Abstract
Background and objectives
In recent years, immune-checkpoint inhibitors (ICIs) particularly atezolizumab is on the rise in treating advanced malignancies. With its increased clinical use, various electrolyte abnormalities have been reported in the literature. In this review, we have addressed the question of significant electrolyte abnormalities associated with atezolizumab.Materials and methods
Following PRISMA guidelines, we performed a thorough literature search in four databases including PubMed, Cochrane Library, Embase, and Clinicaltrials.gov. We included only randomized controlled trials from 2010 till March 2021. After a comprehensive screening of 1587 articles, we selected 14 articles for our review and tabulated the results. Following MeSH terms were used: "electrolyte abnormalities", "immune checkpoint inhibitors", "atezolizumab".Results
Non-small cell lung cancer (n = 1270) and metastatic urothelial carcinoma (n = 1164) were the most common malignancies among 3160 patients. The most common electrolyte abnormality was hypomagnesemia (4.7%). Hyponatremia, hypophosphatemia, hypercalcemia and hypokalemia were found in 2.3%, 0.63%, 0.25% and 0.06% patients respectively. For patients taking atezolizumab, hypomagnesemia was most frequently found in non-small cell lung carcinoma patients (9.4%), while urothelial metastatic carcinoma patients most commonly had hyponatremia (5.15%). Hypokalemia though insignificant was observed only in patients with metastatic renal cell carcinoma (2.85%).Conclusion
Since the use of atezolizumab is on the rise for the treatment of various cancers, more studies need to be conducted to better understand its safety and toxicity profile.Free full text

Electrolyte Abnormalities Associated With the Use of Atezolizumab – A Systematic Review
Abstract
Background and objectives
In recent years, immune-checkpoint inhibitors (ICIs) particularly atezolizumab is on the rise in treating advanced malignancies. With its increased clinical use, various electrolyte abnormalities have been reported in the literature. In this review, we have addressed the question of significant electrolyte abnormalities associated with atezolizumab.
Materials and methods
Following PRISMA guidelines, we performed a thorough literature search in four databases including PubMed, Cochrane Library, Embase, and Clinicaltrials.gov. We included only randomized controlled trials from 2010 till March 2021. After a comprehensive screening of 1587 articles, we selected 14 articles for our review and tabulated the results. Following MeSH terms were used: “electrolyte abnormalities”, “immune checkpoint inhibitors”, “atezolizumab”.
Results
Non-small cell lung cancer (n = 1270) and metastatic urothelial carcinoma (n = 1164) were the most common malignancies among 3160 patients. The most common electrolyte abnormality was hypomagnesemia (4.7%). Hyponatremia, hypophosphatemia, hypercalcemia and hypokalemia were found in 2.3%, 0.63%, 0.25% and 0.06% patients respectively. For patients taking atezolizumab, hypomagnesemia was most frequently found in non-small cell lung carcinoma patients (9.4%), while urothelial metastatic carcinoma patients most commonly had hyponatremia (5.15%). Hypokalemia though insignificant was observed only in patients with metastatic renal cell carcinoma (2.85%).
Conclusion
Since the use of atezolizumab is on the rise for the treatment of various cancers, more studies need to be conducted to better understand its safety and toxicity profile.
1. Introduction
In recent times, immunotherapy has emerged as one of the most influential breakthroughs in cancer treatment. Immune checkpoint inhibitors (ICIs) have become a good right arm of this type of therapy. Although results have been very impressive, they are not without significant adverse effects.1 Most common adverse effects involve colon, liver, lungs, pituitary, thyroid, and skin, although uncommon events involving the heart, nervous system, and other organs also occur.2 Colitis, pneumonitis, hepatitis, and myocarditis are drug-related and arise early during the treatment and these differ with the drug regimen.3 Ocular side effects are seen in 1% of the patients and include dry eyes, uveitis, and myasthenia gravis.4
Electrolyte abnormalities are frequently seen in hospitalized patients. Cancer patients, in particular, are at risk of developing significant electrolyte disturbances due to several etiologies including paraneoplastic syndromes, iatrogenic, poor nutrition, and treatment itself. Hyponatremia, hypernatremia, hypocalcemia, hypercalcemia, and potassium disorders are the major classes.5 The most common electrolyte disturbance overall is hyponatremia. Major groups with the highest prevalence of hyponatremia are patients with prostate cancer (57.1%), pancreatic neoplasms (50%), hepatocellular carcinoma (HCC) (49%), and carcinomas of the lung (40.2%).6 It is an indicator of poor prognosis and increased duration of stay at the hospital.7 It is particularly associated with increased mortality in patients with cancer.6
After hyponatremia, the most common electrolyte disturbances studied are hypernatremia, hypocalcemia, and hypercalcemia.5 Cancer patients are particularly at risk of nephrogenic diabetes insipidus (DI) due to electrolyte disturbances, antibiotics, and chemotherapy drugs.5 Hypocalcemia, although not frequent in cancer patients, is associated with higher mortality in patients with sepsis.8 Hypercalcemia is associated with a large number of cancers including humoral hypercalcemia of malignancy (mediated by Parathyroid Hormone-related Peptide), squamous cell carcinomas, renal cell carcinoma, bladder carcinoma, breast carcinoma, ovarian carcinoma, pheochromocytoma, and lymphomas/leukemias.5 Clinical findings include abdominal pain, nephrolithiasis, depression, psychosis, osteitis fibrosa cystica. Potassium disturbances manifest as muscle weakness and/ or arrhythmias.9 Electrolyte and acid-base abnormalities are the results of metastasis, anticancer therapy, or, a paraneoplastic process seen in many different cancers serving as a poor prognostic indicator.10
Atezolizumab has been approved for the treatment of triple-negative breast cancer (TNBC),11 extensive-stage small-cell lung cancer,12 and advanced bladder cancer.13 It has also shown improved survival in patients with hepatocellular carcinoma (HCC)14 and recently FDA has approved it to be used in a subset of colorectal carcinoma patients.15 Atezolizumab, in combination with chemotherapy, has been successful in achieving the milestone of being the first drug to improve survival in patients with newly diagnosed extensive stage-small-cell lung carcinoma.16 It improved median overall survival when compared with docetaxel (median OS was 13.8 months vs. 9.6 months); in patients with previously treated triple-negative breast cancer17 and is generally well-tolerated in this set of patients.18 Since the use of atezolizumab is on the rise and very few studies have been done so far to look for the associated electrolyte abnormalities, we tried to pinpoint these specific side effects of this immune checkpoint inhibitor to assess the tolerability of atezolizumab.
2. Materials and methods
2.1. Literature search
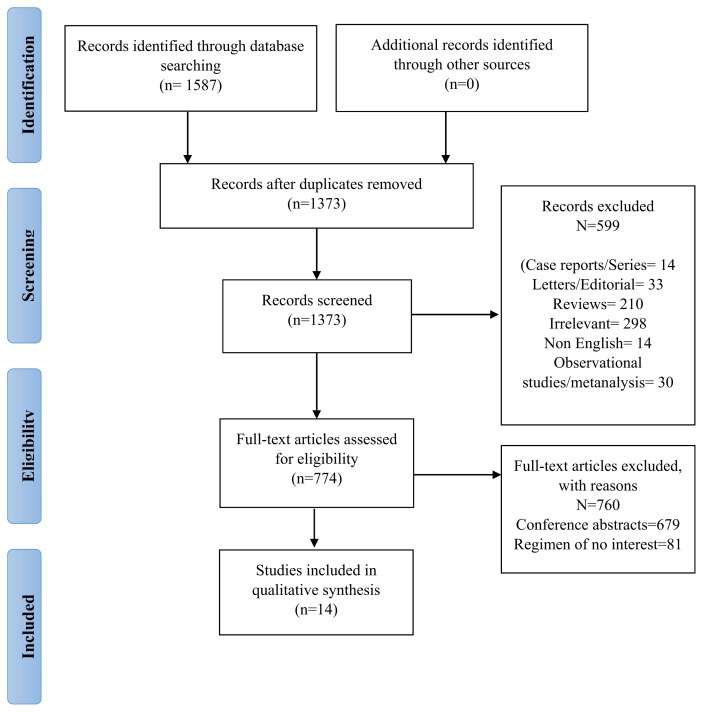
A comprehensive literature search was performed, following the PRISMA guideline (Fig. 1). Relevant studies were looked for in databases including PubMed, Cochrane Library, Embase, and Clinicaltrials.gov by October 15, 2020. No filters of geography, date, or language were applied. We included the following MeSH terms: “electrolyte abnormalities”, “checkpoint inhibitors” and “atezolizumab” in our search.
2.2. Eligibility criteria
We included articles from 2010 onwards and RCTs only. The included articles contained information regarding electrolyte abnormalities of atezolizumab and the classification of these adverse effects in them. We excluded the studies reporting combined results, child studies, meta-analysis, or systematic reviewes.
2.3. Study selection
The overall literature search identified 1587 articles for review. The number was cut short to 1373 after removing duplicate references by screening titles and abstracts of the articles. We excluded 599 articles in our first screening due to the following reasons: case reports/series = 14, letters/editorial = 33, reviews = 210, irrelevant 298, non-English = 14, observational studies/metanalysis = 30. After that 774 full-text articles were assessed for eligibility. We included 14 articles for qualitative synthesis after removing 760 articles because of the following: Conference Abstracts = 679 and Regime of no interest = 81. We did not include any study for quantitative synthesis.
2.4. Data extraction
We used an excel sheet for data extraction, including the following: author, publication year, study design, study duration (months), number of patients, median age, sex, the median duration of follow up (months), patients received atezolizumab, control arm (if any), prior therapy, the median duration of treatment (weeks), type of malignancy, electrolyte abnormality, and hypothyroidism.
3. Results
In this systematic review we aimed to explore toxicity profile; immune-related toxicities and electrolyte abnormalities (ED) related to the use of atezolizumab, a monoclonal antibody, anti–PD-L1/PD-1 agent across the range of cancers, which could have profound impacts on the standard of care. This review summarizes phase I/II/III trials demonstrating the tolerability of atezolizumab, a total of 1587 relevant studies were searched and 14 randomized clinical trials were recruited after excluding 1573 irrelevant studies (Table 1). The most frequent treatment-related abnormalities analyzed in these 14 trials were 6 all-grades hyponatremia, 5 all-grades hypophosphatemia, and 4 all-grades hypomagnesemia in 3 most relevant cancer non-small cell lung cancer, urothelial carcinoma, and squamous cell lung carcinoma (Table 2). Data for other electrolyte disorders hypokalemia, hypercalcemia, and hypernatremia was not significant to mention.
Table 1
Baseline study characteristics.
| Author, year, phase | N | Median age (years) | Malignancy | Median duration of treatment (months) | Follow-up (months) | Prior therapy |
|---|---|---|---|---|---|---|
| McDermott et al. 2016; Ia | 70 | 61 (33–81) | Metastatic RCC | 8 (1–35) | N/A | N/A |
| Petrylak et al. 2018; I | 95 | 66 (36–89) | Metastatic UC | 3 (0–44) | 37.8 (0.7–44.4) | Platinum based chemotherapy |
| Schmid et al. 2019; III | 451 | 55 (46–64) | Metastatic TNBC | N/A | 18.5 (9.6–22.8) | N/A |
| Balar et al. 2017; II | 119 | 73 (51–92) | UC | 15 weeks (0–102) | 17·2 (0·2–23·5) | Radiotherapy, Perioperative chemotherapy |
| Colevas et al. 2018; Ia | 32 | 62 (32–78) | H&N carcinoma | 3.4 (0–30.5) | N/A | N/A |
| Emens et al. 2019; I | 116 | 53 (29–82) | Metastatic TNBC | 2.1 (0–45.6) | 25.3 (0.4–45.6) | Anthracyclines, Taxanes, Bevacizumab, Platinum based chemotherapy |
| Grau et al. 2019; III | N/A | N/A | CC | N/A | N/A | N/A |
| Cathmos et al. 2020; II/III | N/A | 70 (43–81) | UC | 3.6 | 17.3 (15.4–18.1) | Carpoplatin, Cisplatin, etoposide |
| Sternberg et al. 2019; III3b | 997 | 68 | UC 950, SCC 18, Glandular 8, BCD 8, NE 7, N/A 6 | 2.8 | 12.7 (0–19.7) | Gemcitabine, Cisplatin, carboplatin |
| Liu et al. 2018; Ib | 76 | 65 (40–83) | Advanced NSCLC | 6.2 (1–37) and 9.2 (0–26) | 7.2 (1.6–37.6) | N/A |
| Sullivan et al. 2019; Ib | 57 | N/A | Melanomab | N/A | 51.8 (2.8–70.9) and 29.9 (3.3–42.0) | None |
| West et al. 2019; III | 473 | 64 | Stage IV non-squamous NSCLC | N/A | 18.5 (15.2–23.6) | None |
| Lin et al. 2020; II | 40 | 67 (50–83) | Unresectable NSCLC | N/A | 22.5 | N/A |
| Jotte et al. 2020; III | 681a | 65 (23–86) | Stage IV squamous NSCLC | 5.5 (0–37) and 6 (0–37) | 26.8 | N/A |
Abbreviations: N/A: Not available, N: patients who received atezolizumab, RCC: renal cell carcinoma, UC: urothelial carcinoma, TNBC: triple negative breast carcinoma, H&N: head and neck, CC: carcinoma of cervix, SCC: squamous cell carcinoma, NE: neuroendocrine, BCD: Bellini collecting duct carcinoma, NSCLC: non-small cell lung cancer.
Table 2
Electrolyte abnormalities reported in included studies.
| Author | Electrolyte abnormality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Hypophosphatemia | Hypomagnesemia | Hypokalemia | Hyponatremia | Hypercalcemia | ||||||
|
|
|
|
|
| ||||||
| Grade ≤ 2 n (%) | Grade ≥ 3 n (%) | Grade ≤ 2 n (%) | Grade ≥ 3 n (%) | Grade ≤ 2 n (%) | Grade ≥ 3 n (%) | Grade ≤ 2 n (%) | Grade ≥ 3 n (%) | Grade ≤ 2 n (%) | Grade ≥ 3 n (%) | |
| McDermott et al. | 2 (3) (any grade) | 2 (3) | N/A | N/A | 2 (3) | 0 | 2 (3) | 1 (1) | 3 (4) | 1 (1) |
| Petrylak et al. | 1 (3) | 0 | N/A | N/A | N/A | N/A | N/A | N/A | 1 (3) | 0 |
| Schmid et al. | N/A | N/A | 17 (4) | 8(2); 3(1) | N/A | N/A | N/A | N/A | N/A | N/A |
| Balar et al. | 3 (3) | 2 (2) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Colevas et al. | N/A | N/A | N/A | 1 (3); 0 | N/A | N/A | N/A | N/A | N/A | N/A |
| Emens et al. | N/A | N/A | N/A | N/A | N/A | N/A | 3 (3) | 1 (1) | N/A | N/A |
| Grau et al. | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Cathmos et al. | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 1 (4) | N/A | 1/25 (4) |
| Sternberg et al. | N/A | N/A | N/A | N/A | N/A | N/A | 37 (4) | 20(2); 2(0.2) | N/A | N/A |
| Liu et al. | N/A | 3 (4) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Sullivan et al. | N/A | 0 in cohort 1–3, 7/39 (17.9) in cohort 4/B/C | N/A | N/A | N/A | N/A | 2/11 (11.8) in cohort 1–3, 3/39 (7.7) in cohort 4/B/C | N/A | N/A | N/A |
| West et al. | N/A | N/A | 57 (12) | 4(1); 1(<1) | N/A | N/A | N/A | N/A | N/A | N/A |
| Lin et al. | N/A | N/A | N/A | N/A | N/A | N/A | 0 | 1 (10); 0 | 2 (20) | 0 |
| Jotte et al. | N/A | N/A | aACP 20 (6.0); aA + CnP 33 (9.9) | ACP 0; A + CnP 5 (1.5) | N/A | N/A | N/A | N/A | N/A | N/A |
McDermott et al. 2016 reported the use of atezolizumab treatment in metastatic renal cell carcinoma patients.19 This phase Ia study demonstrated the use of atezolizumab in 70 patients with a median age of 61 years (range 33–81). The patients received 16 cycles of atezolizumab for a median duration of 8 months (range 1–35). The incidence of grade three or higher (grade ≥3) electrolyte abnormalities were hypophosphatemia (3%), hyponatremia (1%), and hypercalcemia (1%). The incidence of grade 1–2 electrolyte abnormalities was hypercalcemia (4%), hypophosphatemia (3%), hypokalemia (3%), and hyponatremia (3%).
Petrylak et al. 2018 reported the use of atezolizumab treatment in metastatic urothelial carcinoma patients.20 This phase 1 study demonstrated the use of atezolizumab in 95 patients with a median age of 66 years (range 36–89). The patients received 16 cycles of atezolizumab for a median duration of 3 months (range 0–44). The patients were followed up for a median duration of 37.8 months (range >0.7–44.4). No incidence of grade three or higher (grade ≥3) electrolyte abnormalities was reported. The incidence of grade 1–2 electrolyte abnormalities was hypercalcemia (3%) and hypophosphatemia (3%).
Schmid et al. 2019 reported the use of atezolizumab treatment in metastatic triple-negative breast cancer patients.21 This phase 3 study demonstrated the use of atezolizumab in 451 patients with a median age of 55 years (range 46–64). The patients were followed up for a median duration of 18.5 months (range >9.6–22.8). The incidence of grade three or higher (grade ≥3) electrolyte abnormalities were hypomagnesemia (3%). The incidence of grade 1–2 electrolyte abnormalities was hypomagnesemia (4%).
Balar et al. 2017 reported the use of atezolizumab treatment in urothelial cancer patients. This phase 2 study demonstrated the use of atezolizumab in 119 patients with a median age of 73 years (range 51–92).22 The patients received atezolizumab for a median duration of 15 weeks (range 1–35). The patients were followed up for a median duration of 17.2 months (range 0.2–23.5). The incidence of grade three or higher (grade ≥3) electrolyte abnormalities was hypophosphatemia (3%). The incidence of grade 1–2 hypophosphatemia was in 3% of participants.
Colevas et al. 2018 reported the use of atezolizumab treatment in head and neck cancer patients.23 This phase 1a study demonstrated the use of atezolizumab in 32 patients with a median age of 62 years (range 32–78). The patients received 16 cycles of atezolizumab for a median duration of 3.4 months (range 0–30.5). Grade three or higher (grade ≥3) hypomagnesemia was reported in 3% of the patients.
Emens et al. 2019 reported the use of atezolizumab treatment in metastatic triple-negative breast cancer patients.18 This phase 1 study demonstrated the use of atezolizumab in 116 patients with a median age of 53 years (range 29–89). The patients received 16 cycles of atezolizumab for a median duration of 2.1 months (range 0–45.6). The patients were followed up for a median duration of 25.3 months (range 0.4–45.6). The incidence of grade three or higher (grade ≥3) electrolyte abnormalities was hyponatremia (1%). The incidence of grade 1–2 hyponatremia was 3%.
Grau et al. 2019 reported the use of atezolizumab treatment in carcinoma of the cervix patients in a phase 3 study.24 No incidence of grade three or higher (grade ≥3) or grade 1–2 electrolyte abnormalities were reported.
Cathmos et al. 2020 reported the use of atezolizumab treatment in urothelial carcinoma patients.25 This phase 2/3 study demonstrated the use of atezolizumab in patients with a median age of 70 years (range 43–81). The patients received 6 cycles (range 1–27) of atezolizumab for a median duration of 3.6 months. The patients were followed up for a median duration of 17.3 months (95% CI 15.4–18.1). The incidence of grade three or higher (grade ≥3) electrolyte abnormalities was hyponatremia (4%) and hypercalcemia (4%).
Sternberg et al. 2019 reported the use of atezolizumab treatment in urothelial, squamous cell carcinoma, glandular, bellini collecting duct, neuroendocrine cancer patients.26 This phase 3b study demonstrated the use of atezolizumab in 997 patients with a median age of 68 years. The patients received atezolizumab for a median duration of 2.8 months and were followed up for a median duration of 12.7 months (range 0–19.7). The incidence of grade three or higher (grade ≥3) electrolyte abnormalities was hyponatremia (2.2%). The incidence of grade 1–2 electrolyte abnormalities was hyponatremia (4%).
Liu et al. 2018 reported the use of atezolizumab treatment in non-small-cell lung cancer patients.27 This phase 1b study demonstrated the use of atezolizumab in 76 patients with a median age of 65 years (range 40–83). The patients received 4–6 cycles of atezolizumab for a median duration of 6.2 (1–37) and 9.2 (0–26). The patients were followed up for a median duration of 7.2 months (1.6–37.6). The incidence of grade three or higher (grade ≥3) electrolyte abnormalities was hypophosphatemia (4%).
Sullivan et al. 2019 reported the use of atezolizumab treatment in melanoma patients.28 This phase 1b study demonstrated the use of atezolizumab in 57 patients. The patients were followed up for a median duration of 51.8 months (range 2.8–70.9) and 29.9 months (range 3.3–42.0). The incidence of grade three or higher (grade ≥3) electrolyte abnormalities was hypophosphatemia (17.9%). The incidence of grade 1–2 hyponatremia was 19.5%.
West et al. 2019 reported the use of atezolizumab treatment in non-squamous non-small-cell lung cancer patients.29 This phase 3 study demonstrated the use of atezolizumab in 473 patients with a median age of 64 years. The patients were followed up for a median duration of 18.5 months (range 15.2–23.6). The incidence of grade three or higher (grade ≥3) hypomagnesemia was 1%. The incidence of grade 1–2 hypomagnesemia was 12%.
Lin et al. 2020 reported the use of atezolizumab treatment in non-small cell lung cancer patients.30 This phase 2 study demonstrated the use of atezolizumab in 40 patients with a median age of 67 years (range 50–83). The patients received 2 cycles of atezolizumab and were followed up for a median duration of 22.5 months. The incidence of grade three or higher (grade ≥3) electrolyte abnormalities was hyponatremia (10%). The incidence of grade 1–2 hypercalcemia was 20%.
Jotte et al. 2020 reported the use of atezolizumab treatment in squamous non-small-cell lung cancer patients.31 This phase 3 study demonstrated the use of atezolizumab in 681 patients with a median age of 65 years (range 23–86). The patients received 4–6 cycles of atezolizumab in combination with chemotherapy for a median duration of 5.5 months (range 0–37) and 6 months (range 0–37). The patients were followed up for a median duration of 26.8 months. The incidence of grade three or higher (grade ≥3) electrolyte abnormalities was hypomagnesemia (1.5%). The incidence of grade 1–2 hypomagnesemia was 15.9%.
4. Discussion
Over the past decade, immune checkpoint inhibitors targeting programmed cell death protein 1 (PD-1) or its ligand (PD-L1) have been approved as potential first-line therapy for the treatment of a variety of cancers in association with chemotherapy and other antineoplastic agents.32–35 Although atezolizumab has been well established as emerging antineoplastic therapy that prolongs progression-free survival and overall response rates,33,34 the encountered adverse events with its use seem to affect the outcomes and underlines the need for careful consideration in its administration with complex antitumor drug combinations. 34 As per the United States Food and Drug Administration (FDA) approved data in advanced urothelial carcinoma and metastatic non–small cell lung cancer (mNSCLC), the toxicity related to atezolizumab is mainly immune-mediated adverse events (imAE), electrolyte disturbances, infections, and systemic toxicity resulting in dose attenuation, treatment discontinuation and death.35 The reported incidence of common imAE includes hypersensitivity/infusion reaction, hypothyroidism/thyroiditis, colitis, and pneumonitis requiring management with high dose corticosteroids (1–2mg/kg prednisolone) followed by tapering and discontinuation of atezolizumab therapy. 36 Mortality is attributed to infections including sepsis, pneumonitis, and cardiac arrest, myocardial infarction, and respiratory failure. The common causes of treatment discontinuation were fatigue, infections (sepsis, colitis/diarrhea, cellulitis, and osteomyelitis), and dyspnea. Laboratory abnormalities were reported in 2% of patients with the most frequent being hyponatremia, hypophosphatemia, hyperkalemia, and hypomagnesemia.35,37 This systematic review assesses the correlation between the use of atezolizumab and electrolyte abnormalities across the set of malignancies in which it is used with or without chemotherapy. We observed that atezolizumab use is associated with a significantly higher incidence of hyponatremia, hypophosphatemia, and hypomagnesemia.
Most treatment-related electrolyte disorders were of grade 1 or 2 and were manageable. Hyponatremia and hypomagnesemia was more frequent compared to other electrolyte disorders. Data on other electrolyte abnormalities showed no significant difference in the risk of developing treatment-related hypokalemia, hypercalcemia, and hypernatremia.
4.1. Hypomagnesemia
West et al. demonstrated the highest incidence of treatment-related grade 1 or 2 hypomagnesemia (12%) in stage IV non-squamous NSCLC. Schmid et al. study reported 4% grade 1–2, 2% grade 3, and 1% grade 4 hypomagnesemia in metastatic triple-negative breast cancer. Colevas et al. mentioned 3% of grade 1–2 in Head and neck cancer, West et al. showed 12% grade 1–2, 1% grade 3 in Stage IV non-squamous non-small-cell lung cancer, Jotte et al. observed 6% and 9.9% grade 1–2 hypomagnesemia in atezolizumab plus carboplatin and paclitaxel and atezolizumab + carboplatin and nab-paclitaxel (A + CnP)nP group respectively while 1.5% grade 3–4 hypomagnesemia is seen in A + CnP group only in Stage IV Squamous Non-Small-Cell Lung Cancer patients. The main underlying mechanism leading to atezolizumab associated hypomagnesemia is uncertain but it has been proposed that autoimmune kidney injury or gastroenterology losses can contribute to these electrolyte disorders38,39 and concomitant combination therapy can have more frequent involvement. It has been reported that, without preventive measures, reduce magnesium levels can lead to acute or chronic complications including tetany, seizures, paralysis arrhythmia, neurotoxicity, blindness, and cardiovascular disease,40,41 hence need prompt correction.
4.2. Hyponatremia
Hyponatremia is one of the most commonly reported electrolyte disorders with atezolizumab administration.42 Subsequent/Previous studies and clinical trials also linked the association of hyponatremia with atezolizumab therapy as one of the most common laboratory abnormalities.42–45 One of the proposed mechanism by which PD-1 inhibitors leads to hyponatremia includes low levels of adrenocorticotropic hormone (ACTH) secondary to immune-mediated hypophysitis or primary adrenal insufficiency. 46,47 Hyponatremia serves as an important negative prognostic factor for outcome in cancer patients, determines the response to treatment, and overall-mortality in several different malignancies.48 It has been associated with prolonged hospitalization, poor quality of life, and delays in anti-neoplastic therapy administration.7 Hyponatremia caused by anti-PD-1 therapy is noticed to be associated with poor prognostic outcomes49 and correction of serum sodium level leads to substantial improvement and overall survival.50 In our included studies Sullivan et al. and Lin et al. reported the highest incidence of grade 1 or 2 hyponatremia in melanoma (11.8%) and aNSCLC (10%) patients respectively who received atezolizumab while no grade 3 or above events were reported.
4.3. Hypophosphatemia
The product review of atezolizumab in different malignancies showed that the toxicity related to the use of atezolizumab is considered manageable with supportive measures and dose interruption, optimizing the balance between efficacy and safety of this drug. Although the data on electrolyte disorders is rather short and immature, and only a few studies were seen to report the grade-3 hypophosphatemia with atezolizumab.51,52 Hypophosphatemia was the second most commonly reported treatment-related electrolyte abnormality in patients in our included literature who were treated with atezolizumab. Sullivan et al. described grade 3 or 4 hypophosphatemia (17.9%) in melanoma patients treated with atezolizumab + cobimetinib + vemurafenib consequently requiring hospitalization. Regardless of the multifactorial etiology of hypophosphatemia, low phosphorous levels have been associated with poor clinical outcomes as well.53 Prompt reversal is critical to prevent serious consequences like hemolytic anemia, muscle paralysis, and seizures.54 The mechanism through which atezolizumab leads to hypophosphatemia is yet unknown, however, renal injury or immune-mediated toxicity is postulated to contribute to hypophosphatemia with the use of various checkpoint inhibitors.39,55 Nevertheless, routine monitoring of renal function and electrolytes is recommended in these patients.
4.4. Hypokalemia
Hypokalemia with the use of atezolizumab is rare with only 2% grade ≤2 events reported in the literature (McDermott et al.). However, recently a case report of paralysis due to hypokalemia in a patient receiving an immune checkpoint inhibitor has been published.56 The precise mechanism remains unclear but routine laboratory values also included deranged renal functions. Therefore potential etiology of underlying acute kidney injury due to immune checkpoint inhibitor was proposed. Discontinuation of the drug and treatment with steroids and intravenous potassium bicarbonate successfully lead to the resolution of the paralysis.
4.5. Hypercalcemia
Although extremely rare, grade 1 or 2 hypercalcemia has been reported in Lin et al. in unresectable non-small cell lung cancer. Another phase II/III study reported 4% grade ≥3 or higher hypercalcemia in urothelial carcinoma patients treated with atezolizumab. The exact underlying etiology by which atezolizumab leads to hypercalcemia is not yet clear, however, several mechanisms have been proposed including endocrine dysfunction, sarcoid-like granulomatous inflammation, humoral hypercalcemia of malignancy seen with raised parathyroid hormone-related peptide (PTHrP) produced in excess and subsequently released by dying malignant cells, and/or pseudo-progressive disease phenomenon where the tumor initially seems to be expanding before finally shrinking.57 In general, the incidence of hypercalcemia is reported in malignancies much more commonly due to paraneoplastic syndrome (PTHrP induced), metastatic lesions, or excessive vitamin D production from tumor cells, however, raised calcium levels when the cancer is in remission and temporal relation with starting immune checkpoint inhibitors favors the diagnosis of treatment-related hypercalcemia. Prompt diagnosis is crucial and correction is recommended in symptomatic patients or when serum calcium >15 mg/dL.57
Standalone electrolyte abnormalities are rare and mostly occur in the background of immune-mediated adverse events including adrenal insufficiency, thyroid dysfunction, and hypophysitis. In the event of electrolyte disturbance, a workup for immune-mediated adverse events should be pursued promptly.58 A meta-analysis by Cantini et al. (2020) concluded that an increased risk of electrolyte abnormalities particularly all-grade hyponatremia and hypokalemia correlates with the use of immune checkpoint inhibitors in general when compared to chemotherapy alone, however, separate data on atezolizumab was not available.59 The latest American Society of Clinical Oncology clinical practice guidelines does not explicitly address the management of electrolyte abnormalities occurring with the use of immune check-point inhibitors.58 Discontinuation of therapy should only be considered in severe or refractory cases.
The limitations to our study that may affect our interpretation of the findings are pertinent to mention here. Firstly, the data used is from individual studies rather than individual patients due to which the accurate assessment is limited. Secondly, the trials did not report a uniform definition of electrolyte disturbance and their grades of severity which could lead to a misleading conclusion. Thirdly, the overlap of undiagnosed immune-mediated adverse events may lead to a false sense of increased standalone electrolyte abnormalities events. Moreover, the possible synergistic effect on electrolyte disturbance from other chemotherapeutic drugs used in combination with atezolizumab cannot be effectively ruled out.
5. Conclusion
In conclusion, atezolizumab emerged as a potential therapeutic choice and the benefit–risk profile can be optimized with toxicity surveillance and infection prophylaxis. Severe electrolyte-related adverse events including hyponatremia, hypophosphatemia, and hypomagnesemia remain rare and seem to be a relatively unknown complication associated with atezolizumab that was not reported by clinical trials. Since electrolyte abnormalities commonly occur in patients who are getting treatment for malignancies either due to treatment related adverse effects or the malignancy itself, caution should be exercised by the oncologists with the routine observation of these electrolytes level and prompt intervention to prevent serious complications and achieve a swift recovery that may require ICU admissions.
Footnotes
Authorship statement
AR, SA and AK designed the study. All authors performed the study, contributed to data extraction, analyzed the data, wrote the paper, and approved the manuscript.
Conflict of interest
Authors declare that there is no conflict of interest with this manuscript. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Financial disclosure statement
This work did not receive any financial support.
References
Articles from Journal of Community Hospital Internal Medicine Perspectives are provided here courtesy of Greater Baltimore Medical Center
Full text links
Read article at publisher's site: https://doi.org/10.55729/2000-9666.1037
Read article for free, from open access legal sources, via Unpaywall:
https://scholarlycommons.gbmc.org/cgi/viewcontent.cgi?article=1037&context=jchimp
Citations & impact
Impact metrics
Article citations
Exploring potential roles of long non-coding RNAs in cancer immunotherapy: a comprehensive review.
Front Immunol, 15:1446937, 27 Aug 2024
Cited by: 0 articles | PMID: 39257589 | PMCID: PMC11384988
Review Free full text in Europe PMC
Severe Hyponatremia Triggered by Immune Checkpoint Inhibitor Therapy in a Patient With Mulvihill-Smith Syndrome.
AACE Clin Case Rep, 10(3):105-108, 13 Mar 2024
Cited by: 0 articles | PMID: 38799049 | PMCID: PMC11127580
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Hyponatremia and other electrolyte abnormalities in patients receiving immune checkpoint inhibitors.
Nephrol Dial Transplant, 36(12):2241-2247, 01 Dec 2021
Cited by: 23 articles | PMID: 33374011 | PMCID: PMC8643613
The Differences in the Safety and Tolerability of Immune Checkpoint Inhibitors as Treatment for Non-Small Cell Lung Cancer and Melanoma: Network Meta-Analysis and Systematic Review.
Front Pharmacol, 10:1260, 24 Oct 2019
Cited by: 11 articles | PMID: 31708783 | PMCID: PMC6821878
Review Free full text in Europe PMC
Atezolizumab plus bevacizumab versus sorafenib or atezolizumab alone for unresectable hepatocellular carcinoma: A systematic review.
World J Gastrointest Oncol, 13(11):1813-1832, 01 Nov 2021
Cited by: 11 articles | PMID: 34853653 | PMCID: PMC8603457
A drug safety evaluation of atezolizumab in locally advanced or metastatic urothelial carcinoma.
Expert Opin Drug Saf, 19(8):955-960, 13 Jul 2020
Cited by: 3 articles | PMID: 32657630
Review

 a,*,1
a,*,1