Abstract
Objective
This review aimed to: (I) provide a brief overview of some topical areas of current literature regarding applications of wearable sensors in the management of low back pain (LBP); (II) present a vision for a future comprehensive system that integrates wearable sensors to measure multiple parameters in the real world that contributes data to guide treatment selection (aided by artificial intelligence), uses wearables to aid treatment support, adherence and outcome monitoring, and interrogates the response of the individual patient to the prescribed treatment to guide future decision support for other individuals who present with LBP; and (III) consider the challenges that will need to be overcome to make such a system a reality.Background
Advances in wearable sensor technologies are opening new opportunities for the assessment and management of spinal conditions. Although evidence of improvements in outcomes for individuals with LBP from the use of sensors is limited, there is enormous future potential.Methods
Narrative review and literature synthesis.Conclusions
Substantial research is underway by groups internationally to develop and test elements of this system, to design innovative new sensors that enable recording of new data in new ways, and to fuse data from multiple sources to provide rich information about an individual's experience of LBP. Together this system, incorporating data from wearable sensors has potential to personalise care in ways that were hitherto thought impossible. The potential is high but will require concerted effort to develop and ultimately will need to be feasible and more effective than existing management.Free full text

A vision for the future of wearable sensors in spine care and its challenges: narrative review
Abstract
Objective
This review aimed to: (I) provide a brief overview of some topical areas of current literature regarding applications of wearable sensors in the management of low back pain (LBP); (II) present a vision for a future comprehensive system that integrates wearable sensors to measure multiple parameters in the real world that contributes data to guide treatment selection (aided by artificial intelligence), uses wearables to aid treatment support, adherence and outcome monitoring, and interrogates the response of the individual patient to the prescribed treatment to guide future decision support for other individuals who present with LBP; and (III) consider the challenges that will need to be overcome to make such a system a reality.
Background
Advances in wearable sensor technologies are opening new opportunities for the assessment and management of spinal conditions. Although evidence of improvements in outcomes for individuals with LBP from the use of sensors is limited, there is enormous future potential.
Methods
Narrative review and literature synthesis.
Conclusions
Substantial research is underway by groups internationally to develop and test elements of this system, to design innovative new sensors that enable recording of new data in new ways, and to fuse data from multiple sources to provide rich information about an individual’s experience of LBP. Together this system, incorporating data from wearable sensors has potential to personalise care in ways that were hitherto thought impossible. The potential is high but will require concerted effort to develop and ultimately will need to be feasible and more effective than existing management.
Introduction
Wearable sensors have become pervasive in society. Most individuals carry or wear some type of device that measures some aspect of their life. This ranges from simple measures of movement (1) and location (2) made by smart phones, to comprehensive analysis of health measures derived from specialised devices that, in addition to movement, measure biological variables such as heart rate and estimate parameters such as sleep, stress, and activity level (3). Wearable devices and their derived measures are being increasingly implemented to aid management of a diverse array of health conditions including low back pain (LBP) (4). This has most commonly related to evaluation of physical activity (4), but with advances in technology and analysis, more sophisticated assessments are beginning to be possible, such as measurement of posture and movement in the real world (5,6), which has been the topic of several extensive systematic reviews (4,6,7). Wearable sensor technology is rapidly evolving and the potential utility for LBP management is immense. This paper aims to: (I) provide a brief overview of some topical areas of current literature regarding applications of wearable sensors in management of LBP; (II) present a vision for a future comprehensive system that integrates wearable sensors to measure multiple parameters in the real world that contributes data to guide treatment selection (aided by artificial intelligence), uses wearables to aid treatment support, adherence and outcome monitoring, and interrogates the response of the individual patient to the prescribed treatment to guide future decision support for other individuals who present with LBP; and (III) consider the challenges that will need to be overcome to make such a system a reality. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-21-112/rc).
Methods
This narrative review presents a synthesis of available literature regarding a broad range of issues. The intention was not to systematically review the literature for each topic, but to present current opinions, recent findings and identify new directions for application of wearable sensors in spine care.
Overview of current application of wearable sensors in management of LBP
The most common application of wearable sensors in the management of LBP is for activity monitoring (4). In most cases this has been straightforward assessment of activity level of patients for assessment (8) or to monitor outcome after treatments, including surgery (4,9). Wearable sensors have also been used to assess not just whether a person moves, but how they move, such as evaluation of the spinal curvature/posture and movement (5,6) and movement patterns (7,10) during function and sometimes in the real-world, outside the treatment clinic. Several trials have used wearables to provide feedback for training, with variable success (11-14). This has also been integrated into gamified approaches to movement training (15,16). Most research has provided evidence for the validity and potential utility of the devices (17-19), but evidence on whether patient outcomes are improved by such application is scarce (20-22). Trials of interventions that have used wearable sensors to address a single feature in LBP [e.g., improve posture (23)] have had limited success in reducing pain, which is not surprising considering the heterogeneity and complexity of the condition.
More complex applications of wearables to treatment for LBP have been trialled. These include use of activity sensors to monitor and guide progress of a treatment aimed to improve self-management of LBP (24). Data from wearable sensors was used to monitor progress and guide dosage of physical activity, education and exercises for strength and flexibility. Despite the sophistication of the model of application of intervention, the results from this randomised controlled trial, indicated only a slight and probably not clinically important improvement in outcome relative to intervention unsupported by data from wearable sensors (25). This finding concurs with the observation that although self-management approaches that include exercise are more effective than no treatment, there is little difference in outcome between different models of application of this management strategy (26). From one perspective this might suggest that the hype of wearable sensors is unfounded, but from another it might suggest that we are not yet capturing the full potential of wearables.
Measures other than movement are also being developed and evaluated. Recordings of electromyography (EMG) from back muscles with wearable sensors have been available for many years (27) and have been implemented clinical interventions (28). Advances in technology, such as wearable “tattoo” electrodes provide promise (29). Analysis algorithms to automate analysis of data are being developed and tested (30). Whether data derived from wearable EMG can guide effective treatment has not yet been confirmed.
Estimation of sleep parameters using activity monitoring devices is also beginning to provide information regarding the association between sleep and LBP (31,32). Home-based electroencephalography (EEG) measures with wearable devices are also becoming available and applied to provide more detailed evaluation of sleep architecture (33), but not yet in LBP. Also not yet applied to LBP is the use of wearable sensors to evaluate physiological parameters associated with stress such as heart rate variability and pulse transit time from electrocardiography (ECG) and photoplethysmography recordings (34-36) and novel sensors for detection of cortisol in sweat (37,38).
In summary, there has been widespread use of wearables to monitor physical activity in LBP. This provides greater accuracy of monitoring of treatment outcomes and when integrated into a treatment support system, small benefits for treatment outcomes have been achieved. The question that remains is whether there is further potential to generate larger benefits for individuals with spinal complaints using a system that integrates data from multiple wearable sensors to provide a more comprehensive picture of an individual’s presentation that could provide refined information for treatment selection and provision?
A vision for a future comprehensive system using wearable sensors
Advances in technology are beginning to present possibilities to go beyond the use of sensors to simply enhance the application current management practices, and instead lead to a reconceptualization of the entire system for management of LBP. The following provides an overview of some key issues that plague the current management system and what to consider for a vision for an alternative system that might be possible with these technological advances.
Current LBP management
Current management of LBP generally depends on a limited number of assessments that capture narrow view of specific aspects of the state of the individual at a single timepoint (39) or over a limited period of time (40), or require recall of events/exposures (41). These assessments are generally made in an artificial setting (i.e., in a clinical facility when the individual is aware they are being scrutinised) (42,43). This is unlikely to replicate the context within which an individual lives (7) or the performance of the individual in their usual functional environment (40). The scope of assessments is generally influenced by the discipline of the clinician undertaking the assessment (44). This information is then used to select from a limited array of treatments (or referral to another clinician) that are most commonly applied in a generic one-size-fits-all manner, perhaps with some individualisation of dosage (45). Treatments may include application of an intervention, prescription of a drug, a home program or a supported self-management program (45). The outcome is reviewed to evaluate success or failure, followed by subsequent progression of treatment, change to a different treatment approach (often using a trial-and-error stepped approach) (46), or perhaps referral to another clinician. There are many challenges: assessments are limited in scope and detail and may not reflect actual lived experience (7,42); treatments are narrowly applied (46); treatment effects are challenged by poor adherence (47); and the individual’s response to treatment is not used to inform future decision making for others.
It could be argued that this model of care is unable to address the complexity of LBP and other spinal complaints. It is accepted that LBP is characterised by a huge array of biopsychosocial features that interplay uniquely for each individual who has the condition (48). Adding complexity is the recognition that LBP is a fluctuating condition that varies over time (49,50), and with a unique set of features driving this variation (51,52). These realities imply personalized care is likely to be needed. From one perspective it could be argued that this complexity is too great, and we should search for simple solutions that make some impact, even if small. The alternative perspective is to find ways to develop complex interventions (53) and support the decisions regarding care (54). This will not be straightforward; modelling work suggests that integration of multiple features for personalisation of care quickly becomes impossible once more than a handful of factors are considered (55). Wearable sensors might be part of the solution.
New potential from advances in technology
Advances in technology have enhanced the potential to evaluate features with potential relevance to LBP in the real world and to make these measures over an extended period time (56). These advances are beginning to make it possible to conceptualise the possibility to pervasively collect a broad array of variables across biological, psychological, and social domains in the real world as a person lives their life. These data could be interrogated to identify if and how each (or the interaction between them) relates to an individual’s LBP experience, including any relationship to fluctuation of the condition. Recent work has highlighted that LBP is mostly experienced as an ongoing condition characterised by fluctuations in symptoms (sometimes referred to as flares) (49,57). Qualitative research suggests people often consider themselves to have LBP, even when they are in remission (58). Wearable sensors would lie at the core of analysis of the factors that could explain the fluctuation of the condition, and that are potentially modifiable by treatments.
In the biological domain, research is already providing some insight into the potential utility of data collected in the real world, albeit from a limited set of domains/measures (5,51). For instance, although LBP can interfere with sleep, real-world data from wearable sensors and information that a user inputs into a smartphone application show that for some individuals a night of poor sleep quality (but not quantity) increases risk for a flare of the condition (51). New simplified EEG sensor systems that involve a headband are being applied to evaluate sleep architecture and provide potential for more detailed analysis of this relationship (59). For some individuals, transient exposure to a day of low physical activity is also a risk factor for flare while exposure to moderate activity is protective for flare (51). Potential to evaluate biological features in the real-world might also reveal a relevance of factors for LBP that have not yet been considered (because of inability to make appropriate measures), factors that might be relevant for some individuals but not others, or factors that have been largely dismissed or criticised based on data assessed in artificial settings.
There has been substantial debate regarding the relevance of posture and movement for LBP (60). Several reviews have concluded that there is an absence of supporting evidence (61,62). Although differences in kinematics and posture between individuals with and without pain are common in the literature [e.g., (63,64)], this does not confirm that it is relevant for their condition. Most studies measure spine motion and posture in cross-sectional studies in a laboratory (65) with unclear relevance to the real-world, use measures that have not been validated (66), or rely on subjective reporting of exposure to posture/movements (67). Many studies measure variables such as range of motion which have unclear relevance for interpretation of real-world function which involves consideration of multiple factors such as coordination between segments (68). Most studies use small sample sizes, cannot exclude bias, and measure an enormous variety of parameters that are not consistently applied between studies (60). Further, most studies assume that support for the relevance of a feature of posture or movement to LBP depends on evidence for its presence in individuals with LBP, but not those without LBP (61), and that all individuals with LBP would present in a similar manner (69). These assumptions ignore the reality of individual variation and that the relevance of a posture or movement for an individual’s LBP is likely to depend on the exposure, and other contextual or individual factors. Although individuals without LBP might present with a specific feature (such as flexion of the spine during lifting), that does not preclude the possibility that that this feature of movement is problematic and provocative of symptoms for an individual with LBP. It is well known that individuals with LBP that adopt different movement patterns, and in some cases a cluster of movement and posture features are identified that can be used to allocate individuals to subgroups (64,70,71). It is plausible that continuous assessment of movement and posture in the real-world enabled by advances in wearable technology might reveal an association between specific postures and movements and fluctuations of the condition for an individual and provide meaningful guidance for treatment selection. Algorithms are being developed to classify postures, movements, and transitions in real world settings (72,73).
Although measures in the psychological domain are more challenging to collect in an automated way, advances in technologies and analysis are providing potential to estimate some psychological factors from biological correlates. For instance, algorithms have been developed to estimate stress from measures that include heart rate variability (35). Sensors have been developed, but not yet widely available, to measure cortisol and other molecules from sweat that might provide additional information of stress (74) and immune signalling (75), that both have potential relevance for LBP. Of course, not all psychological features of potential relevance to LBP can be automatically obtained. For measures of many psychological phenomena (e.g., fear of pain/movement; pain catastrophising; mood; depression; self-efficacy) there may be no simple physiological analogue, and user input is likely to be required. Life-logging applications (35,51) present possibility to prompt users to input potentially relevant data at specific times to integrate with wearable sensor data. The individual’s experience of pain itself is not a simple measure and depends on user input.
Although transient exposure to social factors is also likely to require user input into life-logging applications, GPS data from wearable sensors is already being interrogated for information regarding social engagement and broader aspects of function (76). Data from smartphones and wearables related to communication and voice characteristics is also being used to quantify social exchanges (with mechanisms to protect privacy) in mental health conditions (77-79).
Together, there is potential to capture complex data across multiple domains to provide unrivalled data of an individual’s experience. Integration of such data is likely to provide a foundation to shift the paradigm of LBP management towards truly personalised selection of intervention. As a critical steppingstone, the availability of new technologies to provide new insights an individual’s condition, demands a new phase of discovery research to identify relevant factors prior to integration into health care.
A future comprehensive system for management of LBP enabled by wearable sensors
With the potential for collection of detailed ongoing real-world data regarding an array of potentially relevant factors across biological, psychological and social domains using wearable sensors and user input, a new model of management of LBP is possible. A vision for such a system could include: (I) assessment and decision support; (II) treatment support; and (III) ongoing refinement of more precise personalisation of care. An overview of the model is presented in Figure 1.
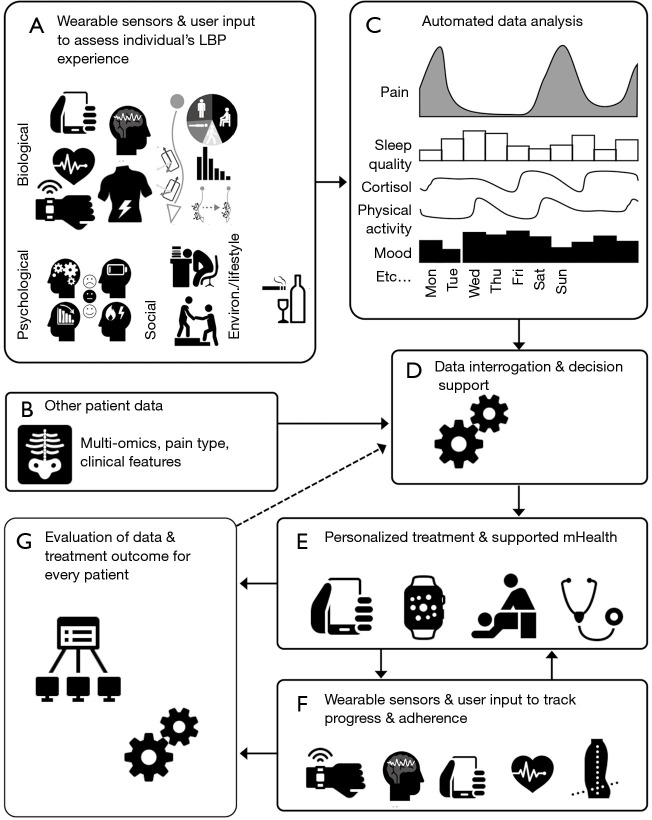
Framework for integration of wearable sensor data to guide personalized management of low back pain. (A) Continuous data from multiple domains are recorded using wearable sensors in the real world. User input data are recorded using apps to record factors that cannot be recorded with wearables (e.g., pain, psychological variables, etc.); (B) other patient data are collected to contribute to decision support. This might include multi-omics data (genomic, transcriptomic, proteomic, metabolomic, etc.), clinical features, imaging, clinically identified primary pain mechanism grouping, etc.; (C) data from wearable sensors are analysed using automated algorithm-based analysis supported by machine learning and combined with user input data; (D) all data inputs are interrogated to evaluation the complex interaction between multiple dimensions and low back pain experience using analytic methods including artificial intelligence to provide decision support for allocation of tailored interventions; (E) personalized management plan is provided that, depending on the individual patient, could include “in person” treatment and a suite of mobile Health (mHealth) solutions for self-management. mHealth solutions might include use of wearable data to support treatment (e.g., biofeedback, motivation, etc.); (F) wearable sensors and user input apps are used to monitor treatment adherence and evaluation of progress. These data would be fed back to a treating clinician; (G) all data from individual patients, the applied treatments and the treatment outcomes are uploaded to a central server to continue to build the accuracy of decision support. LBP, low back pain.
Assessment and decision support
On presentation to a health care provider, the future might include provision of a suite of sensors and a user input interface to the individual with LBP to enable evaluation of an array of features in the real world. Table 1 presents a summary of many of the wearable sensor types that are currently available and their potential utility. These data could be automatically interrogated (using machine learning algorithms, or other data classification methods) to extract measures that relate to an array of variables across domains (e.g., sleep quality; time in specific postures; activity level; variation in stress; social interaction; interaction between the multiple domains; etc.). These data could be integrated with other relevant information of the individual and their condition [e.g., imaging (80,81), history (82), omics (genomic; transcriptomic; proteomic; metabolomic data) (83,84), likely neurobiological mechanisms contributing to pain (85,86)]. The large individual dataset would then be interrogated to identify the factors (and interactions between factors) that are relevant for the individual’s experience of LBP (such as those that fluctuate with the waxing and waning of LBP). These would serve as potential targets for treatments to be selected from an available suite of management options.
Table 1
| Sensors | Measurements | Example clinical information |
|---|---|---|
| Activity | ||
| Accelerometer | Activity level; step count; sedentary time; speed | Physical activity; energy expenditure; sleep |
| Pedometer | Steps count | Physical activity |
| Global positioning system (GPS) | Distance travelled; location | Function; social interaction; participation |
| Movement/posture/muscle activity | ||
| Magnetic (magnetometer), angular rate (gyroscope) and gravity (accelerometer) sensors | Orientation relative to earth | Joint angle/posture or relative angle/posture when 2 sensors are incorporated |
| Strain sensors (e.g., fibre optic; inductance) | Length change | Spine—movement/posture; chest—breathing |
| Goniometer | Angle | Joint angular motion |
| Pressure sensor | Force | Shoe—foot contact; ground reaction forces; inverse dynamics; chest—breathing |
| Electromyography (single channel and grid) | Muscle activity | Contraction/relaxation of muscle |
| Physiological | ||
| Photoplethysmography (PPG) | Heart rate; heart rate variability; heart rate recovery; oxygen saturation; sleep stages; cardiac output | Exercise tolerance; stress |
| Electrocardiography (ECG) | Heart rate; heart rate variability | Exercise tolerance; stress |
| Electroencephalography (EEG) | Brain activity | Sleep; sleep architecture; attention |
| Near Infrared spectroscopy | Muscle oxygenation; Oxy-, deoxy- and total haemoglobin; cerebral oxygenation | Oxygen saturation in muscle |
| Biochemical sensors (e.g., epidermal; sweat; transdermal) | Cortisol; pH; electrolytes; glucose; lactate | Stress; nutrition; fatigue |
| Skin conductance | ||
| Temperature | Body temperature | Temperature; heat stress |
| Other measures | ||
| Video (smart glasses) | Activity; social interaction | |
| Sound | Language analysis; ambient sound | Social interaction; environmental context; social ambience measures |
| Light | Ambient light | Environmental context |
Based on the sheer number of variables to consider, mechanisms for decision support would be necessary. Advances in application of artificial intelligence make this possible. Neither the use of computerised decision support (87), nor the application of artificial intelligence (88) is new in LBP. What is new is the diversity of available data upon which decisions can be made. For this system to be possible the relationship between each factor and its responsiveness to treatments would also need to be known, how they might interact, and whether this responsiveness is affected by other elements of the individual patient’s unique profile. Ideally, prediction of potential effects of matched treatments would be informed by a database built from data accrued from all previous individuals with LBP whose data has been interrogated in this manner (see below). Thus, comprehensive assessment, including that provided by wearables, could provide a foundation for personalisation of care beyond what is even possible to imagine today.
Treatment support
Application of treatment could be supported by data derived from wearable sensors. Wearable sensor data can provide biofeedback (89), alert to problems (12), and monitoring of progress (25). Already data has been interrogated to track improvement of function such as physical activity and gait, and this information has been used to guide refinement of care (25). Data of spine posture and movement has been used to monitor and provide feedback as a component of motor learning interventions to train changes in performance (5). This reinforces the potential for wearable sensors to provide data to support care but, as yet, without comprehensive consideration of other variables.
There has been considerable work undertaken to develop electronic/mobile health (eHealth/mHealth) care resources for LBP that address different elements relevant to the pain experience (90-92) and some of these already incorporate data from wearable sensors (5,93), including social factors (94). Additional work is required to enhance the breadth of features that can be targeted by mHealth interventions, and to incorporate the use of innovative new sensors.
There are considerable advantages to support treatment with data from wearable sensors. First, remote application of treatment through telehealth applications (which currently rely on video assessment) (95) would be facilitated by provision of objective real time data from wearable sensors. Second, a major barrier to treatment efficacy is adherence to care (96); wearable sensors might contribute to strategies to address this issue (e.g., enhanced motivation to promote adherence (97); identification of non-adherence (98). Third, ideally data from the wearable sensors (and life-logging) that is recorded during the management period would be automatically uploaded to a server/cloud, automatically interrogated, used to modify (progress or change) treatments, and fed to clinical providers for review/alert.
Ongoing refinement of more precise personalisation of care
A major potential benefit from a system that provides detailed and automatically analysed data, along with information of applied treatments and the response to these treatments, is that with each new participant, additional data are provided for refinement of decision support. Theoretically, the precision of decision support for the allocation of treatments would likely improve. Similar approaches have been applied in LBP to refine self-management using a limited number of variables (99).
Challenges to overcome to make the system a reality
Although elements of the proposed system are available and have been trialled in LBP, there is considerable work to be done to make it a reality. Major considerations include those that relate to sensors/sensor data, treatment selection, availability of mHealth options, treatment provision and governance/security/privacy and usability of wearable sensors.
In terms of wearable sensors there are physical and technical issues that need to be addressed. From a physical perspective, sensors should not restrict or influence function. An ideal sensor would be one that a participant agrees to wear but is unaware of, once in use. One that a participant has little to do to affix, remove, recharge or to transfer data. How to make this possible is not yet clear, but many attempts have been made to meet these requirements. For instance, simplified EEG system have been designed with electrodes embedded in a headband (59), various sensors have been embedded in clothing (100,101), fibre optic sensors have been used in flexible materials to measure movement (102), and sensors for electrical signals (e.g., EMG and ECG) are available as removable “tattoos” (29). From a technical perspective there are issues of battery life/charging (103) and transmission or recording of data. New electronic designs are being trialled for options such as stretchable materials with integrated energy storage (104). Innovative methods to harvest energy from body heat and kinetic energy from body movement are possible (105). Ideally, data would be automatically uploaded to a server for analysis and interrogation (106). Current technology generally requires transfer to a computer or smartphone as an intermediate step to transfer to permanent storage. Direct transfer is not yet possible. As new technologies and new data management opportunities become available that enable new features to be measured in new ways, it will be necessary to undertake discovery research to evaluate the potential relevance for LBP management. Other health economic evaluation will be critical to consider the balance between costs and benefits of different application methods. For instance, analysis of sleep from EEG data might be more cumbersome and less feasible than use of movement sensors, but this sacrifice of ease might be outweighed by the additional information that can be extracted from analysis of sleep architecture from EEG (59). Even if movement sensors were considered acceptable, there is also the consideration of validity of estimation of sleep parameters from different combinations and placements of accelerometers, some of which are more acceptable than others (107).
Data analysis poses multiple challenges. There are many challenges for analysis of data from sensors in the real world. For instance, position and movement are generally evaluated by fusion of data from accelerometers, gyroscopes and magnetometers, but these measures are impacted by linear accelerations, drift and metal objects, respectively and require application of algorithms to identify and remove the impact of these factors (108-110). Accuracy and validity of measures is paramount and is currently variable (7). There will be challenges with interpretation of data. For motion sensors, any sensor system that involves markers attached to the skin will be influenced by skin motion (111).
Availability of meaningful data is paramount. A major hurdle is the challenge of classification of daily-life behaviours (112-114). For instance, from movement data it is critical to identify different functions and tasks (e.g., sitting, standing, walking and lifting) to reduce the diversity of activities, postures, movements to an interpretable set of meaningful variables (e.g., variation of spine posture in sitting). This requires development of application of mathematical rules/methods that must be validated. In many cases, it is probable that classification and analysis will be facilitated by fusion of data from multiple sources. For instance, interpretation of stress from data of heart rate variability requires fusion with context data [e.g., to differentiate heart rate responses between those induced by physical activity and those induced by stressful events (35)].
Once the challenges of data collection and analysis are overcome the challenge shifts to using this information for selection. This depends on availability of treatments to address the identified parameters, methods to optimise the success of treatments that take advantage of the new technologies, and potential to predict the response to treatments (and the interaction between them). This step might require a reconsideration of the literature. For instance, when considering the suite of treatments, it is plausible that some treatments that have been found to be ineffective when applied in generic manner to the heterogeneous group with LBP might be effective to address specific relevant features for and individual patient. Further, a key element of interventions implemented using wearables is behaviour change. Although change is achievable, it is often discussed that interaction with wearable devices and platforms can be short lived (115,116). Adaptive interventions are required to maintain engagement (117) and pre-empt non-adherence (118).
For a proposed system to be viable, the health system needs to enable the inclusion of this model of care (115). Co-development with clinicians and patients will be necessary to ensure that the sensors and system have utility, and co-development with health services will be necessary to ensure the potential to integrate the model of care. There will be security and governance considerations regarding access to data (3) and cybersecurity tools such as blockchain might provide a solution (119). Models of payment will need to be considered and differ between health systems. It will be critical to consider issues of cost effectiveness, feasibility, and convenience (115). The proposed model of care might not be cost effective to use for all participants. Perhaps a “light” version is necessary for individuals at low risk of poor outcome, and the “full” intensive version is reserved for those at high risk of poor outcome. These decisions might be supported by tools such as StartBack (a LBP screening tool to stratify care) approach to risk stratification (120).
Some of these challenges will need to be considered sequentially, and some in parallel. First it is critical to resolve, with discovery research, whether new wearable sensor technologies provide data that have plausible mechanistic associations with an individual’s condition. Second, there will need to be co-design with clinicians, patients, industry and health services to build the elements of the system to ensure it is feasible and that utility of a system is optimised. Third, the efficacy and cost effectiveness of the system will need to be tested. Fourth, the system will need to be future proofed to embrace new technologies, new knowledge and new methods become available. If effective, the system has great potential for application to other conditions.
Conclusions
This paper aimed to provide an overview of how wearable sensors might be integrated into a model of personalised and supported care for LBP (and other spinal conditions). Major advantages of this system are real world measurement (over time) of data from multiple domains, fusion of data from different sources, decision support (that improves over time as data from more patients are added), and treatment support. Recent technological advances are bringing this closer to reality. Although exciting, it cannot be assumed that this more complex perspective will be more effective than simpler forms of care, that will need to be tested, as will the cost effectiveness of the approach.
Acknowledgments
Funding: PWH was supported by a fellowship from the National Health and Medical Research Council of Australia (APP1194937).
Notes
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Provenance and Peer Review: This article was commissioned by Guest Editors (Ralph J. Mobbs, Pragadesh Natarajan and R. Dineth Fonseka) for the series “Objective Monitoring and Wearable Technologies including Sensor-Based Accelerometers and Mobile Health Applications for the Spine Patient” published in Journal of Spine Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-21-112/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-21-112/coif). The series “Objective Monitoring and Wearable Technologies including Sensor-Based Accelerometers and Mobile Health Applications for the Spine Patient” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
Articles from Journal of Spine Surgery are provided here courtesy of OSS Press
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.21037/jss-21-112
Article citations
Assessing the Reliability and Validity of Inertial Measurement Units to Measure Three-Dimensional Spine and Hip Kinematics During Clinical Movement Tasks.
Sensors (Basel), 24(20):6580, 12 Oct 2024
Cited by: 0 articles | PMID: 39460062 | PMCID: PMC11511509
Preliminary Validity and Acceptability of Motion Tape for Measuring Low Back Movement: Mixed Methods Study.
JMIR Rehabil Assist Technol, 11:e57953, 02 Aug 2024
Cited by: 0 articles | PMID: 39093610 | PMCID: PMC11329853
Lumbo-Pelvic Rhythm Monitoring Using Wearable Technology with Sensory Biofeedback: A Systematic Review.
Healthcare (Basel), 12(7):758, 30 Mar 2024
Cited by: 1 article | PMID: 38610180 | PMCID: PMC11012179
Review Free full text in Europe PMC
Identifying Clinical Phenotypes in People Who Are Hispanic/Latino With Chronic Low Back Pain: Use of Sensor-Based Measures of Posture and Movement, Pain, and Psychological Factors.
Phys Ther, 104(2):pzad185, 01 Feb 2024
Cited by: 0 articles | PMID: 38169435
Concurrent Validity of the Ergotex Device for Measuring Low Back Posture.
Bioengineering (Basel), 11(1):98, 20 Jan 2024
Cited by: 2 articles | PMID: 38275578 | PMCID: PMC10812927
Go to all (7) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The future of Cochrane Neonatal.
Early Hum Dev, 150:105191, 12 Sep 2020
Cited by: 5 articles | PMID: 33036834
Achievements and Challenges for Real-Time Sensing of Analytes in Sweat within Wearable Platforms.
Acc Chem Res, 52(2):297-306, 28 Jan 2019
Cited by: 38 articles | PMID: 30688433
Review
A Promising Wearable Solution for the Practical and Accurate Monitoring of Low Back Loading in Manual Material Handling.
Sensors (Basel), 21(2):E340, 06 Jan 2021
Cited by: 15 articles | PMID: 33419101 | PMCID: PMC7825414
Current Advances in Wearable Devices and Their Sensors in Patients With Depression.
Front Psychiatry, 12:672347, 17 Jun 2021
Cited by: 23 articles | PMID: 34220580 | PMCID: PMC8245757
Review Free full text in Europe PMC
 and
and 


