Abstract
Aim
Monitoring electronic patient-reported outcomes (ePRO) can provide various benefits to cancer patients, such as enhanced quality of life, reduction of hospital admissions, and even prolonged survival. Furthermore, ePRO might offer significant benefits to patients under antineoplastic treatment in the context of the current COVID-19 pandemic. However, evidence on feasibility of ePRO in routine cancer care and barriers met in a real-life setting remains limited.Subject and methods
We conducted a feasibility study among patients diagnosed with multiple myeloma currently under antineoplastic treatment. Patients filled out weekly ePRO questionnaires and were followed up for 6 months. In case of adverse events, an alert was sent to the clinic. We assessed uptake and adherence, as well as subjective perceptions of patients and clinic staff. A semi-structured literature review was conducted to contextualize results.Results
Eleven patients were recruited and followed up for 6 months. Overall adherence was found at a high level and remained stable throughout the study period. Feedback from patients was positive; however, clinic staff expressed disappointment and frustration, criticising an increase of workload while not perceiving any benefit to the oncological treatment. Both findings were backed by evidence we found in literature.Conclusions
Implementation of ePRO monitoring to routine cancer treatment seems to be feasible regarding patients' acceptance and compliance. However, integration of the tool into clinical workflow without increasing workload and deterring clinicians proves to be a major challenge.Supplementary information
The online version contains supplementary material available at 10.1007/s10389-022-01767-3.Free full text

Essential barriers and considerations for the implementation of electronic patient-reported outcome (ePRO) measures in oncological practice: contextualizing the results of a feasibility study with existing literature
Associated Data
Abstract
Aim
Monitoring electronic patient-reported outcomes (ePRO) can provide various benefits to cancer patients, such as enhanced quality of life, reduction of hospital admissions, and even prolonged survival. Furthermore, ePRO might offer significant benefits to patients under antineoplastic treatment in the context of the current COVID-19 pandemic. However, evidence on feasibility of ePRO in routine cancer care and barriers met in a real-life setting remains limited.
Subject and methods
We conducted a feasibility study among patients diagnosed with multiple myeloma currently under antineoplastic treatment. Patients filled out weekly ePRO questionnaires and were followed up for 6 months. In case of adverse events, an alert was sent to the clinic. We assessed uptake and adherence, as well as subjective perceptions of patients and clinic staff. A semi-structured literature review was conducted to contextualize results.
Results
Eleven patients were recruited and followed up for 6 months. Overall adherence was found at a high level and remained stable throughout the study period. Feedback from patients was positive; however, clinic staff expressed disappointment and frustration, criticising an increase of workload while not perceiving any benefit to the oncological treatment. Both findings were backed by evidence we found in literature.
Conclusions
Implementation of ePRO monitoring to routine cancer treatment seems to be feasible regarding patients’ acceptance and compliance. However, integration of the tool into clinical workflow without increasing workload and deterring clinicians proves to be a major challenge.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10389-022-01767-3.
Introduction
Over the past decade, monitoring of electronic patient-reported outcomes (ePRO) has increasingly been considered a promising approach to enhance surveillance and care of cancer patients (Putora 2020). Treatment modalities in outpatient care vary from well-known chemotherapy regimen to sometimes very recently approved targeted therapies. All of them bear the risk of severe, potentially life-threatening adverse events, and timely detection is essential. However, research suggests that physicians often underestimate patients’ burden of symptoms and that patient-reported outcomes are superior to clinicians’ assessment reflecting severity of symptoms and side effects (Basch et al. 2009; Laugsand et al. 2010; Gilbert et al. 2015; Atkinson et al. 2016).
In the context of the current COVID-19 pandemic, ePROs have gained even more attention (Abelson 2020). Cancer patients represent a risk group for severe courses of disease (Liang et al. 2020; Cook et al. 2020; Tian et al. 2020), and every physical contact with health care facilities puts additional risk to acquire a COVID-19 infection (Gosain et al. 2020; Al-Shamsi et al. 2020). However, most cancer patients depend on continuous antineoplastic treatment to prevent progression of disease. Treatment surveillance via ePRO might help solve this dilemma by providing the opportunity of continuous monitoring while minimizing the frequency of clinic visits (Abelson 2020).
Several studies have shown impressive benefits of ePRO, such as enhanced quality of life (Basch et al. 2016), reduction of hospital admissions (Basch et al. 2016), earlier detection of adverse events (Denis et al. 2014), and even prolonged survival (Basch et al. 2017; Denis et al. 2019). In a randomized controlled trial by Basch et al., median survival among patients treated for advanced solid cancer was 31.2 months when being monitored by an ePRO-tool, compared to 26 months with standard care (Basch et al. 2017). Another randomized controlled trial by Denis et al. among lung cancer patients proves earlier relapse detection and improved survival with ePRO (22.5 versus 14.9 months) (Denis et al. 2019).
Nevertheless, evidence on successful implementation of ePROs in a real life setting remains limited, and the process of establishing them as a standard of care is moving at a slow pace (Kotronoulas et al. 2014; Anatchkova et al. 2018; Scheibe et al. 2020). In-depth practical knowledge about potential barriers is essential to avoid failure and frustration, and to enable health care facilities to implement ePRO into their daily routine successfully.
To address this demand, we conducted a feasibility study within a cohort of myeloma patients. Our main focus was to identify barriers met during the process and to investigate adherence and perceptions of patients as well as involved health care personnel. Subsequently, we contextualized our result with evidence and recommendations of a semi-structured literature review.
Methods
Study site and recruitment
During the period of September 2019 until June 2020, we conducted an observational study accompanying the implementation of a commercial ePRO system in an outpatient clinic adjacent to one of the largest hospitals in Berlin. Two oncology nurses and five doctors were involved in the project. No additional staff was employed; however, nurses were specifically trained in introducing and supervising patients using the ePRO system.
The ePRO system was offered to patients diagnosed with multiple myeloma under current treatment. Once the patient consented, one of the nurses would set up an account and give instructions on how to use the application. Patients were then included into the observational study if they provided written informed consent, were over 18 years of age, had access to an electronic mobile device or a private computer and were literate in German or English language.
ePRO system
Patients used a web-based ePRO-monitoring tool on their own mobile device or private computer. The tool included symptom questionnaires based on CTCEA (clinician-based common terminology criteria for adverse events) with graphic display and side effect alert. By a messenger service patients were able to send requests and information to the clinic. On this basis, once a week patient received a symptom questionnaire and a subsequent symptom report was sent to the clinic. The questionnaire collected information on the patient’s general well-being, disease symptoms, and treatment side effects. In addition, the application provides the option to record symptoms in the form of a diary and to send additional reports or contact the clinic directly. The responsible physicians as well as the patients themselves have access to a graphic summary of all reported symptoms when logging on to their account.
Whenever a patient reports a symptom classified as severe by the application, an adverse event message (AEM) is sent to the health care team and to the patient, respectively. Depending on the severity of the symptom, the automated response to the patient would either instruct them to contact the clinic during working hours, or to seek immediate medical help via the emergency department. At the outpatient clinic, the nurses involved are responsible to check the incoming ePRO reports and AEM on a daily basis. In case of an AEM, the nurses are supposed to contact the patient and to inform the treating physician if considered necessary.
Data collection
Patients’ data was pseudonymized and entered into an electronic database using Microsoft Excel. Baseline parameters included age, gender, duration of disease, treatment protocol (first-line versus treatment of recurrence), and ECOG status.
In the further course, patients were followed up for 6 months. Number and causes of AEM were recorded into the database at monthly intervals. For each AEM, information on the subsequent reaction of the clinic (i.e., text message, phone call, unplanned visit at the outpatient clinic or hospital admission) was obtained. In case of several reactions to one AEM (i.e., phone call followed by a visit at the outpatient clinic), all of them were documented. In cases of reactions referring to more than one AEM (i.e., symptoms connected to each other such as fever and weakness), the respective AEM were summarized.
Patient adherence was defined as the percentage of questionnaires submitted by the patient in relation to the number of questionnaires sent to the patient. For each patient, adherence to the ePRO system was determined both on a monthly interval as well as overall adherence regarding the whole study period.
At the end of the study period, perceptions of both patients and the involved medical staff were assessed in the form of a structured interview. The interview was conducted face-to-face or via telephone following a questionnaire (see Table Table2).2). In addition, patients and staff were asked to describe their impressions in their own words. A descriptive analysis of all obtained data was carried out via Microsoft Excel. Percentages are presented rounded without decimal numbers.
Table 2
Results of the questionnaire evaluation for patients and staff in percentages (%) and absolute numbers (n/N)
| Topic of evaluation | Patients response | Staff response |
|---|---|---|
| Time investment | Weekly 80% Daily 10% Median time 10 min | Nurses 15–30min/day Doctors Several times per month or less |
| Was time effort adequate? (patients) | Yes 60% (6/10) No 40% (4/10) | |
Did workload increase? (staff) | Yes 86% (6/7) No 15% (1/7) | |
| Satisfaction with usability | ||
• Very content: • Content: • Rather discontent • Discontent | 20% (2/10) 60% (6/10) 20% (2/10) 0% (0/10) | 0 0 43% (3/7) 57% (4/7) |
| Patients: Was assistance needed? | Yes 30% (3/10) No 70% (7/10) | |
| Technical problems | ||
• Frequently • Occasionally • Not at all | 30% (3/10) 30% (3/10) 40% (4/10) | 57% (4/7) 29% (2/7) 14% (1/7) |
| Were AEM useful/appropriate? | ||
• Yes • No | 0% (0/6) 100% (6/6) | 86% (6/7) 14% (1/7) |
| Positive effect on treatment | ||
• Yes • No | 10% (1/10) 90% (9/10) | 14% (1/7) 86% (6/7) |
| Would you recommend the application to others? | ||
• Yes • No • Not sure | 60% (6/10) 20% (2/10) 20% (2/10) | 0% (0/7) 0% (0/7) 100% (7/7) |
A semi-structured literature search was conducted using MEDLINE via PubMed. The chosen search terms were “epro oncology” combined with the terms “implementation,” “adherence,” “benefits,” “barriers,” and “feasibility,” respectively. References of the identified papers were also considered if relevant (“snow balling”). Randomized-controlled trials and implementation studies focused on ePRO implementation in oncology facilities were taken into account and systematically checked for benefits, barriers, feasibility, and perceptions of patients and staff.
Ethical considerations
The study was approved by the ethics committee of the medical association of Berlin (Ethikkommission der Ärztekammer Berlin).
Results
Baseline data
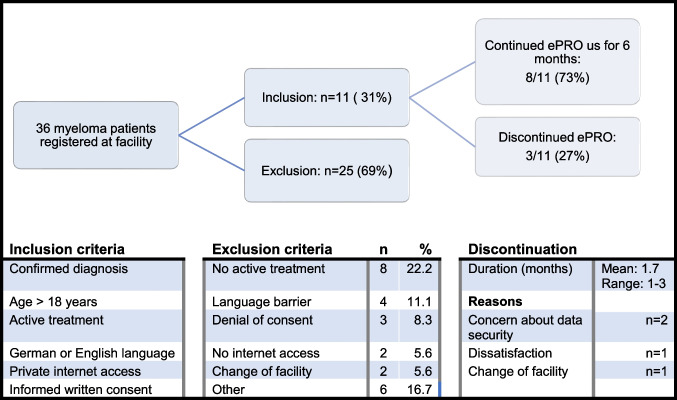
Overall, 36 patients diagnosed with multiple myeloma were identified at the outpatient clinic. Out of these, 11 were enrolled into the observational study. Reasons for not participating included language barrier (11%), not being under active treatment (22%), patient denial (8%) and lack of a mobile electronic device or private computer (6%; see Fig. Fig.11).
When asked to participate in the structured interview at the end of the study, 10/11 patients accepted and 1/11 denied due to poor health condition. The median baseline parameters are shown in Table Table11.
Table 1
Patients baseline data and adherence
| Patient characteristics and adherence | Total (n) or mean | Median or % | Range |
|---|---|---|---|
| Age (years) | 63.8 | 65 | 46–80 |
| Gender | |||
| – Female | 4 | 36% | |
| – Male | 7 | 64% | |
| Duration of disease (months) | 47.3 | 9 | 1–134 |
| ECOG | 1.6 | 1.8 | 0.8–3 |
| Treatment | |||
– First line – Recurrance | 7 4 | 64% 36% | |
| Treatment modification during study period | |||
– Yes – No | 6 5 | 55% 46% | |
| Monthly questionnaires per patient | 6.1 | 4.5 | 2.7–18 |
| Total number of questionnaires received per patient | 24.6 | 26 | 4–52 |
| Total number pf completed questionnaires per patient | 21.8 | 24 | 3–47 |
| Overall adherence | 85.9 | 95% | 44–100% |
Adherence
Of the 11 patients, three patients stopped using the ePRO system in the course of the study period after a mean time of 1.7 months. Reasons were concerns about data security in two cases, although one of these two patients additionally mentioned dissatisfaction with the ePRO system as an additional reason. One patient stopped using the ePRO system after being referred to a different treatment site.
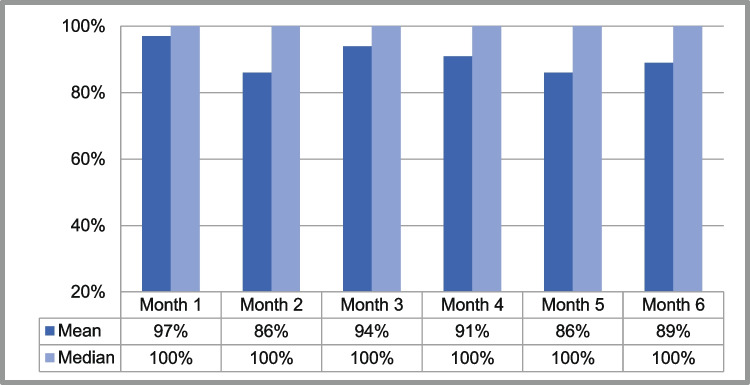
Patients received a median of 4.5 questionnaires per month and a median of 26 questionnaires per patient in the course of the study period. Overall adherence was found at a median level of 95% (per protocol analysis), the median monthly adherence remained on a constant high level, showing no tendency to decline within 6 months (see Table Table11 and Fig. Fig.22).
Adverse event messages
Overall, the medical team received 59 AEM; 64% of patients received at least one AEM. The mean number of AEM per month and patient was 0.92, with a range of 0–5.33 alerts per month.
When investigating the causes of AEM, we found pain to be the most common cause, with almost 50%, followed by cardiopulmonary symptoms and gastrointestinal problems (see Fig. Fig.33).
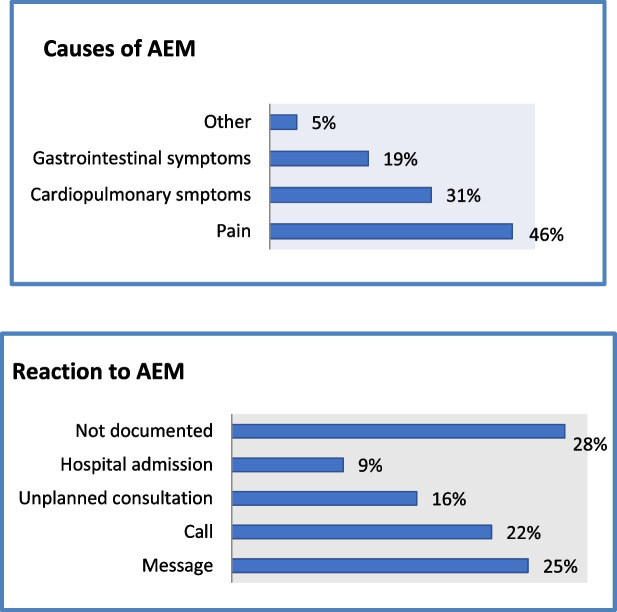
a Causes of adverse event messages in percentages (%) b Reactions to adverse event messages in percentages (%)
Following a received AEM, the involved medical staff would in most cases decide to either send a text message or to call the patient (see Fig. Fig.3b).3b). Only about 15% of AEM resulted in an unplanned visit at the outpatient clinic and 10% led to hospital admission.
Patients’ point of view
Of the patients, 60% judged the required time investment as adequate, although the number of questions was perceived as too high by 60% of patients. However, 80% of patients were content with usability of the app and 70% reported no or few technical issues. Assistance in using the ePRO system was needed by 30% of patients (see Table Table22).
All patients evaluated the automated message as not helpful. Five patients remembered to have been contacted by the nurses or doctors, out of whom three patients thought this was helpful (60%).
In general, patients did not have the impression that using the ePRO tool had a positive impact on their oncological treatment (90%), nor did they feel that their symptoms received more attention by the treating physician (80%). Nevertheless, most patients (60%) stated that they would recommend the ePRO tool to other patients.
When asked for open feedback, most patients gave a positive overall statement. Some mentioned the diary function as helpful, and thought that the ePRO tool gave them an overview of their symptoms and provided them with a sense of security. The option to contact the outpatient clinic via text message was also appreciated.
On the other hand, several patients criticized the redundancy of questions and felt that the answering options did not represent their symptoms accurately. When asked about their overall impressions, two patients answered that it was “a good idea in theory, but has not yet delivered in practice”.
Perceptions of the medical team
Judgement of the medical team was overall negative and differed from patients’ perception in several aspects (see Table Table2).2). Usability was rated unsatisfactory by all staff members, and 57.1% (4/7) reported frequent technical problems. In the opinion of 86%, the system did not display patients’ symptoms in a clear structure and did not provide them with an overview; 86% (6/7) complained about an increase of workload.
AEM were evaluated as mostly inappropriate and unreasonable by 86% (6/7), and no positive impact on treatment was perceived (86%). None of the involved staff members agreed that they would recommend the ePRO system to colleagues.
Nevertheless, the medical team still expressed their belief that ePRO systems in general could offer benefits to the oncological care of outpatients (4/7; 57%). In an open question, the following possible benefits were mentioned: easement of communication (4/7), gaining a better overview on patients’ complaints (4/7), earlier detection of severe events (2/7) and prevention of hospital admissions (1/7). In addition, one of the physicians suggested benefits in regard to pandemic situations.
Literature review
Seventeen relevant publications were identified. The respective content of these publications was analyzed for statements regarding our study questions as mentioned above (see Table Table33).
Table 3
Results of the literature review
| Publication: Author, Title, Journal, Year of publication | Type of study | Benefits | Barriers | Feasibility/adherence | Patients’ perception | Providers’ perception |
|---|---|---|---|---|---|---|
| Basch E, Deal AM, Kris MG et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016 Feb 20;34(6):557–65 | Randomized-controlled trial | • Improved HRQL • Survival benefit • Reduction of hospital admission/ER visits | – | – | – | – |
| Basch E, Barbera L, Kerrigan CL, Velikova G. Implementation of patient-reported outcomes in routine medical care. Am Soc Clin Oncol Educ Book. 2018 May 23;38:122–134 | ASCO session report | • Improvement of communication | • Perceived extra time effort | – | – | – |
| Bennett AV, Jensen RE, Basch E. Electronic patient-reported outcome systems in oncology clinical practice. CA Cancer J Clin. 2012;62(5):337–347 | Review | • Improvement of communication • Potentially time saving • Accuracy of symptom assesssment | – | – | – | – |
| Benze G, Nauck F, Alt-Epping B, et al. PROutine: a feasibility study assessing surveillance of electronic patient reported outcomes and adherence via smartphone app in advanced cancer. Ann Palliat Med. 2019;8(2):104–111 | Feasibility study | – | – | High weekly adherence (87%) | • User friendly • Personal benefit rated good/very good by 53% | • User friendly • Graphic data analysis helpful |
| Denis F, Viger L, Charron A, et al. Detection of lung cancer relapse using self-reported symptoms transmitted via an internet web-application: pilot study of the sentinel follow-up. Support Care Cancer. 2014;22(6):1467–1473. | Feasibility study | • Early detection of relapse | – | High compliance rates (79% weekly, 94% monthly) | • Reassurement • Less anxiety before restaging • User friendly | – |
| Denis F, Basch E, Septans AL, et al. Two-year survival comparing web-based symptom monitoring vs routine surveillance following treatment for lung cancer. JAMA. 2019 Jan 22;321(3):306–307 | Research letter | • Prolonged survival (22 vs. 14 months) | – | – | – | – |
| Friis RB, Hjollund NH, Mejdahl CT, Pappot H, Skuladottir H. Electronic symptom monitoring in patients with metastatic lung cancer: a feasibility study. BMJ Open. 2020;10(6):e035673 | Feasbility study | – | • 69% recruitment • • High adherence (93% weekly) | • High acceptance and usability • Short lenght of questionnaire • Questions seen as relevant • Increased anxiety in some patients (17%) | • Phone calls were seen relevant • Acceptable time effort (with few patients) | |
| Gamper EM, Nerich V, Sztankay M, et al. Evaluation of noncompletion bias and long-term adherence in a 10-year patient-reported outcome monitoring program in clinical routine. Value Health. 2017 Apr;20(4):610–617 | Long-term adherence study | – | – | • Better adherence with ePRO compared to pPRO | – | – |
| Graf J, Moreno B, Wallwiener M, Menzel K, Brucker SY, Simoes E. Praktikabilität und Leistungsfähigkeit von E-Health-Anwendungen bei der Erhebung von patient reported outcomes: Forschungsstand und -bedarf [Practicability and efficiency of E-health applications in patient-reported outcomes: state of and need for research]. Gesundheitswesen. 2018;80(11):953–962 | Review | – | • Lack of knowledge about hurdles, acceptance and usefulness • Lack of legal standards | – | – | – |
| Hans PK, Gray CS, Gill A, Tiessen J. The provider perspective: investigating the effect of the Electronic Patient-Reported Outcome (ePRO) mobile application and portal on primary care provider workflow. Prim Health Care Res Dev. 2018;19(2):151–164 | Qualititative study | – | – | – | – | • Liability concerns • Increased documentation • Workflow disruption |
| Harle CA, Listhaus A, Covarrubias CM, et al. Overcoming barriers to implementing patient-reported outcomes in an electronic health record: a case report. J Am Med Inform Assoc. 2016;23(1):74–79 | Case report (ePRO implemenation in | – | • Uncertain clinical benefit • Time, workload and effort constraints | – | – | • Uncertain clinical benefit • Fear that ePROs divert attention from acute problems |
| Jensen RE, Snyder CF, Abernethy AP, et al. Review of electronic patient-reported outcomes systems used in cancer clinical care. J Oncol Pract. 2014;10(4):e215–e222 | Review ofdifferent ePRO types | – | • Lack of consensus on administration, integration and reporting between system types | – | – | – |
| Judson TJ, Bennett AV, Rogak LJ, et al. Feasibility of long-term patient self-reporting of toxicities from home via the Internet during routine chemotherapy. J Clin Oncol. 2013;31(20):2580–2585 | Feasibility study | – | • Technical burden • Decrease of adherence in patients with high symptom burden | • Monthly: 82% • Weekly 63% | – | – |
| Klagholz SD, Ross A, Wehrlen L, Bedoya SZ, Wiener L, Bevans MF. Assessing the feasibility of an electronic patient-reported outcome (ePRO) collection system in caregivers of cancer patients. Psychooncology. 2018;27(4):1350–1352 | Feasbility study | – | – | – | Caregivers appreciated • User-friendliness • Low time effort • Supportive information | – |
| Locklear T, Miriovsky B, Willig J, et al. Strategies for overcoming barriers to the implementation of patient-reported outcomes measures. NIH Health Systems Research White Paper, 2014 | Reasearch paper | – | • Lack of acceptance among physicians • Disturbance of work flow • Unclear liability for inadequate handling of alerts | – | – | • Fear of overburdening • Unclear clinical benefit |
| Maguire R, McCann L, Miller M, Kearney N. Nurse’s perceptions and experiences of using of a mobile-phone-based Advanced Symptom Management System (ASyMS) to monitor and manage chemotherapy-related toxicity. Eur J Oncol Nurs. 2008;12(4):380–386 | Mixed methods study | – | – | – | – | Benefits: • Early symptom detection • Improved symptom management • High relevance of alerts Problems: • Time effort • Inadequate training |
| McCann L, Maguire R, Miller M, Kearney N. Patients' perceptions and experiences of using a mobile phone-based advanced symptom management system (ASyMS) to monitor and manage chemotherapy related toxicity. Eur J Cancer Care (Engl). 2009;18(2):156–164 | Randomized-coontrolled trial | – | – | – | • Positive experience • User-friendliness • Feeling of security | – |
| Nordan L, Blanchfield L, Niazi S, et al. Implementing electronic patient-reported outcomes measurements: challenges and success factors. BMJ Qual Saf. 2018;27(10):852–856 | Implementation and feasibility study | – | • Reluctance of staff • Previous history of failed ePRO implemetation • Technical problems • Training and supprt required • Patient acceptance • Concerns about data security | – | – | • Disruption of work flow |
| Rotenstein LS, Agarwal A, O'Neil K, et al. Implementing patient-reported outcome surveys as part of routine care: lessons from an academic radiation oncology department. J Am Med Inform Assoc. 2017;24(5):964–968 | Implementation study | Increased attention for symptoms that had been downplayed (i.e. fatigue, depression, sexual disfunction) | • Difficulty in accessing the data and integrating them in EHR • Reluctance of physicians | 86% of providers gave PRO surveys to their patient, but only 34% reviewed them one year after implementation | Frustration when ePROs were filled but not adressed | • PRO data was seen as valuable • PROs increased awareness of topics that had been downplayed • Little delay in workflow • Only 26% saw influence on decision-making |
| Sztankay M, Neppl L, Wintner LM, et al. Complementing clinical cancer registry data with patient reported outcomes: A feasibility study on routine electronic patient-reported outcome assessment for the Austrian Myelome Registry. Eur J Cancer Care (Engl). 2019;28(6):e13154 | Feasibility study | – | – | – | • Few difficulties • No delay in treatment routine • Important to discuss results with doctor • Patients prefer monthly questionnaires | • 80% were quite to very satisfied • No delay in clinical routine • Information was found helpful |
| Taarnhøj GA, Lindberg H, Dohn LH, et al. Electronic reporting of patient-reported outcomes in a fragile and comorbid population during cancer therapy – a feasibility study. Health Qual Life Outcomes. 2020;18(1):225. Published 2020 Jul 11 | Feasbility study | – | Lack of adherence in physicians | • High patient adherece (70–75%) • Low physician adherence (35% of questionnaires viewed) | – | – |
| van Eenbergen MC, van den Hurk C, Mols F, van de Poll-Franse LV. Usability of an online application for reporting the burden of side effects in cancer patients. Support Care Cancer. 2019;27(9):3411–3419 | Feasbility study | – | – | • User-friendliness • 50% would recommend it to other patients • 86% were satisfied with responses of clinic team • 3 drawbacks: technical issues, redundancy of questions, limited topics | – | |
| Wallwiener M, Heindl F, Brucker SY, et al. Implementation and Feasibility of Electronic Patient-Reported Outcome (ePRO) Data Entry in the PRAEGNANT Real-Time Advanced and Metastatic Breast Cancer Registry. Geburtshilfe Frauenheilkd. 2017;77(8):870–878 | Feasibility study | – | – | – | 74% felt comfortable or very comfortable with the questionnaires | 76% found it easy to use |
| Wintner LM, Giesinger JM, Zabernigg A, et al. Evaluation of electronic patient-reported outcome assessment with cancer patients in the hospital and at home. BMC Med Inform Decis Mak. 2015;15:110 | Feasibility study | – | – | Most patients are willing to use ePRO (clinic ePRO: 94,7%, home ePRO 84,4%)) | • ePROS were considered useful • Satisfaction with graphic display and usability | – |
| Wu AW, White SM, Blackford AL, et al. Improving an electronic system for measuring PROs in routine oncology practice. J Cancer Surviv. 2016;10(3):573–582 | Feasibility study | Identification of issues that are otherwise missed | • Low adherence among physicians • Technical issues • Graphical display | – | • Mostly positive feedback • Confusion caused by score presentation | • Primarily positive • Concerns about work load • Difficult to integrate in work flow • Graphic display was important (rather graphs than tables) |
| Zhang R, Burgess ER, Reddy MC, et al. Provider perspectives on the integration of patient-reported outcomes in an electronic health record. JAMIA Open. 2019;2(1):73–80 | Feasibility study | Facilitation of targeted conversation | • Workflow disruption • Technical issues • Reluctance of physicians | – | – | • Time consuming • Workflow disruption • Distraction from discussion with the patient |
Benefits of ePRO were discussed in 6/17 publications. Measureable clinical outcome benefits included enhanced health-related quality of life (Basch et al. 2016), earlier detection of relapse (Denis et al. 2014), decrease in hospital admission (Basch et al. 2016), and prolonged survival (Basch et al. 2016; Denis et al. 2019). Three publications reported positive impacts on communication, such as facilitation of focused discussion (Zhang et al. 2019) and identification of topics that might otherwise be missed or downplayed (Wu et al. 2016; Rotenstein et al. 2017).
Barriers of ePRO implementation were subject to discussion in 6/17 publications. Five out of these six publications found reluctance of physicians to be an important barrier of successful implementation (Wu et al. 2016; Rotenstein et al. 2017; Nordan et al. 2018; Zhang et al. 2019; Taarnhøj et al. 2020). Other frequently mentioned barriers included disruption of workflow (Zhang et al. 2019) and technical issues (Rotenstein et al. 2017; Nordan et al. 2018; Taarnhøj et al. 2020), such as failure to integrate ePRO into the preexisting electronic health files (EHR) (Rotenstein et al. 2017), causing additional effort of time (Harle et al. 2016). Confusing graphical display was additionally found as a barrier (Wu et al. 2016).
Adherence was assessed by 8/17 publications, all of which found high patient adherence (Judson et al. 2013; Denis et al. 2014; Benze et al. 2019; Friis et al. 2020; Taarnhøj et al. 2020). Adherence rates of physicians was measured in only two studies, both of which found roughly about a third of ePRO questionnaires actually being looked at by the doctors (Rotenstein et al. 2017; Taarnhøj et al. 2020). Rotenstein et al. reported that only 34% of providers within their departement would review ePRO results routinely after one year (Rotenstein et al. 2017). Similar results were presented by Taarnhøj et al., who found that only 35% of PRO questionnaires were reviewed by the physician at first consultation after treatment initiation, with physician compliance remaining on a low level (0–52%) throughout the course of treatment (Taarnhøj et al. 2020).
Patients’ perspective was taken into account in 9/17 publications, revealing mostly positive feedback. Most commonly, patients expressed satisfaction with usability and reported a feeling of reassurance (McCann et al. 2009; Denis et al. 2014). Some papers also pointed out drawbacks, one of them being frustration among patients when they noticed that PRO-results were not being reviewed by their physicians (Rotenstein et al. 2017). Also, Friis et al. found some of their participants (17%) to feel more worried about their cancer (Friis et al. 2020). Van Eenbergen et al. received positive overall feedback; however, patients criticized redundancy of questions and limitation of topics (van Eenbergen et al. 2019).
Nurses’ and doctors’ point of view was investigated by 7/17 publications. Conclusions were diverse, with 4/7 giving mainly positive feedback (Wu et al. 2016; Rotenstein et al. 2017; Benze et al. 2019; Friis et al. 2020), 1/7 giving mixed feedback (Maguire et al. 2008), and 2/7 reporting overall negative feedback (Nordan et al. 2018; Zhang et al. 2019).
Positive feedback included perceived relevance of PRO data and AEM (Rotenstein et al. 2017; Friis et al. 2020), as well as an improvement of symptom detection and management (Maguire et al. 2008). Physicians’ impression that PRO data would draw more attention to issues that might have been downplayed otherwise was also mentioned as beneficial (Wu et al. 2016; Rotenstein et al. 2017). In contrast to this finding, Zhang et al. found doctors expressing that PRO assessment would disrupt communication with patients (Zhang et al. 2019). The most important point of criticism was disruption of workflow as well as an increase of time effort and workload (Maguire et al. 2008; Wu et al. 2016; Nordan et al. 2018; Zhang et al. 2019). Out of the five publications reporting mainly positive or mixed feedback, three still mentioned concerns about workload (Maguire et al. 2008; Wu et al. 2016; Friis et al. 2020), and one described “little delay” in workflow (Rotenstein et al. 2017).
Discussion
The results of our study provide insight into some of the challenges met in the process of implementing an ePRO-tool into clinical practice.
Uptake and adherence
General attitude of patients regarding the ePRO technology is a key factor to successful implementation. In our study, we found the majority of patients agreed to participate in the ePRO procedures. Among patients fulfilling all legibility criteria, only three patients denied participation (3/14, 21.4%), which is consistent with refusal rates found in other ePRO trials (Judson et al. 2013; Wu et al. 2016; Taarnhøj et al. 2020).
However, language barrier caused 11.1% of our patients to be excluded. This topic is rarely addressed in literature, even though it is an often stated exclusion criteria (McCann et al. 2009; Judson et al. 2013; Wu et al. 2016; Basch et al. 2016; Sztankay et al. 2019). If mentioned, language barrier accounts for very few patients to be excluded (Wintner et al. 2015; Klagholz et al. 2018). One possible explanation to this might be disparities in the population structure, with our study site located in a very multicultural area in Berlin. Offering an ePRO system translated to a wider range of languages might be necessary in places with higher cultural diversity, though admittedly this presents a challenge.
Among the eight patients continuing until the end of study we found a high-level adherence without any tendency to decline, but three patients dropped out for reasons related to the ePRO tool. High adherence is congruent with previous studies measuring adherence of cancer patients using ePRO tools (Benze et al. 2019; Friis et al. 2020). In some studies, adherence tended to improve with longer intervals of symptom questionnaires (Judson et al. 2013; Denis et al. 2014). However, the “ideal” intervals might depend on the respective setting. For example, Denis et al. found higher monthly adherence among lung-cancer patients in a follow-up situation, using ePRO as a tool for earlier detection of relapse. In this context, close monitoring of severe toxicities is no longer necessary, and patients might prefer not to be reminded of their cancer more than necessary.
Overall, both recruitment and adherence of patients seemed feasible at our facility, but we also identified several barriers that limited general use (e.g., language and lack of IT). Moreover, patients expressed satisfaction with usability of the application, appreciated having a better overview of their symptoms and felt reassured. Again, these findings are similar to previous research, receiving almost entirely positive feedback from patients (McCann et al. 2009; Denis et al. 2014; Wintner et al. 2015; Wu et al. 2016; van Eenbergen et al. 2019). In conclusion, these findings lead to the impression that barriers of ePRO implementation are not primarily to be found on the patients’ side, but lack of perceived benefit certainly jeopardizes patient commitment needed for this tool.
Barriers in a real-life setting
As opposed to that, doctors and nurses at our facility expressed remarkable frustration. In their opinion, ePRO monitoring caused an increase of workload without providing any benefits. Previous evidence on perceptions of clinic staff proves to be diverse, with perceived benefits often compromised by workflow disruption (Maguire et al. 2008; Wallwiener et al. 2017; Benze et al. 2019; Friis et al. 2020). Reluctance of physicians is often mentioned as an important barrier (Wu et al. 2016; Harle et al. 2016; Rotenstein et al. 2017; Nordan et al. 2018; Taarnhøj et al. 2020).
Clear structures and guidelines in how to integrate ePRO into clinical routine might enhance chances of success. However, finding guidelines to integrate ePRO in preexisting work environments proves to be challenging. Each facility has built up their specific individual workflow, and various ePRO systems offer a heterogeneous assortment of functions. It remains unclear which of these functions are actually responsible for the improvement of clinical outcomes. For instance, Denis et al. (2014) proved that AEM sent to clinicians when patients developed signs of disease relapse are very effective in earlier detection of relapse. However, AEM might not be the most important feature in other settings. Basch et al. divided patients into groups according to whether they were computer-experienced or not. Computer-experienced patients reported remotely from home, while computer-inexperienced patients filled out ePRO questionnaires only at clinic visits. In the end, the results did not differ much, and some benefits were even more pronounced in the subgroup of computer-inexperienced patients (Basch et al. 2016). In this context, drawing attention to patients’ subjective burden of disease or gradual deterioration might be the key feature leading to the improvement of clinical outcomes.
Obviously, adapting an ePRO system to an individual setting requires knowledge and effort. Even if it is sometimes suggested that ePRO systems might help save time (Bennett et al. 2012), none of the feasibility studies we assessed confirmed this. On the contrary, additional time and work effort was one of the most frequently reported barriers (Maguire et al. 2008; Harle et al. 2016; Hans et al. 2018; Zhang et al. 2019), even though one scoping review reported no relevant effect on clinical encounters (Howell et al. 2015). Another implementation study found the need for explaining and supporting ePRO completion to be the most time consuming (Nordhausen et al. 2022). In our opinion, it is reasonable to assume that monitoring patients at home, dealing with AEM, and discussing PRO results at consultation is desirable and potentially improves health care, but potential gain in effectiveness is counterbalanced by extra effort needed. Considering the fact that preexisting conditions in health care facilities are already often compromised by shortage of personnel, additional staff might be required at least during the process of implementation. In the long run, optimized procedures and habituation might mitigate initial teething troubles and make continuation possible without additional efforts. However, in the pursuit of this objective, initial commitment and time investment is essential.
Still, the impressive benefits offered by ePRO systems justify putting effort in their implementation. Any novel drug or treatment regimen leading to similar survival benefits would be approved as standard of care almost regardless of additional expenses. It is difficult to argue why a non-pharmaceutical intervention this beneficial to patients’ survival does not deserve the same commitment that any pharmaceutical option would clearly receive.
Limitations
Our study represents a limited in time and scope (single center and patients) intervention and thus results cannot be generalized. Nonetheless, barriers found are minimally addressed in literature and scientific discussions. Also the semi-structured literature search only covered one database and included limited search terms; more detailed literature reviews are available (Howell et al. 2015; van Egdom et al. 2019; Graupner et al. 2021). The described high adherence to the ePRO tool has been found only for those continuing to use it until the end of the study, but we had drop-outs directly related to the tool, reducing factual adherence.
Regardless of the limitations, our study provides needed data about implementation barriers for ePRO, as until today real-life application outside of study settings is scarce, most likely because of the limitations described.
Conclusions
Overall, there is little doubt about the substantial benefits of ePRO monitoring to the care of oncological patients. Our research has shown that the implementation of an ePRO tool is well received by patients, who are willing to participate, presented good compliance, and expressed subjective benefits.
However, difficulties regarding the integration into preexisting workflow routines and an increase of workload leads to frustration on the part of the clinic members. As opposed to the expected effect of saving time, additional commitment is required to overcome initial challenges. Personnel support may be needed to customize the ePRO tool to the respective facility and maintain procedures.
Acknowledgments
We express our gratitude to the cancer society of Berlin (Berliner Krebsgesellschaft) for funding this study. In addition, we would like to thank all participating patients, doctors, and nurses for their participation and engagement.
Authorship contribution statement
All authors whose names appear on the submission made substantial contributions to the conception or design of the work, approved the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Til Ramón Kiderlen drafted the work, did acquisition, analysis and interpretation of data.
Alexandra Schnack contributed to revision, interpretation and write-up.
Funding
A grant was contributed by the Cancer Society of Berlin. Publication was funded by the MHB publication fund supported by DFG.
Availability of data
Data are available at the IT system of the Vivantes Clinic Neukoelln, Berlin, Germany.
Code availability
Not applicable
Declarations
The authors declare that they have no disclosures or competing interests (financial or non-financial) related to this study.
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, version 1996, Somerset West.
The study was approved by the ethics committee of the medical association of Berlin (Ethikkommission der Ärztekammer Berlin).
All participants gave formal written consent to participate in the study.
All participants gave formal written consent data raised may be used for publication.
The ePROM evaluated was provided by Noona® in the context of a test trial in the clinic. There was contract or agreement in regard to the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abelson R (2020) Doctors and patients turn to telemedicine in the coronavirus outbreak. The New York Times
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A Practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936–e945. 10.1634/theoncologist.2020-0213. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Anatchkova M, Donelson SM, Skalicky AM et al (2018) Exploring the implementation of patient-reported outcome measures in cancer care: need for more real-world evidence results in the peer reviewed literature. J Patient Rep Outcomes 2. 10.1186/s41687-018-0091-0 [Europe PMC free article] [Abstract]
- Atkinson TM, Ryan SJ, Bennett AV, et al. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer. 2016;24:3669–3676. 10.1007/s00520-016-3297-9. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Basch E, Jia X, Heller G, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101:1624–1632. 10.1093/jnci/djp386. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557–565. 10.1200/JCO.2015.63.0830. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197–198. 10.1001/jama.2017.7156. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bennett AV, Jensen RE, Basch E. Electronic patient-reported outcome systems in oncology clinical practice. CA Cancer J Clin. 2012;62:337–347. 10.3322/caac.21150. [Abstract] [CrossRef] [Google Scholar]
- Benze G, Nauck F, Alt-Epping B, et al. PROutine: a feasibility study assessing surveillance of electronic patient reported outcomes and adherence via smartphone app in advanced cancer. Ann Palliat Med. 2019;8:104–111. 10.21037/apm.2017.07.05. [Abstract] [CrossRef] [Google Scholar]
- Cook G, Ashcroft AJ, Pratt G et al (2020) Real-world assessment of the clinical impact of symptomatic infection with severe acute respiratory syndrome coronavirus (COVID-19 disease) in patients with Multiple Myeloma receiving systemic anti-cancer therapy. Br J Haematol. 10.1111/bjh.16874 [Europe PMC free article] [Abstract]
- Denis F, Viger L, Charron A, et al. Detection of lung cancer relapse using self-reported symptoms transmitted via an internet web-application: pilot study of the sentinel follow-up. Support Care Cancer. 2014;22:1467–1473. 10.1007/s00520-013-2111-1. [Abstract] [CrossRef] [Google Scholar]
- Denis F, Basch E, Septans A-L, et al. Two-year survival comparing web-based symptom monitoring vs routine surveillance following treatment for lung cancer. JAMA. 2019;321:306–307. 10.1001/jama.2018.18085. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Friis RB, Hjollund NH, Mejdahl CT, et al. Electronic symptom monitoring in patients with metastatic lung cancer: a feasibility study. BMJ Open. 2020;10:e035673. 10.1136/bmjopen-2019-035673. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gilbert A, Ziegler L, Martland M, et al. Systematic review of radiation therapy toxicity reporting in randomized controlled trials of rectal cancer: a comparison of patient-reported outcomes and clinician toxicity reporting. Int J Radiat Oncol Biol Phys. 2015;92:555–567. 10.1016/j.ijrobp.2015.02.021. [Abstract] [CrossRef] [Google Scholar]
- Gosain R, Abdou Y, Singh A et al (2020) COVID-19 and cancer: a comprehensive review. Curr Oncol Rep 22. 10.1007/s11912-020-00934-7 [Europe PMC free article] [Abstract]
- Graupner C, Kimman ML, Mul S, et al. Patient outcomes, patient experiences and process indicators associated with the routine use of patient-reported outcome measures (PROMs) in cancer care: a systematic review. Support Care Cancer. 2021;29:573–593. 10.1007/s00520-020-05695-4. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hans PK, Gray CS, Gill A, Tiessen J. The provider perspective: investigating the effect of the Electronic Patient-Reported Outcome (ePRO) mobile application and portal on primary care provider workflow. Prim Health Care Res Dev. 2018;19:151–164. 10.1017/S1463423617000573. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Harle CA, Listhaus A, Covarrubias CM, et al. Overcoming barriers to implementing patient-reported outcomes in an electronic health record: a case report. J Am Med Inform Assoc. 2016;23:74–79. 10.1093/jamia/ocv085. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Howell D, Molloy S, Wilkinson K, et al. Patient-reported outcomes in routine cancer clinical practice: a scoping review of use, impact on health outcomes, and implementation factors. Ann Oncol. 2015;26:1846–1858. 10.1093/annonc/mdv181. [Abstract] [CrossRef] [Google Scholar]
- Judson TJ, Bennett AV, Rogak LJ, et al. Feasibility of long-term patient self-reporting of toxicities from home via the Internet during routine chemotherapy. J Clin Oncol. 2013;31:2580–2585. 10.1200/JCO.2012.47.6804. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Klagholz SD, Ross A, Wehrlen L, et al. Assessing the feasibility of an electronic patient-reported outcome (ePRO) collection system in caregivers of cancer patients. Psychooncology. 2018;27:1350–1352. 10.1002/pon.4658. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kotronoulas G, Kearney N, Maguire R, et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32:1480–1501. 10.1200/JCO.2013.53.5948. [Abstract] [CrossRef] [Google Scholar]
- Laugsand EA, Sprangers MAG, Bjordal K, et al. Health care providers underestimate symptom intensities of cancer patients: a multicenter European study. Health Qual Life Outcomes. 2010;8:104. 10.1186/1477-7525-8-104. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. 10.1016/S1470-2045(20)30096-6. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Maguire R, McCann L, Miller M, Kearney N. Nurse’s perceptions and experiences of using of a mobile-phone-based Advanced Symptom Management System (ASyMS) to monitor and manage chemotherapy-related toxicity. Eur J Oncol Nurs. 2008;12:380–386. 10.1016/j.ejon.2008.04.007. [Abstract] [CrossRef] [Google Scholar]
- McCann L, Maguire R, Miller M, Kearney N. Patients’ perceptions and experiences of using a mobile phone-based advanced symptom management system (ASyMS) to monitor and manage chemotherapy related toxicity. Eur J Cancer Care (Engl) 2009;18:156–164. 10.1111/j.1365-2354.2008.00938.x. [Abstract] [CrossRef] [Google Scholar]
- Nordan L, Blanchfield L, Niazi S, et al. Implementing electronic patient-reported outcomes measurements: challenges and success factors. BMJ Qual Saf. 2018;27:852–856. 10.1136/bmjqs-2018-008426. [Abstract] [CrossRef] [Google Scholar]
- Nordhausen T, Lampe K, Vordermark D, et al. An implementation study of electronic assessment of patient-reported outcomes in inpatient radiation oncology. J Patient Rep Outcomes. 2022;6:77. 10.1186/s41687-022-00478-3. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Putora PM. Electronic patient-reported outcomes: a new standard of care? OCL. 2020;98:327–328. 10.1159/000494925. [Abstract] [CrossRef] [Google Scholar]
- Rotenstein LS, Agarwal A, O’Neil K, et al. Implementing patient-reported outcome surveys as part of routine care: lessons from an academic radiation oncology department. J Am Med Inform Assoc. 2017;24:964–968. 10.1093/jamia/ocx009. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Scheibe M, Herrmann A, Schmitt J, et al. Implementation of patient-reported outcome assessment in routine cancer care: a systematic review of multicentric programs in Europe. Z Evid Fortbild Qual Gesundhwes. 2020;156–157:11–23. 10.1016/j.zefq.2020.08.001. [Abstract] [CrossRef] [Google Scholar]
- Sztankay M, Neppl L, Wintner LM, et al. Complementing clinical cancer registry data with patient reported outcomes: a feasibility study on routine electronic patient-reported outcome assessment for the Austrian Myelome Registry. Eur J Cancer Care (Engl) 2019;28:e13154. 10.1111/ecc.13154. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Taarnhøj GA, Lindberg H, Dohn LH, et al. Electronic reporting of patient-reported outcomes in a fragile and comorbid population during cancer therapy - a feasibility study. Health Qual Life Outcomes. 2020;18:225. 10.1186/s12955-020-01480-3. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:893–903. 10.1016/S1470-2045(20)30309-0. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- van Eenbergen MC, van den Hurk C, Mols F, van de Poll-Franse LV. Usability of an online application for reporting the burden of side effects in cancer patients. Support Care Cancer. 2019;27:3411–3419. 10.1007/s00520-019-4639-1. [Abstract] [CrossRef] [Google Scholar]
- van Egdom LSE, Oemrawsingh A, Verweij LM, et al. Implementing patient-reported outcome measures in clinical breast cancer care: a systematic review. Value Health. 2019;22:1197–1226. 10.1016/j.jval.2019.04.1927. [Abstract] [CrossRef] [Google Scholar]
- Wallwiener M, Heindl F, Brucker SY, et al. Implementation and feasibility of electronic minimallyatient-Reported Outcome (ePRO) data entry in the PRAEGNANT Real-Time Advanced and Metastatic Breast Cancer Registry. Geburtshilfe Frauenheilkd. 2017;77:870–878. 10.1055/s-0043-116223. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wintner LM, Giesinger JM, Zabernigg A, et al. Evaluation of electronic patient-reported outcome assessment with cancer patients in the hospital and at home. BMC Med Inform Decis Mak. 2015;15:110. 10.1186/s12911-015-0230-y. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wu AW, White SM, Blackford AL, et al. Improving an electronic system for measuring PROs in routine oncology practice. J Cancer Surviv. 2016;10:573–582. 10.1007/s11764-015-0503-6. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhang R, Burgess ER, Reddy MC, et al. Provider perspectives on the integration of patient-reported outcomes in an electronic health record. JAMIA Open. 2019;2:73–80. 10.1093/jamiaopen/ooz001. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1007/s10389-022-01767-3
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007/s10389-022-01767-3.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/137957409
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1007/s10389-022-01767-3
Article citations
Facilitators and barriers to implementing patient-reported outcomes in clinical oncology practice: a systematic review based on the consolidated framework for implementation research.
Implement Sci Commun, 5(1):120, 29 Oct 2024
Cited by: 0 articles | PMID: 39473015 | PMCID: PMC11520578
Review Free full text in Europe PMC
The impact of electronic versus paper-based data capture on data collection logistics and on missing scores in thyroid cancer patients.
Endocrine, 84(2):635-645, 16 Dec 2023
Cited by: 0 articles | PMID: 38103143 | PMCID: PMC11076317
Psychosocial assessment practices for hematopoietic stem cell transplantation: a national survey study.
Bone Marrow Transplant, 58(12):1314-1321, 26 Aug 2023
Cited by: 1 article | PMID: 37634015
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A real-time electronic symptom monitoring system for patients after discharge following surgery: a pilot study in cancer-related surgery.
BMC Cancer, 20(1):543, 10 Jun 2020
Cited by: 33 articles | PMID: 32522163 | PMCID: PMC7285449
Evaluation of electronic patient-reported outcome assessment with cancer patients in the hospital and at home.
BMC Med Inform Decis Mak, 15:110, 23 Dec 2015
Cited by: 30 articles | PMID: 26699708 | PMCID: PMC4690412
Implementation of a cloud-based electronic patient-reported outcome (ePRO) platform in patients with advanced cancer.
J Patient Rep Outcomes, 5(1):91, 15 Sep 2021
Cited by: 10 articles | PMID: 34524558 | PMCID: PMC8443731
Electronic self-reporting of adverse events for patients undergoing cancer treatment: the eRAPID research programme including two RCTs
NIHR Journals Library, Southampton (UK), 10 Feb 2022
Cited by: 0 articles | PMID: 35138783
ReviewBooks & documents Free full text in Europe PMC

 1,2
1,2