Abstract
Free full text

Neutralizing Antibody Correlates of Sequence Specific Dengue Disease in a Tetravalent Dengue Vaccine Efficacy Trial in Asia
Abstract
In the CYD14 trial of the CYD-TDV dengue vaccine in 2–14 year-olds, neutralizing antibody (nAb) titers to the vaccine-insert dengue strains correlated inversely with symptomatic, virologically-confirmed dengue (VCD). Also, vaccine efficacy against VCD was higher against dengue prM/E amino acid sequences closer to the vaccine inserts. We integrated the nAb and sequence data types by assessing nAb titers as a correlate of sequence-specific VCD separately in the vaccine arm and in the placebo arm. In both vaccine and placebo recipients the correlation of nAb titer with sequence-specific VCD was stronger for dengue nAb contact site sequences closer to the vaccine (p=0.005 and p=0.012, respectively). The risk of VCD in vaccine (placebo) recipients was 6.7- (1.80)-fold lower at the 90th vs. 10th percentile of nAb for viruses perfectly matched to CYD-TDV, compared to 2.1- (0.78)-fold lower at the 90th vs. 10th percentile for viruses with five amino acid mismatches. The evidence for a stronger sequence-distance dependent correlate of risk for the vaccine arm indicates departure from the Prentice criteria for a valid sequence-distance specific surrogate endpoint and suggests that the nAb marker may affect dengue risk differently depending on whether nAbs arise from infection or also by vaccination. However, when restricting to baseline-seropositive 9–14 year-olds, the correlation pattern became more similar between the vaccine and placebo arms, supporting nAb titers as an approximate surrogate endpoint in this population. No sequence-specific nAb titer correlates of VCD were seen in baseline-seronegative participants. Integrated immune response/pathogen sequence data correlates analyses could help increase knowledge of correlates of risk and surrogate endpoints for other vaccines against genetically diverse pathogens.
Trial registration:
EU Clinical Trials Register 2014-001708-24; registration date 2014-05-26
Introduction
The four dengue virus serotypes (DENV-1, −2, −3, and −4) share ~80% amino acid sequence homology [1] and co-circulate in tropical/subtropical regions [2]. The clinical spectrum of DENV infection includes asymptomatic infection, dengue fever, and dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) [3], with dengue illness posing substantial health care and economic [4] burdens worldwide. While infection with one serotype confers long-term immunity to that same serotype, individuals remain vulnerable to infection with the other serotypes [5]. Moreover, heterotypic secondary DENV infection is a strong risk factor for DHF/DSS [6, 7], illustrating the complexity of dengue immunity.
The CYD-TDV live attenuated tetravalent dengue vaccine (Sanofi) is four chimeric viruses, each with a common yellow fever virus 17D backbone that expresses the membrane and envelope proteins of a different DENV serotype. CYD-TDV was licensed in 2015 primarily based on the results of two harmonized, randomized, observer-masked, placebo-controlled Phase 3 trials: CYD14 (conducted in children and adolescents aged 2–14 in Asia, NCT01373281 [8]) and CYD15 (conducted in children and adolescents aged 9–16 in Latin America, NCT01374516) [9]. In each trial, CYD-TDV (or placebo) was administered in three doses (Months 0, 6, 12). Estimated vaccine efficacy (VE) against symptomatic virologically confirmed dengue (VCD) of any serotype (DENV-Any) occurring between Month 13 and 25 was 56.5% in CYD14 and 60.8% in CYD15.
In vaccine research, a “correlate of protection” (CoP) is an immune biomarker that statistically associates with – and thus can be used to predict – the level of vaccine efficacy against a clinical endpoint of interest [10–12]. Once validated for a specific prediction application, a CoP can accelerate vaccine research by enabling extrapolation of vaccine efficacy to host and/or viral populations different from those in the efficacy trial(s). An initial step in CoP identification is to look for immune biomarkers that correlate with risk (CoRs, which may or may not be CoPs) of the clinical endpoint in vaccine recipients. There are a variety of statistical frameworks for assessing how well identified CoRs perform as CoPs.
Focusing on the host immune response, Moodie et al. assessed neutralizing antibodies as CoRs of VCD and as CoPs of CYD-TDV protection against VCD in CYD14 and in CYD15, reporting that higher average nAb titer was associated with lower risk of VCD, in each trial and each treatment group [13]. In CYD14, the estimated hazard ratio (HR) of VCD per 10-fold increase in nAb average titer was 0.25 [95% confidence interval (CI) 0.17, 0.38] for the vaccine group and 0.75 (0.57, 1.00) for the placebo group. The stronger association in vaccine recipients indicates some violation of the Prentice criteria for nAb titers being a valid surrogate endpoint for DENV-Any (CoP is a synonym for a valid surrogate) in the whole cohort of 2–14-year-olds [14]; key Prentice criteria are that the biomarker is an inverse CoR in each treatment arm and that the inverse CoR is the same in each treatment arm. Carpp and Fong et al. [15] found that the associations of higher nAb titer with decreased risk of VCD became more similar between the vaccine and placebo groups when restricting to 9–16-year-olds, indicating greater adherence to the Prentice criteria. As the four DENV serotypes have substantial genetic and antigenic variability [16], VE conceivably depends on dengue prM/E amino acid (AA) sequences, especially considering that for vaccines the immune response to one pathogen strain generally tends to be less effective at combating infection or disease with divergent strains. Moreover, if immune responses induced by CYD-TDV are greatest in frequency and magnitude to the vaccine strains vs. genetically divergent dengue viruses, VE may be higher against dengue viruses with sequences closest to the vaccine strains, as differential neutralization can be seen across genotypes [17–19].
Coming from an alternative perspective and focusing on pathogen sequence data, Rabaa et al. [20] and Juraska et al. [21] investigated sequence-dependent VE in CYD14 and CYD15 by analyzing prM/E AA sequences of vaccine and placebo recipients who acquired VCD. Juraska et al. (Fig. 1) found that in CYD14, when considering DENV-Any VCD events across the entire cohort (aged 2–14), without taking account of baseline serostatus, VE against VCD decreased with prM/E AA sequence distance to the vaccine strain, defined as percent residue mismatch to the vaccine sequence of the VCD-causing serotype. Three sets of AA distance were analyzed, which counted mismatches to the vaccine strain in 1) all 661 aligned prM/E positions, 2) 65 nAb contact sites [22], or 3) surface-exposed sites on the mature virion (225–236 positions, depending on serotype). For example, for set 2) estimated VE decreased from 78.0% (95% CI, 65.7 to 85.9%) and 57.0% (95% CI, 41.6 to 68.3%) against a perfectly vaccine-matched DENV3/4 and DENV1/2 sequence, respectively, to VE = 43.5% (95% CI, −16.0 to 72.5%) and −10.7% (95% CI, −138.6 to 48.6%) against DENV3/4 and DENV1/2 sequences with 7 (10.8%) mismatched nAb contact site residues [21]. In contrast, in CYD15 VE did not differ significantly by any of the three dengue AA distances. A potential explanation is that the sequence distances in CYD15 varied over a smaller range than in CYD14 (e.g., for set 1 the range was 0.0045–0.042 in CYD14 versus 0.015–0.033 in CYD15 and for set 2 the range was 0–0.108 in CYD14 versus 0.015–0.077 in CYD15); this lower variability reduces statistical power.
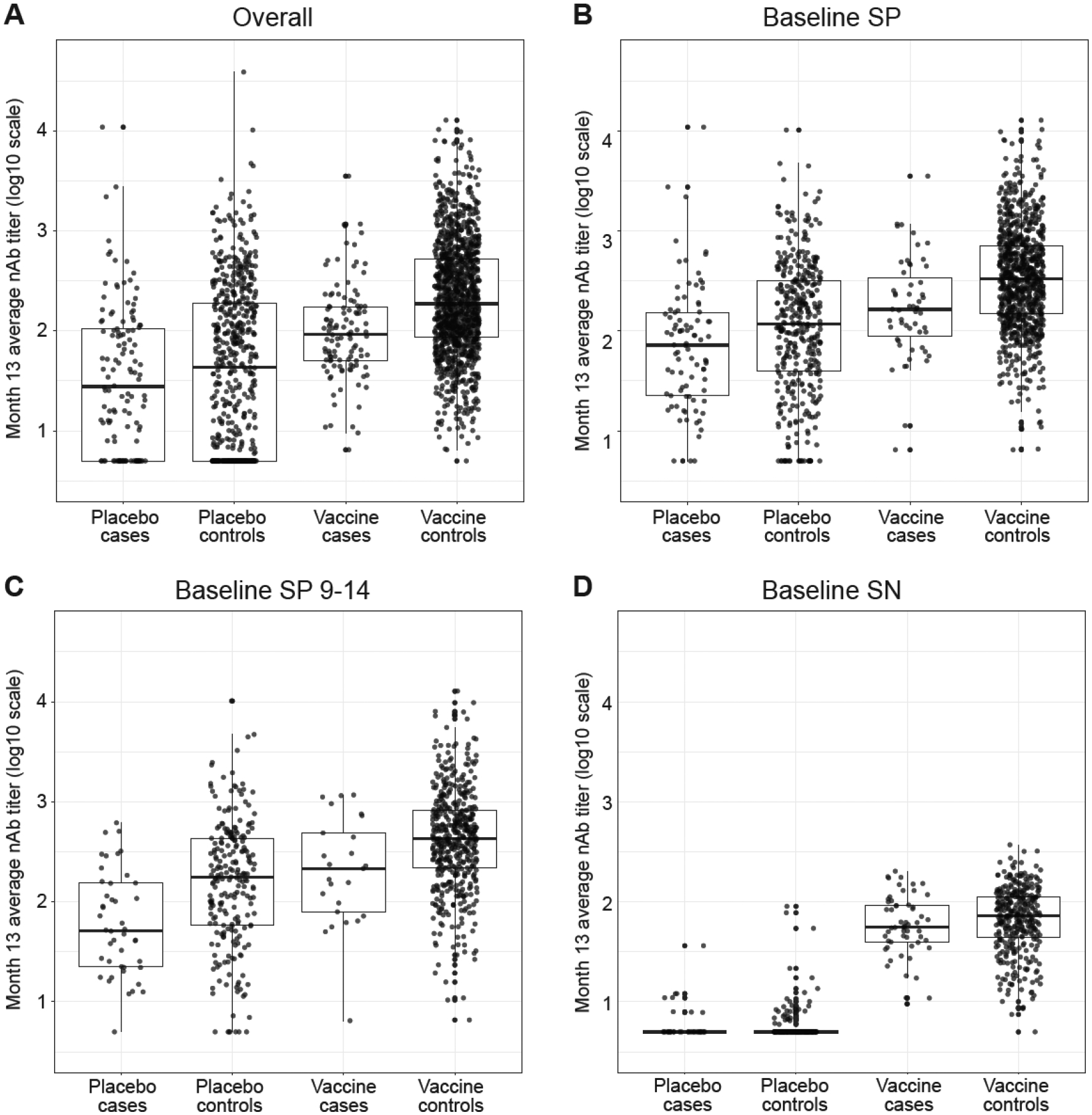
Boxplots for the following cohorts are shown: (A) Overall, (B) Baseline SP, (C) Baseline SP 9–14, and (D) Baseline SN. VCD, symptomatic virologically confirmed dengue. Case = DENV-Any case; Control = completed follow-up Month 25 free of DENV-Any.
Here, we integrated the host immune response (nAb) and pathogen (dengue virus sequence) data types and asked whether the nAb correlates of VCD risk identified previously depended on prM/E AA sequence distances 1)–3). This question is relevant because the extent to which nAb is a mechanistic cause of protection (a “mechanistic correlate of protection” or mCoP as defined by Plotkin and Gilbert) [11] is unclear, and a result where the inverse correlation between nAb titer and VCD risk is stronger against dengue viruses closer to the vaccine (and weaker or non-existent against dengue viruses farther from the vaccine) would increase the evidence for an mCoP. It would also characterize how dengue risk in each treatment group depends jointly on nAb titer and on dengue AA sequence distance, with a variety of applications including refinement of models for bridging vaccine efficacy and evaluation of the extent to which nAb titer is a valid surrogate endpoint for VCD caused by dengue viruses with different AA sequence distances from the vaccine strain (“distance-specific types of VCD”).
The question is addressed for the overall CYD14 cohort as well as stratified by baseline serostatus, a critical variable that can impact both vaccine safety and efficacy [8, 23] [24]. Specifically, three CYD14 subgroups are analyzed: baseline-seropositive participants, 9–14-year-old baseline-seropositive participants, and baseline seronegative participants, for which estimated VE against DENV-Any VCD between Month 13 and Month 25 was 68% (56% to 77%), 69% (50% to 81%), and 23% (−15% to 49%), respectively.
Methods
CYD14 Trial
Healthy 2–14-year-olds in Indonesia, Malaysia, The Philippines, Thailand, and Viet Nam were randomly assigned (2:1) to CYD-TDV vaccine or placebo and immunized at Months 0, 6, and 12, and followed for VCD via active surveillance through Month 25 [8]. The analyses were based on the primary dengue study endpoint assessed previously [8, 9], VCD of any serotype (DENV-Any) occurring between Month 13 and 25.
Case-Cohort Sampling Design
nAb titers were measured according to a case-cohort sampling design [13], in which a random sample of 20% of participants were selected into an immunogenicity subset for measurement of nAb titers at Months 0 and 13 [8]. Month 13 nAb titers were also measured from all cases, defined as participants who experienced the DENV-Any endpoint between Months 13 and Month 25. Month 13 average titer – defined as a participant’s geometric mean nAb titer (PRNT50) to all four serotypes – was assessed as a correlate of DENV-Any in participants who had not experienced the DENV-Any endpoint by Month 13.
PRNT50 Assay
nAb against each serotype was measured as PRNT50 titer, with values below the lower limit of quantification (10) assigned value 5 [13, 25].
Baseline Serostatus Subgroups
Month 13 anti-NS1 titers were used to define baseline serostatus [23]. In brief, participants with a Month 13 NS1 titer < 9 ELISA units (the lower limit of quantitation) were defined as baseline-seronegative; participants with a Month 13 NS1 titer ≥ 9 were defined as baseline-seropositive. This definition was validated to have high sensitivity and specificity for baseline serostatus [23].
Dengue Virus Sequencing
Dengue virus nucleotide sequences from primary endpoint VCD cases were determined using 454 sequencing [20]. The prM/E DENV genes (1,985 base pairs or 1,979 for DENV3) were sequenced and translated into 661 AA positions (659 for DENV3), comprising the complete antigen-coding region of each vaccine insert. The AA sequences were multiply aligned with the four vaccine sequences; no deletions or insertions were observed.
AA Sequence Distance
To calculate the AA sequence distance of a given DENV sequence from a VCD case to the serotype-matched vaccine strain sequence, each aligned position was designated as either “match” or “mismatch” between the case and vaccine sequences. The Hamming distance, a standard metric for evaluating how two binary data strings differ in “information”, is computed as the percent AA mismatch between the case and vaccine sequences, across the AAs present in set 1), 2), or 3) described above. The 65 nAb contact sites in set 2) from [22] are listed in Supplementary Table 1.
Imputation of Missing AA Sequence Distances
Missing AA sequence distances from VCD cases occurred due to a DENV viral titer below the lower limit of quantification, insufficient volume of serum, or lack of participant consent. Five complete AA sequence distance data sets for all (N=244) of the VCD cases with Month 13 nAb titer data were generated by imputing missing distances based on randomly selecting observed sequences from nearest-neighbor VCD cases with observed sequences, with nearest neighbors defined by the five smallest Euclidean distance of standardized z-scores of VCD event time, age, and Month 13 nAb average titer. A second version of the analysis was conducted imputing missing distances the same as in [21], based on a participant’s geographically proximal cases (accounting for clinic, site, and country) with the same serotype of VCD. The first imputation process is maximally statistically efficient if model assumptions hold [26] while the latter is more robust to model misspecification. The results were similar and are reported for the first approach.
Estimation of Sequence-Specific Correlates of VCD Risk
The methods of [26] were used to estimate the AA-distance specific VCD HR per unit change in log10 Month 13 average titer, implemented separately for the vaccine and placebo groups. The methods estimate distance-specific HRs ranging over the spectrum of distance values using nonparametric kernel smoothing, and account for the case-cohort sampling of nAb titers by augmented inverse probability weighting. The methods account for the missing AA sequence distances of some VCD cases using nonparametric hotdeck multiple imputation with 5 imputations from 5-nearest neighborhoods, implemented as described in [26]. The analyses right-censor participants at Month 25 or at dropout if it occurred earlier, and control for sex and age. Results are presented in terms of point and 95% CI estimates of VCD distance-specific HRs per unit change in log10 Month 13 average titer ranging over all observed distance values. The Supplementary Methods provide more details on the statistical methods, the implementation of the proposed estimation and testing procedures, and the the choice of bandwidths for kernel smoothing.
The methods of [26] were also used to estimate probabilities of distance-specific VCD by 25 months (ranging over all distances) within subgroups defined by Month 13 average titer and treatment group.
Hypothesis Testing
The methods of [26] were used to test two different hypotheses that distance-specific HRs varied with AA distance: The first is that the distance-specific HR increased with distance and the second is that the distance-specific HR departed from unity for at least one distance value. See the Supplementary Methods for further details.
Prentice Criteria
There are three main Prentice criteria for validating a biomarker as a surrogate endpoint. First, for a given distance-specific type of VCD, there is some positive vaccine efficacy against the VCD type. Based on Juraska et al. ([21], Figure 1B), the data support this criterion for all VCD types for the nAb contact site distance. Second, for a given VCD type the nAb average titer correlates with DENV-Any in each treatment arm, which can be checked by whether the 95% confidence interval for the distance-specific HR from the Sun et al. method excludes one. Third (and key), the relationship between Month 13 average titer and the probability of distance-specific VCD by 25 months is the same in the vaccine and placebo groups. We evaluate adherence to the second and third Prentice criteria in Results.
Cohort Abbreviations
As baseline dengue serostatus impacts safety and efficacy, we performed analyses in baseline-seronegative and baseline-seropositive subgroups. We use the following abbreviations for the studied cohorts: Overall (all participants), Baseline SN (baseline-seronegative participants), Baseline SP (baseline-seropositive participants), and Baseline SP 9–14 (9–14-year-old baseline-seropositive participants).
Results
Month 13 nAb Titers and VCD Case AA Sequence Distances
The CYD14 cohort is all participants who reached the Month 13 study visit without having experienced the VCD primary endpoint, comprising 6639 vaccine recipients and 3220 placebo recipients. Month 13 nAb titers were measured from immunogenicity subset participants who completed 25-month follow-up without the VCD endpoint (“controls” – 1275 vaccine, 604 placebo), and from all VCD cases (115 vaccine and 129 placebo). Among participants with Month 13 nAb titers measured, 99.6% received all three immunizations. Of the 244 VCD cases with Month 13 nAb titer data, 76 (84) vaccine (placebo) recipients had AA sequence distances 1)–3) measured. Imputation of missing sequence data is described in Methods.
Summary of Results from Host (nAb Titer Correlate of Risk) and Pathogen (More Vaccine-Divergent DENVs in Vaccine vs. Placebo VCD Cases) Data
Table 1/Figure 1 and Table 2/Figure 2 summarize results from the separate host and pathogen data types (nAb titer, AA sequence distance of VCD cases from the vaccine strain), which are integrated in subsequent analyses. Table 1 shows that nAb titers are significantly lower in VCD cases vs. controls in each treatment group (moreso in the vaccine group), for all treatment-cohort groups except Vaccine Baseline SN and Placebo Baseline SN. Figure 1 further shows the individual Month 13 average nAb titers by treatment group and case/control outcome status. As in [13], strong overall inverse associations of nAb average titers with VCD risk are seen in each treatment group in the Overall Cohort (Figure 1A). These inverse associations were evident in the Baseline SP (Figure 1B) and Baseline SP 9–14 (Figure 1C) cohorts (significant p-values in Table 1), and with only a trend for Baseline SN (Figure 1D) (p = 0.19 in Table 1). These results indicate that prior infection with dengue strengthens the inverse correlate of risk; one explanation is the greater vaccine efficacy for Baseline SP. In vaccine recipients the results were similar for the Overall and two Baseline SP cohorts, whereas for placebo recipients, there is greater separation between controls and cases moving from Baseline SN to Baseline SP to Baseline SP 9–14 year-olds (1D to 1B to 1C). In addition, in Baseline SN individuals and each treatment arm, nAb average titers are only slightly higher in controls than cases (Figure 1D).
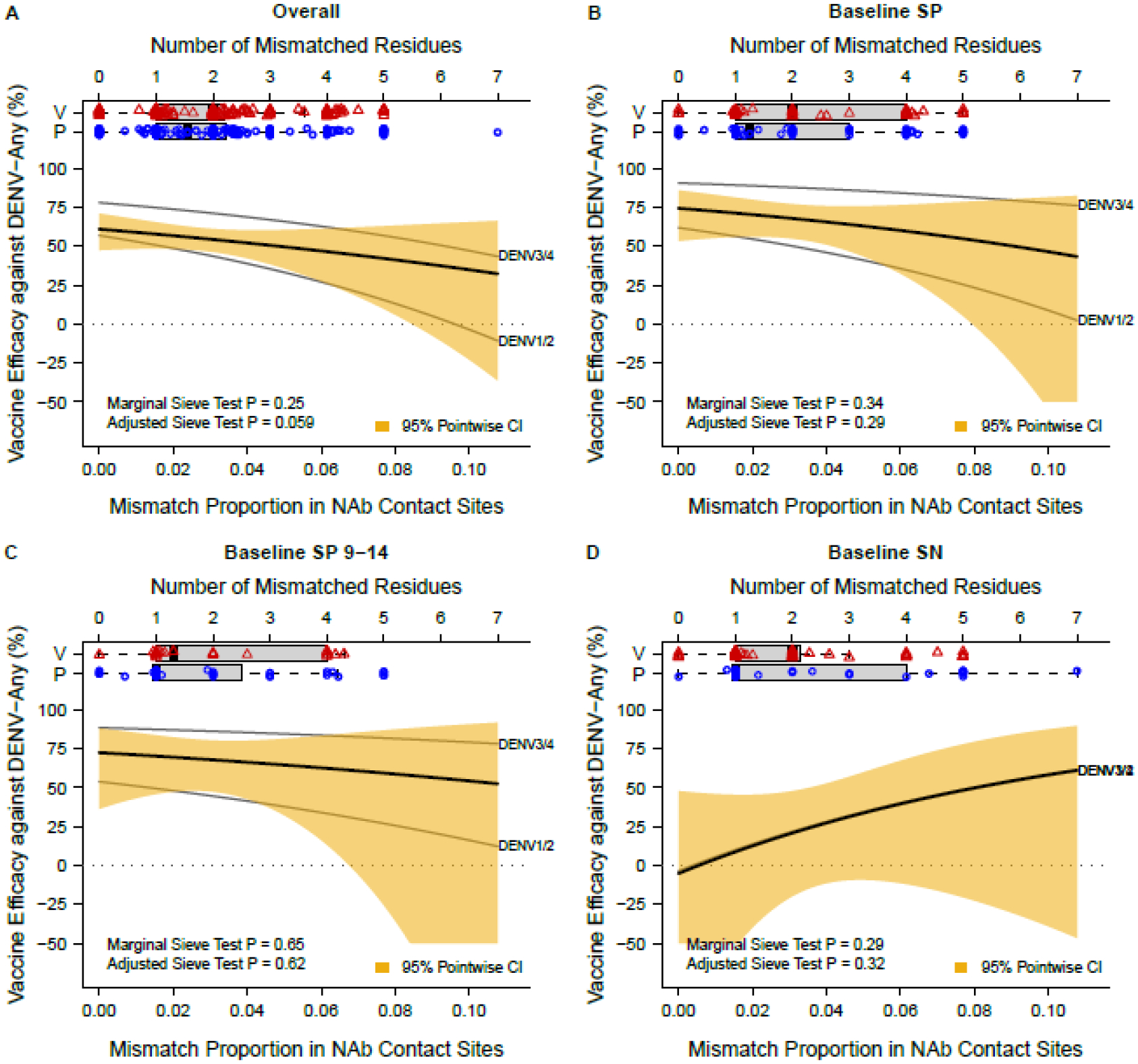
The saffron-shaded area represents the 95% pointwise confidence interval for the marginal VE. P, placebo; V, vaccine. See Juraska et al. [21] for details on the statistical method for the analysis.
Table 1.
CYD14 neutralizing antibody titer data measured at Month 13 by PRNT50
| VCD Cases [Diagnosed with VCD between Month 13 and 25] | VCD Controls [VCD-Free through Month 25] | ||||||
|---|---|---|---|---|---|---|---|
| Treatment Group | No. ppts | No. pos. responders (%)1 | GMAT2 (95% CI) | No. ppts | No. pos. responders (%)1 | GMAT2 (95% CI) | P-value3 |
| Vaccine Overall | 115 | 115 (100) | 98 (12, 818) | 1275 | 1273 (99.8) | 207 (16, 2738) | <0.0001 |
| Placebo Overall | 129 | 93 (72.1) | 32 (1, 841) | 604 | 410 (67.9) | 40 (1, 1685) | <0.0001 |
| Vaccine Baseline SP | 55 | 55 (100) | 171 (18, 1598) | 898 | 898 (100) | 332 (33, 3319) | <0.0001 |
| Placebo Baseline SP | 90 | 85 (94.4) | 68 (4, 1146) | 398 | 372 (93.5) | 106 (5, 2192) | 0.003 |
| Vaccine Baseline SP 9–14 | 25 | 25 (100) | 200 (17, 2329) | 492 | 492 (100) | 422 (44. 4002) | 0.005 |
| Placebo Baseline SP 9–14 | 44 | 43 (97.7) | 60 (6, 604) | 231 | 224 (97.0) | 152 (9, 2681) | <0.0001 |
| Vaccine Baseline SN | 58 | 58 (100) | 58 (14, 233) | 366 | 364 (99.5) | 64 (14, 294) | 0.19 |
| Placebo Baseline SN | 38 | 8 (21.1) | 6 (3, 13) | 201 | 33 (16.4) | 6 (3, 12) | 0.41 |
Table 2.
nAb Contact Site Hamming distances of VCD case dengue sequences to the matched-serotype vaccine insert
| Vaccine Group VCD Cases1 | Placebo Group VCD Cases1 | |||||
|---|---|---|---|---|---|---|
| Cohort | No. ppts | Mean | Range | No. ppts | Mean | Range |
| Overall | 75 | 0.034 | 0–0.077 | 83 | 0.033 | 0–0.108 |
| Baseline SP | 34 | 0.041 | 0.015–0.077 | 59 | 0.030 | 0–0.077 |
| Baseline SP 9–14 | 14 | 0.036 | 0.015–0.062 | 28 | 0.028 | 0–0.077 |
| Baseline SN | 40 | 0.029 | 0–0.077 | 23 | 0.039 | 0.015–0.108 |
Table 2 shows that the mean nAb contact site AA distances are greater in vaccine than placebo group VCD cases for the Baseline SP cohort, but not the Baseline SN cohort. Figure 2 repeats the analysis of Juraska et al. (Figure 1) for each of the four cohorts, providing new results, which support that vaccine efficacy against DENV-Any decreased against viruses with increasing nAb contact site distance in baseline seropositive individuals, although the effect was less marked than in the overall cohort, especially when limiting to Baseline SP 9–14 year-olds. In contrast, a decrease in vaccine efficacy against DENV-Any with increasing nAb contact site distance was not observed in baseline seronegative individuals. A potential explanation for this differential result is the higher vaccine efficacy in baseline seropositive compared to baseline seronegative individuals, which both provides greater statistical power in the sieve analysis to detect sequence-dependent vaccine efficacy (due to more room for wide variability in vaccine efficacy across sequence distances) and implies greater vaccine immune pressure placed on the virus. The correlates results (Table 1, Figure 1) support the greater neutralizing antibody pressure on the virus for baseline-seropositive individuals. Supplementary Table 2 shows the distribution of serotypes, published genotypes, and missing serotypes/genotypes of VCD cases for each of the four cohorts evaluated.
How Well nAb Titer Correlates with Risk Depends on How Well the VCD-Causing Strain Matches the Vaccine Strain at nAb Contact Sites
For each of the three AA distances, treatment groups, and cohorts, we next tested the hypothesis that the distance-specific HR (per 10-fold increase in Month 13 nAb average titer) increased with AA distance. For the Overall cohort, the 1-sided p-values for testing this hypothesis for distance type 1), 2), and 3) were 0.211, 0.005, 0.059 for the vaccine group, and 0.887, 0.012, and 0.384 for the placebo group (Table 3). Thus, there is evidence for a distance-varying HR for each treatment group for the nAb contact site distance. We next tested the hypothesis that the distance-specific HR departed from unity for at least one distance value. For the Overall cohort, the 2-sided p-values for testing this hypothesis for distance type 1), 2), and 3) were <0.001, <0.001, and <0.001 for the vaccine group, and 0.394, 0.116, and 0.476 for the placebo group (Table 3). Thus, nAb average titer correlated with VCD risk for at least some VCD sequences for each distance type in the vaccine group, but with lack of evidence for such associations in the placebo group, except possibly for nAb contact site distances. Results for each of the three subgroup cohorts had larger p-values, due in part to reduced sample size.
Table 3.
Summary results of hypothesis testing for the four cohorts and two treatment arms.
| Testing H201 | Testing H102 | ||
|---|---|---|---|
| prM/E AA sites for Hamming distances of VCD endpoints | Treatment Arm | p-value3 | p-value |
| n=158 VCD cases (75 vaccine, 83 placebo) with observed sequence data | |||
| 1) All aligned sites | Vaccine (h*= 0.285) | 0.211 | <0.001 |
| Placebo (h* = 0.296) | 0.887 | 0.394 | |
| 2) nAb contact sites | Vaccine (h = 0.023) | 0.005 | <0.001 |
| Placebo (h = 0.024) | 0.012 | 0.116 | |
| 3) Surface-exposed sites on the mature virion | Vaccine (h* = 0.278) | 0.059 | <0.001 |
| Placebo (h* = 0.288) | 0.384 | 0.476 | |
| n=93 VCD cases (34 vaccine, 59 placebo) with observed sequence data | |||
| 1) All aligned sites | Vaccine (h* = 0.327) | 0.543 | 0.004 |
| Placebo (h* = 0.346) | 0.301 | 0.610 | |
| 2) nAb contact sites | Vaccine (h = 0.028) | 0.078 | 0.004 |
| Placebo (h = 0.024) | 0.049 | 0.452 | |
| 3) Surface-exposed sites on the mature virion | Vaccine (h* = 0.330) | 0.176 | 0.009 |
| Placebo (h* = 0.339) | 0.060 | 0.334 | |
| n=42 VCD cases (14 vaccine, 28 placebo) with observed sequence data | |||
| 1) All aligned sites | Vaccine (h* = 0.455) | 0.244 | 0.093 |
| Placebo (h* = 0.454) | 0.489 | 0.062 | |
| 2) nAb contact sites | Vaccine (h = 0.035) | 0.105 | 0.054 |
| Placebo (h = 0.030) | 0.164 | 0.116 | |
| 3) Surface-exposed sites on the mature virion | Vaccine (h* = 0.462) | 0.070 | 0.025 |
| Placebo (h* = 0.455) | 0.360 | 0.051 | |
| n=63 VCD cases (40 vaccine, 23 placebo) with observed sequence data | |||
| 1) All aligned sites | Vaccine (h* = 0.509) | 0.842 | 0.684 |
| Placebo (h* = 0.613) | 0.528 | 0.982 | |
| 2) nAb contact sites | Vaccine (h = 0.028) | 0.568 | 0.771 |
| Placebo (h = 0.044) | 0.301 | 0.899 | |
| 3) Surface-exposed sites on the mature virion | Vaccine (h* = 0.506) | 0.888 | 0.586 |
| Placebo (h* = 0.625) | 0.278 | 0.934 | |
In Baseline-Seropositives, nAb Contact Site Sequence-Distance Dependence of the nAb Correlate of Risk is Stronger in the Vaccine (vs. Placebo) Arm
Figures 3 through through55 visualize how the sequence-specific nAb titer correlate of risk varies as the VCD-causing strain increases in mismatch to the vaccine insert at nAb contact sites, for each of the cohorts (Figure 3: Overall; Figure 4: Baseline SP and Baseline SP 9–14; and Figure 5: Baseline SN). Panel A in each figure shows for each treatment group how the estimated AA distance-specific log HR (per log10 increase in nAb average titer) of VCD varies with nAb contact site distance, and panel B in each figure shows for each treatment group estimated ratios of probabilities of nAb contact site distance v-specific VCD for individuals of the median age in the given cohort, where the ratios compare individuals with low (10th percentile), medium (50th percentile), and high (90th percentile) Month 13 nAb average titer responses. In the Overall cohort, estimated HRs in the vaccine group were 0.27 (95% CI 0.15 to 0.47) against VCD caused by a DENV with no nAb contact site mismatches to the serotype-matched vaccine insert versus 0.60 (95% CI 0.32 to 1.11) against VCD caused by a DENV with 5 nAb contact site mismatches (Hamming distance = 0.077) to the serotype-matched vaccine insert (Figure 3A). Figure 3B shows that vaccinees with 90th percentile Month 13 nAb average titer had an estimated 6.72-fold lower risk of VCD caused by a DENV with no nAb contact site mismatches to the serotype-matched vaccine insert compared to vaccinees with 10th percentile nAb response; this ratio decreases to 2.07-fold lower risk for VCD with five nAb contact site mismatches to the serotype-matched vaccine insert. These findings are consistent with a hypothesis that vaccine-induced nAbs best neutralize (and may help mediate protection against) DENVs with nAb contact site profiles most similar to the vaccine insert strains.
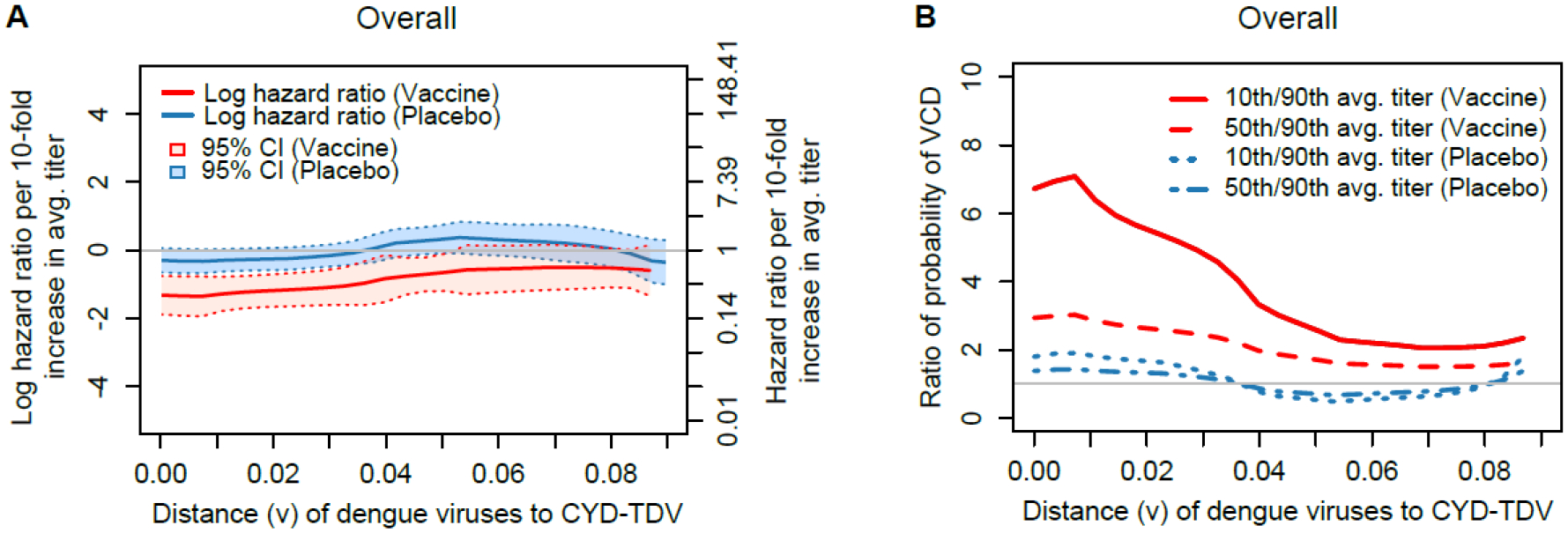
(A) Estimated distance v-specific hazard ratio of VCD from Month 13 to Month 25 per log10 increase in Month 13 average nAb titer by treatment group; (B) Estimated ratios of the probability of distance v-specific VCD from Month 13 to Month 25, where the ratios are for individuals at median age of 8 years for the cohort with 50th vs. 10th and with 90th vs. 50th percentiles of Month 13 average nAb titer, by treatment group. Hamming distances 0.046 and 0.077 correspond to 3 and 5 mismatches in nAb contact sites, respectively.
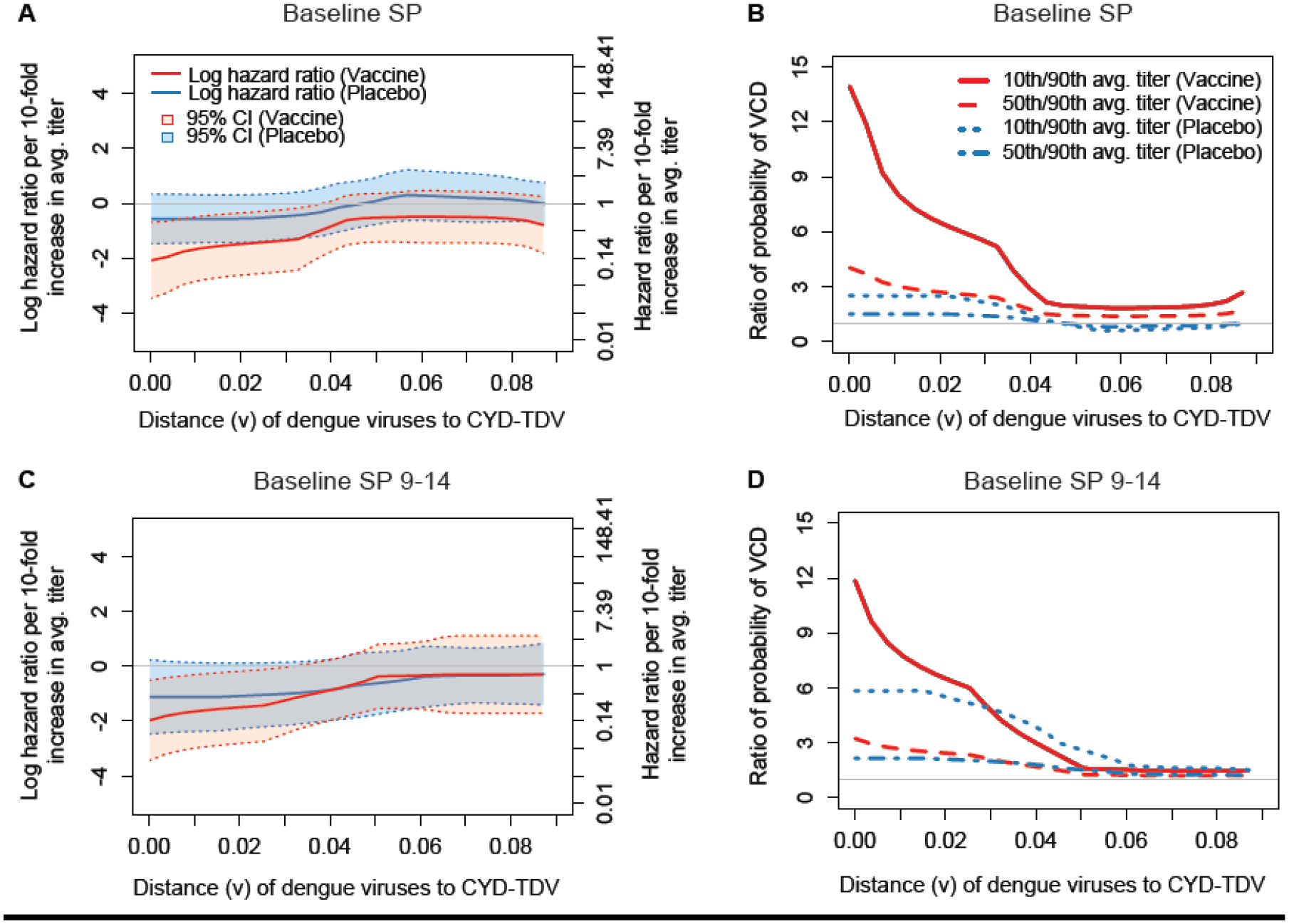
Estimated distance v-specific hazard ratio of VCD from Month 13 to Month 25 per log10 increase in Month 13 average nAb titer by treatment group, for the (A) Baseline SP and (C) Baseline SP 9–14 cohorts; Estimated ratios of the probability of distance v-specific VCD from Month 13 to Month 25, where the ratios are for individuals at median ages of the subcohort with 50th vs. 10th and with 90th vs. 50th percentiles of Month 13 average nAb titer, by treatment group, for the (B) Baseline SP and (D) Baseline SP 9–14 cohorts. The median age of the Baseline SP and of the Baseline SP 9–14 cohort was 9 and 12 years, respectively.
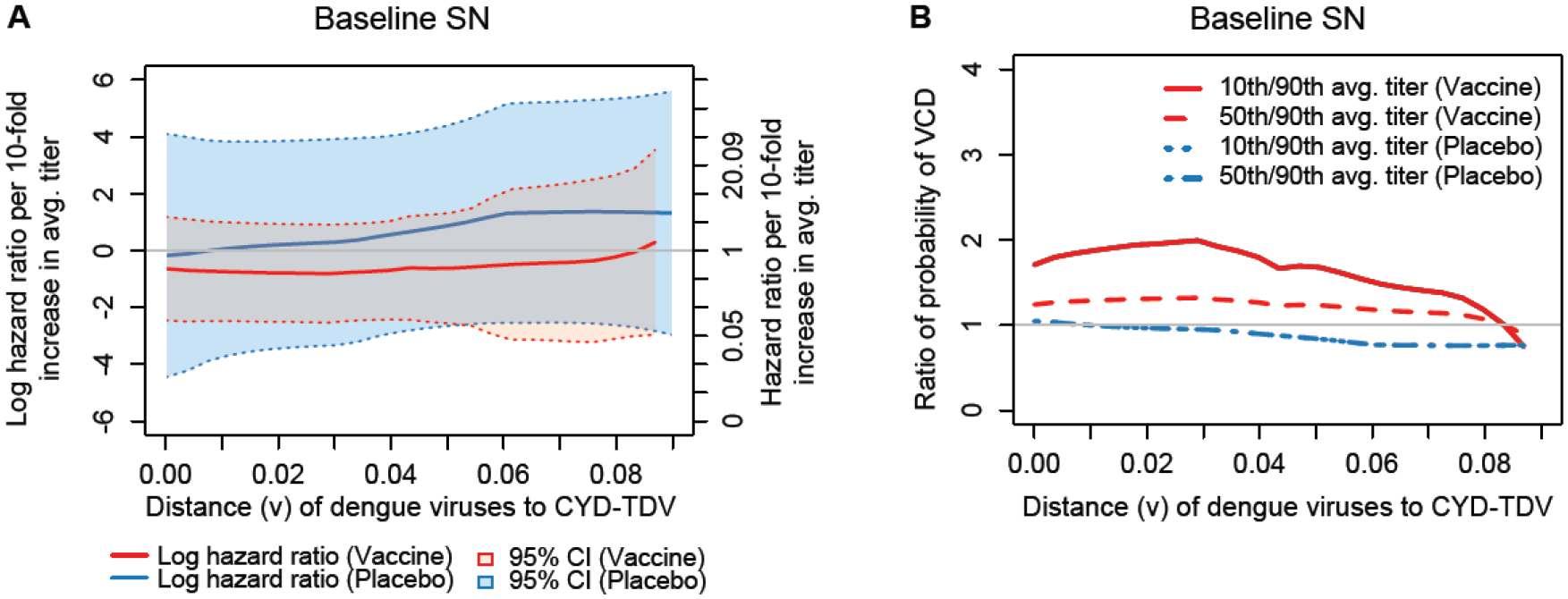
(A) Estimated distance v-specific hazard ratio of VCD from Month 13 to Month 25 per log10 increase in Month 13 average nAb titer by treatment group; (B) Estimated ratios of the probability of distance v-specific VCD from Month 13 to Month 25, where the ratios are for individuals at median age of 6 years for the subcohort with 50th vs. 10th and with 90th vs. 50th percentiles of Month 13 average nAb titer, by treatment group.
For placebo recipients, there was a gradient in the same direction, at lower magnitude [1.80-fold lower risk (90th vs. 10th percentile) of VCD caused by a DENV with no nAb contact site mismatches to the serotype-matched vaccine insert compared to 0.78-fold lower risk (90th vs. 10th percentile) of VCD caused by a DENV with 5 nAb contact site mismatches]. The stronger association for the vaccine group compared to the placebo group indicates departure from Prentice’s third criterion for nAb average titer as a valid surrogate endpoint. In addition, Prentice’s second criterion is supported only for VCD with 0, 1, or 2 mismatches from the vaccine, given that only for Hamming distances up to about 0.03 do the 95% confidence intervals for the HRs exclude one. Thus, there is also departure from Prentice’s second criterion for nAb average titer as a valid surrogate endpoint.
In the Baseline SP and Baseline SP 9–14 cohorts, similar results were seen, with point estimates suggesting stronger sequence-dependency of the correlates of risk. For Baseline SP vaccine recipients, estimated HRs were 0.12 against VCD caused by a DENV with no nAb contact site mismatches to the serotype-matched vaccine insert compared to 0.59 against VCD caused by a DENV with 5 nAb contact site mismatches (Figure 4A). Baseline SP vaccine recipients with 90th percentile nAb average titer had an estimated 14.0-fold lower risk of VCD caused by a DENV with no nAb contact site mismatches to the serotype-matched vaccine insert compared to those with 10th percentile nAb response; this ratio decreased to 1.92-fold for VCD caused by a DENV with 5 nAb contact site mismatches (Figure 4B). Baseline SP placebo recipients with 90th percentile nAb response had an estimated 2.50-fold lower risk of VCD caused by a DENV with no nAb contact site mismatches to the serotype-matched vaccine insert compared to those with 10th percentile nAb response; this ratio decreased to 0.72 for VCD caused by a DENV with 5 nAb contact site mismatches.
For Baseline SP 9–14 vaccine recipients, estimated HRs were 0.14 against VCD caused by a DENV with no nAb contact site mismatches to the serotype-matched vaccine insert compared to 0.74 against VCD caused by a DENV with 5 nAb contact site mismatches (Figure 4C). Baseline SP 9–14 vaccine recipients with 90th percentile nAb response had an estimated 11.9-fold lower risk of VCD caused by a DENV with no nAb contact site mismatches to the serotype-matched vaccine insert compared to those with 10th percentile nAb response; this ratio decreased to 1.45-fold for VCD with 5 nAb contact site mismatches (Figure 4D). For Baseline SP 9–14 placebo recipients, there was an estimated 5.83-fold lower risk for zero mismatches and 1.50-fold lower risk for five mismatches.
Compared to the Overall and Baseline SP cohorts, the results for the Baseline SP 9–14 cohort suggest that the correlation pattern becomes more similar between the placebo and vaccine arms, supporting that nAb average titer adheres better to Prentice’s third criterion when restricting to older seropositive individuals. In addition, the data support that Prentice’s second criterion holds for VCD with 0, 1, or 2 mismatches for Baseline SP and Baseline 9–14, similar to the results for the Overall cohort.
In Baseline-Seronegatives, the nAb Correlate of Risk Does Not Appear to Depend on nAb Contact Site Match of the VCD-Causing Virus
For the Baseline SN cohort, in both treatment groups the estimated HR remained near zero across all nAb contact site distances examined, and the 95% confidence intervals always included zero by a wide margin (Figure 5A). Therefore, in contrast to the Overall and Baseline SP results, the Baseline SN results support that Month 13 nAb average titer is not a correlate of risk of VCD for any Hamming distance for either treatment group, and thus Prentice’s second criterion is not met. However, the confidence intervals are wider than those for the Overall and Baseline SP treatment groups, suggesting that it is not conclusive whether the nAb average titer correlations with VCD risk are absent or whether there could be weak-to-moderate correlations that were not detected due to limited sample size.
Use of the Missing Data Methods Helped Correct for Bias
The sequence of the VCD-causing virus was missing for 35% of all VCD cases. To mitigate potential negative consequences of this missing information, for the results presented in Figures 3–5, we used a nonparametric multiple imputation approach [26] to correct for potential bias and improve efficiency compared to a complete-case analysis that would exclude these cases. To understand the influence of this missing data correction on the results, we repeated the same analyses shown in Figures 3–5 but only including VCD cases with observed sequences. The results are shown in Supplementary Figures 1 and 2. For each cohort and treatment arm, the shape of estimated curves are similar between the original and new analyses, yet the log hazard ratio curves have shifted upwards and the ratios of probabilities of VCD curves have shifted downwards. This means that when ignoring missing sequences the data no longer indicate an inverse correlation of average titer with VCD risk even for acquisition of dengue viruses close to the vaccine strain, and moreover the results indicate a direct correlation of average titer with VCD risk for viruses far from the vaccine strain. This inference of higher nAb titers associating with higher VCD risk is highly likely to not be correct, given the previous analyses that established that these titers are very strongly inversely associated with VCD risk (e.g., [13]). Therefore, we interpret Supplementary Figures 1 and 2 as confirming that it is not recommended to use statistical methods that ignore the missing sequences, as such methods are known to be more likely to yield biased results (as demonstrated in simulation studies [26–28]).
Few VCD Cases Caused by DENVs with High nAb Contact Site Match to Vaccine Strain in Baseline-Seropositive Vaccine Recipients with High nAb Titers
Figure 6 presents individual-level nAb and sequence distance data from VCD cases, to illustrate the relationship between how divergent the VCD-causing DENV was from the vaccine strain at nAb contact sites and the corresponding nAb level in the individual who acquired that DENV. It plots, for each VCD case, the nAb contact site distance versus Month 13 nAb average titer, by treatment group within each cohort. For the Overall cohort, vaccine recipient VCD cases tended to have either low Month 13 nAb average titers or high sequence distances, with few cases with both high titers and short sequence distances. Results were similar for Baseline SP VCD cases (Panels B, C), with apparently slightly increased slope of higher sequence distances with higher titers in vaccine recipients.
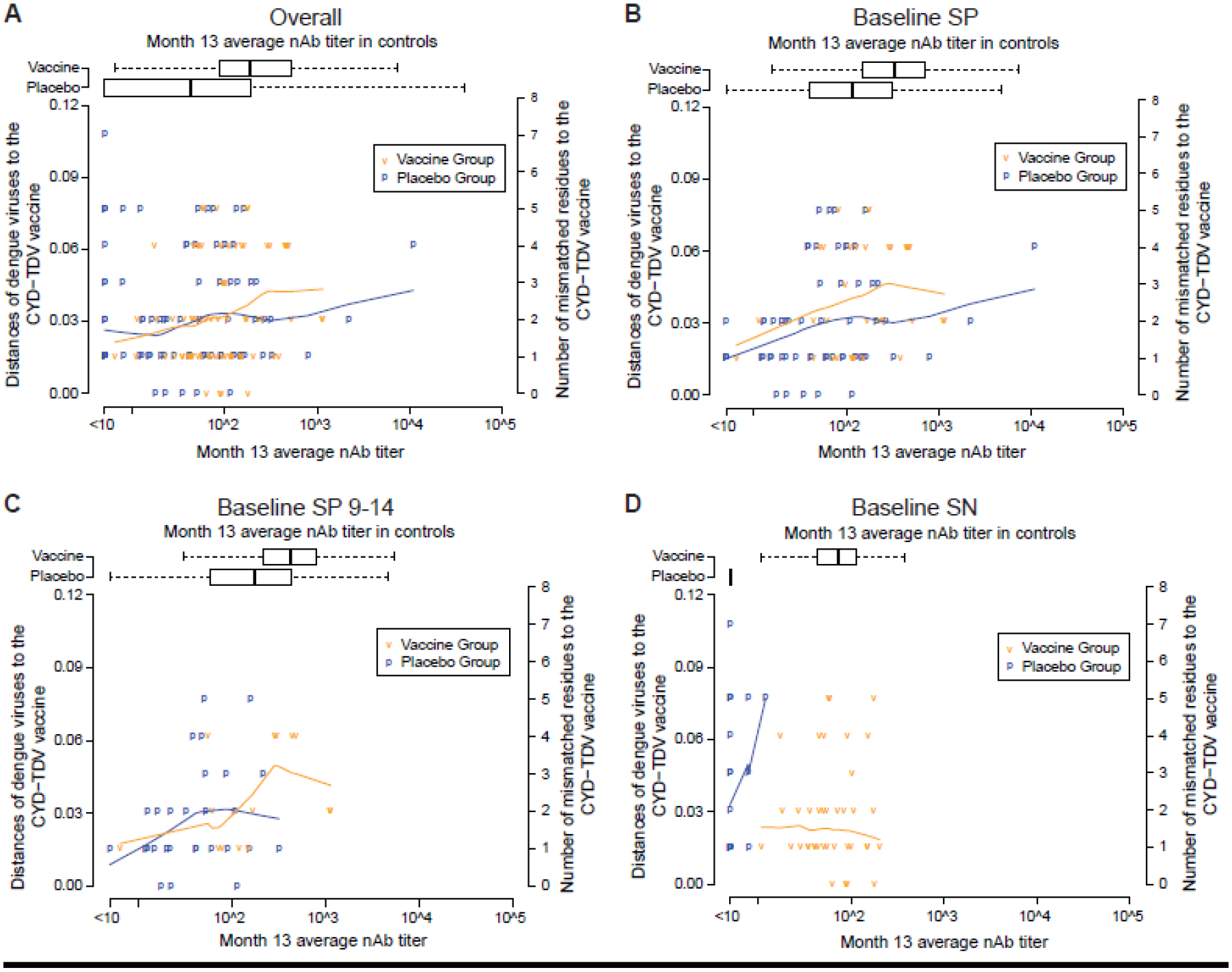
Scatterplots are shown for the following cohorts: (A) Overall, (B) Baseline SP, (C) Baseline SP 9–14, and (D) Baseline SN. nAb contact site distances were averaged over the 20 multiply imputed data sets. The blue and orange lines in each panel are lowess locally-weighted polynomial regression smooth fits to the scatterplot using the R function lowess with default value of the smoother span set to 2/3.
For Baseline SN VCD cases (Panel D), sequence distance did not appear to increase with nAb average titer in vaccine recipients. The result that in VCD cases, nAb contact site distance appears to increase as Month 13 average nAb titer increases in baseline-seropositives (Panels B, C) but not in baseline-seronegatives (Panel D), is consistent with the hypothesis that vaccine-induced nAbs best neutralize DENVs with nAb contact site profiles most similar to the vaccine insert strains in baseline-seropositives.
Discussion
For heterogenous pathogens with characterized serotypes, other immunotypes, or phylogenetic genotypes, typical analyses of immune correlates of risk in vaccine efficacy trials assess the association of immune responses to pathogen strains in the vaccine construct with risk of disease with each specific pathogen type, and study whether the associations are stronger for some pathogen types (e.g., for dengue serotypes in CYD14 [13]). Measurement of amino acid sequences of the pathogens isolated from disease cases, as done in CYD14, allows this normative assessment to be refined by also assessing how immune correlates of risk vary by pathogen genotype defined based on myriad possible sequence features. Assessing correlates of risk by amino acid sequence distance of the pathogen to a vaccine strain may provide a single quantitative pathogen metric (i.e., the distance) that summarizes the strength of the correlate of risk, whereas separate correlates analyses for e.g. each discrete genotype can be highly imprecise if there are only a few outcome cases per genotype/distance value. In addition, this type of analysis enables assessment of the adherence of an immunological marker to Prentice’s second and third criteria for a valid surrogate endpoint, separately for each type of pathogen defined by its sequence distance to the vaccine insert strain or strains.
Our analysis of CYD14 supported that the association of nAb average titer with VCD risk in CYD14 was stronger for dengue viruses closer at nAb contact sites to the vaccine strain for vaccine recipients, which also occurred for placebo recipients, albeit less strongly. Similar results were found for baseline seropositive participants of all ages (2–14 year-olds) and restricting to 9–14 year-olds, with point estimates suggesting greater sequence-specific dependent associations. In contrast, there was not evidence for sequence-dependent correlates of risk in baseline seronegative individuals, who also tended to be younger. These findings suggest that, in older children/adolescents in endemic areas (who have greater prior dengue exposure than younger children [13]), nAbs (particularly vaccine-induced nAbs) against the vaccine strains are maximally protective against closely vaccine-matched strains, with some weakening of their protective function against viruses more dissimilar to the vaccine at nAb contact sites. However, this inference must be interpreted with caution given the analysis is based on associations that may not be causal.
Furthermore, despite any weakening of protective function of nAbs against viruses more dissimilar to the vaccine at nAb contact sites, VE against VCD in baseline seropositive subjects aged 9 or older in CYD14 was observed to be high [79.2% (95%CI 47.2 to 92.7)], and compared favorably to equivalent data from CYD15 [83.7% (95%CI 62.2 to 93.7)] (Hadinegoro et al. [38]), where VE did not differ significantly by any of the three dengue AA distances analyzed in Juraska et al. Moreover, Moodie et al.’s [13] analysis supported that Month 13 PRNT50 nAb titers did not fully mediate vaccine efficacy (VE) against VCD, with implication that neutralizing antibodies not fully represented by the PRNT50 assay or Month 13 time point (e.g., anamnesetic responses), and/or other immune responses [29, 30], must also mediate VE to some extent. Consequently, a degree of weakening of Month 13 nAb titer does not necessarily imply that VE would also be decreased.
One way to determine whether a nAb average titer biomarker becomes more adherent to Prentice’s criteria for a valid surrogate endpoint as the degree of prior dengue exposure increases would be to check whether sequence-distance specific VCD risk became more similar in the vaccine and placebo arms moving from the Baseline SN cohort to the Baseline SP cohort. However, the wide 95% confidence intervals in the Baseline SN cohort results hinder this comparison. We did, however, find that sequence-distance specific VCD risk became more similar in the vaccine and placebo arms moving from the Overall cohort, or from the Baseline SP cohort, to the Baseline SP 9–14 cohort. Baseline seropositivity was defined as nAb titer > LLOQ for at least one serotype; we did not distinguish between single-serotype baseline seropositives and multiple-serotype baseline seropositives. As co-circulation of multiple serotypes was observed at the CYD14 trial sites [20, 21], individuals in the Baseline SP 9–14 cohort were seropositive to a greater number of serotypes than individuals in the Baseline SP cohort. Thus, this result can be tentatively interpreted as the nAb average titer biomarker capturing more of the vaccine effect on dengue outcome in individuals with more prior dengue exposure than in individuals with less prior dengue exposure. In addition, the finding that sequence-distance specific VCD risk became more similar in the vaccine and placebo arms when restricting to Baseline SP 9–14 individuals suggests that as the degree of prior dengue exposure increases, a nAb average titer biomarker becomes more adherent to Prentice’s key third ‘full mediation’ criterion for a valid surrogate endpoint.
Prior dengue exposure has been shown to influence the nature of the antibody response to CYD-TDV vaccination. Specifically, CYD-TDV vaccination of dengue-naïve individuals elicited serotype-specific (especially DENV-4-specific) nAbs, whereas CYD-TDV vaccination of dengue-exposed individuals elicited predominantly broadly reactive cross-serotype nAbs [31]. Given that monoclonal cross-reactive nAbs have been shown to neutralize a broader spectrum of DENV-4 genotypic variants compared to monoclonal DENV-4-specific nAbs [32], it would be logical to hypothesize that the nAb titer correlate of risk would have greater sequence dependency in baseline-seronegative vaccinated individuals, as the combined findings of [31] and [32] suggest that such individuals would have predominantly DENV-4-specific nAbs that would have variable ability to neutralize DENV-4 genotypic variants. However, we did not observe evidence for sequence-dependent correlates of risk in baseline-seronegative individuals (with the caveat of limited precision). A possible explanation for these apparently contradictory results is a combination of: (1) overall VE was low in baseline-seronegative individuals (23% estimate), (2) nAb titer was only a weak correlate of risk when including all VCD endpoints not accounting for sequence distances, and (3) there was little sequence variation at nAb contact sites across the DENV-4 genotypic variant viruses in [32], i.e. most of the sequence variation occurred outside of nAb contact sites, whereas our results are specific to nAb contact site distances. Future work may examine whether serotype-specific and cross-reactive nAbs differ in ability to neutralize across serotype-specific panels of viruses with diversity restricted to nAb contact sites, and whether these results hold for the other three serotypes. Testing additional serotype-specific and cross-reactive nAb neutralization of larger panels of genotypic variants, including those of serotypes other than DENV-4, could also yield insight into the extent of differential neutralization across genotypic variants by serotype-specific nAbs. Moreover, given that DENV-nAb interactions are an area of active research [33–37], future work could look at how the identified nAb correlates of risk depend on sequence distance for expanded sets of nAb contact sites.
Conclusions
Our findings support previous studies that prior dengue immune status influences the nature of the neutralizing antibody response induced by vaccination and highlight the need for further research into the interplay between infection and vaccine immunity. Future similar correlates analyses integrating host immune response data with pathogen sequence data have potentially broader applications in identifying correlates of risk and surrogate endpoints for other vaccines against genetically diverse pathogens.
Supplementary Material
1
Supplementary Methods
Supplementary Table 1. List of the 65 dengue E protein nAb contact sites in set (2) [3].
Supplementary Table 2. Distribution of dengue serotype and published genotype for VCD cases in the CYD14 trial by randomized treatment arm and Cohort [Overall, Baseline Seropositive (SP), Baseline Seropositive 9–14 Years Old (SP 9–14), Baseline Seronegative (SN)].
Supplementary Figure 1. (A) Estimated distance v-specific hazard ratio of VCD from Month 13 to Month 25 per log10 increase in Month 13 average nAb titer by treatment group; (a) Overall, (b) Baseline Seronegative (SN), (c) Baseline Seropositive (SP), (d) Baseline Seropositive 9–14. Distance = nAb contact site Hamming distance. VCD cases without observed sequences were completely excluded from these analyses.
Supplementary Figure 2. (B) Estimated ratios of the probability of distance v-specific VCD from Month 13 to Month 25, where the ratios are for individuals at median age for the subcohort with 50th vs. 10th and with 90th vs. 50th percentiles of Month 13 average nAb titer, by treatment group; (a) Overall, (b) Baseline Seronegative (SN), (c) Baseline Seropositive (SP), (d) Baseline Seropositive 9–14. Distance = nAb contact site Hamming distance. VCD cases without observed sequences were completely excluded from these analyses.
Supplementary References
Acknowledgments
The authors thank the participants, investigators, and sponsors of the CYD14 and CYD15 trials, and Dr. Paul Commander for insightful comments that helped improve the writing.
Funding
This work was supported by Sanofi; the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Department of Health and Human Services [grant number R37AI054165]; and the National Science Foundation [grant number DMS1915829]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Declaration of interests
Peter Gilbert reports financial support was provided by Sanofi. Michal Juraska reports financial support was provided by Sanofi. Lindsay Carpp reports financial support was provided by Sanofi. Zoe Moodie reports financial support was provided by Sanofi. Craig Magaret reports financial support was provided by Sanofi. Li Qi reports a relationship with Sanofi that includes: employment.
List of abbreviations
| AA | amino acid |
| CI | confidence interval |
| DENV | dengue virus |
| DENV-Any | symptomatic virologically confirmed dengue of any serotype |
| DHF/DSS | dengue hemorrhagic fever/dengue shock syndrome |
| HR | hazard ratio |
| nAb | neutralizing antibody |
| LLOQ | lower limit of quantification |
| SN | seronegative |
| SP | seropositive |
| VCD | symptomatic virologically-confirmed dengue |
| VE | vaccine efficacy. |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics approval and consent to participate
The CYD14 protocol was approved by all relevant ethics review boards (The Committee of Medical Research Ethics, Faculty of Medicine, University of Indonesia, Jakarta, Indonesia; The Research and Development Unit Medical Faculty University of Udayana, Sanglah General Hospital, Denpasar, Indonesia; Health Research Ethics Committee, Faculty of Medicine University of Padjadjadrain, Dr Hasan Sadikin Hospital, Bandung, Indonesia; Medical Research and Ethics Committee, Ministry of Health, Malaysia, Kuala Lumpur, Malaysia; Research Institute for Tropical Medicine IRB, Alabang, Muntinlupa City, Philippines; Vicente Sotto Memorial Medical Center EC, Cebu City, The Philippines; Chong Hua Hospital Institutional Review Board, Cebu City, The Philippines; Walter Reed Army Institute of Research International Review Board (WRAIR IRB), MD, USA; The Ethical Review Committee for Research in Human Subjects, Ministry of Public Health, Thailand; Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand; Pasteur Institute EC, Ho Chi Minh City, Viet Nam). Parents or guardians provided written informed consent and older children provided written informed assent before participation, in accordance with local regulations.
Competing interests
LQ was an employee of Sanofi when the work was conducted. MJ, ZM, CAM, LNC, and PBG received a contract from Sanofi to conduct the statistical analysis work and submit the results for publication.
Availability of data and materials
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org/.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.vaccine.2022.08.055
Read article for free, from open access legal sources, via Unpaywall:
http://manuscript.elsevier.com/S0264410X2201043X/pdf/S0264410X2201043X.pdf
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/135613559
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (1 citation) ClinicalTrials.gov - NCT01374516
- (1 citation) ClinicalTrials.gov - NCT01373281
European Clinical Trials
- (1 citation) EU Clinical Trials Register - 2014-001708-24
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Analysis of Neutralizing Antibodies as a Correlate of Instantaneous Risk of Hospitalized Dengue in Placebo Recipients of Dengue Vaccine Efficacy Trials.
J Infect Dis, 225(2):332-340, 01 Jan 2022
Cited by: 2 articles | PMID: 34174082 | PMCID: PMC8915240
Neutralizing Antibody Correlates Analysis of Tetravalent Dengue Vaccine Efficacy Trials in Asia and Latin America.
J Infect Dis, 217(5):742-753, 01 Feb 2018
Cited by: 59 articles | PMID: 29194547 | PMCID: PMC5854020
Microneutralization assay titer correlates analysis in two phase 3 trials of the CYD-TDV tetravalent dengue vaccine in Asia and Latin America.
PLoS One, 15(6):e0234236, 15 Jun 2020
Cited by: 7 articles | PMID: 32542024 | PMCID: PMC7295445
Tetravalent Dengue Vaccine: A Review in the Prevention of Dengue Disease.
Drugs, 76(13):1301-1312, 01 Sep 2016
Cited by: 27 articles | PMID: 27506852
Review
Funding
Funders who supported this work.
NIAID NIH HHS (2)
Grant ID: R37 AI054165
Grant ID: UM1 AI068635
National Institute of Allergy and Infectious Diseases (1)
Grant ID: R37AI054165
National Institutes of Health
National Science Foundation (1)
Grant ID: DMS1915829




