cytometry or for fixation in 10% formalin.
Immunohistochemistry
Formalin-fixed, paraffin-embedded sections (5 μm) were cut from untreated BALB/cJ mouse RENCA tumors. Immunohistochemistry was performed as previously described (31) using the following antibodies: Ki67 (275R-18; Cell Marque), PD-L1 (13684; Cell Signaling Technology, Danvers, MA), and programmed cell death 1 ligand 2 (PD-L2) (82723; Cell Signaling Technology).
Isolation of Tumor-Infiltrating Cells and Flow Cytometry
Tumors and spleens were harvested, mashed against a size 60 mesh stainless steel screen (Sigma-Aldrich), and passed through a 100-µm cell strainer (ThermoFisher Scientific). Red blood cells were lysed using ammonium chloride potassium lysis buffer and cells resuspended in PBS for staining with Zombie Aqua (Biolegend). Nonspecific staining was blocked using mouse γ-globulin (Jackson Immunoresearch Laboratories) and cells were stained in staining buffer (PBS, 3.5% FBS, 1 mM EDTA) with a panel of antibodies purchased from Biolegend, except where indicated: anti–CD45-Alexa Fluor 700 (clone 30-F11), anti–CD11b-APC/Fire 750 (clone M1/70), anti–CD4-PE (clone RM4-), anti–CD19-FITC (clone 6D5), anti–CD25-PE/Cy7 (clone PC61.5; eBiosciences), anti–CD3ε-Brilliant Violet 421 (clone 145-2C11), anti–CD8a-Brilliant Violet 605 (clone 53-6.7). The stained cells were then fixed using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience) and stained for intranuclear staining with anti–FoxP3-APC (clone 3G3; ThermoFisher Scientific). Stained cells were analyzed using a BD LSRII flow cytometer (BD Biosciences, San Jose, CA), and data processed using Flowjo software (Treestar, Ashland, OR). The gating strategy for spleens and tumors is depicted in Supplemental Figure 1 and all populations of immune cells are expressed as a percentage of the total number of single-cell, live CD45+ lymphocytes.
RNA Extraction, Reverse Transcription, and Quantitative PCR
RNA extraction, reverse transcription, and quantitative PCR (qPCR) were performed as previously described (32,33). Primer sequences, annealing temperatures, and efficiency of amplification for NAMPT, NAPRT, Nampt, and Naprt are in Table 1. The (HUMAN)NAMPT primers amplify 9 potential transcripts including the full-length protein coding transcript. The (HUMAN)NAPRT primers amplify 11 potential transcripts including the full-length protein coding transcript. The (MOUSE)Nampt and Naprt primers amplify the single protein coding transcript in each case.
Table 1.
PCR primers used for quantitative PCR
| Gene | Accession Number | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Tm (°C) | E (%) |
| (HUMAN)NAMPT (34) | GCAGAAGCCGAGTTCAACATC | TGCTTGTGTTGGGTGGATATTG | 64 | 98 | |
| (HUMAN)NAPRTa | NM_145201.5 | TCCCTGGGTGGCGTCTATAA | ATGAGTGGAGACCCGTCAGA | 64 | 96 |
| (MOUSE)Nampt (35) | TCGGTTCTGGTGGCGCTTTGCTAC | AAGTTCCCCGCTGGTGTCCTATGT | 66 | 81 | |
| (MOUSE)Naprta | NM_172607.3 | AGAAGGGCAGTGAGGTGAATG | TCCAGTAGCAAAGACCCATCG | 60 | 86 |
| Rpl13a (33) | GACCTCCTCCTTTCCCAGGCG | GCCTCGGCCATCCAATACCAGAA | 66 | 92 | |
| Eef2 (33) | TCTGAGAATCCGTCGCCATCCG | TCAGAGAGCTCGTAGAAGAGGGA | 66 | 80 | |
| RNA18S5&Rn18S (36) | ACGGCTACCACATCCAAGGA | CCAATTACAGGGCCTCGAAA | 60 | 70 | |
| PPIA (37) | ACCGCCGAGGAAAACCGTGTA | TGCTGTCTTTGGGACCTTGTCTGC | 64 | 82 | |
| RPS13 (37) | TCGGCTTTACCCTATCGACGCAG | ACGTACTTGTGCAACACCATGTGA | 64 | 83 |
Tm, annealing temperature; E, efficiency of amplification.
Immunoblotting
Immunoblotting was performed as previously described (38). Briefly, tumors and kidneys were homogenized in T-PER buffer (ThermoFisher Scientific). Polyvinylidene difluoride membranes were blocked in 5% milk and probed with appropriate primary and secondary antibodies. Signal was detected with enhanced chemiluminescence using either a Fuji Imager, or x-ray film and ImageJ to quantify band intensity. Phospho-β-catenin and PAK4 antibodies (Cell Signaling Technology) were probed at 1:1000. Rabbit anti-NAMPT (Bethyl Laboratories, Montgomery, TX) was probed at 1:2000. Rabbit polyclonal anti-NAPRT (PA5-70595; ThermoFisher Scientific) was probed at 1:500. Mouse anti–β-actin (Sigma-Aldrich) was probed at 1:4000. Rabbit anti–β-actin (Cell Signaling Technology) was probed at 1:2000.
Oxidized and Reduced β-Nicotinamide Adenine Dinucleotide Assay
Total oxidized (NAD+) and reduced β-Nicotinamide adenine dinucleotide (NADH; NAD+NADH) was quantified in tumor extracts using the NAD/NADH Glo Assay kit (Promega) following the manufacturer’s instructions. Briefly, 10–30 mg of tumor tissue was homogenized in 1–5 ml of a 50:50 mixture of PBS, pH 7.4, and bicarbonate base buffer with dodecyltrimethylammonium bromide (DTAB; Sigma-Aldrich) (1% DTAB, 100 mM sodium carbonate, 20 mM sodium bicarbonate, 10 mM nicotinamide [Sigma-Aldrich], 0.05% Triton X-100). Four dilutions of the tumor homogenates were assayed against a standard curve of NAD+ (Sigma-Aldrich), prepared in a 50:50 mixture of PBS/bicarbonate base buffer with DTAB (0–400 nM). NAD+NADH values were normalized to protein concentrations that were measured using the DC Protein Assay (Bio-Rad).
Thiazolyl Blue Tetrazolium Bromide Assay
Cells (1.4×103) were plated in 96-well plates and the next day KPT-9274 was added to fresh media in a range of concentrations (0–10 µM). Three days later, cells were stained with Thiazolyl Blue Tetrazolium Bromide as previously described (39).
Bisulfite Genomic DNA Modification, Methylation-Specific PCR, and Quantitative Methylation-Specific PCR
Genomic DNA (gDNA) was extracted from ccRCC tumors; RENCA tumors (untreated mice); normal kidneys (human and BALB/cJ mice); and the RCC cell lines Caki-1, 786-O, and ACHN. Each human gDNA sample (1 µg) was methylated using CpG Methyltransferase following the manufacturer’s instructions (New England Biolabs, Ipswich, MA). For each human sample, an equal mass of both 100% methylated gDNA and untreated gDNA (range of 376–826 ng) were bisulfite converted along with mouse gDNA (500 ng) using the Zymo EZ DNA Methylation kit (Zymo Research), and eluted in 20 μl. MethPrimer (40) predicted CpG islands in the first exon of both (HUMAN)NAPRT and (MOUSE)Naprt and primers were designed for both methylated and unmethylated (MOUSE)Naprt and for methylated (HUMAN)NAPRT (Table 2). The (MOUSE)Naprt primers were located in exon 1, 26–27 bp upstream and 78–79 bp downstream of the translation start site. The (HUMAN)NAPRT primers were located in exon 1, 56–123 bp downstream of the translation start site. The methylation-specific PCR (MSP) reactions were performed on mouse gDNA using EpiMark Hot Start Taq Polymerase (New England Biolabs) on 2 μl of bisulfite-modified DNA with 200 nM of primers specific for methylated (M) or unmethylated (U) (MOUSE)Naprt. PCR reactions were heated to 95°C for 30 seconds, then 40 cycles of 95°C for 15 seconds, annealing for 30 seconds (unmethylated) or 60 seconds (methylated), and 68°C for 30 seconds, followed by a final extension of 68°C for 5 minutes. The quantitative methylation specific PCR (qMSP) reactions were performed on 100% methylated and untreated human gDNA using Power SYBR Green Master Mix (ThermoFisher) on 1 μl of bisulfite-converted gDNA with 400 nM of primers specific for methylated (HUMAN)NAPRT or 250 nM of (HUMAN)ACTB primers. PCR reactions were heated to 95°C for 10 minutes, then 40 cycles of 95°C for 15 seconds, and annealing/extension for 1 minute. The percentage methylation of each human gDNA sample was calculated using the 2−ΔΔCT method. (HUMAN)NAPRT methylation (Ct) was corrected for (HUMAN)ACTB (Ct) and then the fraction of methylation in untreated gDNA (if greater than zero) was calculated relative to the 100% methylated gDNA for each sample.
Table 2.
Methylation-specific primers for (HUMAN)NAPRT and (MOUSE)Naprt used on bisulfite-converted genomic DNA
| Accession Number | Gene | M or U | Forward Primers (5′-3′) | Reverse Primers (5′-3′) | Tm (°C) |
| NM_145201.5 | (HUMAN) NAPRTa | M | ACCTCTACCAAACCACCATAACG | GAATTCGGCGGCGTTTC | 65 |
| (HUMAN) ACTB (41) | M | GGGTGGTGATGGAGGAGGTT | TTAACCACCACCCAACACACAAT | 66 | |
| NM_172607.3 | (MOUSE) Naprtb | U | AGTTGGGAGTTTGTGTATTTTGTGG | CACCATAATAACCTAATAAAAATCAAT | 59 |
| NM_172607.3 | (MOUSE) Naprtb | M | TAGTCGGGAGTTTGTGTATTTCGC | ACGCCATAATAACCTAATAAAAATCGAT | 64 |
M, methylated sequence; U, unmethylated sequence; Tm, annealing temperature.
Statistical Analyses
Statistical analyses of data were performed with SAS software, version 9.3 (SAS Institute, Cary, NC). Data were transformed to achieve normality and homogeneity of variance before statistical analysis. Outliers in the flow cytometry data sets were assessed using Prism8 (GraphPad Software). Tumor growth and size data were analyzed using mixed-effects models with repeated measures followed by a post hoc Tukey test while controlling for multiple testing. Treatment, day, and batch were considered fixed effects; cage was considered a random effect. Similarly, data for in situ and ex vivo tumor sizes on individual days and flow cytometry data were analyzed using a mixed-effects model followed by a post hoc Tukey test. Immunoblotting and NAD+NADH assay data were analyzed for main effects of PD1 antibody (present/absent), KPT-9274 treatment (present/absent), and its interaction using a two-way factorial ANOVA (PROC GLM) and a post hoc Tukey test. Two-tailed P values <0.05 were considered statistically significant as appropriate.
Results
NAMPT and NAPRT Expression in Human and Murine Kidney Cancers
We have previously demonstrated growth attenuation of human ccRCC cell line murine xenografts in response to KPT-9274 administration (3), however we did not assess NAMPT and NAPRT expression. Herein, we evaluated these genes in archived human ccRCC tumors and normal kidneys using qPCR. No significant differences were observed in either NAMPT (Figure 1A) or NAPRT mRNA (Figure 1B) expression between normal kidneys and ccRCC tumors of grades 1–4. However, six out of 19 (31%) ccRCC tumors ranging from grades 1 to 4 had at least 25% lower NAPRT mRNA expression than the lowest-expressing normal kidney sample (Figure 1B). These low-expressing NAPRT tumors were found across all four grades of ccRCC. We next examined the methylation status of the NAPRT promoter in normal kidney samples, ccRCC tissue, and cell lines. We used qMSP to measure the relative percentage of methylation and found that the NAPRT promoter was unmethylated in normal human kidneys. However, NAPRT was hypermethylated in all ccRCC tumors and cell lines with low expression of NAPRT mRNA, but only minimally methylated in ccRCC tumors with high expression of NAPRT (Figure 1C).

(HUMAN)NAPRT is downregulated at the level of transcription, which in some renal cell carcinoma tumors is associated with high levels of promoter methylation. RNA was extracted from normal kidneys and renal cell carcinoma (RCC) tumors of grades 1–4. (A) Extracted RNA was reverse transcribed and subjected to quantitative PCR for (HUMAN)NAMPT mRNA (corrected for PPIA, RPS13, and RNA18S5 mRNA levels). Data are means±SD (n=3–9). (B) Extracted RNA was reverse transcribed and subjected to quantitative PCR for (HUMAN)NAPRT mRNA as in (A). The red line is located below the lowest NAPRT mRNA value for normal kidneys. Data are means±SD (n=3–9). (C) The relative percentage of methylation of the (HUMAN)NAPRT promoter. Bisulfite-converted, untreated genomic DNA (gDNA) and bisulfite-converted 100% methylated gDNA from human kidneys (NHK; n=3), RCC tumors (n=8), and RCC cell lines (ACHN, Caki-1, 786-O) was analyzed by quantitative methylation-specific PCR using primers specific for methylated NAPRT sequences 56–123 bp downstream of the NAPRT translation start site, and normalized against ACTB. Samples were divided into low (blue bars) and high NAPRT expression (red bars) based on the data in (B) and from Abu Aboud et al. (3). hNARPT, (HUMAN)NARPT.
We chose the RENCA mouse model of RCC (32) to test the effects of KPT-9274 in combination with an immunotherapeutic because these cells represent a syngeneic tumor in an immunocompetent mouse, whereas “standard” immunodeficient xenografted nude mice would be ineffective for evaluating an immunotherapeutic. The RENCA tumors were found to be highly proliferative in the mouse as evidenced by Ki67 staining, associated with a relatively high level of PD-L1 expression, with PD-L2 expression confined to stromal cells (Figure 2). We next examined the expression of both (MOUSE)Nampt and (MOUSE)Naprt in RENCA tumors and normal mouse kidneys and found that RENCA tumors expressed Nampt at a similar level to that in the normal kidney, both at the protein and mRNA levels (Figure 3A). However, RENCA tumors have downregulated expression of NAPRT compared with normal kidney tissue (Figure 3A), whereas Naprt mRNA expression is more than sixfold lower in RENCA tumors versus kidneys (Figure 3B). These protein expression data are consistent with what we previously observed in human RCC cell lines (3).

RENCA tumors express both programmed cell death 1 ligand 1 (PD-L1) and PD-L2 and are highly proliferative. Subcutaneous RENCA tumors from untreated Balb/cJ mice were fixed, paraffin-embedded, sectioned, and stained with hematoxylin and eosin (H&E) or subjected to immunohistochemistry for Ki67, PD-L1, and PD-L2 using 3,3'-diaminobenzidine as the chromagen and a hematoxylin counterstain.

(MOUSE)NAPRT is downregulated at the level of transcription in RENCA tumors where the Naprt promoter is methylated. Protein and RNA were extracted from kidneys and subcutaneous RENCA tumors from untreated Balb/cJ mice. (A) Proteins were immunoblotted for NAMPT, NAPRT, and β-actin. Immunoblots are representative of at least two repeats. (B) RNA was extracted, reverse transcribed, and subjected to quantitative PCR for Nampt or Naprt mRNA (corrected for Eef2, Rpl13a, and Rn18S mRNA levels). Data are means±SEM (n=3 kidneys; n=4 tumors). a,b,cP<0.05. (C) Bisulfite-converted gDNA from Balb/cJ mouse kidneys or RENCA tumors was amplified by PCR using primers specific for either unmethylated (U) or methylated (M) sequences to amplify sequences between 26–27 bp upstream and 78–79 bp downstream of the Naprt translation start site. Data are representative of two independent PCR reactions. Neg, mouse gDNA.
We next examined whether the observed downregulation of (MOUSE)NAPRT was due to methylation of the promoter, as we have already demonstrated was present in the human ccRCC tumors. A CpG island was found in exon 1 of the Naprt gene, in a similar location to the NAPRT gene. The gDNA from RENCA cells was subjected to MSP analysis and found to contain methylated Naprt promoter sequences (data not shown). When we analyzed gDNA from three mouse kidneys using MSP, we found they contained unmethylated Naprt gDNA, whereas three RENCA tumors were found to contain both unmethylated and methylated Naprt gDNA (Figure 3C). Thus, as with human ccRCC, in RENCA tumors the downregulation of Naprt at the level of transcription is also associated with methylation of the promoter.
We measured the effect of KPT-9274 on RENCA-luc cell growth in vitro and found the viability of RENCA-luc cells was attenuated by KPT-9274 in a concentration-dependent manner (Supplemental Figure 2). This is evidence that the loss of the NAPRT pathway through epigenetic changes not only sensitizes human RCC to NAMPT inhibition but may be directly recapitulated in this syngeneic mouse model of RCC. Based on our in vitro data as well as in vivo data on subcapsular growth of RENCA in the syngeneic mouse previously described (15), we expected that the subcutaneous RENCA tumor model would respond well to NAMPT inhibition with KPT-9274 (3).
RENCA Tumor Growth Is Attenuated by KPT-9274
For the in vivo model, cultured RENCA-luc cells were injected subcutaneously in BALB/cJ mice. After palpable tumors appeared (in 10 days), the mice were then treated for 21 days with either KPT-9274, anti-PD1 antibody, or a combination of both agents (see Materials and Methods). The tumors were measured with calipers every 2–3 days (Figure 4A, Supplemental Figure 3). An analysis of the tumor growth data using repeated measures over time demonstrated there were significant effects of day and treatment on tumor sizes (P<0.001), but no interaction between day and treatment (P=0.98). The combined treatment with KPT-9274 and anti-PD1 gave significantly smaller tumors than KPT-9274 alone (P=0.001), anti-PD1 alone (P<0.001), or control treatments (P<0.001). When tumor sizes were analyzed at each day of measurement, there was an effect of KPT-9274 on tumor growth on days 14 (P=0.01), 17 (P=0.01), 19 (P=0.02), and 21 (P=0.004) and there was also an interaction between anti-PD1 and KPT-9274 to reduce tumor growth on days 19 (P=0.02) and 21 (P=0.01; Figure 4A). No significant changes in weight were observed due to treatment, suggesting a lack of general toxicity of the treatments (Figure 4B).

The combination of KPT-9274 and anti–programmed cell death 1 has a significant inhibitory effect on tumor growth. (A) Subcutaneous measurements of RENCA tumor growth in 10-week-old male Balb/cJ mice over the 21 days of treatment. RENCA-luc cells (250,000) were injected subcutaneously in 30% matrigel and treatments started 10 days after injection. Data are mean±SEM (n=8–10 per treatment). a,bIndicate significant differences within one day’s measurements (P<0.05). *P<0.05, **P<0.005 for main effect of KPT-9274. (B) The antitumor treatments did not affect mouse health as determined by body weights. Male Balb/cJ mice bearing RENCA tumors were 10 weeks old when treatments were started. Mice were weighed at the start of the treatment period and once a week thereafter. Data are means±SEM (n=9–10 per treatment). *P<0.05 compared to control at that time point. (C) Volume of RENCA tumors measured ex vivo at euthanasia after 21 days of treatment with either KPT-9274, anti–programmed cell death 1 antibody (PD1), or a combination of both. Data are mean±SD (n=8–10 per treatment). a,bP<0.05. Control, isotype control.
The RENCA tumors were removed and measured at necropsy 21 days after starting treatment. In this analysis, we found there was an effect of KPT-9274 on tumor size (P=0.001) and evidence of a possible interaction between anti-PD1 and KPT-9274 (P=0.088). The combination treatment (KPT-9274 and anti-PD1) was more effective at decreasing tumor growth than either anti-PD1 alone (P=0.001) or KPT-9274 alone (P=0.016; Figure 4C).
RENCA Tumors Were Highly Necrotic
Formalin-fixed, paraffin-embedded tissue sections stained with hematoxylin and eosin were evaluated by a pathologist (K.-Y.J). All tumor samples showed pronounced areas of geographic necrosis admixed with areas of viable tumor, which did not correlate with specific treatments (Supplemental Figure 4).
Effects of Treatments on Tumor-Infiltrating T Cell Populations
RENCA tumors were dispersed to single cells and stained to detect live CD45+ lymphocytes that were further gated on CD11b, CD19, CD3, CD4, CD8, CD25, and FoxP3 (Supplemental Figure 1, A and B). Data are expressed as a percentage of live CD45+ lymphocytes. The gating is described in Supplemental Figure 1. The infiltration of CD3lo and CD3high cells into tumors was heterogenous in all treatment groups (Figure 5A). They had a bimodal distribution (either <20% CD3+ or >40% CD3+) which was most accentuated in the tumors from KPT-9274–treated mice. The infiltration of CD8+ cells was low in most tumors and heterogeneous with a bimodal distribution (either <1% CD8+ or >2% CD8+). This bimodal distribution was accentuated by the addition of KPT-9274 and anti-PD1, resulting in significantly more CD8+ cells in the combination treatment group compared with KPT-9274 alone (Figure 5, A and B). The infiltration of regulatory T cells was also very low (<2% of all CD45+ tumor-infiltrating lymphocytes), although they too had a bimodal distribution in different tumors (<0.35% and >0.5%) which was accentuated somewhat in the combination treatment group (Figure 5A).

T cell infiltration into RENCA tumors showed a bimodal distribution in mice treated with KPT-9274 or KPT-9274 plus anti-PD1. (A) CD3+, CD4+, CD8+, and regulatory T cell (Tregs) infiltration into syngeneic RENCA tumors. Data are individual tumor data points with mean±SD. Statistically determined outliers for CD8+ cells in the KPT-9274–treated mice (3.8%, 8.1%) and in the anti-PD1–treated mice (31.2%) were omitted to enable clarity of presentation. (B) Representative two-dimensional contour plots (with outliers) for CD4+ and CD8+ T cells in one tumor from each treatment group.
Tumor Expression of PAK4 and Phospho-β-Catenin Was Reduced by KPT-9274
KPT-9274 treatment of RCC cells is known to reduce PAK4 protein levels and interfere with the WNT/β-catenin signaling pathways (3). Proteins from the RENCA tumors were immunoblotted to examine levels of PAK4 and phospho-β-catenin (Figure 6, Supplemental Figure 5). We measured PAK4 and phospho-β-catenin in the tumors from 24 mice in the four treatment groups (n=6 per group; Supplemental Figure 5). As expected, there was an effect of KPT-9274 causing a reduction in PAK4 expression levels (P=0.04; Figure 6A) as well as inhibiting the phosphorylation of β-catenin (P=0.02; Figure 6B).

KPT-9274 decreased phosphorylation of β-catenin and total PAK4 expression. Protein was extracted from RENCA tumors in mice treated with PD1 antibody (anti-PD1), KPT-9274, or a combination of both and was immunoblotted for (A) PAK4, (B) phospho-β-catenin (P-βcatenin), and β-actin. ImageJ quantification of phospho-β-catenin and PAK4 expression, each corrected for β-actin, are underneath a representative immunoblot from one tumor per mouse. Data are means±SEM (n=6). a,bP<0.05.
Tumor Levels of NAD+NADH Were Reduced by KPT-9274 and Increased by PD1 Antibody
In addition to reducing levels of PAK4, KPT-9274 also inhibits the enzymatic activity of NAMPT and represses NAD biosynthesis. To confirm NAMPT inhibition in vivo, we examined the NAD+NADH levels in RENCA tumors from mice. When we examined NAD+NADH levels in response to each treatment individually, the NAD+NADH levels in RENCA tumors were lower in mice treated with KPT-9274 compared to anti-PD1 (P=0.01; Figure 7). We also found, as in other models, that total NAD+NADH was decreased by a main effect of KPT-9274 treatment (P=0.02; Figure 7). Interestingly, the NAD+NADH levels in RENCA tumors were increased by a main effect of the anti-PD1 antibody (P=0.02; Figure 7).

KPT-9274 reduces and anti-PD1 increases NAD+NADH concentrations in tumors. RENCA tumors from Balb/cJ mice were harvested 12–18 hours after the last dose of either KPT-9274 or vehicle and subjected to assays of total NAD+NADH as described in Materials and Methods. Mice treated with KPT-9274 had lower total NAD+NADH in RENCA tumors than mice treated with anti-PD1. Data are means±SD (n=7). a,bP<0.05. NAD+NADH, total oxidized (NAD+) and reduced β-Nicotinamide adenine dinucleotide (NADH).
Discussion
In this study, we found a significant interaction between the dual PAK4 and NAMPT inhibitor, KPT-9274, and an antibody against PD1 that slowed the growth of RENCA tumors in a syngeneic mouse model of renal cancer. This is in line with a recent report describing mouse models of melanoma and colon adenocarcinoma, where KPT-9274 improves the response to anti-PD1 therapy to reduce tumor growth (24). This was accompanied by a KPT-9274–driven decrease in PAK4 expression and β-catenin activation. Interestingly, although KPT-9274 reduced total NAD+NADH as expected, there was not an overall decrease in total NAD+NADH in the combination treatment (KPT-9274 and anti-PD1), possibly due to the inhibitory effect of KPT-9274 on energy metabolism being offset by a stimulatory effect of anti-PD1. To our knowledge, this is the first evidence that PD1 antibody therapy may be linked to increases in NAD+. Therefore, two mechanisms may be occurring independently in these tumors: first, the inhibition of (MOUSE)NAMPT activity by KPT-9274 which decreases NAD+NADH in the tumor cells (because the RENCA cells are lacking NAPRT) and, second, the PD1 antibody may have re-engaged T cell activation and possibly increased NAD+NADH levels even in the presence of KPT-9274, because T cells have normal levels of NAPRT. Increased NAD+ may actually enhance the antitumor T cell response (42), another salutary effect of this combination therapy.
Data from breast, colorectal, non-small cell lung, and RCC tumors (26) has suggested that combination therapies in which activated β-catenin is inhibited simultaneously with targeting PD1 can be particularly efficacious. In the RENCA model, we found the effects of PD1 blockade was unpredictable with only approximately 25% of tumors responding (Figure 4C). The effect of anti-PD1 on T cell infiltrates was minimal but the combination of anti-PD1 and KPT-9274 increased CD8+ infiltration in approximately 50% of animals (Figure 5A). However, the tumors that responded to the anti-PD1 with KPT-9274 treatment were not the tumors with increased T cell infiltration (data not shown). Similarly, the tumor size response to treatment (Figure 4A, Supplemental Figure 3) did not display the same bimodal distribution observed in the infiltrated T cells (Figure 5). Thus the effectiveness of the combination treatment is possibly associated with direct antitumor effects of KPT-9274 perhaps stimulating more robust T cell activity which may be enhanced by anti-PD1. Further experiments would greatly assist in exploring these possibilities.
NAMPT transfers a phosphoribosyl residue from 5-phosphoribosyl-1-pyrophosphate to nicotinamide which produces nicotinamide mononucleotide. Nicotinamide mononucleotide adenylyltransferase converts nicotinamide mononucleotide into NAD+ (43). An alternative NAD biosynthesis pathway employs the three step Preiss–Handler pathway that starts with NAPRT acting on nicotinic acid to generate nicotinic acid mononucleotide, which is then converted to nicotinic acid adenine dinucleotide and subsequently to NAD+ (44,45). This pathway can also salvage NAD+ from nicotinamide by using a gut bacterial nicotinamidase to convert nicotinamide to nicotinic acid (13). A subset of non-small cell lung carcinoma, small cell lung carcinoma, breast cancer, pancreatic cancer, glioma, and fibrosarcoma cell lines have epigenetic downregulation of the NAPRT promoter, which correlates with low NAPRT expression in these lines (14,46), as we found in a subset of human ccRCC patients (Figure 1) and RENCA cells (Figure 3). In these patients, NAMPT inhibitors may be particularly efficacious at inhibiting tumor growth because one salvage pathway for NAD biosynthesis is severely reduced. The supplementation of nicotinic acid to patients can mitigate the potential toxic side effects of NAMPT inhibition in nontarget tissues that still express NAPRT, while the tumor cells that have reduced NAPRT expression will still be affected by the NAMPT inhibitor despite nicotinic acid supplementation (14). Low expression of NAPRT (<7.62 fragments per kilobase of transcript per million) in renal cancer is an unfavorable prognostic marker (https://www.proteinatlas.org/ENSG00000147813-NAPRT/pathology/tissue/renal+cancer). This has not been found in other malignancies and has not been reported elsewhere, and the data suggest a personalized-medicine approach in which those patients with RCC who are identified to have low expression of NAPRT mRNA associated with methylation of the NAPRT promoter (see Figure 1) would likely be particularly appropriate candidates for KPT-9274 therapy with nicotinic acid supplementation.
A high level of variation in tumor growth rates was observed in the control mice, despite these being clonal tumors in syngeneic hosts. In all treatment groups, there were mice whose tumors grew very little over the 21-day treatment period (Supplemental Figure 3), whereas some mice grew very large tumors in the anti-PD1 and control groups only. The innate immune system is capable of both promoting tumor growth as well as inhibiting tumor growth (47). If the combination treatment KPT-9274 and anti-PD1 is inhibiting tumor growth via recruitment of more tumor-inhibitory than tumor-promoting elements of the immune system, this suggests that there are stochastic events which control the immune response to tumors that we may not be able to measure or easily control. However, we can conclude that the combination treatment did not cooperate to recruit more T cells to the tumors in our experiments.
In summary, we have extended our previous work on a dual PAK4/NAMPT inhibitor in kidney cancer to show that therapy with this drug in combination with the anti-PD1 is more effective at inhibiting growth of the tumors when compared with the single therapies. These data indicate that this combination should be evaluated in patients who are unresponsive to immune CPI alone; our work may thus broaden the populations that show a robust response with these novel antibody treatments.
Disclosures
This work was in part supported by grants from Dialysis Clinics Incorporated (DCI), but DCI had no influence on the experiments, data collection, or manuscript writing. E. Baloglu, H. Chang, Y. Landesman, and W. Senapedis were employees of Karyopharm Therapeutics while the work was being carried out and report having patent WO 2017031213 issued, but they did not influence the experiments or conclusions of the paper. Karyopharm provided KPT-9274 and an unrestricted gift for purchase of some reagents. W. Senapedis has stock and options in Karyopharm Therapeutics Inc. O. Abu Aboud, K. Anderson, K. Jen, K. Kim, B. McLaughlin, J. Modiano, R. Pili, J. Trott, and R. Weiss have nothing to disclose.
Funding
This work was supported by National Institutes of Health (NIH) National Cancer Institute (NCI) grant 1R03CA181837-01, NIH National Institute of Diabetes and Digestive and Kidney Diseases grant 1R01DK082690-01A1, and the Medical Service of the US Department of Veterans Affairs, all to R. Weiss. This work was also supported in part by DCI (to J. Trott and R. Weiss). K. Anderson was supported by the NCI Ruth L. Kirschstein National Research Service Award F30 CA195973. J. Modiano was supported by the Perlman Chair in Animal Oncology. This project was also supported by the University of California Davis Flow Cytometry Shared Resource Laboratory with funding from NCI grant P30 CA093373 (Cancer Center) and NIH National Center for Research Resources grant C06-RR12088. A nonrestricted gift for research purposes was provided by Karyopharm Therapeutics.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/10.34067/KID.0000282019/-/DCSupplemental.
Supplemental Figure 1
Gating strategy for flow cytometry. Download Supplemental Figure 1, PDF file, 668 KB
Supplemental Figure 2
RENCA-luc cell viability is reduced by KPT-9274 in a dose-dependent manner. Download Supplemental Figure 2, PDF file, 668 KB
Supplemental Figure 3
Subcutaneous measurements of tumor growth of RENCA-luc cells in 10- week-old male Balb/cJ mice over the 21 days of treatment. Download Supplemental Figure 3, PDF file, 668 KB
Supplemental Figure 4
Sections of Balb/c RENCA tumors excised 21 days after treatment with combinations of KPT-9274 and/or anti-PD1 antibody. Download Supplemental Figure 4, PDF file, 668 KB
Supplemental Figure 5
KPT-9274 decreased phosphorylation of β-catenin and total PAK4 expression. Download Supplemental Figure 5, PDF file, 668 KB
Acknowledgments
We thank Lauren Hirao for assistance with planning the flow cytometry experiments.
Author Contributions
O. Abu Aboud, K. Anderson, J. Modiano, W. Senapedis, J. Trott, and R. Weiss conceptualized the study; O. Abu Aboud, K. Jen, K. Kim, B. McLaughlin, and J. Trott were responsible for formal analysis; Y. Landesman, W. Senapedis, J. Trott, and R. Weiss were responsible for funding acquisition; O. Abu Aboud, H. Chang, B. McLaughlin, and J. Trott were responsible for investigation; O. Abu Aboud, K. Anderson, B. McLaughlin, J. Modiano, and J. Trott were responsible for methodology; O. Abu Aboud, B. McLaughlin, J. Modiano, J. Trott, and R. Weiss wrote the original draft; O. Abu Aboud, K. Anderson, H. Chang, K. Jen, K. Kim, Y. Landesman, B. McLaughlin, J. Modiano, W. Senapedis, J. Trott, and R. Weiss reviewed and edited the manuscript; E. Baloglu, R. Pili, and R. Weiss were responsible for resources; H. Chang conducted immunohistochemistry experiments; Y. Landesman and W. Senapedis provided advice; R. Weiss provided supervision.
Footnotes
Present address: Dr. Katie L. Anderson, North Carolina State University College of Veterinary Medicine, Raleigh, North Carolina.
References
Articles from Kidney360 are provided here courtesy of American Society of Nephrology
Full text links
Read article at publisher's site: https://doi.org/10.34067/kid.0000282019
Read article for free, from open access legal sources, via Unpaywall:
https://kidney360.asnjournals.org/content/kidney360/1/5/376.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.34067/kid.0000282019
Article citations
Development of a PAK4-targeting PROTAC for renal carcinoma therapy: concurrent inhibition of cancer cell proliferation and enhancement of immune cell response.
EBioMedicine, 104:105162, 28 May 2024
Cited by: 1 article | PMID: 38810561 | PMCID: PMC11154127
Channeling Nicotinamide Phosphoribosyltransferase (NAMPT) to Address Life and Death.
J Med Chem, 67(8):5999-6026, 05 Apr 2024
Cited by: 0 articles | PMID: 38580317 | PMCID: PMC11056997
Review Free full text in Europe PMC
Advances in NAD-Lowering Agents for Cancer Treatment.
Nutrients, 13(5):1665, 14 May 2021
Cited by: 34 articles | PMID: 34068917 | PMCID: PMC8156468
Review Free full text in Europe PMC
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Ensembl Genome Browser
- (1 citation) Ensembl - ENSG00000147813
RefSeq - NCBI Reference Sequence Database (2)
- (3 citations) RefSeq - NM_172607.3
- (2 citations) RefSeq - NM_145201.5
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Dual and Specific Inhibition of NAMPT and PAK4 By KPT-9274 Decreases Kidney Cancer Growth.
Mol Cancer Ther, 15(9):2119-2129, 07 Jul 2016
Cited by: 72 articles | PMID: 27390344 | PMCID: PMC5010932
KPT-9274, an Inhibitor of PAK4 and NAMPT, Leads to Downregulation of mTORC2 in Triple Negative Breast Cancer Cells.
Chem Res Toxicol, 33(2):482-491, 09 Jan 2020
Cited by: 13 articles | PMID: 31876149 | PMCID: PMC9316853
Dual PAK4-NAMPT Inhibition Impacts Growth and Survival, and Increases Sensitivity to DNA-Damaging Agents in Waldenström Macroglobulinemia.
Clin Cancer Res, 25(1):369-377, 11 Sep 2018
Cited by: 18 articles | PMID: 30206161 | PMCID: PMC6320280
Check point inhibitors a new era in renal cell carcinoma treatment.
Med Oncol, 35(6):85, 04 May 2018
Cited by: 23 articles | PMID: 29728867
Review
Funding
Funders who supported this work.
Dialysis Clinics Inc
Karyopharm Therapeutics Inc.
Medical Service of the US Department of Veterans' Affairs
NCI NIH HHS (3)
Grant ID: F30 CA195973
Grant ID: R03 CA181837
Grant ID: P30 CA093373
NCRR NIH HHS (1)
Grant ID: C06 RR012088
NIDDK NIH HHS (2)
Grant ID: R01 DK082690
Grant ID: R01 DK107416
National Cancer Institute (3)
Grant ID: 1R03CA181837-01
Grant ID: P30 CA093373
Grant ID: F30 CA195973
National Center for Research Resources (1)
Grant ID: NCRR C06-RR12088
National Institute of Diabetes and Digestive and Kidney Diseases (1)
Grant ID: 1R01DK082690-01A1
 1
1