Abstract
Purpose of review
To discuss emerging understandings of adolescent long COVID or post-COVID-19 conditions, including proposed clinical definitions, common symptoms, epidemiology, overlaps with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and orthostatic intolerance, and preliminary guidance on management.Recent findings
The recent World Health Organization clinical case definition of post-COVID-19 condition requires a history of probable or confirmed SARS-CoV-2 infection, with symptoms starting within 3 months of the onset of COVID-19. Symptoms must last for at least 2 months and cannot be explained by an alternative diagnosis. Common symptoms of the post-COVID-19 condition include, but are not limited to, fatigue, shortness of breath, and cognitive dysfunction. These symptoms generally have an impact on everyday functioning. The incidence of prolonged symptoms following SARS-CoV-2 infection has proven challenging to define, but it is now clear that those with relatively mild initial infections, without severe initial respiratory disease or end-organ injury, can still develop chronic impairments, with symptoms that overlap with conditions like ME/CFS (profound fatigue, unrefreshing sleep, post-exertional malaise, cognitive dysfunction, and orthostatic intolerance).Summary
We do not yet have a clear understanding of the mechanisms by which individuals develop post-COVID-19 conditions. There may be several distinct types of long COVID that require different treatments. At this point, there is no single pharmacologic agent to effectively treat all symptoms. Because some presentations of post-COVID-19 conditions mimic disorders such as ME/CFS, treatment guidelines for this and related conditions can be helpful for managing post-COVID-19 symptoms.Supplementary information
The online version contains supplementary material available at 10.1007/s40124-022-00261-4.Free full text

Long-Term COVID 19 Sequelae in Adolescents: the Overlap with Orthostatic Intolerance and ME/CFS
Abstract
Purpose of Review
To discuss emerging understandings of adolescent long COVID or post-COVID-19 conditions, including proposed clinical definitions, common symptoms, epidemiology, overlaps with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and orthostatic intolerance, and preliminary guidance on management.
Recent Findings
The recent World Health Organization clinical case definition of post-COVID-19 condition requires a history of probable or confirmed SARS-CoV-2 infection, with symptoms starting within 3 months of the onset of COVID-19. Symptoms must last for at least 2 months and cannot be explained by an alternative diagnosis. Common symptoms of the post-COVID-19 condition include, but are not limited to, fatigue, shortness of breath, and cognitive dysfunction. These symptoms generally have an impact on everyday functioning. The incidence of prolonged symptoms following SARS-CoV-2 infection has proven challenging to define, but it is now clear that those with relatively mild initial infections, without severe initial respiratory disease or end-organ injury, can still develop chronic impairments, with symptoms that overlap with conditions like ME/CFS (profound fatigue, unrefreshing sleep, post-exertional malaise, cognitive dysfunction, and orthostatic intolerance).
Summary
We do not yet have a clear understanding of the mechanisms by which individuals develop post-COVID-19 conditions. There may be several distinct types of long COVID that require different treatments. At this point, there is no single pharmacologic agent to effectively treat all symptoms. Because some presentations of post-COVID-19 conditions mimic disorders such as ME/CFS, treatment guidelines for this and related conditions can be helpful for managing post-COVID-19 symptoms.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40124-022-00261-4.
Introduction
As of January 2022, there had been over 300 million confirmed cases of SARS-CoV-2 globally and over 5.4 million deaths [1]. While the vast majority of surviving patients return to their baseline health [2••], it has been evident from early in the pandemic that a proportion of patients experience chronic health impairments. Some of these conditions are sequelae of more severe acute COVID-19 such as acute respiratory distress syndrome, post-ICU syndrome, myocarditis, thrombosis, renal injury, stroke, and multisystem inflammatory syndrome in children (MIS-C). Sequelae of MIS-C and the more organ-specific complications have been discussed elsewhere [3–5, 6•]. The focus of this review is on adolescents who have developed long-term symptoms, including those with mild respiratory or systemic illnesses in the acute phase. These individuals have been described as having post-COVID-19 conditions, also referred to as long COVID [7•]. In this paper, we review proposed definitions of post-COVID-19 chronic conditions, discuss the epidemiology of pediatric long COVID, and the overlaps with orthostatic intolerance and other post-infectious illnesses like myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Based largely on our experience treating these overlapping conditions, we offer preliminary recommendations on management.
Search Strategy
In addition to papers and materials known to the authors, we conducted a search of PubMed, Embase, Scopus, World Health Organization (WHO) COVID database (which contains preprints from bioRxiv and medRxiv), and I Love Evidence. The search was conducted in mid-November 2021 and used multiple methods of identifying pediatric and adolescent articles, along with various synonyms for long COVID or post-COVID conditions (described in detail in Supplemental File 1).
Definitions
Definitions for post-COVID conditions and other disorders discussed in this review can be found in Table Table1.1. We will use the terms post-COVID-19 condition, post-COVID conditions, and long COVID interchangeably throughout.
Table 1
Definitions for post-COVID condition and related disorders
| Post-COVID-19 Condition | |
|---|---|
| WHO | In October 2021, the WHO published a clinical case definition of the post-COVID-19 condition [2••], following a Delphi consensus process that included patients, patient researchers, external experts, WHO staff, and others. According to this case definition, the post-COVID-19 condition occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually within 3 months of the onset of COVID-19. Symptoms must last for at least 2 months and cannot be explained by an alternative diagnosis. Common symptoms of the post-COVID-19 condition include, but are not limited to, fatigue, shortness of breath, and cognitive dysfunction. These symptoms may follow an initial recovery period or persist from the initial COVID-19 infection and generally have an impact on everyday functioning. Other groups have used a variable duration of prolonged symptoms, commonly 1 month |
| CDC | The CDC presents post-COVID conditions as the failure to return to a previous state of health following a SARS-CoV-2 infection [7•]. As of July 2021, the CDC used post-COVID conditions as an umbrella term for a variety of health conditions in which patients of all ages present with new, returning, or ongoing symptoms, at least 4 weeks post-SARS-CoV-2 infection. Symptoms may return after a period of recovery following initial infection and may occur regardless of the severity of acute infection. Characteristic and persistent symptoms include, but are not limited to, dyspnea, fatigue, post-exertional malaise/poor endurance, cognitive impairment (“brain fog”), cough, chest pain, and headache |
| Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) | |
The 2015 Institute of Medicine definition of ME/CFS [56••] requires the following core symptoms to be present at least half the time and with at least moderate severity, with the assumption that other causes of these symptoms have been excluded: 1. A substantial reduction or impairment in the ability to engage in pre-illness levels of occupational, educational, social, or personal activities that persists for more than 6 months and is accompanied by fatigue, which is often profound, is of new or definite onset (not lifelong), is not the result of ongoing exertion, and is not substantially alleviated by rest 2. Post-exertional malaise 3. Unrefreshing sleep Plus at least one of the following: 4a. Cognitive impairment 4b. Orthostatic intolerance | |
| Orthostatic intolerance (OI) | |
| Orthostatic intolerance refers to a group of circulatory disorders defined by the provocation of symptoms with assuming or maintaining upright posture and the improvement in symptoms with recumbency [108]. Some orthostatic symptoms, such as fatigue and brain fog, can persist after lying down, but symptoms of lightheadedness typically respond promptly | |
| Initial orthostatic hypotension (IOH) | Transient drop of > > 40 mm Hg in SBP or 40 mm Hg in SBP or > > 20 mm Hg DBP within 15 s of standing, accompanied by lightheadedness and reflex tachycardia, with hypotension lasting for less than 1 min [109]. Syncope is uncommon. This condition is more common in adolescents. Pharmacologic treatment is not necessary unless there is impairment in overall function 20 mm Hg DBP within 15 s of standing, accompanied by lightheadedness and reflex tachycardia, with hypotension lasting for less than 1 min [109]. Syncope is uncommon. This condition is more common in adolescents. Pharmacologic treatment is not necessary unless there is impairment in overall function |
| Classical orthostatic hypotension (cOH) | A sustained 20 mm Hg drop in systolic or a 10 mm Hg drop in diastolic blood pressure within the first 3 min of standing or head-up tilt [110]. This is more common in adults, but can be seen in children with acute dehydration, anorexia nervosa, or in response to certain medications (e.g., tricyclic antidepressants, prochlorperazine, quetiapine) |
| Delayed orthostatic hypotension (dOH) | dOH involves the same drop in blood pressure as in classical OH but occurring after 3 min upright [110] |
| Neurally mediated hypotension (NMH) | Characterized by an abrupt drop in blood pressure and frequently a relative bradycardia at the time of hypotension, often associated with recurrent syncope or pre-syncope in day-to-day life. However, syncope in daily life is not universal in this group, so we refer to this circulatory pattern as NMH, the physiology of which is identical to reflex syncope, often termed vasovagal syncope, neurocardiogenic syncope, and neurally mediated syncope [111–114] Symptoms can be present soon after assuming an upright posture, but hypotension usually is not detected unless the orthostatic stress is prolonged. Fatigue is common for hours following a vasovagal syncope or NMH episode |
| Postural tachycardia syndrome (POTS) | In adolescents, POTS is characterized by a sustained heart rate increment of at least 40 beats/minute within 10 min of standing or head-up tilt testing, in the absence of orthostatic hypotension in the first three minutes upright, and in association with chronic orthostatic symptoms. The onset is insidious for some, but it often appears after infection, immunization, surgery, and trauma [47••] |
| Inappropriate sinus tachycardia | Characterized by a sinus rhythm with a heart rate greater than 100 bpm at rest [100]. The symptoms are similar to those of POTS |
| Low orthostatic tolerance | Characterized by prominent orthostatic symptoms without the heart rate and blood pressure changes of OH, NMH, or POTS [66••] |
Long COVID Symptoms
Long COVID is a spectrum of disease, likely with multifactorial etiologies. Children and adolescents with long COVID are presenting with a variety of complaints, although some patterns are beginning to emerge. Long COVID symptoms can occur in hospitalized [8, 9] or non-hospitalized children [10, 11•, 12]. As in adults, many pediatric patients present with symptoms of long COVID after experiencing only mild or asymptomatic acute infections [11•, 12].
The time course and constellation of symptoms may vary in adults and children with long COVID. As acknowledged in the WHO definition, some have persistent symptoms that linger after an acute infection, while others develop new or a relapse of symptoms after complete recovery from the initial infection [13•, 14]. Some of the fluctuation in symptom frequency and intensity may relate to post-exertional exacerbation in symptoms (discussed below). The literature describing pediatric long COVID remains sparse [15, 16], and there is a lack of consistency in how symptoms are elicited. Additional studies are needed to clarify the nature and duration of long COVID symptoms in pediatric populations.
Fatigue or low energy is one of the most common symptoms reported in children with long COVID, with recent studies suggesting that up to 87% of affected children report fatigue [10, 11•, 14, 17•, 18–22, 23••]. Fatigue in this population often leads to difficulty with physical and cognitive activity, which can limit participation in school, extracurricular activities, and sports. Excessive sleep, problems initiating or maintaining sleep, or non-refreshing sleep often accompany fatigue in pediatric long COVID. Fatigue may persist even with an improvement in sleep patterns.
Post-exertional malaise (PEM) is also common in long COVID. PEM refers to an exacerbation not just of fatigue, but of many symptoms, including lightheadedness, cognitive fogginess, sensory sensitivity, headaches, and pain, occurring after relative increases in physical activity or cognitive demands. Orthostatic stress and neuromuscular strain are additional triggers of PEM in ME/CFS and may also be capable of causing symptom exacerbations in long COVID [24, 25]. Sometimes typical activities of daily life like participation in a full day of school can lead to substantial PEM, thereby contributing to functional impairment and distress in these patients.
Cognitive difficulties or “brain fog” are also commonly reported by children with long COVID [10, 11•, 18, 19, 21, 23••]. Cognitive difficulties, while inconsistently ascertained in the literature, tend to include problems with concentration, short-term memory, and school performance [10, 11•, 17•, 18, 23••]. Similar to physical fatigue and PEM, cognitive difficulties or “brain fog” can also be exacerbated by mental exertion, such as schoolwork or studying for examinations.
Headaches are also commonly reported both in the acute and post-acute phase of COVID in children [10, 11•, 17•, 19–22, 23••, 26]. Additional studies are needed to characterize the nature and specific types of headaches experienced, as well as the best treatment options. Patients with a history of headaches prior to COVID infection may develop more severe or more frequent headaches, but these can arise as a new symptom. Headaches usually are not attributable to any secondary cause (brain lesion, brain injury, etc.).
Orthostatic symptoms: Many patients also report orthostatic symptoms, including lightheadedness or dizziness, syncope, blurred vision, exercise intolerance, dyspnea, chest discomfort, palpitations, tremulousness, anxiety, diaphoresis, and nausea [10, 17•, 22]. In some cases, patients meet criteria for postural tachycardia syndrome (POTS) or other forms of orthostatic intolerance (OI) [27]. Many patients with POTS also have overlapping symptoms with long COVID, including fatigue, cognitive difficulties, headaches, gastrointestinal symptoms, anxiety, and mood concerns. Other patients have heart rate changes that do not meet the threshold for POTS, but still experience significant orthostatic symptoms that may represent a spectrum of dysautonomia [17•].
Cardiopulmonary symptoms: Many adolescents have reported a variety of cardiopulmonary symptoms including dyspnea, chest pain or tightness, and cough [10, 11•, 17•, 21, 22, 23••, 28, 29]. In the absence of severe acute pulmonary disease, cardiopulmonary work-up in these patients tends to be negative [28, 29]. The underlying pathology for these symptoms is unclear at this point.
Mental health and behavioral symptoms also are prominent in this population, with anxiety and depression being the most prevalent [17•, 21, 30]. Whether this is directly related to the effects of the virus, effects of physical symptoms of long COVID or effects of the pandemic in general is not completely clear. However, recent studies in both adults and children suggest that mood and cognitive functioning after SARS-CoV-2 infection are impaired when compared to controls with similar pandemic-related experiences [31, 32].
Changes in taste and smell including anosmia, ageusia, parosmia, and dysgeusia are reported with acute SARS-CoV-2 infection in children [20] and adults [33]. Some children with long COVID also experience persistent alterations to taste and smell, impacting appetite and potentially leading to weight loss [10, 21, 23••, 26, 34]. These sensory alterations tend to cluster closer to the earlier phase of the illness.
Long COVID is a broad umbrella term that encompasses many symptoms. The symptoms described here are a subset of the ones that have been reported. Evidence is beginning to emerge that different phenotypes may exist within long COVID. We have found that adolescents experiencing prolonged symptoms after mild, acute COVID infection often report a phenotype that overlaps with OI or ME/CFS.
Epidemiology
The reported rates of prolonged symptoms following COVID-19 vary based on the age of the participants, the duration of follow-up (4 weeks vs 3 months vs 6–12 months), and the design of the study. Differences in design include whether the study population was clinic based or population based, whether there was a clinical evaluation to exclude other causes of symptoms, whether ascertainment of symptoms and function relied on validated questionnaires or on self- or parent-report, and on the precision with which specific symptoms were investigated. Few studies differentiate the rates of prolonged symptoms after SARS-CoV-2 infection from more general symptoms caused by the pandemic itself [32].
Currently, published studies on long COVID have used various designs to answer different epidemiologic questions. For instance, some ask: in patients who have had COVID-19 infection, what is the risk of developing long COVID? In hospital and clinic-based studies, 45–70% of patients with SARS-CoV-2 infection report prolonged symptoms for a variable duration after infection, but many of these studies do not have a comparison group, are subject to referral biases, and have relatively small sample sizes (reviewed by Zimmermann et al. [23••]). Larger studies and those with comparison groups have the potential to more accurately estimate symptom prevalence in patients with confirmed versus suspected SARS-CoV-2 infection. For example, the CLoCK study in the United Kingdom (UK) surveyed individuals aged 11–17-year-old 3 months after a positive PCR test and also enrolled controls who had a negative PCR test (performed due to symptoms, anxiety, contact, or other reasons) [22]. The prevalence of at least one symptom at 3 months of follow-up was 66.5% in test-positives versus 53.4% in test-negatives and 30.3% versus 16.2% respectively for three or more symptoms. These results must be interpreted with appropriate caution, as only 13% of the eligible test-positive and test-negative population responded to the survey, a limitation that also complicates interpretation of the incidence data from a nationwide study in Denmark [35].
Other studies have asked: what proportion of the population is experiencing symptoms suggestive of long COVID at this time? For example, a study by the National Office for Statistics in the UK reports a much lower prevalence of post-COVID conditions [36••]. The study randomly surveyed over 350,000 UK residents living in private households, defining long COVID as still experiencing symptoms 4 or more weeks after infection, including pre-existing symptoms that worsened after COVID. As illustrated in Fig. 1, post-COVID symptoms at 12 weeks and 12 months were least common in those ages 2–11 (0.21% vs 0.12%), increasing in 12–16-year-old (0.82% vs 0.26%) and approximating adult rates in the 17–24-year-old group (1.31% vs 0.48%).
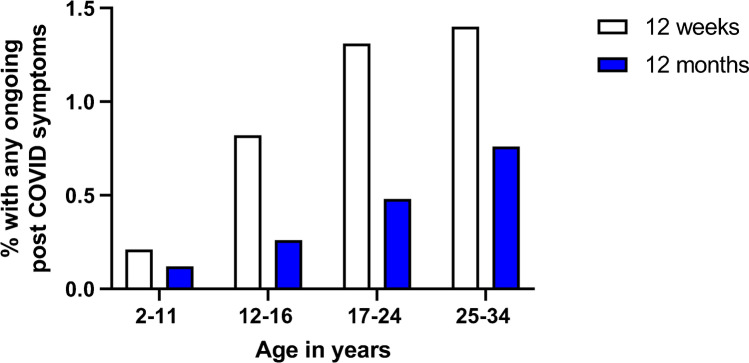
Estimated percentage of UK respondents at different ages reporting persistent COVID-19 symptoms at 12 weeks and 12 months after suspected or confirmed infection (from reference 37, United Kingdom Office of National Statistics data collected in the 4 weeks preceding December 6, 2021, published January 6, 2022)
Several additional caveats are germane. Ascertainment of the full range of post-COVID symptoms was incomplete in early studies; many early post-COVID studies focused primarily on respiratory or infectious symptoms (e.g., shortness of breath, fever, congestion). Notably, symptoms associated with OI were not initially recognized, described, or assessed as being common [37•]. Moreover, attribution of individual symptoms to specific causes has been problematic in some studies. Labeling problems with attention, processing, and short-term memory as psychiatric symptoms could be misleading given that orthostatic stress can provoke cognitive problems in the absence of classical psychiatric conditions [38]. Similarly, in OI, orthostatic dyspnea can occur [39, 40], and the hyperadrenergic response to reductions in cerebral blood flow can be misinterpreted as anxiety [41].
Future large scale longitudinal studies with comparison groups are needed in order to understand the full constellation of symptoms, risk factors, and prevalence of long COVID in children and adolescents.
Orthostatic Intolerance After COVID-19 Infection
The common forms of OI are listed in Table Table1.1. Beginning with the report of Miglis [42•], and followed by reports from a variety of centers (reviewed by Bisaccia et al.), it became clear that syndromes of OI were common in association with COVID-19 [43•]. Information on pediatric post-COVID OI is more limited. One case report describes a previously healthy 12-year-old girl who contracted COVID-19 in March 2020; orthostatic symptoms progressed until she became bedbound by July 2020 [44]. Testing revealed severely symptomatic OI associated with a drop in blood pressure and resting tachycardia. Another case series describes a 19-year-old male with confirmed COVID-19 who developed orthostatic symptoms within the first 2 weeks of infection [45]. Orthostatic testing 3 months into the illness revealed a striking 70-bpm increase in heart rate (HR) from supine to standing, consistent with POTS. In our pediatric post-COVID clinic, we perform a 10-min passive standing test (Table (Table2)2) in all patients. In a case series describing the initial cohort of patients seen at the Kennedy Krieger Institute Pediatric Post-COVID-19 Rehabilitation Clinic, two of eight patients met criteria for POTS, and all but one experienced increased symptoms during the 10 min upright, even though they did not meet formal HR criteria for POTS [17•]. In a case series of 20 adults who developed circulatory dysfunction after COVID-19 infection, many experienced improvement in symptoms with treatment targeted to OI [46], emphasizing the importance of recognizing OI as a treatable complication of COVID-19 infection.
Table 2
Clinical evaluation of post-COVID conditions/long COVID
| Suggested procedures | ||
|---|---|---|
Detailed history Physical examination Neurologic examination The Beighton Score for joint hypermobility [115] Physical therapy screening tests to look for limitations in symptom-free range of motion of the limbs and spine [116] Laboratory tests Complete blood count, with platelet count and differential white blood cell count Serum chemistries including electrolytes, urea, creatinine, total protein, albumin, calcium, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase T4 free, thyroid-stimulating hormone Erythrocyte sedimentation rate or C-reactive protein Ferritin or other measures of iron deficiency Vitamin B12, vitamin D Celiac disease screening Urinalysis Electrocardiogram Orthostatic testing (see below) Other testing is dependent on the history and physical examination (e.g., consider quantitative immunoglobulins in those with a history of recurrent, severe, or persistent infections; consider plasma histamine, and other tests for mast cell activation syndrome in those with a strong history of allergic inflammation or signs and symptoms of facial flushing, pruritis, or urticaria [117]) | ||
| Questionnaires | ||
Supplemental questionnaires can provide more information vital to evaluating the impact of the patient’s symptoms on their daily life. We recommend the following instruments in children and adolescents, all of which have the advantage of being brief and imposing only a minimal cognitive burden on patients: •Functional Disability Inventory [118] •Pediatric Quality of Life Inventory (Peds QL) [119] (Questionnaires exist for both the patient and an adult proxy, but a direct report from the patient is important) •Peds QL Multidimensional Fatigue Scale [120] •Wood Mental Fatigue Inventory [121] •Hospital Anxiety and Depression Scale [122] or Beck Depression Inventory [123] Our recommended battery for neuropsychologic evaluation that can be performed in person or via telehealth has been published elsewhere [17•] | ||
| Orthostatic testing | ||
| In all individuals with chronic fatigue, and at this stage of the investigation of long COVID, we recommend orthostatic testing of at least 10 min duration. This can be accomplished using either a passive standing test or a head-up tilt test | ||
| Passive standing test [95] | Laboratory head-up tilt table test [124, 125] | |
5 min supine—> 10 min of quiet standing with the upper back against the wall and heels 2–6 inches away from the wall—> 10 min of quiet standing with the upper back against the wall and heels 2–6 inches away from the wall—> 2 min supine 2 min supine | Heart rate and blood pressure were measured during a 70-degree head-up tilt 10-min tests are sufficient for diagnosing POTS and OH Prolonged testing of 40–45 min is usually required to identify neurally mediated hypotension or delayed OH | |
| Record | ||
| Each minute | Heart rate and blood pressure *To calculate the HR increment between lowest supine and peak standing, select the lowest supine HR value from either the 5 min pre-test or the 2 min post-test | |
| The end of the first supine phase and each minute standing | Symptoms on a 0–10 scale (0 Presence of acrocyanosis | |
Similar to the observed patterns of long COVID in children of different ages, OI affects adolescents more than pre-pubertal children, and females are more likely to be affected than males. OI can also be triggered by immunizations, pregnancy, surgery, or trauma [47••]. Symptom burden can be significant, resulting in limited ability to participate in school or work. POTS itself is heterogeneous, with several proposed mechanisms including autoimmunity [48], increased sympathetic activity [49], hypovolemia, and peripheral sympathetic noradrenergic denervation [50].
The development of OI following COVID-19 infection is not surprising. Prior to the pandemic, those with POTS specifically or OI in general often reported a history of infection closely preceding the onset of their orthostatic symptoms [51]. Among patients who develop lightheadedness and other orthostatic symptoms in the first 2 weeks of COVID-19 infection, it would be reasonable to postulate a direct effect of the virus on central autonomic networks [52]. The short time frame also argues against deconditioning or inactivity as an etiology of symptoms [45, 53]. For those developing symptoms beyond 2 weeks, after the emergence of antibodies directed at SARS-CoV-2, an autoimmune pathogenesis has been proposed, consistent with pre-pandemic observations that POTS may have an autoimmune etiology [54, 55].
It remains unclear whether OI is more prevalent or different in some manner after COVID-19 compared to other infections, and how long orthostatic symptoms after COVID-19 will persist [53]. As the number of patients affected by post-COVID OI increases, one challenge will be to meet the clinical demand, as the number of physicians treating OI was insufficient for the existing patient volume prior to the pandemic [46].
Is Long COVID a Unique Illness or Is SARS-CoV-2 Another Trigger for ME/CFS?
The illness formerly termed chronic fatigue syndrome is now referred to by the US National Institutes of Health and the Centers for Disease Control and Prevention as ME/CFS (Table (Table1)1) [56••]. While evidence of classical encephalomyelitis is not present, evidence of disturbed cognitive function and autonomic nervous system control are prominent. Early in the COVID-19 pandemic, it became clear that a subset of patients with prolonged symptoms had features consistent with ME/CFS. It remains to be determined whether these patients with long COVID persisting more than 6 months meet the criteria for ME/CFS or if long COVID is in some way distinctive. Our preliminary observations suggest that SARS-CoV-2 is emerging as a common trigger for ME/CFS.
As is true for the pathogenesis of ME/CFS, the cause or causes of long COVID remain uncertain. There may be different phenotypes of long COVID, and causes of long COVID are likely to be multifactorial in some patients. For certain patients, acute COVID-19 might exacerbate pre-existing ME/CFS. Prominent hypotheses for ME/CFS pathophysiology include autoimmunity [57–59], a physiologic stress response that does not attenuate once the acute infection or stressor has resolved [60], a chronic inflammatory response to an initial infection [61, 62] (including glial cell activation [63–65]), viral reactivation, or a hypo-metabolic cellular response. Any postulated mechanism must also explain the presence of circulatory dysfunction and reduced cerebral blood flow [66••] as a prominent component of the persistence of ME/CFS symptoms. OI has a prevalence of over 95% in pediatric patients with ME/CFS [56••, 67]. Recent evidence using extracranial Doppler echography of the vertebral and internal carotid arteries demonstrates that 90% of adults with ME/CFS experience significant reductions in cerebral blood flow during head-up tilt, confirming OI even when heart rate and blood pressure responses might be normal [66••].
Some of the current hypotheses for the development of long COVID include SARS-CoV-2 tropism for the brainstem [68•, 69], renin-angiotensin system dysfunction leading to central and peripheral circulatory abnormalities [70], chronic immune activation [71, 72], mast cell activation [70, 73•], persistence of whole virus or remnants of SARS-CoV-2 [74], the ability of the virus to lead to hemostatic imbalance, reactivation of Epstein-Barr virus (EBV) [75, 76] and other viruses, interference with fibrinolysis and promotion of micro-thrombi [77, 78], and the development of auto-antibodies [79–81].
Our experience in a single center suggests that a subset of adolescents with a moderate or severe burden of long COVID symptoms and impaired health-related quality of life have features consistent with ME/CFS [45]. One recent case–control study in adults shows that cerebral blood flow reductions during upright posture in long COVID patients are at least as severe as the reductions in comparison groups with ME/CFS and POTS and ME/CFS with a normal heart rate and blood pressure response to upright posture [82•]. In the 10 long COVID patients, all of whom had POTS, cerebral blood flow fell 33% over the 30 min upright, comparable to the 20 with ME/CFS and POTS (29%) and the 20 with ME/CFS and a normal heart rate and blood pressure response (25%), all significantly different than the 4% reduction in cerebral blood flow for the 20 healthy controls.
Whether the risk factors and co-morbid clinical conditions in long COVID are similar to those in pediatric ME/CFS remains to be determined. Prominent biological risk factors previously identified for pediatric ME/CFS include age [83•] (adolescents more affected than pre-pubertal children), sex (females are affected 3–4 times more commonly [84]), and joint hypermobility [85] (seen in 60% with ME/CFS versus 20–24% of age and sex-matched controls). One trial of IVIG for pediatric ME/CFS identified cutaneous anergy in 21% [86]. Conditions found more commonly in ME/CFS (possibly a consequence of the initial infectious or inflammatory trigger but also possibly preceding the illness and creating a risk of prolonged impairment) include OI in >
> 95% [67, 87], allergic inflammation [88], mast cell activation syndrome (MCAS) in a subset [89], and restrictions in symptom-free range of motion in
95% [67, 87], allergic inflammation [88], mast cell activation syndrome (MCAS) in a subset [89], and restrictions in symptom-free range of motion in >
> 80% [24, 90]. Further research is needed to determine whether treatment for co-morbid conditions in pediatric ME/CFS is relevant to and improves function in pediatric long COVID.
80% [24, 90]. Further research is needed to determine whether treatment for co-morbid conditions in pediatric ME/CFS is relevant to and improves function in pediatric long COVID.
Management
There may be several distinct types of post-COVID conditions that require different treatments. At this time, there is no single pharmacologic agent for all variations of post-COVID symptoms nor is there a uniform treatment approach. Several groups have authored recommendations for investigation and management [91•, 92, 93, 94•]. The provisional CDC guidance states that some presentations of post-COVID conditions mimic disorders such as ME/CFS, MCAS, and OI [93]. Treatment guidelines for these conditions can be helpful for managing post-COVID symptoms. At present, the management of post-COVID conditions focuses primarily on addressing symptoms. Based on our clinical experience evaluating pediatric OI and ME/CFS, our approach includes the diagnostic testing in Table Table2.2. Below we offer evaluation and management suggestions for specific symptoms:
Orthostatic Intolerance
Although lightheadedness/dizziness has been ascertained in some long COVID studies [10, 26], OI is not ascertained in a consistent manner in the majority of the pediatric long COVID literature. It is important to ask about specific conditions that can provoke orthostatic symptoms, and not simply to ask about lightheadedness, as adolescent patients may not be aware that what they experience is abnormal. Typical situations that provoke symptoms in those with OI include standing in line, standing at a reception or religious service, shopping, showering, and being in hot environments. To elicit symptoms of OI, practitioners might ask questions such as “How long can you stand still before having to sit down?” and “Do you fidget and move around when standing, study in a reclining position, or sit with your knees to your chest or with your feet under you?”.
The CDC guidance recommends testing post-COVID patients with a 3-min standing test [93]. However, a 3-min standing test will miss 43% of adolescent and young adult patients with POTS [95]. While 10 min is insufficient to document NMH, most patients with NMH or low orthostatic tolerance will be quite symptomatic during the first ten minutes upright. An at-home 10-min standing test using a heart rate monitor might be a helpful screening measure, but needs to be supervised because of the potential for developing syncope. Formal tilt-table testing is expensive and not always available, but might be warranted in specific situations or research studies [96].
If the patient presents with OI, non-pharmacological management can be initiated with simple treatments like avoiding aggravating conditions, increasing dietary salt and fluid intake, using cooling garments, and employing postural counter-maneuvers and wearing compression garments to reduce gravitational pooling of blood and improve the blood return to the heart [97, 98]. Gradual increases in activity, designed to avoid provoking PEM, are part of the overall approach [99, 100]. If non-pharmacologic management alone is insufficient, a variety of medications can be used, such as vasoconstrictors (e.g., midodrine, stimulants), agents that improve blood volume (e.g., hormonal birth control therapy, fludrocortisone, desmopressin acetate), and medicines that control sympathetic tone, heart rate, or the effect and release of catecholamines (e.g., beta-blockers, clonidine, SSRIs/SNRIs, pyridostigmine bromide, ivabradine). Doses are published elsewhere [83•]. Medications can be selected based on a variety of clinical factors, including the resting heart rate and blood pressure, and whether two problems can be treated with a single medication. For example, a beta-blocker might be appropriate in the presence of a relatively high resting heart rate or in someone with headaches. Fludrocortisone might be a better first choice in somebody with a low resting blood pressure or a very high salt appetite. Stimulants or clonidine can treat both OI and cognitive dysfunction. OI is one of the more treatable components of ME/CFS and may prove to have similar benefits in long COVID.
PEM and Managing Activity
Exercise intolerance was identified as a common symptom in some pediatric long COVID studies [8, 101•], but few have ascertained for PEM. We recommend utilizing pacing techniques for many post-COVID symptoms. Pacing as a management technique can be helpful to avoid exacerbating symptoms. Gradual return to activities should be approached with caution and modified to accommodate the severity of each patient’s condition. For patients who are moderately to severely impaired and cannot tolerate exercise while seated or standing, exercise should occur while lying down. Start with stretching for 1–2 min and increase the duration of activity gradually as long as PEM is not provoked. For the mildly impaired, start with 5–15 min of walking. Manual forms of physical therapy can be a bridge to tolerating exercise. Sometimes exercise will not be tolerated until OI is adequately treated [83•].
Cognitive Dysfunction
Cognitive impairments can be demonstrated on baseline neuropsychiatric testing, but may also emerge with more complex tasks and in response to upright tilt-table testing [38]. Aside from treating OI as mentioned above, helpful strategies include dividing work into smaller and more manageable sections, performing mental work lying down, snacks and regular fluid intake, and reducing stressors [83•]. In some instances, stimulants may be helpful. A gradual transition back to learning is recommended, with educational and environmental accommodations as needed.
Behavioral Symptoms
Referral to a psychologist can be helpful for those who are struggling to cope with the effects of the illness or who have true depression or anxiety disorders. A behavioral psychologist can also work with patients to incorporate the lifestyle modifications that are recommended for management of long COVID, OI, or ME/CFS. It remains to be seen whether behavioral symptoms in long COVID will be similar to ME/CFS. While adolescents with ME/CFS might be frustrated and demoralized by their illness, they are less likely to endorse the primary features of depression such as feelings of worthlessness, guilt, and low self-esteem, or a lack of interest in friendships, relationships, or activities they previously enjoyed [83•]. ME/CFS differs from depression in that individuals with depression often feel better after exercise, while untreated ME/CFS patients can have a prolonged post-exertional increase in symptoms. Patients with ME/CFS have plans for the future and would like to participate in school and other activities but are often physically limited by their symptoms [83•].
Headaches
For those experiencing headaches, lifestyle modifications can be effective for many. These include stress management, adequate hydration, identifying and avoiding headache triggers, and satisfactory sleep patterns [102–104]. Neuroimaging may be warranted if abnormalities are present on a neurological examination or if there are red flags on history that are concerning for increase intracranial pressure or secondary causes of headache [103, 104]. Medications for preventing headaches can include magnesium, riboflavin, cyproheptadine, beta-adrenergic antagonists, tricyclic antidepressant medications, anti-convulsants, and, for those with migraines, calcitonin gene receptor antagonists [102, 104].
Sleep Disturbances
Patients experiencing sleep disturbances can benefit from a specified, regular bedtime, avoiding daytime naps where possible, and avoiding caffeine late in the day. Using phones, computers, or other electronics after “lights out” can aggravate fatigue and should be avoided after bedtime. White noise or meditative phone apps can be helpful. Parents may need to awaken those with hypersomnolence after 12 h of sleep to ensure better hydration. Individuals can go back to sleep if needed, but long periods of uninterrupted sleep promote low blood volume and can aggravate OI. If insomnia is impressive and unresponsive to relaxation techniques and standard sleep hygiene measures, pharmacologic treatment may be needed.
MCAS and Allergic Phenomenon
MCAS has emerged as a co-morbid and potentially causal factor in patients with OI and ME/CFS and has been hypothesized as a pathophysiologic influence on the severity of COVID-19 [105•]. Infections of all types as well as physical and chemical stimuli can activate mast cells, leading to degranulation and release of multiple mediators, including histamine and cytokines [106]. Clinical suspicion for MCAS increases in those with recurring rashes, pruritus, urticaria, facial flushing, and an intolerance of multiple foods and medications. Treatment consists of avoidance of triggers, as well as the addition of antihistamines and medications to stabilize mast cell membranes such as cromolyn, leukotriene inhibitors, and others.
Some allergic phenomena may be addressed with dietary changes. For example, up to 31% of ME/CFS adolescents in one study met the criteria for a delayed cow’s milk protein hypersensitivity, which can be recognized by a triad of upper gastrointestinal symptoms that include epigastric pain, gastroesophageal reflux, and early satiety, sometimes associated with recurrent aphthous ulcers [88]. A diet free of cow’s milk protein in those with milk protein intolerance usually improves the local upper gastrointestinal symptoms, and in some can improve overall well-being, fatigue, and orthostatic symptoms.
Individualized Approach
Mononucleosis can cause persistent fatigue in 13% of adolescents at 6 months post-infection [107]. As with mononucleosis, there is likely to be some spontaneous improvement over time in those with milder post-COVID symptoms. Consequently, some patients may not need intensive intervention and can expand activities as tolerated. Similarly, recommendations cover a broad range of symptoms and may not apply to each patient. Although a patient may meet long COVID diagnostic criteria, we recommend that patients be evaluated on a case-by-case basis. A standing test or pharmacological intervention will not be necessary for every presenting patient. In a setting of limited resources, practitioner discretion will help to avoid inundating long COVID clinics with those who do not require extensive care and will leave resources for individuals with increasingly severe impairments. We recommend that follow-up time and treatment be decided according to the impact long COVID has on the patient’s quality of life.
Conclusion
Emerging data confirm that prolonged symptoms can develop following even mild or asymptomatic initial SARS-CoV-2 infection. The most common symptoms are fatigue, cognitive dysfunction, and headaches. As ascertainment for orthostatic intolerance in these patients improves, lightheadedness is becoming more commonly recognized. A proportion of long COVID patients meet the criteria for ME/CFS at 6 months. At present, management of post-COVID conditions focuses primarily on addressing symptoms, borrowing management strategies from conditions like OI and ME/CFS.
Compliance with Ethical Standards
The authors declare no competing interests.
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Adolescent Medicine
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Full text links
Read article at publisher's site: https://doi.org/10.1007/s40124-022-00261-4
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007/s40124-022-00261-4.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/124417491
Article citations
Using a novel methodology to map Post-COVID services for children and young people in England: a web-based systematic search.
BMC Health Serv Res, 24(1):863, 29 Jul 2024
Cited by: 0 articles | PMID: 39080694 | PMCID: PMC11288108
Researching COVID to enhance recovery (RECOVER) pediatric study protocol: Rationale, objectives and design.
PLoS One, 19(5):e0285635, 07 May 2024
Cited by: 3 articles | PMID: 38713673 | PMCID: PMC11075869
A Multidisciplinary Approach: Management and Rehabilitation of Children With Pediatric Post-COVID-19 Condition.
Pediatr Infect Dis J, 43(9):880-884, 29 May 2024
Cited by: 0 articles | PMID: 38808972 | PMCID: PMC11319073
Neuropsychological functioning of pediatric patients with long COVID.
Clin Neuropsychol, 38(8):1855-1872, 25 Apr 2024
Cited by: 0 articles | PMID: 38664068
Severe pediatric COVID-19: a review from the clinical and immunopathophysiological perspectives.
World J Pediatr, 20(4):307-324, 06 Feb 2024
Cited by: 0 articles | PMID: 38321331 | PMCID: PMC11052880
Review Free full text in Europe PMC
Go to all (26) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Long COVID in pediatrics-epidemiology, diagnosis, and management.
Eur J Pediatr, 183(4):1543-1553, 27 Jan 2024
Cited by: 5 articles | PMID: 38279014 | PMCID: PMC11001657
Review Free full text in Europe PMC
Analysis of post COVID-19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome.
J Adv Res, 40:179-196, 26 Nov 2021
Cited by: 53 articles | PMID: 36100326 | PMCID: PMC8619886
Review Free full text in Europe PMC
Orthostatic Symptoms and Reductions in Cerebral Blood Flow in Long-Haul COVID-19 Patients: Similarities with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.
Medicina (Kaunas), 58(1):28, 24 Dec 2021
Cited by: 38 articles | PMID: 35056336 | PMCID: PMC8778312
[Post-COVID syndrome with fatigue and exercise intolerance: myalgic encephalomyelitis/chronic fatigue syndrome].
Inn Med (Heidelb), 63(8):830-839, 13 Jul 2022
Cited by: 11 articles | PMID: 35925074 | PMCID: PMC9281337
Review Free full text in Europe PMC

 4
4


