Abstract
Free full text
Genome Mining Enabled by Biosynthetic Characterization Uncovers a Class of Benzoxazolinate‐Containing Natural Products in Diverse Bacteria
Abstract
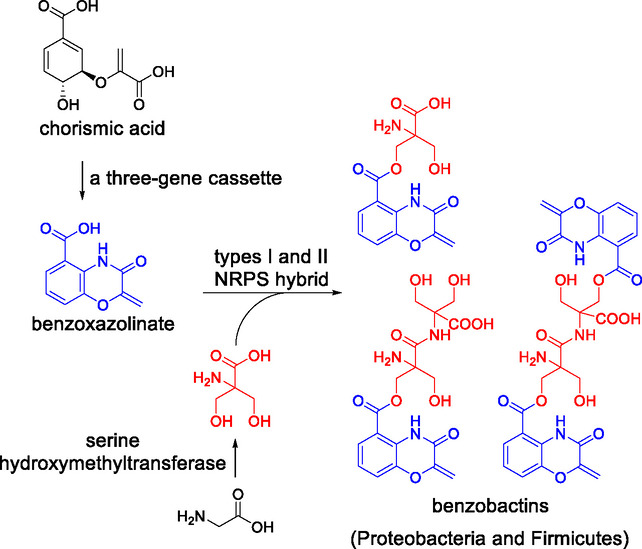
Benzoxazolinate is a rare bis‐heterocyclic moiety that interacts with proteins and DNA and confers extraordinary bioactivities on natural products, such as C‐1027. However, the biosynthetic gene responsible for the key cyclization step of benzoxazolinate remains unclear. Herein, we show a putative acyl AMP‐ligase responsible for the last cyclization step. We used the enzyme as a probe for genome mining and discovered that the orphan benzobactin gene cluster in entomopathogenic bacteria prevails across Proteobacteria and Firmicutes. It turns out that Pseudomonas chlororaphis produces various benzobactins, whose biosynthesis is highlighted by a synergistic effect of two unclustered genes encoding enzymes on boosting benzobactin production; the formation of non‐proteinogenic 2‐hydroxymethylserine by a serine hydroxymethyltransferase; and the types I and II NRPS architecture for structural diversity. Our findings reveal the biosynthetic potential of a widespread benzobactin gene cluster.
Abstract
Benzoxazolinate is a rare bis‐heterocyclic moiety that interacts with proteins and DNA. It was found that a putative acyl AMP‐ligase mediates the last cyclization step to afford benzoxazolinate. The enzyme was then used as a probe for genome mining, which led to the discovery of a new class of benzoxazolinate‐containing compounds in diverse bacteria.
Introduction
Benzoxazolinate (1) moiety is a rare feature in natural products and was first found in C‐1027 (Figure 1), [1] endowing C‐1027 with remarkable biological activities. The bis‐heterocyclic moiety not only stabilizes the enediyne chromophore of C‐1027 during biosynthesis and secretion by noncovalently binding to the CagA apoprotein,[ 2 , 3 ] but also intercalates DNA and positions the enediyne core in the minor groove [4] to induce DNA cleavage. [5] The biosynthetic genes responsible for synthesizing 1 were first identified in Streptomyces globisporus. [6] Subsequent in vitro functional characterization [7] of two key enzymes encoded by the sgc biosynthetic gene cluster (BGC; Figure 2a) revealed that SgcD, an anthranilate synthase component I homolog, unexpectedly converts chorismic acid into 2‐amino‐2‐deoxyisochorismic acid (ADIC; Figure 3a), which is devoid of cleaving pyruvate activity as usually observed for anthranilate synthase component I. Then, an FMN‐dependent and [Fe−S]‐containing enzyme, SgcG, dehydrogenates the dihydroaromatic ring of ADIC to an aromatic scaffold without loss of the enolpyruvate, leading to 3‐O‐enolpyruvoylanthranilic acid (OPA) formation. However, the enzyme catalyzing intramolecular attack of the NH2 on the carboxylate of the enolpyruvate to afford a 1,4‐oxazolinate ring remains undefined.
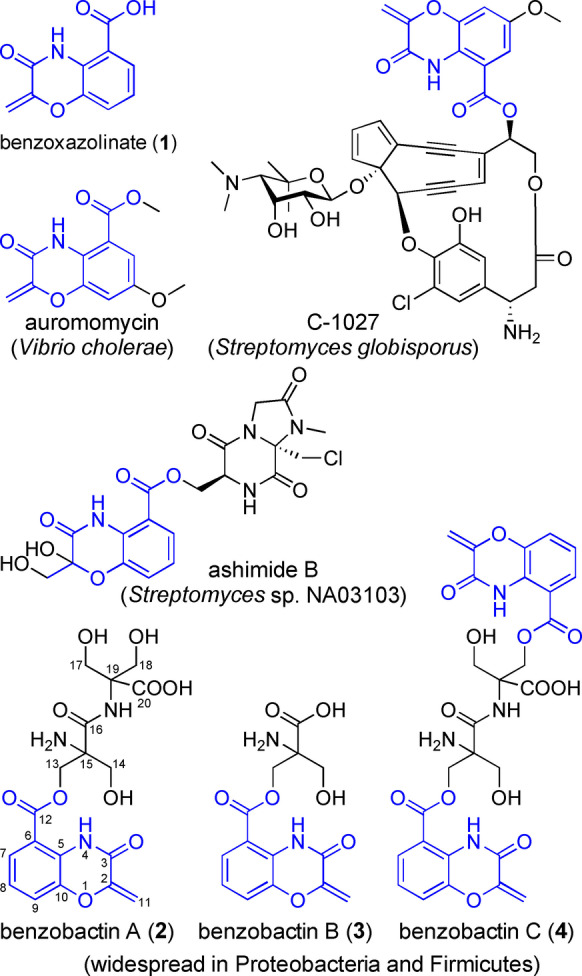
Representative natural products containing a benzoxazolinate moiety reported thus far, as well as previously unknown benzobactins B (3) and C (4).
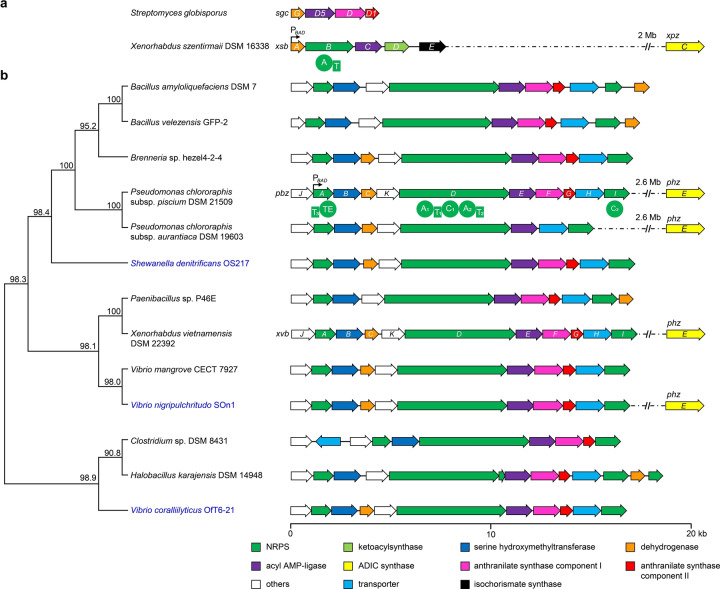
BGCs encoding the biosynthesis of benzoxazolinate and benzobactins in different strains. a) BGCs encoding the biosynthesis of 1 from S. globisporus (sgc) and X. szentirmaii DSM 16338 (xsb). b) Phylogenetic tree and gene organization of the pbz and xvb BGCs and their representative homologs. The tree is based on protein sequences of putative benzobactin BGCs. Numbers next to the branch indicate the percentage of replicate trees in which this topology was reached using a bootstrap test of 1000 replicates. Microbes that originate from marine resources are highlighted in blue. ADIC synthases are encoded by xpzC and phzE, which are unclustered with the benzoxazolinate and benzobactin BGCs, and their distances from the BGC in the genome are indicated if available. A black arrow in the BGC shows the position where an arabinose‐inducible promoter P BAD is inserted. A, adenylation; T, thiolation; C, condensation; and TE, thioester domain. kb, kilobase.
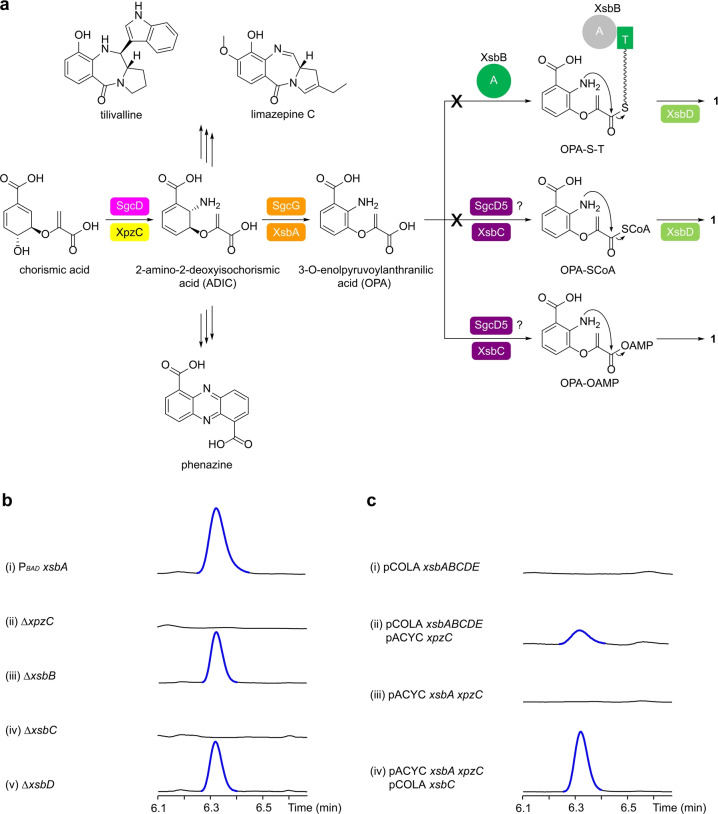
Characterization of the benzoxazolinate biosynthesis in X. szentirmaii DSM 16338. a) Biosynthetic pathway of 1 in X. szentirmaii (xsb) and S. globisporus (sgc). ADIC serves as a key branching point for the biosynthesis of tilivalline, limazepine, and phenazine. The color codes enzymes are corresponding to the encoded genes in Figure 2. A, adenylation; and T, thiolation domains. b) HPLC‐UV analysis of (i) the constructed promoter exchange mutant X. szentirmaii P BAD xsbA and (ii‐v) deletion mutants. Deletions were carried out in the X. szentirmaii P BAD xsbA mutant. Mutants expressing xsb BGC were induced by l‐arabinose. c) HPLC‐UV analysis of (i‐iv) heterologous expression of xsb and xpz genes in E. coli BL21(DE3) induced by IPTG. 1 (blue) is highlighted in traces.
Auromomycin [8] and ashimides [9] (Figure 1) are two additional classes of benzoxazolinate‐containing natural products, of which auromomycin is a nonbacteridical, potent inhibitor against biofilm formation in Vibrio. [8] Cytotoxic benzobactin A (2) is the product of the xvb pathway in the entomopathogenic bacterium Xenorhabdus vietnamensis, [10] featuring two non‐proteinogenic amino acid residues, 2‐hydroxymethylserines, that are a rare building block only found in antrimycin [11] thus far. The xvb BGC coding for types I and II non‐ribosomal peptide synthetase (NRPS) hybrid [12] was identified by extensive mapping of Xenorhabdus and Photorhabdus BGCs to the MIBiG database, [10] and the biosynthetic machinery of 2 is yet to be characterized. To date, benzoxazolinate‐containing natural products were only accessible with these four bacterial producers. Here, we present that genome mining enabled by gene functional characterization of benzoxazolinate biosynthesis provides a prolific producer for a class of benzoxazolinate derivatives, whose BGC is widely distributed across Gram‐negative and Gram‐positive strains.
Results and Discussion
Biosynthetic Crosstalk
To explore potential alternative benzoxazolinate‐producing hosts among Xenorhabdus and Photorhabdus, we used the protein sequence of SgcG as a probe to search for putative BGCs. We found that in addition to XvbC encoded by the known xvb BGC in X. vietnamensis, [10] another two SgcG homologs present in the genomes of X. szentirmaii DSM 16338 and X. szentirmaii US are located in two highly similar gene clusters, of which the BGC in X. szentirmaii DSM 16338 is designated as xsb. The xsb BGC (Figure 2a and Table S4) is strikingly different from the part of sgc BGC encoding benzoxazolinate biosynthesis (hereafter “sgc BGC” refers to the benzoxazolinate cluster in S. globisporus). The xsb BGC lacks an SgcD homolog, and only xsbA (encoding a dehydrogenase) and xsbC (encoding a phenylacetate‐CoA ligase family enzyme) contain a counterpart in the sgc BGC. The remaining xsb BGC is made up of xsbB which encodes an NRPS with an adenylation (A) and a thiolation (T) domain, xsbD that encodes a 3‐oxoacyl‐ACP synthase, and xsbE that encodes an isochorismate synthase.
To explore the biosynthetic theme of xsb BGC, we inserted an arabinose‐inducible P
BAD
promoter
[13]
in front of xsbA and found that the induced P
BAD
xsbA mutant yielded a single peak (Figure 3b, trace i) via untargeted metabolite searches. The compound was isolated and determined to be 1 by HRMS (Table S5) and NMR spectroscopy (Table S6 and Figure S1). However, in contrast to the homologous expression of xsb BGC, the heterologous expression of xsbABCDE in Escherichia coli BL21(DE3) did not produce 1 (Figure 3c, trace i). These results suggest that the xsb BGC alone is insufficient to fulfill production of 1 and that most likely, an enzyme encoded by a discrete gene for ADIC synthesis is required. Given that ADIC is a common precursor of phenazine
[14]
and pyrrolobenzodiazepine
[15]
(e.g. tilivalline and limazepines) (Figure 3a), we postulated that the deficiency of xsb BGC in synthesizing ADIC precursor is complemented by the phenazine or pyrrolobenzodiazepine pathway. The genome of X. szentirmaii does not encode pyrrolobenzodiazepine‐related pathways, and therefore it is highly likely that the xsb BGC “borrows” ADIC derived from the phenazine pathway (xpz), which has a high expression level in the wild‐type strain.
[16]
Consistent with our hypothesis, co‐expression of xsbABCDE with the ADIC synthase encoding gene xpzC (homologous to PhzE
[17]
) from a typical phenazine operon
[16]
yielded 1 (Figure 3c, trace ii). Interestingly, XpzC which is the only ADIC synthase present in X. szentirmaii DSM 16338 is functionally identical to SgcD, but both share a low sequence identity (14.5 %), consistent with previously described.
[7]
Two putative anthranilate synthase component I encoding genes, xsze_03053 and xsze_03255, were found when using SgcD as a probe to search for homologs in the genome of X. szentirmaii DSM 16338. We were concerned that Xsze_03053 and Xsze_03255 could also synthesize ADIC and such genetic redundancy within X. szentirmaii DSM 16338 could functionally complement XpzC. Therefore, we deleted xpzC and the obtained P
BAD
xsbA ΔxpzC mutant lost production of 1 (Figure 3b, trace ii), which points to the essential role of xpzC from a phenazine operon in generating the ADIC precursor for the xsb pathway (Figure 3a).
%), consistent with previously described.
[7]
Two putative anthranilate synthase component I encoding genes, xsze_03053 and xsze_03255, were found when using SgcD as a probe to search for homologs in the genome of X. szentirmaii DSM 16338. We were concerned that Xsze_03053 and Xsze_03255 could also synthesize ADIC and such genetic redundancy within X. szentirmaii DSM 16338 could functionally complement XpzC. Therefore, we deleted xpzC and the obtained P
BAD
xsbA ΔxpzC mutant lost production of 1 (Figure 3b, trace ii), which points to the essential role of xpzC from a phenazine operon in generating the ADIC precursor for the xsb pathway (Figure 3a).
Cyclization of OPA Intermediate
We next explored the cyclization step of OPA to give 1 in the xsb BGC. Upon closer examination of the gene component, we notice that xsbB features an A domain that might adenylate non‐amino acid substrates, due to the lack of a conserved aspartate residue in motif A4 that typically interacts with the α‐NH2 of amino acid substrates
[18]
(Figure S2). Also, a 3‐oxoacyl‐ACP synthase encoded by xsbD has a high sequence identity (83.6 %) with XpzS
[16]
that catalyzes amide‐ and ester‐bond formation between the carrier‐protein‐bound aminoacyl with phenazines. Therefore, there are three conceivable pathways to achieve OPA cyclization (Figure 3a): 1) the A domain of XsbB adenylates OPA for loading onto the T domain, and subsequently, XsbD catalyzes intramolecular amide‐bond formation; 2) XsbC thioesterifies the carboxylate of enolpyruvate with a CoASH, followed by amide‐bond formation under XsbD catalysis; or 3) the cyclization occurs in a non‐thiotemplated fashion, during which XsbC activates the carboxylate of enolpyruvate through ATP‐dependent adenylation, followed by direct nucleophilic attack of the NH2 on the carboxylate without forming a CoA‐bound thioester intermediate, as observed during the biosyntheses of coumermycin A1,
[19]
dapdiamide,
[20]
and yatakemycin.
[21]
To confirm the involvement of genes in OPA cyclization, we individually deleted xsbB, xsbC, and xsbD. While the P
BAD
xsbA ΔxsbB and P
BAD
xsbA ΔxsbD mutants did not affect the product profile (Figure 3b, traces iii and v), 1 was completely absent in the P
BAD
xsbA ΔxsbC mutant (Figure 3b, trace iv), revealing xsbC being solely responsible for the formation of 1 from OPA. We then verified the function of XsbC by in vivo experiments. E. coli BL21(DE3) harboring xsbA, xpzC, and xsbC produced 1, while no production of 1 was observed in E. coli expressing xsbA and xpzC (Figure 3c, traces iii and iv).
%) with XpzS
[16]
that catalyzes amide‐ and ester‐bond formation between the carrier‐protein‐bound aminoacyl with phenazines. Therefore, there are three conceivable pathways to achieve OPA cyclization (Figure 3a): 1) the A domain of XsbB adenylates OPA for loading onto the T domain, and subsequently, XsbD catalyzes intramolecular amide‐bond formation; 2) XsbC thioesterifies the carboxylate of enolpyruvate with a CoASH, followed by amide‐bond formation under XsbD catalysis; or 3) the cyclization occurs in a non‐thiotemplated fashion, during which XsbC activates the carboxylate of enolpyruvate through ATP‐dependent adenylation, followed by direct nucleophilic attack of the NH2 on the carboxylate without forming a CoA‐bound thioester intermediate, as observed during the biosyntheses of coumermycin A1,
[19]
dapdiamide,
[20]
and yatakemycin.
[21]
To confirm the involvement of genes in OPA cyclization, we individually deleted xsbB, xsbC, and xsbD. While the P
BAD
xsbA ΔxsbB and P
BAD
xsbA ΔxsbD mutants did not affect the product profile (Figure 3b, traces iii and v), 1 was completely absent in the P
BAD
xsbA ΔxsbC mutant (Figure 3b, trace iv), revealing xsbC being solely responsible for the formation of 1 from OPA. We then verified the function of XsbC by in vivo experiments. E. coli BL21(DE3) harboring xsbA, xpzC, and xsbC produced 1, while no production of 1 was observed in E. coli expressing xsbA and xpzC (Figure 3c, traces iii and iv).
To support our hypothesis that XsbC belonging to the phenylacetate‐CoA ligase family mediates OPA cyclization via an ATP‐dependent adenylating manner (Figure 3a), we pursued a maximum‐likelihood‐based phylogenetic analysis
[22]
to infer the relationships between XsbC and enzymes in the ANL (acyl‐CoA synthetases, NRPS adenylation domains, and luciferase enzymes) superfamily. The resultant tree (Figure S3) showed that XsbC is distantly related to NatL2 and BomJ, both of which are acyl‐AMP ligases involved in intermolecular ester‐bond formation.[
23
,
24
] Although a multi‐sequence alignment indicated a low sequence identity (19.8 %) of XsbC with NatL2, a closer inspection of the alignment (Figure S4) and homology modeling (Figures S5–8) revealed that XsbC harbors diagnostic binding sites and conserved motifs that are observed in NatL2.
[23]
The Zn2+‐binding tetrad Cys263‐His269‐Cys321‐Cys323 is a unique feature in distinguishing NatL2 from canonical Mg2+‐binding CoA ligases and is well conserved in XsbC (Figures S4 and S6). Most importantly, a C‐terminal extension with Lys429 in NatL2 is also present in XsbC. Lys429 from the other monomer forms a salt bridge with the AMP (Figure S7), and thus C‐terminal extension crossing to the other monomer is proposed to lock the functional homodimer in the “close” conformation, which prevents a CoA from entering the binding pocket.
%) of XsbC with NatL2, a closer inspection of the alignment (Figure S4) and homology modeling (Figures S5–8) revealed that XsbC harbors diagnostic binding sites and conserved motifs that are observed in NatL2.
[23]
The Zn2+‐binding tetrad Cys263‐His269‐Cys321‐Cys323 is a unique feature in distinguishing NatL2 from canonical Mg2+‐binding CoA ligases and is well conserved in XsbC (Figures S4 and S6). Most importantly, a C‐terminal extension with Lys429 in NatL2 is also present in XsbC. Lys429 from the other monomer forms a salt bridge with the AMP (Figure S7), and thus C‐terminal extension crossing to the other monomer is proposed to lock the functional homodimer in the “close” conformation, which prevents a CoA from entering the binding pocket.
Taking all these data into account, we propose a three‐gene cassette consisting of an anthranilate synthase component I homolog (e.g. SgcD) or ADIC synthase (e.g. XpzC), an FMN‐dependent and [Fe−S]‐containing dehydrogenase (e.g. SgcG and XsbA), and an acyl‐AMP ligase (e.g. XsbC) being responsible for benzoxazolinate biosynthesis.
Benzobactin BGC in Diverse Bacteria
To expand the biosynthetic repertoire of benzoxazolinate‐containing natural products, we carried out a survey of all bacterial genomes available in the NCBI database using the protein sequence of XsbC as a query. Besides C‐1027 [6] and ashimides [9] homologous BGCs found in Actinobacteria as we expected, the Blast result revealed that the orphan xvb BGC responsible for benzobactin biosynthesis in entomopathogenic bacteria Xenorhabdus and Photorhabdus [10] abounds in Pseudomonas, and is prevalent across two phyla of bacteria (Figure 2b), the Proteobacteria (e.g. Pseudomonas, Vibrio, Shewanella, and Brenneria) and Firmicutes (e.g. Bacillus, Paenibacillus, Halobacillus, and Clostridium). Such extensive similarities to the xvb BGC suggest that benzoxazolinate‐containing natural products are more widespread in diverse bacteria—from ocean to land—than we thought, and that Pseudomonas being the most abundant genus among all these strains might be prolific producers for these rare natural products.
We selected different Pseudomonas strains for homologously expressing the target BGC by a promoter‐exchange strategy. [25] A BGC, designated as pbz, from Pseudomonas chlororaphis subsp. piscium DSM 21509 was successfully activated as indicated by the significant distinction of HPLC profiles between induced and non‐induced mutants (Figure 4a, traces i and ii). In addition to 2 produced in a high amount, the P. chlororaphis P BAD pbzA mutant yielded five more benzobactin derivatives with masses ranging from 305 to 1012 (Table S5) as determined by untargeted analysis, and therefore we attempted to isolate them for structure elucidation. The compound with a mass of 305, designated as benzobactin B (3) confirmed by NMR spectroscopy (Table S6 and Figure S1), has one 2‐hydroxymethylserine residue less than 2. Compounds with larger masses (e.g. 609, 628, and 1012) were not isolated due to their low production levels and instability. Among them, the structure of a compound with a mass of 609, designated as benzobactin C (4), was tentatively assigned to be a dimer of 3 by tandem MS/MS and isotope labeling experiments (Figures S9 and S10a). Based on the sum formula (Table S5), we proposed that benzobactins‐628 and 1012 are a dimer and tetramer of 3, respectively, while their exact chemical structures cannot be formulated at the current stage.
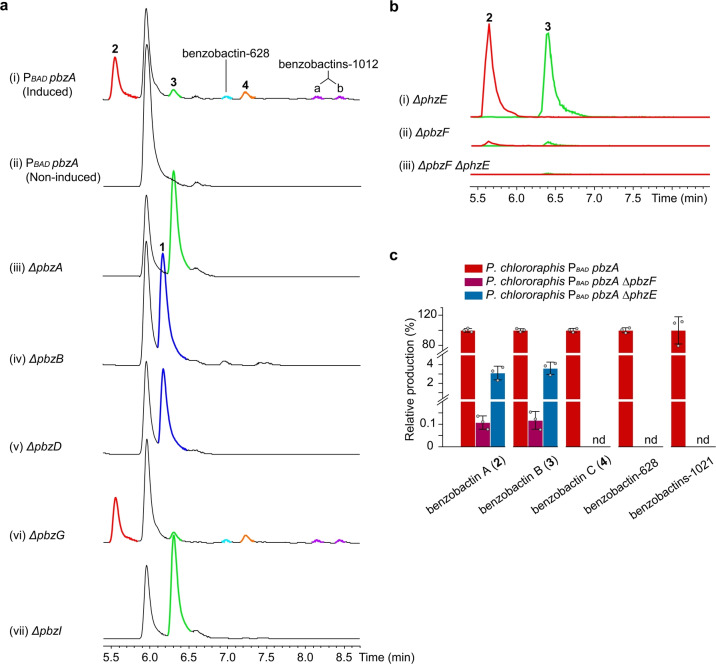
HPLC analysis of P. chlororaphis subsp. piscium DSM 21509 mutants. a) HPLC‐UV analysis of (i) the constructed promoter exchange mutant P. chlororaphis P BAD pbzA and (iii–vii) deletion mutants. Deletions were carried out in the P BAD pbzA mutant. Mutants expressing the pbz BGC were induced by l‐arabinose. 1 (dark blue) and 2 (red), 3 (green), and 4 (orange), as well as the as‐yet‐uncharacterized benzobactins‐628 (light blue) and 1021 (purple), are highlighted in the traces. (i) The P BAD pbzA and (vi) P BAD pbzA ΔpbzG mutants shared an identical metabolite profile, indicating that PbzG (homologous to anthranilate synthase component II) might not be involved in benzobactin biosyntheses, or that its function could be complemented by other genes in the genome. b) HPLC‐MS analysis of (i–iii) the ΔpbzF, ΔphzE, and ΔpbzF ΔphzE mutants for benzobactin production. Deletions were carried out in the P. chlororaphis P BAD pbzA mutant. Shown are the EICs of 2 (red) and 3 (green) in deletion mutants. c) Production of benzobactins in the ΔpbzF and ΔphzE mutants. The production of individual compounds at each deletion mutant is relative to its production in the P BAD pbzA mutant (in percentage, %). nd=not detected.
Synergistic Effect of ADIC and Anthranilate Synthases
The pbz BGC contains a typical three‐gene benzoxazolinate cassette pbzCEF. An ADIC synthase (PhzE) in a phenazine biosynthetic operon that is 2.6 megabase pairs away from the pbz BGC is also present in the genome (Figure 2b). Again, consistent with the speculation that the ADIC precursor could be synthesized by PhzE and PbzF, both of which are functionally complementary, the P BAD pbzA ΔphzE and P BAD pbzA ΔpbzF mutants still yielded 2 and 3 (Figure 4b, traces i and ii), while the products completely lost in the P BAD pbzA ΔpbzF ΔphzE mutant (Figure 4b, trace iii). Surprisingly, the production titers of 2 and 3 decreased ≈30‐fold in the P BAD pbzA ΔphzE mutant and ≈900‐fold in the P BAD pbzA ΔpbzF mutant, compared to that of the P BAD pbzA mutant (Figure 4c). This suggests that PbzF and PhzE are not only functionally complementary but also have a synergistic positive effect on production level of the downstream benzobactin products, presumably because the ADIC metabolic flux is dramatically increased by both enzymes. The coexistence of pbzF and phzE also occurs in the genomes of X. vietnamensis DSM 22392 and Vibrio nigripulchritudo SOn1 (Figure 2b). These observations perhaps are unsurprising, because phenazines, which often have a high expression level being the pigmentation phenotype of wild‐type strains, are much more widely distributed in bacteria (particularly in Pseudomonas)[ 26 , 27 ] than benzoxazolinate derivatives.
Unusual Serine Hydroxymethyltransferase
The bimodular NRPS, PbzD, appears to be responsible for loading and elongating 1 (Figure 5a), since the A1 domain is non‐amino‐acid substrate specific, as indicated by its motif A4 (Figure S2). This is supported by the P BAD pbzA ΔpbzD mutant that abolished production of benzobactins but accumulated that of 1 (Figure 4a, trace v). It is proposed that the incorporation of a 2‐hydroxymethylserine into the benzoxazolinate moiety is performed by the second module of PbzD with the assistance of PbzB which is a putative serine hydroxymethyltransferase. Pyridoxal‐5′‐phosphate‐dependent (PLP) serine hydroxymethyltransferases were reported to catalyze the transfer of a hydroxymethyl group from 5,10‐methylenetetrahydrofolate (mTHF) to glycine or alanine to afford l‐serine or α‐methylsereine, as found during the biosyntheses of ashimides [9] and JBIR‐34/35 (ref. [28]). PbzB has high sequence similarity with AsmD [9] and FmoH, [28] and contains a conserved PLP‐binding loop (Figure S11) in which the lysine residue forms a Schiff base with PLP. [29] Therefore, we assumed that PbzB could catalyze mono‐hydroxymethylation of serine or/and bis‐hydroxymethylation of glycine to afford 2‐hydroxymethylserine.
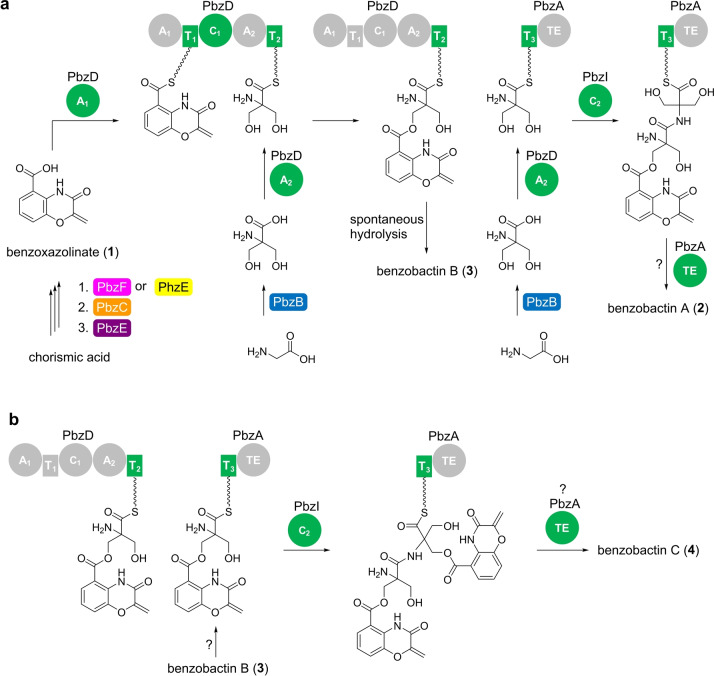
Proposed biosynthesis of benzobactins in P. chlororaphis subsp. piscium DSM 21509. a) Proposed biosynthetic pathways of 2 and 3. b) Proposed biosynthetic pathway of 4. Domain involved in the corresponding reaction step is highlighted in green. The color codes enzymes are corresponding to the encoded genes in Figure 2. A, adenylation; T, thiolation; C, condensation; and TE, thioester domains.
To verify the origin of building blocks in vivo, we cultivated the P BAD pbzA mutant in 15N media supplemented with l‐serine and glycine. Exemplified by compound 3, while no inverse mass shifts were obtained in the 15N medium supplemented with l‐serine, we observed—1 Da mass shift in the 15N medium supplemented with glycine, which resulted from the incorporation of a glycine residue (Figure S10b). Phylogenetic analysis revealed that the PbzD‐A2 domain unexpectedly falls into the clade of A domains with cysteine specificity and is separate from those with glycine or serine specificity (Figure S12). This suggests that PbzD‐A2 domain might be evolved for larger substrates and adenylate non‐glycine substrates for loading onto PbzD‐T2 domain. Thus, we propose that PbzB catalyzes the conversion of glycine into 2‐hydroxymethylserine prior to being subjected to the PbzD‐A2 domain activation (Figure 5a).
To verify the conversion of glycine into 2‐hydroxymethylserine by PbzB, we expressed and purified PbzB with a His‐SUMO‐tag from E. coli BL21(DE3) (Figure S13a) and conducted in vitro enzymatic assays (Figure 6a). Incubation of PbzB with glycine and cofactors, PLP and mTHF, in the potassium phosphate buffer at pH 7.5 showed the formation of 2‐hydroxymethylserine and a trace amount of serine (Figure 6b, trace ii). This finding motivated us to examine whether PbzB can also catalyze the conversion of d‐/l‐serine into 2‐hydroxymethylserine. Surprisingly, under the same enzymatic reaction condition, the production of 2‐hydroxymethylserine that was converted from d‐/l‐serine was much higher than that from glycine (Figure 6c). While glycine was the authentic building block in vivo, serine was a more favored in vitro substrate for PbzB than glycine. Two possible explanations for the seemingly conflicting results are that 1) in in vitro, the one‐step conversion of serine to 2‐hydroxymethylserine is faster than the two‐step conversion of glycine to 2‐hydroxymethylserine; 2) in in vivo, the degradation of serine to glycine catalyzed by the serine hydroxymethyl transferase from primary metabolism is faster than the formation of serine to 2‐hydroxymethylserine catalyzed by PbzB. We then carried out a steady‐state kinetic analysis for the formation of 2‐hydroxymethylserine using d‐/l‐serine (Figure 6d and e), which suggested that the affinity of PbzB for l‐serine is 9‐fold higher than that for d‐serine as indicated by the K m values, and that PbzB is 22‐fold more efficient at catalyzing the formation of 2‐hydroxymethylserine with l‐serine than that with d‐serine based on the k cat/K m values.
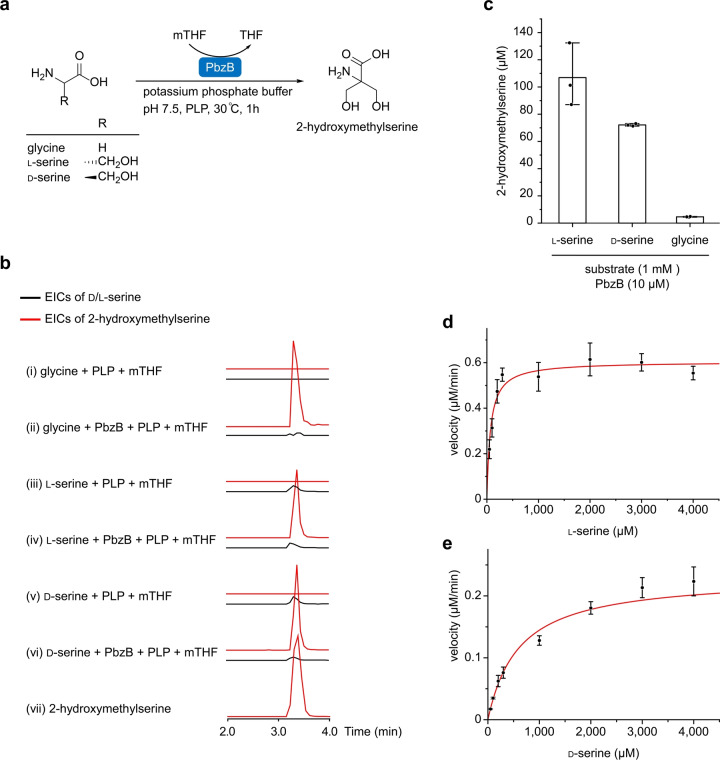
In vitro characterization of 2‐hydroxymethylserine conversions. a) Enzymatic conversion of glycine and d‐/l‐serine to 2‐hydroxymethylserine using the recombinant serine hydroxymethyltransferase PbzB. b) EICs of d‐/l‐serine (black, 106.0492 [M+H]+) and 2‐hydroxymethylserine (red, 136.0604 [M+H]+) are shown, while the mass of glycine is too small to be detected. c) Quantification of 2‐hydroxymethylserine production with d‐/l‐serine and glycine substrates. Steady‐state kinetics of formation of 2‐hydroxymethylserine from d) l‐serine (K m=69.52±0.03 μM, k cat=0.151±0.006 min−1) and e) d‐serine (K m=602.90±56.67 μM, k cat=0.058±0.003 min−1).
Bioinformatics and Structure of PbzB
Serine hydroxymethyltransferases involved in the central metabolism have been studied in various organisms.
[30]
While GlyA from E. coli, one of the best‐studied examples, catalyzes reversible conversion between serine and glycine and thereby affords mTHF as the major source of C1 unit in cells,
[31]
the newly identified PbzB involved in the benzobactin biosynthesis catalyzes production of 2‐hydroxymethylserine using l‐serine, d‐serine, or glycine. Sequence alignment analysis (Figure S11b) of PbzB and its homologs AsmD,
[9]
FmoH,
[28]
and XvbB
[10]
(PbzB type) in comparison with structurally and biochemically characterized GlyA‐type enzymes revealed a high sequence similarity of over 42 %. Almost all residues involved in coordinating N‐pyridoxyl‐glycine‐5‐phosphate (PLG) and mTHF were conserved among the analyzed proteins (Figure S11b). While GlyA‐type enzymes contain a conserved YAEG— ‐RYY motif, where the two tyrosine residues (Y65 and Y55) are crucial for efficient substrate conversion and the formation of cation π‐interaction with the ligand,[
32
,
33
] the PbzB‐type enzymes harbor a TAEG— ‐RYH motif (Figure S11b).
%. Almost all residues involved in coordinating N‐pyridoxyl‐glycine‐5‐phosphate (PLG) and mTHF were conserved among the analyzed proteins (Figure S11b). While GlyA‐type enzymes contain a conserved YAEG— ‐RYY motif, where the two tyrosine residues (Y65 and Y55) are crucial for efficient substrate conversion and the formation of cation π‐interaction with the ligand,[
32
,
33
] the PbzB‐type enzymes harbor a TAEG— ‐RYH motif (Figure S11b).
To gain an insight into the substituted residues involved in substrate binding in PbzB, we set out to structurally analyze its apo‐ and substrate‐bound form. While crystals of the apo‐form diffracted to 2.8 Å, we only obtained poorly diffracting anisotropic crystals for PbzB co‐crystallized with l‐serine and mTHF. The crystal structure of apo‐PbzB (Figure 7a; Table S7; PDB 7QWZ) shared the overall fold of GlyA‐type enzymes with an RMSD value of 2.86 to GlyA (Figure 7b and c). PbzB, same as GlyA‐type enzymes, forms homodimers with two active sites shared between the two monomers (Figure 7a and b). However, the residues coordinating PLG and mTHF were not visible in the PbzB structure, since they were probably too flexible in the absence of a ligand (Figure 7d), and therefore, we modeled PbzB using Alphafold.
[34]
The simulated structural model had a confidence of over 95 % and an RMSD of 0.97 and 0.84 for the crystal structures of apo‐PbzB and GlyA from E. coli,
[31]
respectively (Figure 7c). Given that GlyA binds PLP catalyzing formation of PLG in presence of glycine, we postulated that in addition to the formation of PLG, PbzB generates N‐pyridoxyl‐serine‐5‐phosphate (PLS) as an external aldimine formed by PLP and l‐serine (Figure 7e). An in‐depth analysis of the active sites revealed that all residues involved in substrate binding within GlyA and PbzB are superimposable (Figure 7f). Even Y55 and Y65 in GlyA are superimposable with T68 and H78 in PbzB, but these alterations might enlarge the binding pocket of PbzB. While Y55 and Y65 in GlyA perfectly coordinate PLG, T68 and H78 in PbzB open the space for the binding of PLS. Particularly, the substitution of Y55 by T68 may lead to a reduction of negative charges in the binding pocket and thus enable coordination of serine bound to PLP (Figure 7g–i). We constructed a site‐directed mutant of PbzB to investigate the key residues and attempt to shrink the capacity of the binding pocket. T68 and H78 were both mutated to Tyr as in GlyA (Figure S13). However, the PbzB T68Y H78Y mutant did not catalyze formation of any detectable amount of serine or 2‐hydroxymethylserine, suggesting that T68 and H78 residues are crucial to maintaining the function of PbzB.
% and an RMSD of 0.97 and 0.84 for the crystal structures of apo‐PbzB and GlyA from E. coli,
[31]
respectively (Figure 7c). Given that GlyA binds PLP catalyzing formation of PLG in presence of glycine, we postulated that in addition to the formation of PLG, PbzB generates N‐pyridoxyl‐serine‐5‐phosphate (PLS) as an external aldimine formed by PLP and l‐serine (Figure 7e). An in‐depth analysis of the active sites revealed that all residues involved in substrate binding within GlyA and PbzB are superimposable (Figure 7f). Even Y55 and Y65 in GlyA are superimposable with T68 and H78 in PbzB, but these alterations might enlarge the binding pocket of PbzB. While Y55 and Y65 in GlyA perfectly coordinate PLG, T68 and H78 in PbzB open the space for the binding of PLS. Particularly, the substitution of Y55 by T68 may lead to a reduction of negative charges in the binding pocket and thus enable coordination of serine bound to PLP (Figure 7g–i). We constructed a site‐directed mutant of PbzB to investigate the key residues and attempt to shrink the capacity of the binding pocket. T68 and H78 were both mutated to Tyr as in GlyA (Figure S13). However, the PbzB T68Y H78Y mutant did not catalyze formation of any detectable amount of serine or 2‐hydroxymethylserine, suggesting that T68 and H78 residues are crucial to maintaining the function of PbzB.
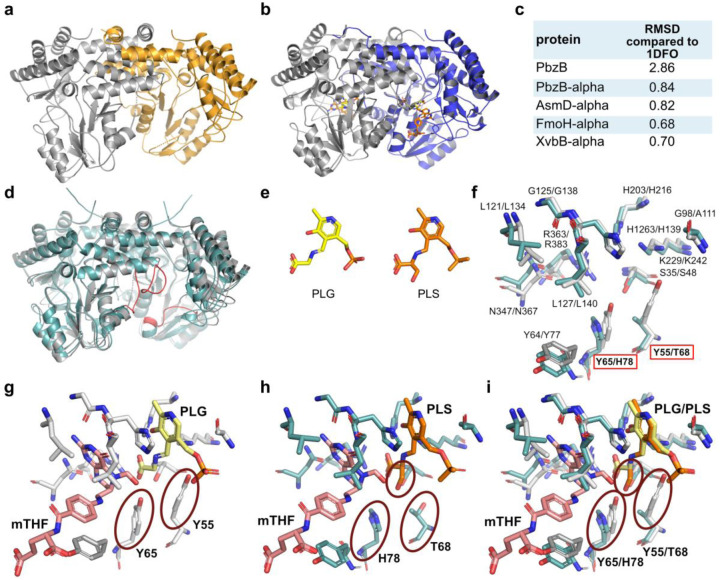
Structural and bioinformatical analysis of PbzB and its homologs. a) Overall‐fold of the crystal structure of apo PbzB. The individual monomers of the dimeric complex are shown in grey and orange (PDB 7QWZ). b) Overall‐fold of the crystal structure of GlyA in complex with PLG (yellow) and mTHF (pink). The two monomers of the GlyA dimer are shown in grey and blue (PDB 1DFO). [31] c) Root mean square deviation (RMSD) of PbzB apo structure (PDB 7QWZ), PbzB model, AsmD model, FmoH model, and XvbB model (models obtained by Alphafold) in comparison to the crystal structure of GlyA (PDB 1DFO). [31] d) Overlay of the PbzB Alphafold model (green) and the PbzB apo crystal structure (grey). Undissolved regions of the PbzB crystal structure are highlighted with red in the PbzB Alphafold model. e) Comparison of the PLP‐bound substrates PLG and PLS. f) Overlay of the residues present in the active site of PbzB (Alphafold model, green) and GlyA (crystal structure, PDB 1DFO, grey). g) Detailed view of the GlyA active site coordinating mTHF (pink) and PLG (yellow). h) Modeled view of the PbzB active site coordinating mTHF (pink) and PLS (orange). i) Overlay of the active sites of GlyA and the PbzB Alphafold model. In (g)–(i), the residues with red circles are critical for fitting the amino acid substrate into the active site. In i, possible stereo hinderance between serine and Y65/Y55 in GlyA is indicated.
Consistent with a general observation that central metabolism enzymes have higher k
cat and k
cat/K
m values than secondary metabolism enzymes,
[35]
the k
cat and k
cat/K
m values of GlyA
[36]
is 4200‐fold and 360 000‐fold larger than those of PbzB for l‐serine as a substrate. However, the K
m value of PbzB is ≈9‐fold lower than GlyA, suggesting that PbzB has a better affinity for l‐serine than GlyA. Although PbzB is much less efficient than GlyA, a low flux of 2‐hydroxymethylserine formation can be maintained by PbzB that satisfies the requirement of benzobactin production without disturbing primary metabolisms. Therefore, one could assume that benzobactin‐producing bacteria remodel an enzyme from the central amino acid metabolism to deliver a building block for the NRPS biosynthetic pathway. Since only 1 and no benzobactins were observed in the P
BAD
pbzA ΔpbzB mutant (Figure 4a, trace iv), 2‐hydroxymethylserine furnished by PbzB is crucial for the downstream assembly line.
000‐fold larger than those of PbzB for l‐serine as a substrate. However, the K
m value of PbzB is ≈9‐fold lower than GlyA, suggesting that PbzB has a better affinity for l‐serine than GlyA. Although PbzB is much less efficient than GlyA, a low flux of 2‐hydroxymethylserine formation can be maintained by PbzB that satisfies the requirement of benzobactin production without disturbing primary metabolisms. Therefore, one could assume that benzobactin‐producing bacteria remodel an enzyme from the central amino acid metabolism to deliver a building block for the NRPS biosynthetic pathway. Since only 1 and no benzobactins were observed in the P
BAD
pbzA ΔpbzB mutant (Figure 4a, trace iv), 2‐hydroxymethylserine furnished by PbzB is crucial for the downstream assembly line.
Atypical Condensation Domain/Enzyme
The PbzD‐C1 domain is proposed to catalyze ester‐bond formation between benzoxazolinyl‐S‐T1 and 2‐hydroxymethylserinyl‐S‐T2 (Figure 5a). Interestingly, PbzD‐C1 is homologous to a heterocyclization domain as indicated by the phylogenetic analysis (Figure S14a) and sequence alignment (Figure S14b). Moreover, although 2‐hydroxymethylserine is a substrate containing both free NH2 and OH, the PbzD‐C1 domain appears to have a preference for using the OH as a nucleophile, which might result from the primary hydroxyl group having less steric hindrance than the α‐tertiary amine. The off‐loading of benzoxazolinyl‐2‐hydroxymethylserinyl‐S‐T2 to afford 3 is irrelevant to the PbzA‐TE domain but instead might be achieved by spontaneous hydrolysis, as indicated by the P BAD pbzA ΔpbzA mutant that still produced 3 (Figure 4a, trace iii).
The absence of 2 in the P BAD pbzA ΔpbzA mutant (Figure 4a, trace ii) also validated the necessity of the PbzA‐T3 domain for the incorporation of a second 2‐hydroxymethylserine into benzoxazolinyl‐2‐hydroxymethylserinyl‐S‐T2 (Figure 5a). Since no extra A domains are encoded within the BGC, presumably, the PbzA‐T3 domain “borrows” the activity of PbzD‐A2 domain that activates 2‐hydroxymethylserine (Figure 5a). Subsequently, the condensation between benzoxazolinyl‐2‐hydroxymethylserinyl‐S‐T2 and a 2‐hydroxymethylserinyl‐S‐T3 occurs under a putative free‐standing condensation enzyme encoded by pbzI, which is supported by the deletion of pbzI that led to loss of 2 (Figure 4a, trace vii). Interestingly, benzobactin C (4), as well as the as‐yet‐uncharacterized benzobactins‐628 and 1012, were lost in both the P BAD pbzA ΔpbzA and P BAD pbzA ΔpbzI mutants (Figure 4a, traces iii and vii). This indicates that the free‐standing NRPSs might be used iteratively resulting in dimerization or even tetramerization (Figure 5b).
From a functional point of view, PbzI appears to be a canonical condensation enzyme catalyzing amide‐bond formation and regioselectively uses the α‐tertiary amine with steric hindrance as a nucleophile, which differs from the PbzD‐C1 domain. However, phylogenetic analysis showed that PbzI, together with the other homologs that are encoded by putative benzobactin‐related BGCs, falls into a clade that is distinct from all other condensation domains/enzymes (Figure S14a). In particular, a closer inspection of the core motif C3 [xH(D)HxxxDxx] revealed that PbzI and its homologs do not have any histidine or aspartic acid residues in the second and third positions (Figure S14b). PbzI as a free‐standing enzyme with an atypical core motif C3 might be a new example to understand the catalytic mechanism of condensation domains/enzymes.
Conclusion
We first characterized the biosynthesis of benzoxazolinate (xsb) in the entomopathogenic bacterium X. szentirmaii DSM 16338 (Figure 3a): the xsb BGC devoid of an ADIC synthase encoded gene “borrows” the ADIC precursor from the phenazine pathway (xpz); an FMN‐dependent and [Fe−S]‐containing dehydrogenase (XsbA, homologous to SgcG) is assumed to convert ADIC into OPA as previously described; [7] and 1 is formed by intramolecular cyclization of OPA under the mediation of a putative acyl AMP‐ligase (XsbC). Given the structural uniqueness and the fascinating biological function of benzoxazolinate and benzoxazolinate‐containing natural products, we then performed a targeted BGC‐based genome mining by using the protein sequence of XsbC, and discovered that the orphan benzobactin pathway (xvb) in entomopathogenic bacteria Xenorhabdus and Photorhabdus [10] exists in diverse bacteria across Proteobacteria and Firmicutes, as exemplified by the pbz BGC in P. chlororaphis subsp. piscium DSM 21509 (Figure 2b). We homologously overexpressed the pbz BGC and discovered two new benzobactins (3 and 4), as well as benzobactins‐628 and 1012 that are yet to be characterized. Our recent whole‐genome‐sequencing of X. vietnamensis DSM 22392 revealed that the xvb cluster resides on a 51‐kb plasmid and is flanked with transposase and integrases, while the pbz cluster resides on the chromosome. This suggests that the benzobactin BGC is likely to spread horizontally in bacteria at a great evolutionary distance.
During the biosyntheses of benzobactins A–C (2–4), we observe that the anthranilate synthase component I (PbzF in the benzobactin pathway) and the ADIC synthase (PhzE in the phenazine pathway), which share low protein sequence similarity, have a synergistic positive effect on the production level of benzobactins (Figure 4c). Furthermore, our findings might indicate the immense biosynthetic potential of the pbz BGC, in which the serine hydroxymethyltransferase (PbzB) catalyzes mono‐ and bis‐hydroxymethylation to afford 2‐hydroxymethylserine which is an unusual building block in natural products (Figures 5a and and6a),6a), and the free‐standing NRPS gene organization and non‐canonical condensation domain/enzyme (Figure S14) might have an iterative function.
Conflict of interest
The authors declare no conflict of interest.
1.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information
Acknowledgements
We acknowledge the help of Viktor Lehmann from the Philipps University of Marburg during the crystallization process of PbzB and the constructive suggestion on in vitro pathway reconstructions of Dr. Henrike Niederholtmeyer from the Max Planck Institute for Terrestrial Microbiology. Yi‐Ming Shi was supported by the Alexander von Humboldt Foundation. This work is supported by the LOEWE Centre for Translational Biodiversity Genomics (LOEWE TBG) and the ERC advanced grant (835108) to Helge B. Bode. Open Access funding enabled and organized by Projekt DEAL.
Notes
Y.-M. Shi, J. J. Crames, L. Czech, K. A. J. Bozhüyük, Y.-N. Shi, M. Hirschmann, S. Lamberth, P. Claus, N. Paczia, C. Rückert, J. Kalinowski, G. Bange, H. B. Bode, Angew. Chem. Int. Ed. 2022, 61, e202206106; Angew. Chem. 2022, 134, e202206106. [Europe PMC free article] [Abstract]
Contributor Information
Yi‐Ming Shi, Email: [email protected].
Helge B. Bode, Email: [email protected].
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
References
Full text links
Read article at publisher's site: https://doi.org/10.1002/anie.202206106
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/anie.202206106
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/136831757
Article citations
Fragment-Guided Genome Mining of Octacyclic Cyclophane Alkaloids from Fungi.
J Am Chem Soc, 146(34):23933-23942, 14 Aug 2024
Cited by: 0 articles | PMID: 39140852
Genome Mining Enabled by Biosynthetic Characterization Uncovers a Class of Benzoxazolinate-Containing Natural Products in Diverse Bacteria.
Angew Chem Int Ed Engl, 61(51):e202206106, 17 Nov 2022
Cited by: 2 articles | PMID: 36198080 | PMCID: PMC10098953
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
Protein structures in PDBe (2)
-
(3 citations)
PDBe - 7QWZView structure
-
(3 citations)
PDBe - 1DFOView structure
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
An Orphan MbtH-Like Protein Interacts with Multiple Nonribosomal Peptide Synthetases in Myxococcus xanthus DK1622.
J Bacteriol, 200(21):e00346-18, 10 Oct 2018
Cited by: 5 articles | PMID: 30126939 | PMCID: PMC6182236
The expanding spectrum of diketopiperazine natural product biosynthetic pathways containing cyclodipeptide synthases.
Org Biomol Chem, 17(9):2305-2314, 01 Feb 2019
Cited by: 38 articles | PMID: 30688950
Review
New insights into the echinocandins and other fungal non-ribosomal peptides and peptaibiotics.
Nat Prod Rep, 31(10):1348-1375, 01 Oct 2014
Cited by: 37 articles | PMID: 25156669
Review
Enzymatic Synthesis of Diverse Heterocycles by a Noncanonical Nonribosomal Peptide Synthetase.
ACS Chem Biol, 16(12):2776-2786, 12 Nov 2021
Cited by: 4 articles | PMID: 34767712 | PMCID: PMC8917869
Funding
Funders who supported this work.
Alexander von Humboldt-Stiftung
European Research Council (1)
Grant ID: 835108

 1
,
2
,
+
1
,
2
,
+