Abstract
Background/objective
Immune checkpoint inhibitors (ICI), including Programmed Cell Death 1, Programmed Cell Death Ligand 1, and Cytotoxic T-lymphocyte Associated Antigen 4 inhibitors, upregulate T-cell responses against tumor cells and are becoming a cornerstone in the treatment of various advanced solid and hematological cancers. Mulvihill-Smith Syndrome (MSS) is a rare genetic syndrome that has been associated with metabolic abnormalities and early-onset tumors, including malignancies. We report the first known case of ICI-induced hyponatremia attributable to syndrome of inappropriate antidiuretic hormone ADH release (SIADH) in a patient with MSS.Case report
A 23-year-old female patient with MSS and hepatocellular carcinoma presented with recurrent hyponatremia. Assessment of fluid status and electrolytes revealed a euvolemic, hypotonic process consistent with SIADH shortly after initiating adjuvant therapy with atezolizumab, a Programmed Cell Death Ligand 1 inhibitor.Discussion
Endocrine etiologies for euvolemic hypotonic hyponatremia, including adrenal insufficiency and hypothyroidism, were excluded. The diagnosis of SIADH was confirmed based on electrolyte and osmolality studies. Sodium levels normalized with fluid restriction. Given the onset of hyponatremia 30 days after atezolizumab initiation, we posit that atezolizumab triggered severe hyponatremia due to SIADH.Conclusion
With the expanding utilization of ICIs, including in patients predisposed to malignancies such as MSS, vigilant monitoring for ICI-mediated electrolyte imbalances is crucial. Monitoring for hyponatremia and SIADH in the setting of ICI therapy is recommended.Free full text
Severe Hyponatremia Triggered by Immune Checkpoint Inhibitor Therapy in a Patient With Mulvihill-Smith Syndrome
Associated Data
Abstract
Background/Objective
Immune checkpoint inhibitors (ICI), including Programmed Cell Death 1, Programmed Cell Death Ligand 1, and Cytotoxic T-lymphocyte Associated Antigen 4 inhibitors, upregulate T-cell responses against tumor cells and are becoming a cornerstone in the treatment of various advanced solid and hematological cancers. Mulvihill-Smith Syndrome (MSS) is a rare genetic syndrome that has been associated with metabolic abnormalities and early-onset tumors, including malignancies. We report the first known case of ICI-induced hyponatremia attributable to syndrome of inappropriate antidiuretic hormone ADH release (SIADH) in a patient with MSS.
Case Report
A 23-year-old female patient with MSS and hepatocellular carcinoma presented with recurrent hyponatremia. Assessment of fluid status and electrolytes revealed a euvolemic, hypotonic process consistent with SIADH shortly after initiating adjuvant therapy with atezolizumab, a Programmed Cell Death Ligand 1 inhibitor.
Discussion
Endocrine etiologies for euvolemic hypotonic hyponatremia, including adrenal insufficiency and hypothyroidism, were excluded. The diagnosis of SIADH was confirmed based on electrolyte and osmolality studies. Sodium levels normalized with fluid restriction. Given the onset of hyponatremia 30 days after atezolizumab initiation, we posit that atezolizumab triggered severe hyponatremia due to SIADH.
Conclusion
With the expanding utilization of ICIs, including in patients predisposed to malignancies such as MSS, vigilant monitoring for ICI-mediated electrolyte imbalances is crucial. Monitoring for hyponatremia and SIADH in the setting of ICI therapy is recommended.
Introduction
Immune checkpoint inhibitors (ICI) have revolutionized cancer therapy and are increasingly utilized in the treatment of numerous solid and liquid malignancies.1 Cytotoxic T-lymphocyte Associated Antigen 4, Programmed Cell Death 1 (PD-1), and Programmed Cell Death Ligand 1 (PD-L1) inhibitors invigorate effective T-cell antitumor activity but have also been associated with immune-related adverse events (irAEs).1,2 Commonly described endocrine irAEs include thyroiditis and hypophysitis, while hyponatremia is the most frequently reported electrolyte abnormality.1,3,4
Mulvihill-Smith Syndrome (MSS) is a rare genetic disorder of which there are only 11 reported cases.5 In addition to the syndromic features of pinched facies, short stature, multiple pigmented nevi, developmental delay, hearing loss, and immune deficiencies, the syndrome is associated with early onset malignant tumors as well as endocrine and metabolic abnormalities including diabetes mellitus, hyperlipidemia, hypogonadism, and subcutaneous fat redistribution.5, 6, 7, 8, 9, 10 We present a case of syndrome of inappropriate antidiuretic hormone ADH release (SIADH) triggered by atezolizumab, a PD-1 inhibitor, in a patient with MSS undergoing treatment for hepatocellular carcinoma (HCC).
Case Report
A 23-year-old woman presented with asymptomatic severe hyponatremia to 116 mEq/L (reference range [RR] 135-145 mEq/L). Her medical history was significant for MSS, HCC, type 2 diabetes mellitus with insulin resistance, hyperlipidemia, primary hypothyroidism, spinal hemangioma, and thyroid nodules. Based on phenotypic criteria, the patient was clinically diagnosed with MSS in early childhood, a progeria-like syndrome without a yet identified causative gene.5,6 Subsequent chromosomal microarray, whole exon sequencing, and chromosomal breakage studies were unrevealing. At 19 years, she was diagnosed with HCC after a 14.8 cm liver mass biopsy and underwent a right hepatectomy and transarterial chemoembolization. She received PD-1 inhibitor immunotherapy with nivolumab in late 2019, tyrosine kinase inhibitor (TKI) therapy with lenvatinib in 2020, and cabozantinib in early 2021. Due to disease progression, she began concomitant treatment with atezolizumab (PD-L1 inhibitor) and bevacizumab (vascular endothelial growh factor inhibitor) in October 2021. In March 2022, she was transitioned to monotherapy with atezolizumab. Persistent hyponatremia was first noted 4 weeks after initiating atezolizumab infusions with sodium levels ranging between 126 and 134 mEq/L in November 2021 and March 2022.
The patient was first hospitalized in March 2022 with myoclonus and severe hyponatremia (118 mEq/L), which improved with fluid restriction. She was readmitted in July 2022 with severe hyponatremia 121 mEq/L (corrected 132 mEq/L), hyperglycemia (770 mg/dL), and acute kidney injury (creatinine 1.36 mg/dL). She was treated for volume depletion in the setting of hyperglycemia (without diabetic ketoacidosis or hyperosmolarity), and her insulin regimen was optimized with subsequent renal and sodium normalization. Ten days after discharge, she presented with recurrent asymptomatic severe hyponatremia of 116 mEq/L, leading to the hospitalization event that will be the focus of this discussion.
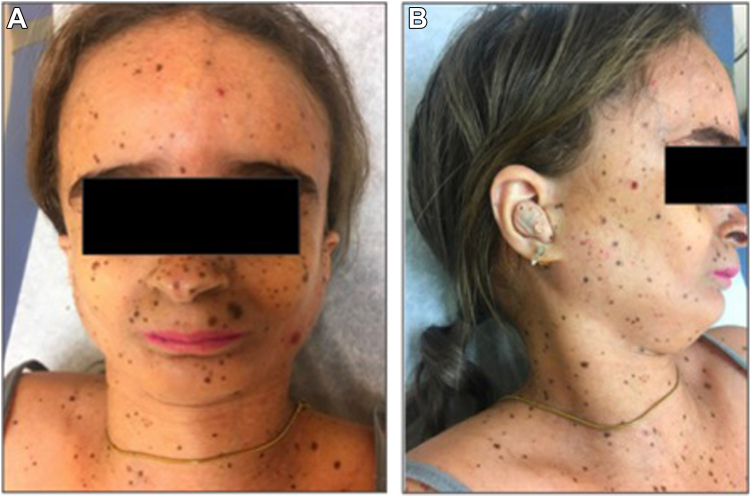
At presentation, she was afebrile, tachycardic (116 beats/min), and tachypneic (21 breaths/min). Examination revealed MSS syndromic features, including small stature (23.6 kg, BMI 15.9 kg/m2), microcephaly, bird-like facies, high-pitched voice, diffuse pigmented nevi, and loss of subcutaneous facial fat (Fig. 1). There was no ascites or peripheral edema. Laboratory evaluation revealed hyperglycemia of 200 mg/dL and moderate hypertriglyceridemia of 327 mg/dL with a corrected sodium of 118 mEq/L, excluding pseudohyponatremia. The serum osmolality of 275 mOsm/kg (RR 275-295) with a urine osmolality of 295 mOsm/kg and urine sodium of 61 mEq/L was consistent with euvolemic hypotonic hyponatremia. An 8 AM cortisol of 27.6 ug/dL ruled out adrenal insufficiency. A normal free T4 of 0.9 ng/dL (RR 0.6-1.5 ng/dL) and a suppressed thyroid stimulating hormone (TSH) of <0.05 μU/mL (RR 0.5-4.0 μU/mL) did not reveal hypothyroidism. The undetectable TSH was likely iatrogenic with a possible component of nonthyroidal illness. Post-ICI hypophysitis was considered but unlikely as the patient was free of headaches or vision complaints, had an intact hypothalamic-pituitary-adrenal axis, and maintained monthly menses. Furthermore, the patient’s dose of levothyroxine 88 mcg was significantly higher than her weight-based requirement, and after a dose reduction, subsequent TSHs have since normalized. Laboratory evaluation, along with her euvolemic status implicated SIADH as the most likely etiology of her hyponatremia. She was fluid-restricted to 1 L of water intake daily, after which sodium levels normalized within 2 weeks of hospital discharge. As of late July 2023, the atezolizumab was discontinued due to progression of HCC and recurrent episodes hyponatremia. The patient’s sodium levels have thus remained stable in the normal range since October 2023.
Discussion
MSS is a complex and rare disorder that is believed to be caused by a single gene mutation. Only 11 cases have thus been reported in the literature, and early onset of malignant tumors are described in 4 identified cases of MSS, suggesting an association with increased malignancy risk.5, 6, 7, 8, 9, 10
ICIs have markedly changed the clinical course of malignant cancers by inhibiting various immunologic pathways responsible for T-cell anergy.1,2 The binding of PD-L1 to PD-1 serves as a mechanism for tumors to evade an antigen-specific T-cell response. PD-1 and PD-L1 inhibitors disrupt this suppression and enhance endogenous antitumor immunity. ICIs have been linked to several endocrine irAEs and electrolyte disturbances.1,3 We report the first known case of severe hyponatremia secondary to atezolizumab in a patient with MSS.
Hyponatremia is the most frequently encountered electrolyte and fluid disturbance, affecting up to 30% of hospitalized patients.11 The first step in diagnosis begins with the categorization of the serum tonicity and the exclusion of pseudohyponatremia. Our patient was determined to have euvolemic hypotonic hyponatremia based on volume status, a low serum osmolality and an inappropriately high urine osmolality and sodium. Once true euvolemic hyponatremia is established, endocrinopathies including adrenal insufficiency, hypothyroidism, and SIADH must be investigated. Our patient’s robust cortisol and normal free T4 levels ruled out adrenal dysfunction and hypothyroidism, prompting further evaluation for an underlying etiology of SIADH.
Determining the etiology of our patient’s SIADH required consideration of her other medications including the antineoplastic agents used for HCC treatment. Between October 2019 and January 2020, the patient received nivolumab, a PD-1 inhibitor that has been associated with hyponatremia.3,11 One study found that severe hyponatremia typically occurs within 1 year of ICI initiation3; another reported onset as early as 9 days after initiation.11 As the patient’s first episode of severe hyponatremia in November 2021 was noted 22 months after the discontinuation of nivolumab, we concluded that nivolumab was an unlikely trigger for the recurrent hyponatremia. The patient also received lenvatinib therapy between February and August 2020, cabozantinib between November 2020 and May 2021, and bevacizumab between October 2021 and March 2022. Although there are no reported cases of SIADH induced by these specific antineoplastic agents, other TKIs and vascular endothelial growh factor inhibitors have been associated with hyponatremia.11 One report described the onset of SIADH within 1 week of initiating imatinib12 while another reported the onset of hyponatremia within 2 to 4 weeks after initiating a series of TKIs.13 Lenvatinib and cabozantinib are therefore unlikely to have caused the onset of SIADH several months after their discontinuation. Similarly, it is unlikely that bevacizumab triggered persistent, severe hyponatremia several months after its discontinuation.
The patient was taking no other medications known to be associated with hyponatremia or SIADH. The temporal onset of hyponatremia merely 30 days after starting atezolizumab and its persistent recurrence months after the discontinuation of bevacizumab strongly suggests an atezolizumab-induced etiology (Fig. 2). Whether MSS contributes to the risk or severity of ICI-induced hyponatremia is unclear.
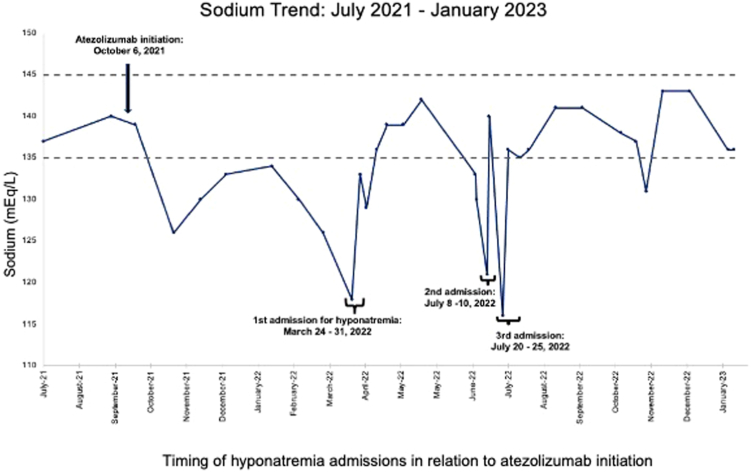
Timing of hyponatremia admissions in relation to atezolizumab initiation. Atezolizumab was initiated on 10/16/2021 with first hyponatremic episode documented on 11/5/2021. Severe hyponatremia first noted during March 2022 hospitalization with a recurrent hospitalization in July 2022 for a sodium of 116 mEq/L. Sodiums gradually stabilize with treatment after discharge.
While mild-moderate hyponatremia has been described as the most common ICI-induced electrolyte abnormality with 1 meta-analysis estimating a significant relative risk of 1.78, ICI-induced severe hyponatremia is rare.4 An observational study of 2458 patients reported that the overall incidence of hyponatremia among a cohort of patients on ICI therapy was 62% while severe hyponatremia (Na < 124 mEq/L) only occurred in 6%.3 Incidence rates for atezolizumab-triggered hyponatremia have generally ranged 3% to 4% for grades 1 to 2 (as defined by the National Cancer Institute’s common terminology criteria of adverse events) hyponatremia and 1% to 4% for grades 3 to 4 hyponatremia with higher rates noted for melanoma and nonsmall cell lung cancer.14 It has been proposed that the hyponatremia may be due to renal tubular dysfunction as PD-L1 is expressed on renal tubular epithelial cells and may play a role in ICI-induced kidney injury.15 SIADH was the most common cause of ICI-associated severe hyponatremia and was implicated in 36% of these cases.
Conclusion
We report the first known case of severe hyponatremia secondary to SIADH as an irAE in a patient with MSS being treated with ICI therapy, atezolizumab. MSS is a genetic syndrome that carries an inherent propensity for metabolic abnormalities and is associated with early-onset tumors including malignancies. Due to the rarity of MSS, there is currently inadequate clinical experience and data to conclude if MSS increases the risk and/or severity of hyponatremia. As the indications for ICIs continue to broaden, it is important for clinicians to be aware of its metabolic adverse effects and the need to monitor for hyponatremia and SIADH.
Author Contributions
TT, JS, PM, and HL contributed to the writing of the manuscript.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Disclosure
The authors have no multiplicity of interest to disclose.
Footnotes
T.T. and J.M.S. have contributed equally to this manuscript and share first authorship.
Informed Patient Consent for Publication: Signed informed consent obtained directly from the patient’s relatives or guardians.
References
Articles from AACE Clinical Case Reports are provided here courtesy of American Association of Clinical Endocrinology
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Hyponatremia in the postoperative craniofacial pediatric patient population: a connection to cerebral salt wasting syndrome and management of the disorder.
Plast Reconstr Surg, 108(6):1501-1508, 01 Nov 2001
Cited by: 28 articles | PMID: 11711918
Chronic idiopathic hyponatremia in an elderly patient due to inappropriate antidiuretic hormone secretion (SIADH) syndrome.
Hippokratia, 23(1):42-44, 01 Jan 2019
Cited by: 1 article | PMID: 32256039 | PMCID: PMC7124873
Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH) Associated with Mediastinal Schwannoma.
Electrolyte Blood Press, 15(2):42-46, 31 Dec 2017
Cited by: 3 articles | PMID: 29399023 | PMCID: PMC5788814
Management of euvolemic hyponatremia attributed to SIADH in the hospital setting.
Minerva Endocrinol, 39(1):33-41, 01 Mar 2014
Cited by: 9 articles | PMID: 24513602
Review