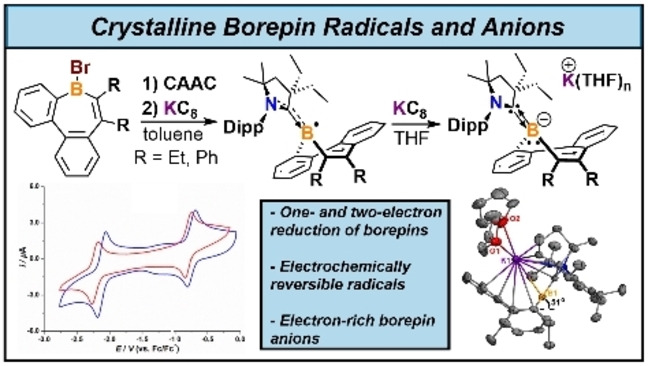
Abstract
Free full text
Isolation of Stable Borepin Radicals and Anions
Abstract
Borepin, a 7‐membered boron‐containing heterocycle, has become an emerging molecular platform for the development of new materials and optoelectronics. While electron‐deficient borepins are well‐established, reduced electron‐rich species have remained elusive. Herein we report the first isolable, crystalline borepin radical (2 a, 2
a, 2 b) and anion (3
b) and anion (3 a, 3
a, 3 b) complexes, which have been synthesized by potassium graphite (KC8) reduction of cyclic(alkyl)(amino) carbene‐dibenzo[b,d]borepin precursors. Borepin radicals and anions have been characterized by EPR or NMR, elemental analysis, X‐ray crystallography, and cyclic voltammetry. In addition, the bonding features have been investigated computationally using density functional theory.
b) complexes, which have been synthesized by potassium graphite (KC8) reduction of cyclic(alkyl)(amino) carbene‐dibenzo[b,d]borepin precursors. Borepin radicals and anions have been characterized by EPR or NMR, elemental analysis, X‐ray crystallography, and cyclic voltammetry. In addition, the bonding features have been investigated computationally using density functional theory.
Abstract
The first examples of reduced borepins have been structurally characterized and offer new platforms for chemical reactivity.
Boron‐doped polycyclic aromatic hydrocarbons (B‐PAHs) have been studied in a variety of different subfields of chemistry. [1] Most notably, they have been used as a platform to elicit a wide range of optical and electronic properties in π‐conjugated materials. [1] Borepin, a 6π‐electron B‐PAH, has recently received attention in materials chemistry, [2] and as a means to understand fundamental issues of aromaticity in heterocyclic molecules. [3] With few exceptions, nearly all structurally characterized tricoordinate borepins feature a neutral boron atom in a 7‐membered ring with three B−R single bonds (Figure 1A, R=C or N atom). Several neutral dibenzo‐ [4] and dithieno‐fused borepins[ 3e , 5 ] have been isolated and their optoelectronic properties have been investigated.[ 2 , 4 , 5 ] In 2019, we reported the molecular structures of the first examples of cationic borepins, borepinium ions (Figure 1B). [6] Recently, fused diborepinium ions were synthesized and exhibited luminescent properties as a result of extended conjugation. [7] Despite the ongoing interest in boron radicals [8] and anions, [9] the chemical synthesis and structural authentication of reduced, nucleophilic borepins are hitherto unknown (Figure 1C). This is likely due to their inherent high reactivity and the challenging nature of the experimental chemistry. Herein, we report the synthesis and isolation, X‐ray crystal structures, and theoretical studies of borepin radicals and anions—the first structurally characterized and storable examples of reduced borepin compounds. Unlike borepins in Figure 1A and B which are electron‐deficient, borepins in Figure 1C are electron‐rich, and the non‐planar conformation of the heterocycles result in a progressive concentration of electron density around boron and the formation of an exocyclic B=C double bond.
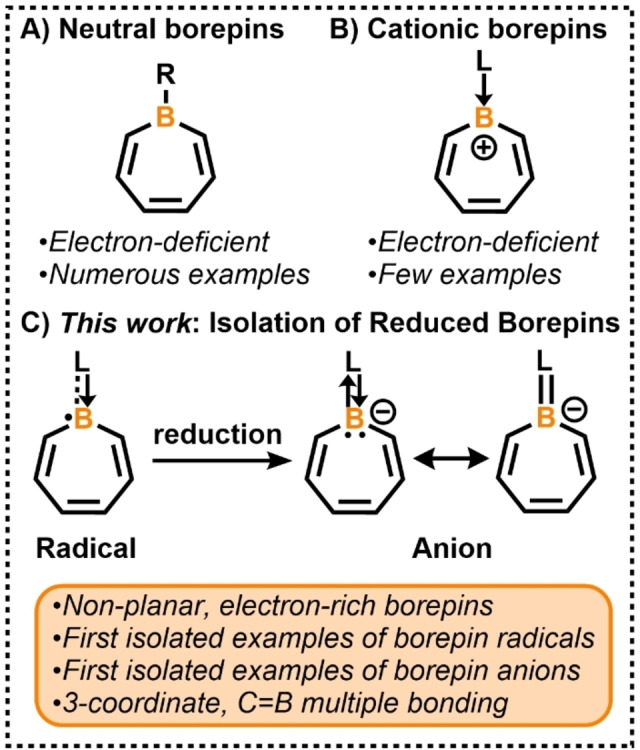
A) Neutral tricoordinate borepins. B) Electron‐deficient cationic borepins. C) First isolated examples of reduced borepins.
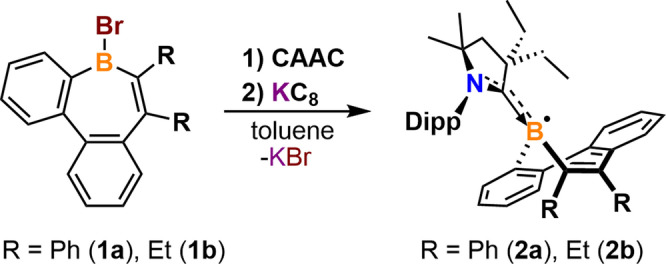
We began our studies by synthesizing dibenzo[b,f]borepin
[6]
as a platform for reduction chemistry. However, numerous attempts at isolating radicals or anions proved challenging, and relevant compounds were prone to decomposition. Thus, the more sterically demanding neutral borepin 1 a
[10]
was synthesized and allowed to react with 2,6‐(diisopropylphenyl)‐4,4‐diethyl‐2,2‐dimethyl‐pyrrolidin‐5‐ylidene (CAAC)
[11]
in toluene to yield a carbene‐supported tetracoordinate borepin complex. However, after stirring at room temperature overnight, the colorless solution turned brown, and NMR analysis of an aliquot of the reaction mixture indicated that there was a substantial amount of 1
a
[10]
was synthesized and allowed to react with 2,6‐(diisopropylphenyl)‐4,4‐diethyl‐2,2‐dimethyl‐pyrrolidin‐5‐ylidene (CAAC)
[11]
in toluene to yield a carbene‐supported tetracoordinate borepin complex. However, after stirring at room temperature overnight, the colorless solution turned brown, and NMR analysis of an aliquot of the reaction mixture indicated that there was a substantial amount of 1 a remaining. The difficulty in achieving complete conversion is likely due to the sterically hindered boron center. Despite these synthetic challenges, the CAAC‐stabilized diphenyl‐substituted‐dibenzo[b,d]borepin radical (2
a remaining. The difficulty in achieving complete conversion is likely due to the sterically hindered boron center. Despite these synthetic challenges, the CAAC‐stabilized diphenyl‐substituted‐dibenzo[b,d]borepin radical (2 a) was isolated as a yellow crystalline solid (Figure S1A) in 56
a) was isolated as a yellow crystalline solid (Figure S1A) in 56 % yield via a one‐pot synthesis in which 1
% yield via a one‐pot synthesis in which 1 a and CAAC were combined with one equivalent of KC8 in toluene at room temperature (Scheme 1). Following a similar procedure, the CAAC‐stabilized diethyl‐substituted‐dibenzo[b,d]borepin radical (2
a and CAAC were combined with one equivalent of KC8 in toluene at room temperature (Scheme 1). Following a similar procedure, the CAAC‐stabilized diethyl‐substituted‐dibenzo[b,d]borepin radical (2 b) was isolated as an orange crystalline solid (Figure S1B) in 61
b) was isolated as an orange crystalline solid (Figure S1B) in 61 % yield. Both radicals were obtained as analytically pure solids, which are stable in the solid‐state for at least six months. The radicals are also stable in hexanes under inert atmosphere for at least three months.
% yield. Both radicals were obtained as analytically pure solids, which are stable in the solid‐state for at least six months. The radicals are also stable in hexanes under inert atmosphere for at least three months.
Air‐ and moisture‐sensitive single‐crystals of 2 a and 2
a and 2 b suitable for X‐ray crystallographic analysis were obtained by recrystallization from concentrated hexanes solutions at −37
b suitable for X‐ray crystallographic analysis were obtained by recrystallization from concentrated hexanes solutions at −37 °C (Figure 2).
[12]
For 2
°C (Figure 2).
[12]
For 2 a, two chemically equivalent but crystallographically distinct molecules were observed in the unit cell (Figures 2 and S8). A planar geometry was observed for the tricoordinate boron center in both 2
a, two chemically equivalent but crystallographically distinct molecules were observed in the unit cell (Figures 2 and S8). A planar geometry was observed for the tricoordinate boron center in both 2 a and 2
a and 2 b, with the borepin rings adopting a boat‐shaped conformation. The boron centers lie 0.7576(51) Å above the plane containing C30−C23−C48−C43 in 2
b, with the borepin rings adopting a boat‐shaped conformation. The boron centers lie 0.7576(51) Å above the plane containing C30−C23−C48−C43 in 2 a and 0.7276(21) Å above the C28−C23−C36−C35 plane in 2
a and 0.7276(21) Å above the C28−C23−C36−C35 plane in 2 b. The CAACC−B bond lengths in 2
b. The CAACC−B bond lengths in 2 a [1.517(5) Å] and 2
a [1.517(5) Å] and 2 b [1.544(2) Å] are significantly shorter than that of the reported CAAC‐stabilized borepinium ion [1.637(8) Å].
[6]
This is consistent with partial delocalization of the unpaired π‐electron onto the CAAC moiety in the radicals.
b [1.544(2) Å] are significantly shorter than that of the reported CAAC‐stabilized borepinium ion [1.637(8) Å].
[6]
This is consistent with partial delocalization of the unpaired π‐electron onto the CAAC moiety in the radicals.
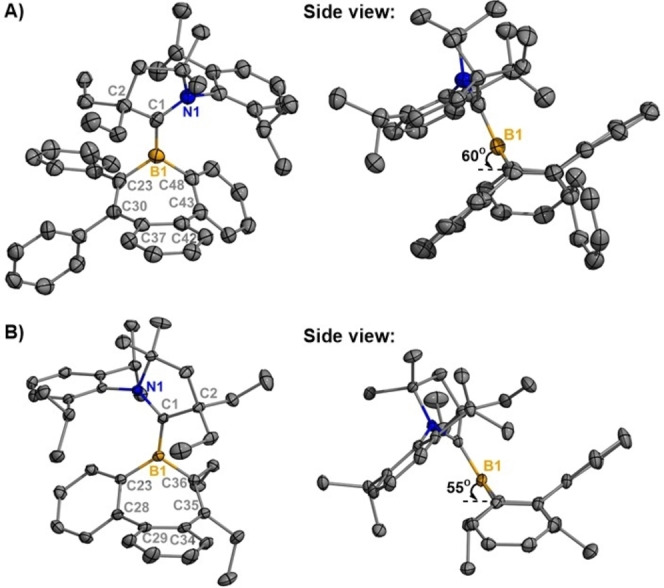
Molecular structures of 2 a (A) and 2
a (A) and 2 b (B) (thermal ellipsoids at 50
b (B) (thermal ellipsoids at 50 % probability; H atoms were omitted for clarity, for 2
% probability; H atoms were omitted for clarity, for 2 a: only one of two crystallographically independent molecules shown). Selected bond lengths [Å] and angles [°]: 2
a: only one of two crystallographically independent molecules shown). Selected bond lengths [Å] and angles [°]: 2 a: B1−C1 1.517(5), B1−C23 1.596(5), B1−C48 1.574(5); C1‐B1‐C23 122.7(3), C1‐B1‐C48 127.6(3), C23‐B1‐C48 109.2(3); 2
a: B1−C1 1.517(5), B1−C23 1.596(5), B1−C48 1.574(5); C1‐B1‐C23 122.7(3), C1‐B1‐C48 127.6(3), C23‐B1‐C48 109.2(3); 2 b: B1−C1 1.544(2), B1−C23 1.591(2), B1−C36 1.594(2); C1‐B1‐C23 128.92(12), C1‐B1‐C36 121.48(12), C23‐B1‐C36 109.38(11).
b: B1−C1 1.544(2), B1−C23 1.591(2), B1−C36 1.594(2); C1‐B1‐C23 128.92(12), C1‐B1‐C36 121.48(12), C23‐B1‐C36 109.38(11).
The radical nature of compounds 2 a and 2
a and 2 b was confirmed by EPR spectroscopy in toluene at room temperature, which displays different multiple‐line spectra centered at g=2.0019 (2
b was confirmed by EPR spectroscopy in toluene at room temperature, which displays different multiple‐line spectra centered at g=2.0019 (2 a) and 1.9993 (2
a) and 1.9993 (2 b) with complex hyperfine splitting (Figure 3). Simulations of the EPR spectra for 2
b) with complex hyperfine splitting (Figure 3). Simulations of the EPR spectra for 2 a and 2
a and 2 b are well‐reproduced with a single spin system consisting of atoms N1, C1, and B1. Spin density plots reveal the unpaired electron in 2
b are well‐reproduced with a single spin system consisting of atoms N1, C1, and B1. Spin density plots reveal the unpaired electron in 2 a and 2
a and 2 b is predominantly associated with the N1−C1−B1 π‐system (Figure 3). In complex 2
b is predominantly associated with the N1−C1−B1 π‐system (Figure 3). In complex 2 a, the spin population is B1 (0.315, 31
a, the spin population is B1 (0.315, 31 %), C1 (0.421, 42
%), C1 (0.421, 42 %), and N1 (0.245, 24
%), and N1 (0.245, 24 %), with minor contributions from the ligand and borepin heterocycle. In 2
%), with minor contributions from the ligand and borepin heterocycle. In 2 b, the unpaired spin distribution is similar to 2
b, the unpaired spin distribution is similar to 2 a [B1 (0.306, 30
a [B1 (0.306, 30 %), C1 (0.437, 44
%), C1 (0.437, 44 %), N1 (0.237, 24
%), N1 (0.237, 24 %) (see ESI Table S3 for spin populations).
%) (see ESI Table S3 for spin populations).
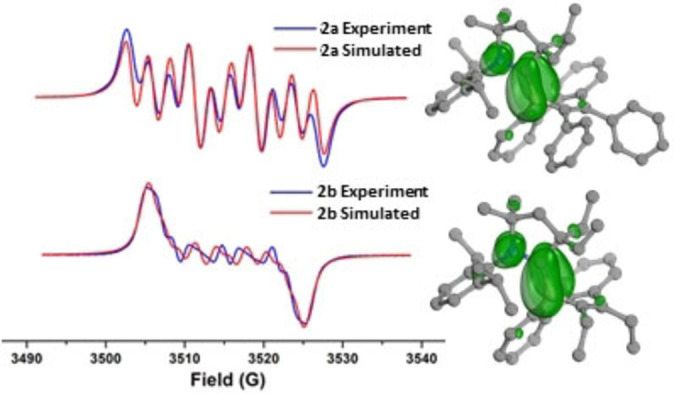
Continuous wave X‐band EPR spectrum and spin density plots of 2 a (top) and 2
a (top) and 2 b (bottom) in toluene solution at 298 K.
b (bottom) in toluene solution at 298 K.
Cyclic voltammetry (CV) experiments were carried out in THF to investigate the redox properties of 2 a and 2
a and 2 b (Figure 4). Both radicals give rise to two reversible waves. The diphenyl‐substituted dibenzo[b,d]borepin radical 2
b (Figure 4). Both radicals give rise to two reversible waves. The diphenyl‐substituted dibenzo[b,d]borepin radical 2 a exhibits slightly more positive reduction and oxidation potentials than the analogous diethyl‐substituted radical 2
a exhibits slightly more positive reduction and oxidation potentials than the analogous diethyl‐substituted radical 2 b. For the reduction of 2
b. For the reduction of 2 a and 2
a and 2 b to their corresponding anions, reduction potentials are observed at E
1/2=−2.12 and −2.22 V, respectively (referenced against the ferrocene/ferrocenium (Fc/Fc+) redox couple). All cationic species are stable in THF with reversible oxidation waves at E
1/2=−0.74 and −0.79 V for 2
b to their corresponding anions, reduction potentials are observed at E
1/2=−2.12 and −2.22 V, respectively (referenced against the ferrocene/ferrocenium (Fc/Fc+) redox couple). All cationic species are stable in THF with reversible oxidation waves at E
1/2=−0.74 and −0.79 V for 2 a and 2
a and 2 b, respectively. In contrast, the electrochemical features of neutral mesityl‐substituted dibenzo[b,f]borepin and mesityl‐substituted tetrabenzo[bc,ef]borepin
[4a]
are significantly different, with quasi‐reversible reduction potentials at E
1/2=−2.20 V and −2.56 V (THF, vs. Fc/Fc+), respectively, representing reduction to the corresponding radical anions. The reversible nature of borepin radicals 2
b, respectively. In contrast, the electrochemical features of neutral mesityl‐substituted dibenzo[b,f]borepin and mesityl‐substituted tetrabenzo[bc,ef]borepin
[4a]
are significantly different, with quasi‐reversible reduction potentials at E
1/2=−2.20 V and −2.56 V (THF, vs. Fc/Fc+), respectively, representing reduction to the corresponding radical anions. The reversible nature of borepin radicals 2 a and 2
a and 2 b highlights the uniqueness of tricoordinate borepins stabilized by neutral carbene ligands. The UV/Vis absorption spectra of 2
b highlights the uniqueness of tricoordinate borepins stabilized by neutral carbene ligands. The UV/Vis absorption spectra of 2 a and 2
a and 2 b in toluene reveals that both radicals exhibit strong absorption in the visible regions (Figure S2). TD‐DFT calculations (ωB97XD/def2‐SVP, SMD toluene) of UV/Vis absorbance agree with the experimental observations (Figure S13).
b in toluene reveals that both radicals exhibit strong absorption in the visible regions (Figure S2). TD‐DFT calculations (ωB97XD/def2‐SVP, SMD toluene) of UV/Vis absorbance agree with the experimental observations (Figure S13).
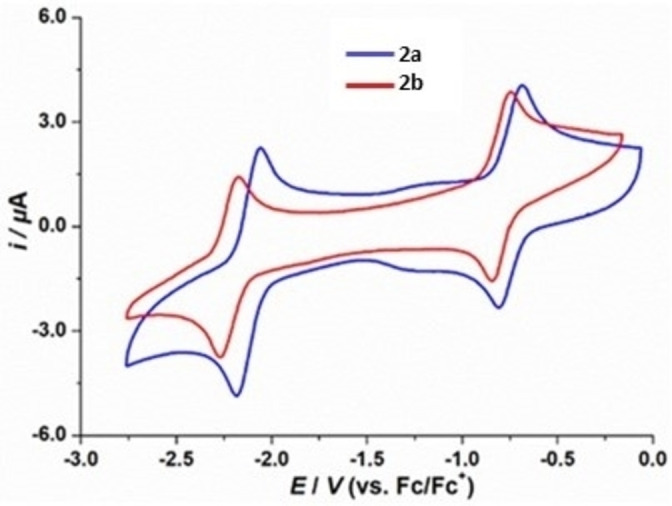
Cyclic voltammograms of 2 a and 2
a and 2 b in THF/0.1 M [nBu4N][PF6] at room temperature. Scan rate: 100 mV
b in THF/0.1 M [nBu4N][PF6] at room temperature. Scan rate: 100 mV s−1.
s−1.
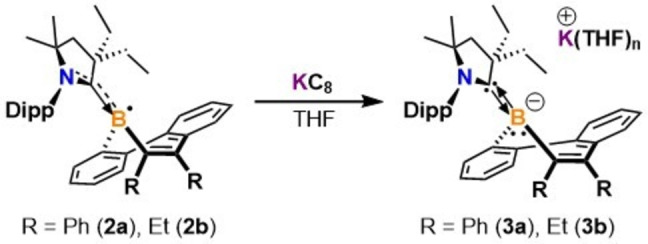
Due to the appearance of a reversible reduction wave in the borepin radical CV experiments, we then sought to synthesize borepin anions by subsequent one‐electron chemical reduction. The CAAC‐stabilized diphenyl‐substituted dibenzo[b,d]borepin anion (3 a) was obtained as dark red crystals (Figure S1C) in 87
a) was obtained as dark red crystals (Figure S1C) in 87 % yield after stirring a mixture of 2
% yield after stirring a mixture of 2 a with 2 equivalents of KC8 in THF at room temperature and recrystallizing from a THF/hexanes mixture at −37
a with 2 equivalents of KC8 in THF at room temperature and recrystallizing from a THF/hexanes mixture at −37 °C (Scheme 2). The use of excess reducing agent resulted in cleaner conversion to 3
°C (Scheme 2). The use of excess reducing agent resulted in cleaner conversion to 3 a. Attempts to obtain NMR data on multiple batches of 3
a. Attempts to obtain NMR data on multiple batches of 3 a proved to be challenging, with inseparable trace impurities of 2
a proved to be challenging, with inseparable trace impurities of 2 a resulting in a paramagnetic NMR in the majority of reaction trials.
[13]
However, in two instances, sufficiently resolved 1H and 11B{1H} NMR spectra could be obtained and the solid was analytically pure by elemental analysis. The 11B{1H} resonance at 21.7 ppm is significantly upfield compared to the neutral borepins bearing tricoordinate boron centers (δ 48.0–68.0 ppm),
[2a]
and can be attributed to a negatively charged, electron‐rich boron center. Following a similar synthetic procedure, the CAAC‐stabilized diethyl‐substituted dibenzo[b,d]borepin anion (3
a resulting in a paramagnetic NMR in the majority of reaction trials.
[13]
However, in two instances, sufficiently resolved 1H and 11B{1H} NMR spectra could be obtained and the solid was analytically pure by elemental analysis. The 11B{1H} resonance at 21.7 ppm is significantly upfield compared to the neutral borepins bearing tricoordinate boron centers (δ 48.0–68.0 ppm),
[2a]
and can be attributed to a negatively charged, electron‐rich boron center. Following a similar synthetic procedure, the CAAC‐stabilized diethyl‐substituted dibenzo[b,d]borepin anion (3 b) was obtained as a red/orange solid (Figure S1D) in 71
b) was obtained as a red/orange solid (Figure S1D) in 71 % yield by reacting 2
% yield by reacting 2 b with 1.1 equivalents of KC8. Compound 3
b with 1.1 equivalents of KC8. Compound 3 b was fully characterized by NMR spectroscopy and elemental analysis. The 1H NMR spectrum in C6D6 shows a septet resonance at 4.10 ppm for the methine proton of the CAAC Dipp group, which is downfield compared to the free CAAC (3.18 ppm). The 11B{1H} NMR resonance at 22.4 ppm is consistent with the electron‐rich tricoordinate boron signal observed for 3
b was fully characterized by NMR spectroscopy and elemental analysis. The 1H NMR spectrum in C6D6 shows a septet resonance at 4.10 ppm for the methine proton of the CAAC Dipp group, which is downfield compared to the free CAAC (3.18 ppm). The 11B{1H} NMR resonance at 22.4 ppm is consistent with the electron‐rich tricoordinate boron signal observed for 3 a.
a.
Single crystals of 3 a and 3
a and 3 b were obtained by recrystallization from a THF/hexanes mixture at −37
b were obtained by recrystallization from a THF/hexanes mixture at −37 °C. Crystallographic analyses of 3
°C. Crystallographic analyses of 3 a and 3
a and 3 b reveal that both molecules possess a trigonal planar geometry around the boron centers (Figure 5). No contacts are observed between the borepin core and potassium cation in 3
b reveal that both molecules possess a trigonal planar geometry around the boron centers (Figure 5). No contacts are observed between the borepin core and potassium cation in 3 a, whereas the cation in 3
a, whereas the cation in 3 b has contacts with both the borepin and CAAC ligand. Similar to radicals 2
b has contacts with both the borepin and CAAC ligand. Similar to radicals 2 a and 2
a and 2 b, the borepin rings in 3
b, the borepin rings in 3 a and 3
a and 3 b adopt a boat‐shaped conformation where the boron centers lie 0.7690 Å above the plane containing C30−C23−C48−C43 (3
b adopt a boat‐shaped conformation where the boron centers lie 0.7690 Å above the plane containing C30−C23−C48−C43 (3 a) and 0.7531 Å above the plane containing C28−C23−C35−C38 (3
a) and 0.7531 Å above the plane containing C28−C23−C35−C38 (3 b). Significant shortening of the CAACC−B bond is observed upon reduction of 2
b). Significant shortening of the CAACC−B bond is observed upon reduction of 2 a [1.517(5) Å] and 2
a [1.517(5) Å] and 2 b [1.544(2) Å] to their anions 3
b [1.544(2) Å] to their anions 3 a [1.461(5) Å] and 3
a [1.461(5) Å] and 3 b [1.465(4) Å]. This observation is consistent with the formation of an exocyclic B=C double bond, similar to the bonding observed in methyleneboranes.
[14]
b [1.465(4) Å]. This observation is consistent with the formation of an exocyclic B=C double bond, similar to the bonding observed in methyleneboranes.
[14]
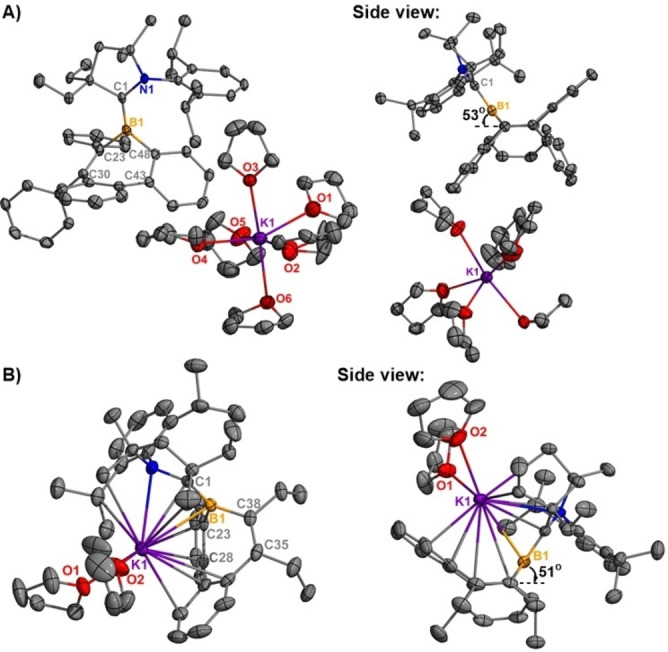
Molecular structures of 3 a (A) and 3
a (A) and 3 b (B) (thermal ellipsoids at 30
b (B) (thermal ellipsoids at 30 % probability, respectively; H atoms were omitted for clarity, one uncoordinated THF omitted for clarity (3
% probability, respectively; H atoms were omitted for clarity, one uncoordinated THF omitted for clarity (3 a), Selected bond lengths [Å] and angles [°]: 3
a), Selected bond lengths [Å] and angles [°]: 3 a: B1−C1 1.461(5), B1−C23 1.624(5), B1−C48 1.597(5); C1‐B1‐C23 121.9(3), C1‐B1‐C48 129.6(3), C23‐B1‐C48 106.2(3); 3
a: B1−C1 1.461(5), B1−C23 1.624(5), B1−C48 1.597(5); C1‐B1‐C23 121.9(3), C1‐B1‐C48 129.6(3), C23‐B1‐C48 106.2(3); 3 b: B1−C1 1.465(4), B1−C23 1.610(4), B1−C38 1.614(4), C1‐B1‐C23 129.3(2), C1‐B1‐C38 124.2(2), C23‐B1‐C38 106.54(19).
b: B1−C1 1.465(4), B1−C23 1.610(4), B1−C38 1.614(4), C1‐B1‐C23 129.3(2), C1‐B1‐C38 124.2(2), C23‐B1‐C38 106.54(19).
Theoretical calculations were performed to probe the electronic structure and bonding of compounds 2 a, 2
a, 2 b, 3
b, 3 a and 3
a and 3 b. In addition, radical and anionic CAAC‐stabilized unsubstituted borepins were also examined to assess the conformational effects of borepin. Both radical and anionic unsubstituted borepins exhibit planar geometries with highly localized borepin π contributions to the HOMO (Figure S19). The deviation from planarity in substituted borepins is due to low‐frequency vibrations of the annulated rings and Et/Ph substituents. Although anionic borepins 3
b. In addition, radical and anionic CAAC‐stabilized unsubstituted borepins were also examined to assess the conformational effects of borepin. Both radical and anionic unsubstituted borepins exhibit planar geometries with highly localized borepin π contributions to the HOMO (Figure S19). The deviation from planarity in substituted borepins is due to low‐frequency vibrations of the annulated rings and Et/Ph substituents. Although anionic borepins 3 a and 3
a and 3 b are formally anti‐aromatic, the sizable displacement from planarity results in reduced π‐character of the borepin moiety and increased B1–C1 π‐character. Therefore, compounds 3
b are formally anti‐aromatic, the sizable displacement from planarity results in reduced π‐character of the borepin moiety and increased B1–C1 π‐character. Therefore, compounds 3 a and 3
a and 3 b are best described as non‐aromatic systems. The HOMO of the anionic compounds display significant B1−C1 π‐bonding character with no contributions from the π‐system of the borepin ring (Figures S16 and S17). Hirshfield‐CM5 atomic charges were computed to confirm the radical and anionic character of the reduced borepins. Negative charges are assigned to the boron atoms in 2
b are best described as non‐aromatic systems. The HOMO of the anionic compounds display significant B1−C1 π‐bonding character with no contributions from the π‐system of the borepin ring (Figures S16 and S17). Hirshfield‐CM5 atomic charges were computed to confirm the radical and anionic character of the reduced borepins. Negative charges are assigned to the boron atoms in 2 a (−0.167 e) and 2
a (−0.167 e) and 2 b (−0.171 e), with an increased negative charge in 3
b (−0.171 e), with an increased negative charge in 3 a (−0.296 e) and 3
a (−0.296 e) and 3 b (−0.297 e), consistent with one‐ and two‐electron reductions. The carbene carbon is positively charged in singly‐reduced complexes (2
b (−0.297 e), consistent with one‐ and two‐electron reductions. The carbene carbon is positively charged in singly‐reduced complexes (2 a +0.129 e, 2
a +0.129 e, 2 b +0.125 e) while almost neutral in doubly‐reduced species 3
b +0.125 e) while almost neutral in doubly‐reduced species 3 a (+0.028 e) and 3
a (+0.028 e) and 3 b (+0.022 e), highlighting the polarization towards boron in anionic borepin.
b (+0.022 e), highlighting the polarization towards boron in anionic borepin.
Energy decomposition analysis in combination with natural orbitals for chemical valence (EDA‐NOCV)
[15]
and calculations of Wiberg bond indices (WBI) were performed to shed light on the nature of bonding in 2 a and 2
a and 2 b and their anionic analogues. WBI values for B1−C1 indicate that both radical (WBI=1.342 (2
b and their anionic analogues. WBI values for B1−C1 indicate that both radical (WBI=1.342 (2 a), 1.340 (2
a), 1.340 (2 b)) and anionic (WBI=1.533 (3
b)) and anionic (WBI=1.533 (3 a), 1.525 (3
a), 1.525 (3 b)) compounds possess electron populations associated with delocalized bonds. In order to reflect the most suitable bonding situation in these complexes, we considered CAAC and borepin with different charges and electronic states (Tables S4–S7). In cases where more than one partitioning scheme is available, the size of the orbital interaction, ΔE
orb, can be used as a probe to understand which one best describes the bonding situation.
[16]
In 2
b)) compounds possess electron populations associated with delocalized bonds. In order to reflect the most suitable bonding situation in these complexes, we considered CAAC and borepin with different charges and electronic states (Tables S4–S7). In cases where more than one partitioning scheme is available, the size of the orbital interaction, ΔE
orb, can be used as a probe to understand which one best describes the bonding situation.
[16]
In 2 a and 2
a and 2 b, doublet borepin radical binding with singlet CAAC through a donor–acceptor interaction was the most suitable bonding description. In 3
b, doublet borepin radical binding with singlet CAAC through a donor–acceptor interaction was the most suitable bonding description. In 3 a and 3
a and 3 b, doublet borepin radical binding with doublet anionic [CAAC]− through an electron‐sharing π bond and dative σ bond was a marginally preferred bonding description compared to singlet borepin anion interacting with CAAC through donation/back‐donation. However, the small difference in ΔE
orb (ΔΔE
orb≈2.7 kcal
b, doublet borepin radical binding with doublet anionic [CAAC]− through an electron‐sharing π bond and dative σ bond was a marginally preferred bonding description compared to singlet borepin anion interacting with CAAC through donation/back‐donation. However, the small difference in ΔE
orb (ΔΔE
orb≈2.7 kcal mol−1) for the two models indicates that either description could reasonably be employed. The detailed numerical results of EDA‐NOCV for the preferred interacting scheme for radical and anionic complexes are provided in Table S8. The intrinsic interaction (ΔE
int) between CAAC and borepin is quite strong in the anionic system (ΔE
int (kcal
mol−1) for the two models indicates that either description could reasonably be employed. The detailed numerical results of EDA‐NOCV for the preferred interacting scheme for radical and anionic complexes are provided in Table S8. The intrinsic interaction (ΔE
int) between CAAC and borepin is quite strong in the anionic system (ΔE
int (kcal mol−1)=−202.6 (3
mol−1)=−202.6 (3 a), −195.5 (3
a), −195.5 (3 b)) as two electrons are involved in the π bond in contrast to the single electron in the neutral radicals (ΔE
int (kcal
b)) as two electrons are involved in the π bond in contrast to the single electron in the neutral radicals (ΔE
int (kcal mol−1)=−155.1 (2
mol−1)=−155.1 (2 a), −161.1 (2
a), −161.1 (2 b)). The bonds between CAAC and borepin are slightly more covalent (ΔE
orb≈49–52
b)). The bonds between CAAC and borepin are slightly more covalent (ΔE
orb≈49–52 %) than electrostatic (ΔE
elstat≈42–44
%) than electrostatic (ΔE
elstat≈42–44 %) in nature. Dispersion interaction accounts for 5–7
%) in nature. Dispersion interaction accounts for 5–7 % of the total attraction. The breakdown of the ΔE
orb into pairwise orbital interactions shows that the strongest orbital interaction, ΔE
orb(1), originates from [borepin]←[CAAC] σ donation (51–60
% of the total attraction. The breakdown of the ΔE
orb into pairwise orbital interactions shows that the strongest orbital interaction, ΔE
orb(1), originates from [borepin]←[CAAC] σ donation (51–60 % of total ΔE
orb), whereas the next most important orbital term, ΔE
orb(2), is due to [borepin]→[CAAC] π backdonation for the neutral system, and [borepin]—[CAAC]− electron‐sharing π bond formation for anionic complexes, which account for 23–33
% of total ΔE
orb), whereas the next most important orbital term, ΔE
orb(2), is due to [borepin]→[CAAC] π backdonation for the neutral system, and [borepin]—[CAAC]− electron‐sharing π bond formation for anionic complexes, which account for 23–33 % of total ΔE
orb.
% of total ΔE
orb.
In conclusion, the first isolated examples of reduced borepin compounds have been synthesized as crystalline radicals and anions. The combined experimental and simulated EPR spectroscopic data for the radicals indicate that the chemical environment of the unpaired electron can be tuned based on the nature of the borepin substituents. Synthesis and isolation of stable organic radicals such as these species have potential applications in energy storage and conversion devices (e.g., organic batteries, photovoltaic devices, thermoelectric systems, optoelectronics). [17] Redox‐flexible radicals are particularly interesting because of their ambipolar behavior—the ability to be selectively oxidized to closed shell cations or reduced to the respective anions—an important prerequisite for many energy‐relevant applications. [17a] Reduction to the borepin anions not only serves as a key fundamental discovery, but these molecules could serve as chemical synthons for new types of materials with unusual bonding. Studies of this variety are currently underway in our laboratory and will be reported in due course.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information
Acknowledgements
The authors acknowledge the University of Virginia and the National Science Foundation Chemical Synthesis (CHE2046544) and Major Research Instrumentation (CHE2018870) programs for support of this work. R.J.G. acknowledges additional laboratory support through a Sloan Research Fellowship provided by the Alfred P. Sloan Foundation and a Beckman Young Investigator Award from the Arnold and Mabel Beckman Foundation. Generous allocation of computing resources from the National Computational Infrastructure (NCI), Intersect, and La Trobe University are also acknowledged. G.F. and S.P. thank the German Research Foundation (DFG) for funding.
Notes
K. K. Hollister, W. Yang, R. Mondol, K. E. Wentz, A. Molino, A. Kaur, D. A. Dickie, G. Frenking, S. Pan, D. J. D. Wilson, R. J. Gilliard, Angew. Chem. Int. Ed. 2022, 61, e202202516; Angew. Chem. 2022, 134, e202202516. [Europe PMC free article] [Abstract]
Contributor Information
Dr. Sudip Pan, Email: ed.grubliam-inu.eimehc@snap.
Prof. Dr. David J. D. Wilson, Email: [email protected].
Prof. Dr. Robert J. Gilliard, Jr., Email: ude.ainigriv@s8gjr.
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
References
 a by EPR spectroscopy. See detailed comment in Supporting Information.
a by EPR spectroscopy. See detailed comment in Supporting Information.Full text links
Read article at publisher's site: https://doi.org/10.1002/anie.202202516
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/anie.202202516
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/124655139
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1002/anie.202202516
Article citations
Pentacyclic fused diborepinium ions with carbene- and carbone-mediated deep-blue to red emission.
Chem Sci, 05 Aug 2024
Cited by: 0 articles | PMID: 39156927 | PMCID: PMC11325318
Solvatochromic and Aggregation-Induced Emission Active Nitrophenyl-Substituted Pyrrolidinone-Fused-1,2-Azaborine with a Pre-Twisted Molecular Geometry.
J Mater Chem C Mater, 11(40):13740-13751, 13 Sep 2023
Cited by: 0 articles | PMID: 38855717 | PMCID: PMC11160477
A linear Di-coordinate boron radical cation.
Nat Commun, 13(1):7051, 17 Nov 2022
Cited by: 0 articles | PMID: 36396646 | PMCID: PMC9671878
Gauging Radical Stabilization with Carbenes.
Angew Chem Int Ed Engl, 61(37):e202206390, 04 Aug 2022
Cited by: 10 articles | PMID: 35796423 | PMCID: PMC9545232
Isolation of Stable Borepin Radicals and Anions.
Angew Chem Int Ed Engl, 61(23):e202202516, 05 Apr 2022
Cited by: 5 articles | PMID: 35289046 | PMCID: PMC9324096
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Redox- and Charge-State Dependent Trends in 5, 6, and 7-Membered Boron Heterocycles: A Neutral Ligand Coordination Chemistry Approach to Boracyclic Cations, Anions, and Radicals.
Acc Chem Res, 57(10):1510-1522, 06 May 2024
Cited by: 0 articles | PMID: 38708938
Benzo[b]thiophene Fusion Enhances Local Borepin Aromaticity in Polycyclic Heteroaromatic Compounds.
J Org Chem, 82(24):13440-13448, 28 Nov 2017
Cited by: 7 articles | PMID: 29136463
Isolation of a neutral boron-containing radical stabilized by a cyclic (alkyl)(amino)carbene.
Angew Chem Int Ed Engl, 53(28):7360-7363, 10 Jun 2014
Cited by: 58 articles | PMID: 24917474
Persistent Borafluorene Radicals.
Angew Chem Int Ed Engl, 59(10):3850-3854, 03 Feb 2020
Cited by: 20 articles | PMID: 31816143 | PMCID: PMC7064902
Funding
Funders who supported this work.
Deutsche Forschungsgemeinschaft
German Research Foundation
National Science Foundation (1)
Grant ID: CHE2046544 and CHE2018870

 3
3