Abstract
Background
Transient diaphragm dysfunction is common during the first week after cardiac surgery; however, the precise incidence, risk factors, and outcomes of persistent diaphragm dysfunction are not well described.Methods
In a single-centre prospective cohort study, we included all consecutive patients over 18 yr who underwent elective cardiac surgery. Diaphragm function was evaluated with ultrasound (M-mode) by recording the excursion of both hemidiaphragms at two different time points: preoperatively and after the seventh postoperative day in patients breathing without assistance. Significant diaphragm dysfunction after the seventh day of the index cardiac surgery was defined as a decrease in diaphragm excursion below the lower limit of normal: at rest, < 9 mm for women and < 10 mm for men; after a sniff test, < 16 mm for women and < 18 mm for men.Results
Overall, 122 patients were included in the analysis. The median [interquartile range (IQR)] age was 69 [59-74] years and 96/122 (79%) were men. Ten (8%) patients had diaphragm dysfunction after the seventh postoperative day. We did not identify risk factors for persistent diaphragm dysfunction. Persistent diaphragm dysfunction was associated with a longer median [IQR] duration of noninvasive (8 [0-34] vs 0 [0-0] hr; difference in medians, 8 hr; 95% confidence interval [CI], 0 to 22; P < 0.001) and invasive mechanical ventilation (5 [3-257] vs 3[2-4] hr; difference in medians, 2 hr; 95% CI, 0.5 to 41; P = 0.008); a higher reintubation rate (4/10, 40% vs 1/112, 0.9%; relative risk, 45; 95% CI, 7.1 to 278; P < 0.0001), a higher incidence of pneumonia (4/10 [40%] vs 7/112 [6%]; relative risk, 6; 95% CI, 2 to 16; P < 0.001), and longer median [IQR] length of stay in the intensive care unit (8 [5-29] vs 4 [2-6] days; difference in medians, 4 days; 95% CI, 2 to 12; P = 0.002).Conclusion
The incidence of persistent diaphragm dysfunction was 8% in patients undergoing elective cardiac surgery and was associated with adverse respiratory outcomes.Study registration
ClinicalTrials.gov (NCT04276844); prospectively registered 19 February 2020.Free full text

Persistent diaphragm dysfunction after cardiac surgery is associated with adverse respiratory outcomes: a prospective observational ultrasound study
Association entre dysfonctionnement persistant du diaphragme après une chirurgie cardiaque et issues respiratoires indésirables : une étude échographique prospective observationnelle
Abstract
Background
Transient diaphragm dysfunction is common during the first week after cardiac surgery; however, the precise incidence, risk factors, and outcomes of persistent diaphragm dysfunction are not well described.
Methods
In a single-centre prospective cohort study, we included all consecutive patients over 18 yr who underwent elective cardiac surgery. Diaphragm function was evaluated with ultrasound (M-mode) by recording the excursion of both hemidiaphragms at two different time points: preoperatively and after the seventh postoperative day in patients breathing without assistance. Significant diaphragm dysfunction after the seventh day of the index cardiac surgery was defined as a decrease in diaphragm excursion below the lower limit of normal: at rest, < 9 mm for women and < 10 mm for men; after a sniff test, < 16 mm for women and < 18 mm for men.
Results
Overall, 122 patients were included in the analysis. The median [interquartile range (IQR)] age was 69 [59–74] years and 96/122 (79%) were men. Ten (8%) patients had diaphragm dysfunction after the seventh postoperative day. We did not identify risk factors for persistent diaphragm dysfunction. Persistent diaphragm dysfunction was associated with a longer median [IQR] duration of noninvasive (8 [0–34] vs 0 [0–0] hr; difference in medians, 8 hr; 95% confidence interval [CI], 0 to 22; P < 0.001) and invasive mechanical ventilation (5 [3–257] vs 3[2–4] hr; difference in medians, 2 hr; 95% CI, 0.5 to 41; P = 0.008); a higher reintubation rate (4/10, 40% vs 1/112, 0.9%; relative risk, 45; 95% CI, 7.1 to 278; P < 0.0001), a higher incidence of pneumonia (4/10 [40%] vs 7/112 [6%]; relative risk, 6; 95% CI, 2 to 16; P < 0.001), and longer median [IQR] length of stay in the intensive care unit (8 [5–29] vs 4 [2–6] days; difference in medians, 4 days; 95% CI, 2 to 12; P = 0.002).
Conclusion
The incidence of persistent diaphragm dysfunction was 8% in patients undergoing elective cardiac surgery and was associated with adverse respiratory outcomes.
Study registration
ClinicalTrials.gov (NCT04276844); prospectively registered 19 February 2020.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12630-022-02360-8.
Résumé
Contexte
Un dysfonctionnement transitoire du diaphragme est fréquent au cours de la première semaine après une chirurgie cardiaque. Toutefois, l’incidence précise, les facteurs de risque et les devenirs liés à un dysfonctionnement persistant du diaphragme ne sont pas bien décrits.
Méthode
Dans une étude de cohorte prospective monocentrique, nous avons inclus tous les patients consécutifs de plus de 18 ans qui ont bénéficié d’une chirurgie cardiaque non urgente. La fonction du diaphragme a été évaluée à l’échographie (mode M) en enregistrant l’excursion des deux hémidiaphragmes à deux moments différents : avant l’opération et après le septième jour postopératoire chez les patients respirant sans assistance. Un dysfonctionnement significatif du diaphragme après le septième jour de la chirurgie cardiaque initiale a été défini comme une diminution de l’excursion diaphragmatique en dessous de la limite inférieure de la normale, soit : au repos, < 9 mm pour les femmes et < 10 mm pour les hommes; après un test de reniflement, < 16 mm pour les femmes et < 18 mm pour les hommes.
Résultats
Au total, 122 patients ont été inclus dans l’analyse. L’âge médian des patients (écart interquartile [ÉIQ]) était de 69 ans [59-74] ans et 96/122 (79 %) étaient des hommes. Dix (8 %) patients ont présenté un dysfonctionnement du diaphragme après le septième jour postopératoire. Nous n’avons pas identifié de facteurs de risque de dysfonctionnement persistant du diaphragme. Un dysfonctionnement persistant du diaphragme était associé à : une durée médiane [ÉIQ] de ventilation non invasive (8 [0–34] vs 0 [0–0] h; différence dans les médianes, 8 heures; intervalle de confiance [IC] à 95 %, 0 à 22; P < 0,001) et de ventilation mécanique invasive (5 [3–257] vs 3[2–4] h; différence dans les médianes, 2 heures; IC 95 %, 0,5 à 41; P = 0,008) plus longues, un taux de réintubation plus élevé (4/10, 40 % vs 1/112, 0,9 %; risque relatif, 45; IC 95 %, 7,1 à 278; P < 0,0001), une incidence plus élevée de pneumonie (4/10 [40 %] vs 7/112 [6 %]; risque relatif, 6; IC 95 %, de 2 à 16; P < 0,001), et une durée de séjour médiane [ÉIQ] plus longue à l’unité de soins intensifs (8 [5-29] vs 4 [2–6] jours; différence en médianes, 4 jours; IC 95 %, 2 à 12; P = 0,002).
Conclusion
L’incidence de dysfonctionnement persistant du diaphragme était de 8 % chez les patients bénéficiant d’une chirurgie cardiaque non urgente et était associée à des issues respiratoires indésirables.
Enregistrement de l’étude
ClinicalTrials.gov (NCT04276844); enregistrée prospectivement le 19 février 2020.
Diaphragm dysfunction (DD) after cardiac surgery is closely related to frequent respiratory complications.1,2 It has been associated with pneumonia and difficulty weaning from mechanical ventilation in critical ill patients3,4 and in cardiac surgery patients.5–7
Its incidence is unclear, ranging from 1% to 60%.8 It may be the consequence of mechanical ventilation leading to both diaphragmatic atrophy and injury leading to contractile dysfunction.9,10 It may also occur during cardiac surgery, for example, because of phrenic nerve injury caused by ice-cardioplegia solution or the surgical technique. There may also be an ischemic mechanism with ligation of the diaphragmatic blood supply during internal mammary dissection.11
Early postoperative DD has been found to be frequent, but data are controversial regarding its consequence on short-term and mid-term outcomes.5,12–16
Ultrasonography is now recognized as an easy and accurate method to noninvasively evaluate diaphragmatic function at the bedside in the intensive care unit (ICU) for assessment and monitoring of DD.17 Nevertheless, the definition of DD assessed by ultrasound is not standardized and the methods of diagnosis are variable. The latter include diaphragmatic excursion after the sniff test or after deep inspiration in M-mode, measuring the thickness of the diaphragm and the thickening fraction, and, recently, tissue Doppler.18
Our main objective was to investigate the incidence of DD one week after cardiac surgery, assessed by diaphragm excursion measured by ultrasound at the bedside. We hypothesized that DD was frequently persistent after cardiac surgery and could worsen respiratory outcomes.
Methods
This observational, single-centre prospective study was approved by the responsible ethics committee (8 February 2020; Comité de Protection des Personnes Nord-Ouest III, CNRIPH: 20.01.08.52326) and registered at ClinicalTrials.gov (NCT04276844). Informed consent was obtained from all participants.
Patients
All consecutive patients admitted to the cardiothoracic surgery department of the Clinique Ambroise Paré (Neuilly-Sur-Seine, France) prior to elective cardiac surgery were screened for enrollment; all adult patients who underwent elective cardiac surgery were eligible for inclusion. Exclusion criteria included contraindication to preoperative functional respiratory assessment, pregnancy, active COVID-19 pneumonia, and inability to give consent. All variables, demographic data, comorbidities, surgical data, and postoperative data were prospectively recorded.
Surgical and perioperative management
Perioperative management was standardized. All patients were fitted with a right internal jugular central venous catheter. All procedures involved a full sternotomy approach. Mammary arteries and saphenous veins were used as coronary bypass grafts. Myocardial protection was provided using normothermic continuous blood cardioplegia solution. During surgery (except the cross-clamping period), mechanical ventilation was set at a tidal volume of 6 mL·kg-1 of predicted body weight and a positive end-expiratory pressure (PEEP) level of 5 cm H2O, the respiratory rate was adjusted to maintain normocapnia, and the inspired fraction of oxygen (FIO2) was set to keep the arterial partial pressure of oxygen (PaO2) below 100 mm Hg. In the ICU, the ventilation protocol was lung-protective with a tidal volume of 6 mL·kg-1 of the ideal body weight, a PEEP of 5 cm H2O, an FIO2 set to obtain a PaO2 above 100 mm Hg, and an inspiration/expiration time ratio of 1:2. Physicians were free to adjust the ventilatory parameters according to patient needs, including adjustment of PEEP level in case of postoperative hypoxia due to atelectasis. Other therapies were left to the discretion of the ICU intensivists. After surgery, patients were transferred to the ICU where absence of bleeding, respiratory and hemodynamic stability, and normothermia were determined before stopping sedatives drugs and performing a spontaneous breathing trial.
Ultrasound measurements
The excursion of both hemidiaphragms was measured sequentially in each patient at two different time points: preoperatively (the day before surgery) and from the seventh postoperative day in patients breathing without assistance (extubated or on pressure support mode with zero PEEP); if patients were not breathing without assistance, the ultrasound evaluation was deferred. In case of respiratory failure with clinical suspicion of DD, a supplementary echography evaluation was performed before the seventh postoperative day. Ultrasound examinations of diaphragm excursion were performed at the bedside with the patient in a 45° semirecumbent position during quiet and unassisted breathing, using an ultrasound platform (Philips CX50®, Amsterdam, The Netherlands) connected to a 1–5-MHz phased-array transducer. Diaphragm excursion was evaluated at rest and after a sniff test, using anatomical motion (M)-mode through a lateral approach from the midaxillary line.14,19 As an exploratory measurement, we also examined the value of each hemidiaphragm peak after the sniff test using tissue doppler imaging velocity. The excursion value of each hemidiaphragm was the average measurement of three consecutive respiratory cycles (separately at rest and after the sniff test) (see example on Fig. Fig.1).1). Diaphragm dysfunction after the seventh day after cardiac surgery was defined, in M-mode, by a decrease in amplitude of the movement of the diaphragm below the lower limit of normal for at least one of the following measurements: 1) at rest, < 9 mm for women and < 10 mm for men; and 2) after the sniff test, < 16 mm for women and < 18 mm for men.20
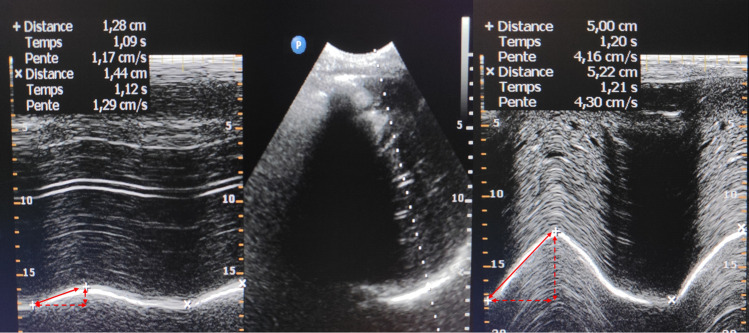
Right hemidiaphragm ultrasound. Visualization of the right hemidiaphragm in B-mode from the subcostal view (Panel B). Then, application of an M Mode to record diaphragm motion. Panel A shows normal inspiratory diaphragm excursion at rest while panel B shows normal inspiratory diaphragm excursion after the sniff test.
Outcomes
The main outcome was the incidence of persistent postoperative DD after seven days following the index surgery. Secondary outcomes included respiratory complications (pneumonia, atelectasis requiring bronchial clearing by fibroscopy, reintubation rate, prolonged mechanical ventilation [> 24 hr], or prolonged noninvasive ventilation [> 48 hr]), length of ICU stay, and pulmonary function tests in patients diagnosed with persistent postoperative DD.
Statistical analysis
Continuous variables are expressed as mean (standard deviation [SD]) when normally distributed or median [interquartile range (IQR)] elsewhere. Categorical variables are expressed as n (%) and were compared using Fisher’s exact test. Shapiro–Wilk tests were used to test the normality of the distribution of the studied variables. Differences between the two groups were compared using Student’s t test for normally distributed data and the Mann–Whitney U test for nonnormally distributed numerical data. Comparisons of median diaphragmatic excursion between the day before surgery and after seven days following surgery were performed using a paired Wilcoxon test, to test for within-group differences. Since this nonparametric test works with ranks, it is usually not possible to obtain a confidence interval (CI) with exactly 95% confidence of the difference in medians. The statistical software computes an approximate confidence level, which is reported in Tables Tables22 and and33 as 95% CI of difference in medians. The significance threshold adopted was P < 0.05.
Table 2
Evolution of diaphragm excursion before and one week after surgery
| No DD N = 112 | Persistent DD N = 10 | |||||||
|---|---|---|---|---|---|---|---|---|
| D-1 | D+7 | Difference in medians (95% CI) | P value | D-1 | D+7 | Difference in medians (95% CI) | P value | |
| Left hemidiaphragm | ||||||||
| Diaphragm excursion at rest (mm), median [IQR] | 25 [18–29] | 21 [16–27] | -2 (-3 to -0.2) | 0.03a | 28 [15–33] | 12 [8–17] | -16 (-21 to 8) | 0.04a |
| Diaphragm excursion after sniff test (mm), median [IQR] | 42 [34–52] | 34 [28–43] | -8 (-10 to -4) | < 0.001a | 37 [33–49] | 16 [12–23] | -25 (-35 to 9) | 0.02a |
| TDI after sniff test (cm·s-1), median [IQR] | 12 [9–15] | 10 [8–13] | -2 (-2 to -1) | 0.001a | 11 [7–13] | 9 [6–13] | 1 (-5.7 to 5) | 1.00a |
| Right hemidiaphragm | ||||||||
| Diaphragm excursion at rest (mm), median [IQR] | 24 [18–30] | 20 [17–24] | -3 (-5 to -1) | < 0.001a | 20 [17–25] | 16 [10–23] | -6.5 (-14 to 7) | 0.28a |
| Diaphragm excursion after sniff test (mm), median [IQR] | 42 [32–49] | 33 [26–41] | -6 (-10 to -4) | < 0.001a | 41 [24–49] | 24 [12–42] | -7.5 (-36 to 18) | 0.16a |
| TDI after sniff test (cm·s-1), median [IQR] | 11 [9–14] | 10 [7–14] | -1 (-2 to -0.9) | 0.03a | 10 [8–14] | 16 [6–20] | 2.5 (-7 to 21) | 0.40a |
Each hemidiaphragmatic excursion was evaluated in unassisted patients in M-mode at rest, i.e., during calm spontaneous breathing, and after the sniff test. Each hemidiaphragmatic function was evaluated in unassisted patients in TDI mode after the sniff test.
aPaired Wilcoxon test
CI = confidence interval; D-1 = day before surgery; D+7 = after the seventh postoperative day in patients during unassisted breathing; DD = diaphragmatic dysfunction; IQR = interquartile range; TDI = tissue doppler imaging
Table 3
Secondary outcomes
| Outcome | No DD N = 112 | Persistent DD N = 10 | P value | |
|---|---|---|---|---|
| Relative risk (95% CI) | ||||
| NIV or HFO > 48 hr (days), n/total N (%) | 3/112 (3%) | 4/10 (40%) | 15 (4 to 52) | < 0.001a |
| Mechanical ventilation > 24 hr, n/total N (%) | 1/112 (1%) | 4/10 (40%) | 45 (7 to 278) | < 0.001a |
| Pneumonia, n/total N (%) | 7/112 (6%) | 4/10 (40%) | 6 (2 to 16) | 0.006a |
| Reintubation, n/total N (%) | 1/112 (1%) | 4/10 (40%) | 45 (7 to 278) | < 0.001a |
| Atelectasis requiring bronchoscopy, n/total N (%) | 2/112 (2%) | 3/10 (30%) | 17 (4 to 75) | 0.004a |
| Mortality, n/total N (%) | 0/112 (0%) | 1/10 (10%) | - | 0.08a |
| Difference in medians (95% CI) | ||||
|---|---|---|---|---|
| Duration of NIV or HFO (hr), median [IQR] | 0 [0–0] | 8 [0–34] | 8 (0 to 22) | < 0.00b |
| Duration of mechanical ventilation (hr), median [IQR] | 3 [2–4] | 5 [3–257] | 2 (1 to 41) | 0.008b |
| Postoperative Us-troponin peak, median [IQR] | 2.4 [1.2–4.3] | 2.4 [1.3–5.0] | 0.1 (-1.3 to 1.9) | 0.82b |
| Postoperative creatine kinase, median [IQR] peak | 495 [340–809] | 666 [357–1,114] | 171 (-151 to 477) | 0.38b |
| Length of stay in ICU (days), median [IQR] | 4 [2–6] | 8 [5–29] | 4 (2 to 12) | 0.002b |
| Length of stay in hospital (days), median [IQR] | 10 [8–12] | 11 [8–29] | 0.5 (-1 to 12) | 0.24b |
aFisher’s exact test
bMann–Whitney test
CI = confidence interval; DD = diaphragm dysfunction; HFO = high-flow oxygen; ICU = intensive care unit; IQR = interquartile range; NIV = noninvasive ventilation
The sample size was estimated by hypothesizing a 30% incidence of persistent DD after seven days according to a previously published study.13 We powered this study to detect the same incidence in elective patients. We needed 100 participants to obtain an effect size of 30%, an incidence with 80% power, and a two-tailed alpha of 0.05. Statistical analysis was performed using MedCalc® version 14 (MedCalc Software Ltd, Ostend, Belgium).
Results
During the study period, we included 157 patients, 122 of whom were included in the final analysis (33 patients were excluded because of incomplete pre- or postoperative echographic measurements including poor echogenicity; one patient withdrew his consent; and one patient was not operated) (Electronic Supplementary Material [ESM] eFigure).
In the whole cohort, the median [IQR] age was 69 [59–74] years, 96/122 (79%) were men, the median [IQR] body mass index was 25.6 [23.0–28.8] kg·m-2, and 16/122 (13%) patients had chronic obstructive pulmonary disease. No patient had COVID-19 pneumonia. Baseline characteristics and surgical data are displayed in Table Table1.1. Overall, 10/122 (8%) patients had DD after the seventh day following surgery: six with left hemidiaphragm dysfunction, three with right hemidiaphragm dysfunction, and one with bilateral dysfunction.
Table 1
Baseline characteristics and surgical data
| No DD N = 112 | Persistent DD N = 10 | P value | |
|---|---|---|---|
| Baseline characteristic | |||
| Age, median [IQR] | 69 [59–75] | 65 [56–74] | 0.50a |
| Male sex, n/total N (%) | 88/112 (79%) | 8/10 (80%) | 1.00b |
| BMI (kg·m-2), median [IQR] | 25.6 [23.0–28.7] | 27.1 [23.4–35.6] | 0.26a |
| Diabetes, n/total N (%) | 33/112 (30%) | 5/10 (50%) | 0.32b |
| Dyslipidemia, n/total N (%) | 65/112 (58%) | 4/10 (40%) | 0.44b |
| Arterial hypertension, n/total N (%) | 67/112 (60%) | 6/10 (60%) | 0.74b |
| Smoker status, n/total N (%) | 46/112 (41%) | 4/10 (40%) | 0.79b |
| COPD, n/total N (%) | 16/112 (14%) | 0/10 (0%) | 0.36b |
| Surgery | |||
| Isolated CABG, n/total N (%) | 60/112 (54%) | 6/10 (60%) | 0.95b |
| Isolated valve surgery, n/total N (%) | 31/112 (28%) | 1/10 (10%) | 0.45b |
| AVR, n/total N (%) | 16/112 (14%) | 1/10 (10%) | 1.00b |
| MVR or MVP, n/total N (%) | 15/112 (13%) | 0/10 (0%) | 0.61b |
| Tricuspid valve surgery, n/total N (%) | 0/112 (0%) | 0/10 (0%) | - |
| Ascendant aortic surgery, n/total N (%) | 2/112 (2%) | 0/10 (0%) | 1.00b |
| AVR plus ascendant aortic surgery, n/total N (%) | 3/112 (3%) | 1/10 (10%) | 0.29b |
| Combined CABG + valve surgery, n/total N (%) | 7/112 (6%) | 1/10 (10%) | 0.51b |
| Combined valve surgery, n/total N (%) | 9/112 (8%) | 1/10 (10%) | 0.59b |
| Number of grafts, median [IQR] | 3 [2, 3] | 3 [2–4] | 0.39a |
| Left mammary artery, n/total N (%) | 66/112 (59%) | 7/10 (70%) | 0.73b |
| Right mammary artery, n/total N (%) | 59/112 (53%) | 5/10 (50%) | 0.87b |
| Saphenous vein, n/total N (%) | 1/112 (1%) | 0/10 (0%) | 1.00b |
| CPB time (min), median [IQR] | 73 [56–87] | 71 [59–118] | 0.61a |
| ACC time (min), median [IQR] | 59 [46–68] | 60 [52–95] | 0.32a |
| Pleural drains after surgery, n/total N (%) | 63/112 (56%) | 7/10 (70%) | 0.61b |
aMann–Whitney test
bFisher’s exact test
ACC = aortic cross clamping; AVR = aortic valve replacement; BMI = body mass index; CABG = coronary artery bypass grafting; CPB = cardiopulmonary bypass; DD = diaphragm dysfunction; IQR = interquartile range; MVP = mitral valvuloplasty; MVR = mitral valve replacement
Risk factors for developing diaphragm dysfunction after surgery
We compared patients who developed DD with those who did not. There was no difference in preoperative characteristics in patients who developed DD compared with those who did not. Procedural variables were not different either (median [IQR] cardiopulmonary bypass time, 73 [58–88] min and median [IQR] aortic cross-clamping time, 59 [46–70] min) in both groups). The type of procedure did not affect the incidence of postoperative DD either. There was no difference in the occurrence of DD between the different surgeons (surgeon 1: 5/82 [6%]; surgeon 2: 2/17 [12%]; surgeon 3: 3/23 [13%]; P = 0.48).
Evolution of hemidiaphragm excursion over time
In patients without DD, according to the chosen definition, there was a significant decrease in both left and right hemidiaphragm excursion at rest and after the sniff test on the seventh postoperative day compared with the day before surgery (Table (Table2,2, Fig. Fig.22).
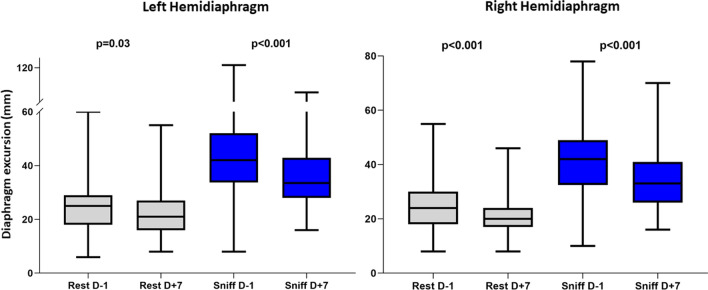
Evolution of diaphragmatic excursion of each hemidiaphragm before and one week after surgery in patients without persistent diaphragm dysfunction. Each hemidiaphragm excursion was evaluated in patients during unassisted breathing at rest, i.e., during calm spontaneous breathing, and after sniff test. P values are from paired Wilcoxon tests for comparisons of excursion evolution within left and right hemidiaphragm groups, between D-1 and D+7. D-1 = day before surgery; D+7 = after the seventh postoperative day in patients during unassisted breathing
In patients with persistent DD, the excursion of the impaired hemidiaphragm was severely decreased (Table (Table2,2, Fig. Fig.33).
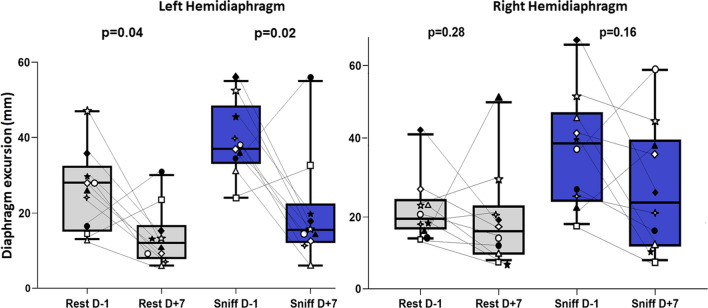
Evolution of diaphragmatic excursion of each hemidiaphragm before and one week after surgery in patients with persistent diaphragm dysfunction. Each hemidiaphragm excursion was evaluated in patients during unassisted breathing in M-mode at rest, i.e., during calm spontaneous breathing, and after sniff test. Each symbol represents the same patient for the evaluation of the two hemidiaphragms. P values are from paired Wilcoxon tests for comparisons of excursion evolution within left and right hemidiaphragm groups, between D-1 and D+7. D-1 = day before surgery; D+7 = after the seventh postoperative day in patients with unassisted breathing
Secondary clinical outcomes
Secondary outcomes are displayed in Table Table3.3. Patients with persistent DD after the seventh postoperative day experienced a significant impairment of postoperative respiratory outcomes. Persistent DD was associated with a longer median duration of noninvasive and mechanical invasive ventilation, a higher reintubation rate, more frequent episodes of pneumonia, and a longer median length of stay in the ICU. Mortality did not significantly differ between both groups.
In patients with persistent DD, a pulmonary function test was performed before and after surgery in 4/10 patients; the median [IQR] forced vital capacity post/pre ratio was 0.81 [0.67–0.90], the median [IQR] forced expiratory volume in the first second post/pre ratio was 0.77 [0.61–0.84], and the median [IQR] total lung capacity post/pre ratio was 0.85 [0.82–1.10] (n = 3) (see ESM eTable).
Discussion
In this prospective observational cohort study in cardiac surgery patients, we observed an incidence of 8% of persistent DD after the postoperative seventh day, which was associated with worsened respiratory outcomes compared to patients without DD. Overall, patients presented an average 15% decrease in diaphragm excursion at rest and 19% decrease after sniff test on day 7. Overall, DD was associated with a 33% incidence of respiratory complications. We identified no preoperative and procedural risk factors for persistent DD.
Most patients undergoing cardiac surgery show an early and transient DD.5,15,16 Indeed, transient diaphragm impairment can be caused by many factors, including sternotomy pain, mechanical factors affecting the chest and mediastinum, electrolyte disorders (such as hypophosphatemia), and pleural or pericardial effusion.
Our findings revealed two important issues associated with DD in cardiac surgery patients. First, we found that a significant proportion of these patients developed severe postoperative DD, which was persistent after the seventh postoperative day. Most studies have evaluated DD in the early perioperative days.5,15,16 Only one study by Pasero et al. assessed, in a prospective and observational study of 24 patients, the diaphragmatic thickness fraction at 24 hr before elective surgery and within one week after surgery. The authors reported 21% and 25% right and left DD, respectively.13 This difference in incidence compared to our study could be explained by the use of ice-cold cardioplegia solution in Pasero’ study and the different methods of ultrasound assessment (diaphragmatic thickness vs excursion).
Second, whether persistent DD worsens respiratory outcomes is still under debate. Here, we report poorer respiratory outcomes in these patients as DD was associated with a longer duration of noninvasive and mechanical invasive ventilation, a higher reintubation rate, higher episodes of pneumonia, and longer length of stay in the ICU.
We did not observe differences in terms of preoperative characteristics in patients with and without persistent DD, especially the incidence of diabetes. Moreover, there was no significant difference in the incidence of DD between patients undergoing coronary bypass grafting procedures compared with those undergoing other procedures. Nevertheless, we found that, even in patients without severe persistent DD, there was a significant decrease of both hemidiaphragm excursion after the seventh postoperative day, which suggests a bilateral involvement of the muscle, excluding an exclusive surgical phrenic nerve injury.
In this work, we chose to evaluate diaphragmatic excursion after the sniff test, as described by Boussuges et al.20 to define DD because this approach matched the expertise of the investigators and is known to be a reproducible method. Excursion and the thickening fraction are frequently used and accurately examined diaphragm function in critically ill individuals during a spontaneous breath trial.17 Ultrasonographic diaphragmatic excursion and the thickening fraction are correlated in cardiac surgery patients during unassisted breathing.16 Nevertheless, excursion seems to be a more feasible and reproducible method in this population (interobserver reliability yielded a bias below 0.1 cm with limits of agreement [LOA] of ± 0.3 cm for excursion and −2% with LOA of ± 21% for thickening fraction).16 Finally, while ultrasound accurately evaluates diaphragm function, the electrical activity of the diaphragm is still considered the gold standard diagnosis method.11,21
We acknowledge several limitations to this study. First, the single-centre nature of this study may impact the generalizability of the results. Nevertheless, the existence of persistent DD and its consequences are consistent with the literature. Moreover, we previously showed consistency with external cohorts of patients who are treated in our centre.12,22 Second, we observed a lower incidence of DD at day 7 than was reported by Pasero et al., who described a 30% incidence but used diaphragm thickness criteria.13 While our study may have been underpowered to determine risk factors of persistent DD, we observed significant differences between both groups, in terms of postoperative complications. The exclusion of patients with incomplete data from the final analysis due to poor echogenicity may also have led to an underestimation of DD incidence. Third, a significant number of patients were excluded from the study because of the COVID-19 pandemic, which reduced operator or machine availability. Furthermore, we did not report the incidence of pleural effusion on day 7, which could be linked to DD. Finally, we chose to define DD as a decrease of excursion under the lower limit of at least one measurement of spontaneous breathing or the sniff test, but not both. Although there is no consensus definition of DD, this may reduce the generalization of our results.
Conclusion
In this observational ultrasound study, persistent DD after the seventh postoperative day, evaluated utrasonographically by diaphragmatic excursion, occurred in 8% of patients undergoing elective cardiac surgery. This was associated with prolonged mechanical ventilation and length of stay in the ICU. Further larger studies are needed to fully explore the risk factors and consequences of DD after cardiac surgery.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Driss Laghlam, Cecile Naudin, and Philippe Estagnasié have substantially contributed to the conception and the design of the study and were major contributors in writing the original manuscript. Lee S. Nguyen and Pierre Squara have also substantially contributed to the conception and the design of the study and were contributors in revision of the manuscript. Driss Laghlam, Alexandre Srour, Raphael Monsonego, Ghilas Rahoual, and Julien Malvy have substantially contributed to the acquisition and analysis of the data.
Acknowledgements
Thank you to all the team of the Department of Clinical Research, particularly to Elefteria Sideris, Fatma Bouaziz, Djamiath, Thiamiyou, Steve Novak, and Messaouda Merzoug.
Disclosures
The authors declare that they have no competing interests.
Funding statement
Support was provided solely from institutional and/or departmental sources from the department of Clinical Research, CMC Ambroise Paré, Neuilly-sur-Seine, France.
Data availability statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Editorial responsibility
This submission was handled by Dr. Philip M. Jones, Deputy Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s12630-022-02360-8
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007/s12630-022-02360-8.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/144955431
Article citations
Early diaphragm dysfunction assessed by ultrasonography after cardiac surgery: a retrospective cohort study.
Front Cardiovasc Med, 11:1457412, 09 Oct 2024
Cited by: 0 articles | PMID: 39444548 | PMCID: PMC11496164
Liver autotransplantation and atrial reconstruction on a patient with multiorgan alveolar echinococcosis: a case report.
BMC Infect Dis, 24(1):659, 02 Jul 2024
Cited by: 1 article | PMID: 38956482 | PMCID: PMC11218102
Diaphragmatic dysfunction is associated with postoperative pulmonary complications in the aged patients underwent radical resection of esophageal cancer: a prospective observational study.
J Thorac Dis, 16(6):3623-3635, 13 Jun 2024
Cited by: 0 articles | PMID: 38983161
Diagnosis of hemidiaphragm paralysis: refine ultrasound criteria.
Front Med (Lausanne), 11:1416520, 23 May 2024
Cited by: 1 article | PMID: 38846144 | PMCID: PMC11153810
Ultrasound assessment of diaphragmatic dysfunction in non-critically ill patients: relevant indicators and update.
Front Med (Lausanne), 11:1389040, 18 Jun 2024
Cited by: 0 articles | PMID: 38957305 | PMCID: PMC11217340
Review Free full text in Europe PMC
Go to all (8) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (2 citations) ClinicalTrials.gov - NCT04276844
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Diaphragmatic Dysfunction After Elective Cardiac Surgery: A Prospective Observational Study.
J Cardiothorac Vasc Anesth, 34(12):3336-3344, 17 Jun 2020
Cited by: 10 articles | PMID: 32653270
Preoperative Diaphragm Function Is Associated With Postoperative Pulmonary Complications After Cardiac Surgery.
Crit Care Med, 47(12):e966-e974, 01 Dec 2019
Cited by: 17 articles | PMID: 31609771
Ultrasonographic diagnostic criterion for severe diaphragmatic dysfunction after cardiac surgery.
Chest, 135(2):401-407, 27 Aug 2008
Cited by: 88 articles | PMID: 18753469
Ultrasound assessment of ventilator-induced diaphragmatic dysfunction in mechanically ventilated pediatric patients.
Paediatr Respir Rev, 40:58-64, 23 Feb 2021
Cited by: 10 articles | PMID: 33744085
Review

 1,3
1,3 


