Abstract
Free full text

Understanding the role of GRE3 in the erythritol biosynthesis pathway in Saccharomyces uvarum and its implication in osmoregulation and redox homeostasis
Abstract
Erythritol is produced in yeasts via the reduction of erythrose into erythritol by erythrose reductases (ERs). However, the genes codifying for the ERs involved in this reaction have not been described in any Saccharomyces species yet. In our laboratory, we recently showed that, during alcoholic fermentation, erythritol is differentially produced by Saccharomyces cerevisiae and S. uvarum species, the latter being the largest producer. In this study, by using BLAST analysis and phylogenetic approaches the genes GRE3, GCY1, YPR1, ARA1 and YJR096W were identified as putative ERs in Saccharomyces cerevisiae Then, these genes were knocked out in our S. uvarum strain (BMV58) with higher erythritol biosynthesis compared to control S. cerevisiae wine strain, to evaluate their impact on erythritol synthesis and global metabolism. Among the mutants, the single deletion of GRE3 markedly impacts erythritol production, although ΔYPR1ΔGCY1ΔGRE3 was the combination that most decreased erythritol synthesis. Consistent with the increased production of fermentative by‐products involved in redox balance in the Saccharomyces uvarum strain BMV58, erythritol synthesis increases at higher sugar concentrations, hinting it might be a response to osmotic stress. However, the expression of GRE3 in the S. uvarum strain was found to peak just before the start of the stationary phase, being consistent with the observation that erythritol increases at the start of the stationary phase, when there is low sugar in the medium and nitrogen sources are depleted. This suggests that GRE3 plays its primary function to help the yeast cells to maintain the redox balance during the last phases of fermentation.
Abstract
The expression of the gene GRE3, encoding the main erythritol reductase of S. uvarum, is induced at the entry into the stationary phase, which suggests that it plays its primary function in helping yeast cells to maintain the redox balance during the stationary phase when nitrogen sources are depleted.
INTRODUCTION
Erythritol is a non‐caloric and rapidly absorbed four‐carbon polyol presenting a sweetening power of approximately 0.7 in comparison to sucrose (Carly & Fickers, 2018). Such properties make it a suitable sucrose alternative for food and nutraceutical formulation. Due to the increasing popularity of erythritol in the food industry, the biotechnological production of this polyol using bacteria or fungi, including yeasts (Moon et al., 2010), has been explored as a less expensive and more sustainable method than the conventional chemical synthesis.
In lactic acid bacteria, erythritol synthesis starts from glucose 6‐phosphate, which is isomerized by a phosphohexose to fructose 6‐phosphate, and this is cleaved into erythrose‐4‐phosphate and acetyl‐phosphate by a phosphoketolase. Erythrose‐4‐phosphate is then reduced to erythritol‐4‐phosphate, which is hydrolysed to erythritol by a phosphatase (Veiga‐Da‐Cunha et al., 1993). In yeasts, erythritol production is achieved at the level of the pentose phosphate pathway in a two‐step reaction: D‐erythrose 4‐phosphate is first dephosphorylated to erythrose by the polyol phosphatase PYP1 (Xu et al., 2018), which is subsequently reduced to erythritol by NAD(P)H‐dependant erythrose reductases (ERs). While the genes encoding for the ERs involved in this reaction have been described and exploited in other yeast species (e.g. Yarrowia lipolytica), they have not been described in Saccharomyces cerevisiae, the most important yeast specie in the production of fermented food and beverages, nor any other species of the Saccharomyces genus.
Saccharomyces cerevisiae is particularly well‐adapted to the variety of stresses that occur during winemaking, except for low temperatures (Querol et al., 2018). For this reason, cryotolerant Saccharomyces species, like S. uvarum, have recently appeared as a suitable alternative for the production of low‐temperature wines while, at the same time, meeting the wine market's expectation: lower ethanol yield, higher glycerol synthesis and wider aromatic profile (Pérez et al., 2022; Pérez‐Torrado et al., 2018). Among other interesting properties of this species, a recent metabolomic comparison performed under winemaking conditions between S. cerevisiae and S. uvarum showed that both species produced erythritol during fermentation, but that S. uvarum strains also produced more erythritol than their S. cerevisiae counterpart (Minebois et al., 2020a, 2020b).
Different functions for erythritol synthesis in yeasts have been proposed (Carly & Fickers, 2018). One of them is a role in osmotic tolerance in the presence of high concentrations of sugar and salt. Therefore, the role of erythritol could be the response to hyperosmotic stress because this molecule is a compatible solute as different polyols such as glycerol, ribitol, arabinitol, xylitol, sorbitol, mannitol, galactitol and others, that can act as a protectant against osmotic stress (Blomberg & Adler, 1992). Moreover, ERs, as well as other enzymes of the Aldo‐keto reductase (AKR) family to which they belong, are upregulated during osmotic stress in Y. lipolytica and Moniliella megachiliensis (Carly & Fickers, 2018). In S. cerevisiae, the expression of AKR genes has also been observed in response to stress (Chang et al., 2007) and their promotors contain stress‐response elements (STRE) (Garay‐Arroyo & Covarrubias, 1999). However, the regulatory mechanisms involved in the production of erythritol in response to osmotic stress have not been described yet. In Y. lipolytica, the production of erythritol in response to osmotic stress is independent of the Hog pathway (Rzechonek et al., 2020).
In addition to its putative osmotolerant role, erythritol has also been associated in yeasts with certain biological functions such as a cofactor balancing and storage compound (Brown, 1978). For instance, during wine fermentation, ethanol production is overall neutral in terms of redox homeostasis. However, different other cellular processes like the one associated with anabolism (i.e. amino acids and nucleotide synthesis) and detoxification (e.g. free radical scavenging via glutathione‐glutaredoxin reactions) require reducing power in the form of NAD(P)H equivalents, which have to be produced elsewhere in the metabolism. In this aspect, erythritol production could contribute to the correct performance of fermentation by supplying part of this reductive power, for instance, balancing the GABA shunt. S. uvarum produces a high amount of succinate (Minebois et al., 2020a, 2020b), and thanks to the models (Henriques et al., 2021), it has been proved that a significant fraction of this succinate would come from the GABA shunt, which leads to the production of NADPH. Furthermore, the largest succinate fraction in S. uvarum occurs after stationary phase entry (Henriques et al., 2021). Similarly, in S. uvarum, erythritol is produced when cells enter the stationary phase (Minebois et al., 2020a). Both pathways (GABA shunt and erythritol synthesis) are cytoplasmic and, hence, it is likely that they complement each other at a redox level: erythritol synthesis recycles the NADPH produced at the GABA shunt pathway.
As mentioned, erythritol production in Saccharomyces is poorly understood and putative ERs have only been described in a few species, such as Candida magnoliae (Lee et al., 2003) and Yarrowia lipolytica (Cheng et al., 2018; Janek et al., 2017). Interestingly, although the synthesis of erythritol was observed in S. cerevisiae and S. uvarum yeasts during wine fermentation (Minebois et al., 2020a), the metabolic pathway of erythritol has never been described in the Saccharomyces genus and the putative ERs involved are unknown. Therefore, this study aims to discover the genes involved in erythritol production to decipher their biological function.
EXPERIMENTAL PROCEDURES
Strains and media
Two commercial (Lallemand, Montreal) wine yeast strains, selected in our laboratory, were used as representative strains in this study, and correspond to those used in the comparative metabolome study of Minebois et al. (2020a). The representative S. cerevisiae strain is Lalvin T73 (from now T73), isolated from Alicante, Spain and the S. uvarum strain is Velluto BMV58 (BMV58), isolated from Requena, Spain. BMV58 is our high erythritol producer during wine fermentation according to Minebois et al. (2020a). All yeast strains were preserved in 15% glycerol at −80°C and cultivated in GPY solid media (2% glucose, 0.5% peptone, 0.5% yeast extract and 2% agar) when needed.
Bioinformatic and phylogenetic analyses
The genomes of T73 (NCBI SRA ref. SRX4791864) and BMV58 (NCBI SRA ref. SRX4086831) have previously been sequenced, assembled and annotated in our laboratory (Macías et al., 2021; Morard et al., 2019). From them, the sequences of the putative genes of this study were obtained. Y. lipolytica ER‐encoding gene sequences were obtained from the National Center for Biotechnology Information (NCBI) (http://blast.ncbi.nlm.nih.gov/Blast) and their homologous genes in Saccharomyces were searched with Basic Local Alignment Search Tool (BLAST) using ‘The BLAST Sequence Analysis Tool’. Other yeast AKR sequences were obtained from the AKR superfamily homepage (Perelman School of Medicine, University of Pennsylvania, www.med.upenn.edu/akr), maintaining the same references used in this database. Sequences were translated into amino acids and aligned with MUSCLE (Edgar, 2004) implemented in the MEGA v. 11.0.13 program (Tamura et al., 2021). The best evolutionary protein models based on empirical frequencies of amino acid replacements were selected with ProtTest 3.4.2 (Darriba et al., 2011), and the best‐fitting model was the LG model (Le & Gascuel, 2008) with gamma‐distributed rates with an α shape parameter of 1.57 and the observed amino acid frequencies. Maximum‐Likelihood phylogenies were also obtained by using the MEGA v. 11.0.13 program (Tamura et al., 2021).
Knockout production by CRISPR‐Cas9
CRISPR‐Cas9 was used to knock out the putative genes in S. uvarum BMV58 following the protocols described for Saccharomyces (Dicarlo et al., 2013). The gRNA for each gene was designed using Geneious software to reduce the off‐target probability and maximize correct Cas9 nuclease guidance. This gRNA was inserted into pRCC‐K Cas9 plasmid (Generoso et al., 2016) by PCR using the appropriate primers (Table S1) and Phusion™ High‐Fidelity DNA Polymerase (Thermo Fisher) followed by DpnI treatment (Thermo Fisher) to remove the original E. coli plasmid. Donor ssDNA was designed to be used as a template during homologous recombination, this sequence contains homologous regions upstream and downstream of the ORF of each gene and includes the stop codon (Stovicek et al., 2017). Forward and reverse ssDNAs (Table S1) were incubated and dsDNA was obtained. The S. uvarum strain was transformed using the lithium acetate method for DNA extraction (Gietz & Woods, 2006) with the plasmid containing each gRNA and with each donor DNA. Mutants were grown on GPY plates containing G418 antibiotics for the selection because pRCC‐K has a neomycin‐resistance marker. After 3 days, the obtained colonies were tested to verify the deletion with each specific primer set for each gene (Table S1) and with genomic DNA extracted using the lithium acetate method (Lõoke et al., 2011). Colonies that were confirmed as positive knockouts were plated on GPY without G418 to get rid of the plasmid. Finally, verified mutants were stored in 15% glycerol. When double (or multiple) knockouts were done, the single (or multiple) mutants were used as the initial strain, and the same procedure was followed.
days, the obtained colonies were tested to verify the deletion with each specific primer set for each gene (Table S1) and with genomic DNA extracted using the lithium acetate method (Lõoke et al., 2011). Colonies that were confirmed as positive knockouts were plated on GPY without G418 to get rid of the plasmid. Finally, verified mutants were stored in 15% glycerol. When double (or multiple) knockouts were done, the single (or multiple) mutants were used as the initial strain, and the same procedure was followed.
Fermentations
To determine if the putative ERs were responsible for the erythrose reduction to erythritol, single and multiple knockout strains of each gene were used in fermentation at 25°C, and the amount of erythritol produced was measured. Fermentations were performed in 100‐mL bottles with 70 mL media of synthetic must (Rossignol et al., 2003) with different concentrations of sugar (glucose and fructose) ranging from a total of 200–260
mL media of synthetic must (Rossignol et al., 2003) with different concentrations of sugar (glucose and fructose) ranging from a total of 200–260 g/L and two YAN (Yeast Assimilable Nitrogen) concentrations, 200 or 300
g/L and two YAN (Yeast Assimilable Nitrogen) concentrations, 200 or 300 mg/L. Overnight precultures of each strain (wild type and mutants) were used to inoculate in triplicate the synthetic grape must at 2
mg/L. Overnight precultures of each strain (wild type and mutants) were used to inoculate in triplicate the synthetic grape must at 2 ×
× 106
106 C/mL using a Guava® Muse® Cell Analyser (Merck) and bottles were incubated at 25°C with continuous orbital shaking (100
C/mL using a Guava® Muse® Cell Analyser (Merck) and bottles were incubated at 25°C with continuous orbital shaking (100 rpm). Weight loss of the bottles was measured to follow the fermentation phases and samples were taken at the end of the fermentation to measure the metabolites.
rpm). Weight loss of the bottles was measured to follow the fermentation phases and samples were taken at the end of the fermentation to measure the metabolites.
Extracellular metabolite quantification
The quantification of the sugars and fermentative by‐products (glycerol, ethanol, 2,3 butanediol, erythritol, succinic, acetic and lactic acids) was done by High‐Performance Liquid Chromatography (HPLC) (Thermo Fisher Scientific) that has a refraction index detector and UV/VIS (210 nm) detector equipped with a HyperREZTM XP Carbohydrate H+ 8
nm) detector equipped with a HyperREZTM XP Carbohydrate H+ 8 mm column (Thermo Fisher Scientific) and HyperREZTM XP Carbohydrate Guard (Thermo Fisher Scientific). Regarding the analysis conditions, the eluent was 1.5
mm column (Thermo Fisher Scientific) and HyperREZTM XP Carbohydrate Guard (Thermo Fisher Scientific). Regarding the analysis conditions, the eluent was 1.5 mM of sulfuric acid at a flux of 0.6
mM of sulfuric acid at a flux of 0.6 mL/min and the oven temperature was 50°C. The samples were diluted, filtered through a 0.22‐μm nylon filter (Symta) and injected. To quantify the metabolites of interest, the retention times of the eluted peaks were compared to those produced by commercial analytical standards with known concentrations (Sigma‐Aldrich).
mL/min and the oven temperature was 50°C. The samples were diluted, filtered through a 0.22‐μm nylon filter (Symta) and injected. To quantify the metabolites of interest, the retention times of the eluted peaks were compared to those produced by commercial analytical standards with known concentrations (Sigma‐Aldrich).
Gene expression analysis
To correlate if the putative ERs were expressed at a higher level when erythritol production increased, the relative mRNA levels of these genes were determined. The fermentation was performed at 25°C using 500 mL bottles with 400
mL bottles with 400 mL of synthetic must (Rossignol et al., 2003) with 240
mL of synthetic must (Rossignol et al., 2003) with 240 g/L glucose + fructose and 300
g/L glucose + fructose and 300 mg/L YAN.
mg/L YAN.
1 ×
× 108 cells were immediately harvested after 0, 1, 4, 24, 48, 72, 96 and 192
108 cells were immediately harvested after 0, 1, 4, 24, 48, 72, 96 and 192 h by centrifugation, then cells were washed and frozen in liquid nitrogen. RNA from the frozen cells was extracted using RNeasy Mini Kit (Qiagen). This RNA was converted to cDNA by adding 0.8
h by centrifugation, then cells were washed and frozen in liquid nitrogen. RNA from the frozen cells was extracted using RNeasy Mini Kit (Qiagen). This RNA was converted to cDNA by adding 0.8 mM of the four dNTPs, 80
mM of the four dNTPs, 80 pmol Oligo (dT) to 2
pmol Oligo (dT) to 2 μg of RNA in 13
μg of RNA in 13 μL. This was heated at 65°C for 5
μL. This was heated at 65°C for 5 min and incubated on ice for 1
min and incubated on ice for 1 min. Then in a 20‐μL reaction, 5
min. Then in a 20‐μL reaction, 5 mM dithiothreitol (DTT), 50
mM dithiothreitol (DTT), 50 U of RNase inhibitor (Invitrogen), First Strand Buffer (Invitrogen), and 200
U of RNase inhibitor (Invitrogen), First Strand Buffer (Invitrogen), and 200 U Superscript III (Invitrogen) were added and incubated at 50°C for 60
U Superscript III (Invitrogen) were added and incubated at 50°C for 60 min and then, inactivated at 70°C during 15
min and then, inactivated at 70°C during 15 min. The desired genes (GCY1, YPR1, ARA1, GRE3, YJR096 and GPD1) were quantified by qRT‐PCR (quantitative real‐time PCR) by using gene‐specific primer sets (Table S2) and the Light Cycler FastStart DNA MasterPLUS SYBR green (Roche Applied Science) in a LightCycler® 2.0 System (Roche Applied Science). The actin ACT1, and 18S ribosomal RNA RDN18‐1, genes were used as constitutive genes to normalize the amount of mRNA and guarantee accuracy, repeatability and reproducibility (Starovoytova et al., 2013).
min. The desired genes (GCY1, YPR1, ARA1, GRE3, YJR096 and GPD1) were quantified by qRT‐PCR (quantitative real‐time PCR) by using gene‐specific primer sets (Table S2) and the Light Cycler FastStart DNA MasterPLUS SYBR green (Roche Applied Science) in a LightCycler® 2.0 System (Roche Applied Science). The actin ACT1, and 18S ribosomal RNA RDN18‐1, genes were used as constitutive genes to normalize the amount of mRNA and guarantee accuracy, repeatability and reproducibility (Starovoytova et al., 2013).
In parallel, the metabolites produced during fermentation at the same sampling times were measured by HPLC as previously described.
Osmotic and cold stress assays
T73, BMV58 and the selected knockouts were cultured in 50 mL of GPY at 25°C. Overnight cultures were centrifuged, washed and resuspended in water. The initial cell concentration of the preculture was measured and adjusted using a Guava® Muse® Cell Analyzer (Merck) so that 50
mL of GPY at 25°C. Overnight cultures were centrifuged, washed and resuspended in water. The initial cell concentration of the preculture was measured and adjusted using a Guava® Muse® Cell Analyzer (Merck) so that 50 μL were inoculated (in triplicates) into 950
μL were inoculated (in triplicates) into 950 μL media, on a 24‐well plate, to reach 1
μL media, on a 24‐well plate, to reach 1 ×
× 106 cells/mL.
106 cells/mL.
For the determination of tolerance to sorbitol, experiments were carried out in minimal medium YNB with 20 g/L glucose as a control condition and different sorbitol concentrations as test conditions: 1, 2 and 3
g/L glucose as a control condition and different sorbitol concentrations as test conditions: 1, 2 and 3 M. The microplates were incubated inside a camera that maintains 75% humidity at 25°C. The settings of the microplates were shaking in an orbital mode for 20
M. The microplates were incubated inside a camera that maintains 75% humidity at 25°C. The settings of the microplates were shaking in an orbital mode for 20 s each half an hour at 300
s each half an hour at 300 rpm. In the case of cold stress, the same settings were used although the temperature was 12°C. 1
rpm. In the case of cold stress, the same settings were used although the temperature was 12°C. 1 mM erythritol was added to each stress condition (cold or different sorbitol concentrations), to know if its addition was useful for yeast growth. Moreover, for cold stress conditions, additions of 5 and 10
mM erythritol was added to each stress condition (cold or different sorbitol concentrations), to know if its addition was useful for yeast growth. Moreover, for cold stress conditions, additions of 5 and 10 mM erythritol were also tested. Besides, 10
mM erythritol were also tested. Besides, 10 mM glycerol was used as a control due to its known properties to protect against stress. Yeast growth in each different medium was followed by measuring the OD600 in Stacker Microplate Handling System attached to plate readers SPECTROstar Omega (BMG LABTECH). The OD600 results were analysed by the Growth Curve Analysis Tool (GCAT) (Bukhman et al., 2015) using the sigmoid model option GCAT selected between Richards, Gompertz and logistic sigmoid curve models. Then, the reparametrized formulas (Zwietering et al., 1990) were used to represent growth in terms of essential growth curve characteristics: lag time, growth rate and asymptotic growth value, and these were analysed.
mM glycerol was used as a control due to its known properties to protect against stress. Yeast growth in each different medium was followed by measuring the OD600 in Stacker Microplate Handling System attached to plate readers SPECTROstar Omega (BMG LABTECH). The OD600 results were analysed by the Growth Curve Analysis Tool (GCAT) (Bukhman et al., 2015) using the sigmoid model option GCAT selected between Richards, Gompertz and logistic sigmoid curve models. Then, the reparametrized formulas (Zwietering et al., 1990) were used to represent growth in terms of essential growth curve characteristics: lag time, growth rate and asymptotic growth value, and these were analysed.
Statistical analyses and graphs
The statistical analysis of data obtained was performed using IBM SPSS Statistics (version 23.0, IBM Co.). One‐way analysis of variance (anova) was performed to detect if significant differences existed. If there were significant differences, a post‐hoc range test was applied to determine which means were different. Tukey finds differences during pairwise comparison, so it is useful in confirmatory research and may be used when group sizes are unequal and smaller (Lee & Lee, 2018). Therefore, Tukey's ‘Honestly significant difference’ (HSD) was used in this study. Means were considered significantly different when <0.05. Graphs were constructed using GraphPad Prism 7.0 (GraphPad Software).
RESULTS
Homology search and phylogenetic analysis of erythrose reductases
In those few yeasts that have been studied, erythritol is produced by a reduction of erythrose catalysed by an ER. However, until the study of Minebois et al. (2020a), the putative production of erythritol in Saccharomyces yeast has been disregarded, and hence, the identification of those genes encoding enzymes potentially involved in this synthesis was neglected. Aiming to discover putative genes in Saccharomyces involved in erythrose reduction, the ERs described in Y. lipolytica were used as references for a homology search in the Saccharomyces genomes. So far, the ER‐encoding genes described in Y. lipolytica are YALI0F18590 (ER27), YALI0D07634g (ER10), YALI0C13508g (ER25) and YALI0B07117 (Cheng et al., 2018; Janek et al., 2017; Szczepańczyk et al., 2021). Their sequences were used as the query sequence for a BLAST analysis and the results showed some sequences with high similarities in the Saccharomyces genomes (Table S3). These sequences correspond to genes encoding enzymes of the AKR family, involved in the conversion of carbonyl compounds into their corresponding alcohol products (Chang et al., 2007).
A phylogenetic analysis was done using the genes identified in Y. lipolytica and the sequences of the genes selected in different species of Saccharomyces. Furthermore, all known AKR sequences described (Jez & Penning, 2001) and updated on the AKR superfamily homepage (Perelman School of Medicine, University of Pennsylvania, www.med.upenn.edu/akr) were used to obtain a maximum‐likelihood phylogenetic reconstruction (Figure 1). Of the Saccharomyces genes homologous to the reference yeast ERs, the gene YDL124W seems to be pseudogenized in S. uvarum because the homologous syntenic region has lost the start codon and contains several frameshift mutations. Moreover, its encoded enzyme is not related to ERs; therefore, this gene was not taken into consideration in this study.

Maximum‐Likelihood phylogeny based on amino acid sequences of the AKR family including the selected genes that encode for ERs in Saccharomyces obtained after a multiple sequence alignment.
Accordingly, we decided to study in detail genes YOR120W (GCY1), YDR368W (YPR1), YBR149W (ARA1), YHR104W (GRE3) and YJR096W. A phylogenetic analysis only including S. cerevisiae T73, S. uvarum BMV58 and the reference ER protein sequences (Figure 1 and Figure S1), showed that Saccharomyces enzymes encoded by genes YHR104W (GRE3) and YJR096W, belonging to the mannose and xylose reductases group, are closely related to ER25 from Y. lipolytica. Proteins encoded by genes YBR149W (ARA1), YOR120W (GCY1) and YDR368W (YPR1) appear within the group of Yeast AKRs, together with the remaining ERs from Yarrowia lipolytica, Magnaporthe grisea, Candida magnolia and Trichosporonoides megachiliensis. ARA1p appears as closely related to ER10 from Y. lipolytica, which was shown to be closely related to L‐arabinose reductase from the fungus Magnaporthe grisea. ER10 catalyzes the reduction of erythrose into erythritol, but also the reverse reaction in the presence of NADP+ (Cheng et al., 2018).GCY1 encodes a glycerol dehydrogenase involved in an alternative pathway for glycerol catabolism, and YPR1 is its paralog, generated during a whole genome duplication event (WGD) that occurred in an ancestor of the Saccharomyces clade (Wolfe, 2015).
Knockout mutant generation
To determine the role of the Saccharomyces genes with potential ER activity, we decided to obtain knockout mutants of these genes in BMV58, as this is the strain with the highest erythritol production reported in our laboratory (Minebois et al., 2020a, 2020b). Single, double and triple knockouts were confirmed by PCR with the corresponding primers (Table S1) followed by electrophoresis on an agarose gel. When each gene was present in the WT, there was a band of GCY1 (1495 bps), YPR1 (1544
bps), YPR1 (1544 bps), GRE3 (1190
bps), GRE3 (1190 bps), ARA1 (1180
bps), ARA1 (1180 bps) and YJR096W (1151
bps) and YJR096W (1151 bps). In the knockout mutants, these amplification bands were smaller GCY1 (559
bps). In the knockout mutants, these amplification bands were smaller GCY1 (559 bps), YPR1 (608
bps), YPR1 (608 bps), GRE3 (209
bps), GRE3 (209 bps), ARA1 (148
bps), ARA1 (148 bps) and YJR096W (305
bps) and YJR096W (305 bps), indicating that the genes were successfully deleted. The efficiency of the transformation ranged between 40% and 60%. The successful knockouts (Table S4) were plated on GPY with G418 to ensure that they were not able to grow, confirming they lost the pRCC‐K plasmid.
bps), indicating that the genes were successfully deleted. The efficiency of the transformation ranged between 40% and 60%. The successful knockouts (Table S4) were plated on GPY with G418 to ensure that they were not able to grow, confirming they lost the pRCC‐K plasmid.
Erythritol synthesis during fermentation
To test if the selected genes are involved in the synthesis of erythritol, the mutants were used in fermentation in synthetic must. Wild‐type (WT) S. cerevisiae T73 and S. uvarum BMV58 strains were also inoculated in the same conditions as controls, and erythritol was measured at the end of fermentation. The production of erythritol has been reported to increase at high sugar concentrations in Y. lipolytica (Janek et al., 2017). Therefore, we performed fermentations at 25°C using different sugar and nitrogen concentrations to know which condition led to the highest erythritol yield. When using 200 g/L sugars and 200
g/L sugars and 200 mg/L YAN, we obtained a relatively low amount of erythritol, and the knockouts did not significantly reduce its production (Figure S2). However, when using 240
mg/L YAN, we obtained a relatively low amount of erythritol, and the knockouts did not significantly reduce its production (Figure S2). However, when using 240 g/L sugars and 300
g/L sugars and 300 mg/L YAN, the production of erythritol was the highest, being (0.72
mg/L YAN, the production of erythritol was the highest, being (0.72 ±
± 0.02
0.02 g/L) (Table S5). Thus, this medium was selected to test the effect of the deletion of the putative ER genes. Strikingly, we observed that the WT strain of S. uvarum produced more than twice the amount of erythritol than S. cerevisiae in this medium (Figure 2).
g/L) (Table S5). Thus, this medium was selected to test the effect of the deletion of the putative ER genes. Strikingly, we observed that the WT strain of S. uvarum produced more than twice the amount of erythritol than S. cerevisiae in this medium (Figure 2).
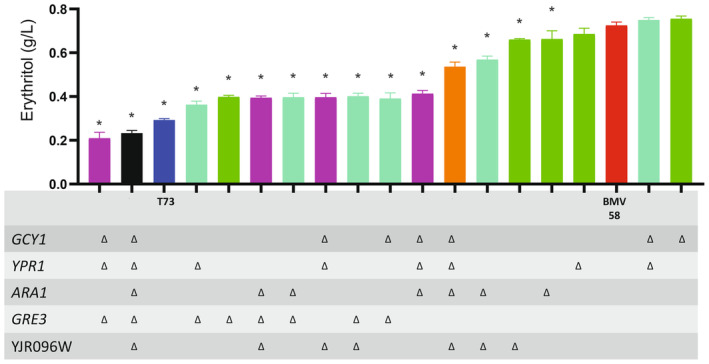
Concentrations of erythritol produced by Saccharomyces cerevisiae T73 (dark blue), S. uvarum BMV58 (red) as WT, the single mutants (green), double mutants (light blue), triple mutants (purple), quadruple mutant (orange) and quintuple mutant, with all selected genes deleted (black). This concentration was measured after fermentation on SM with 240 g/L glucose + fructose and 300
g/L glucose + fructose and 300 mg/L YAN. Standard deviation is shown, and the asterisk represents significant differences at the level of 0.05 in comparison with the WT (BMV58).
mg/L YAN. Standard deviation is shown, and the asterisk represents significant differences at the level of 0.05 in comparison with the WT (BMV58).
When using 240 g/L sugars and 300
g/L sugars and 300 mg/L YAN, ΔGRE3 was the single mutant showing the largest reduction in erythritol synthesis (0.4
mg/L YAN, ΔGRE3 was the single mutant showing the largest reduction in erythritol synthesis (0.4 ±
± 0.01
0.01 g/L). The double ΔARA1ΔGRE3, ΔYJR096WΔGRE3 and triple ΔYJR096WΔGRE3ΔARA1 knockout mutants significantly produced less erythritol than the WT, but the same amount as the single mutant ΔGRE3. Therefore, the combination of the deleted genes YJR096W and ARA1 did not decrease the production more than ΔGRE3. The double deletant ΔARA1ΔYJR096W significantly reduced erythritol synthesis compared to the WT, although less than ΔGRE3. In the case of the paralogous GCY1 and YPR1, neither the single mutants, ΔYPR1 and ΔGCY1, nor the double mutant ΔYPR1ΔGCY1 decreased erythritol production. The double deletion of GCY1 or YPR1 with GRE3, ΔYPR1ΔGRE3 and ΔGCY1ΔGRE3, decreased erythritol synthesis to a similar level to ΔGRE3. Nevertheless, the combined deletion ΔYPR1ΔGCY1ΔGRE3 is the triple knockout exhibiting the highest decrease in erythritol production (0.21
g/L). The double ΔARA1ΔGRE3, ΔYJR096WΔGRE3 and triple ΔYJR096WΔGRE3ΔARA1 knockout mutants significantly produced less erythritol than the WT, but the same amount as the single mutant ΔGRE3. Therefore, the combination of the deleted genes YJR096W and ARA1 did not decrease the production more than ΔGRE3. The double deletant ΔARA1ΔYJR096W significantly reduced erythritol synthesis compared to the WT, although less than ΔGRE3. In the case of the paralogous GCY1 and YPR1, neither the single mutants, ΔYPR1 and ΔGCY1, nor the double mutant ΔYPR1ΔGCY1 decreased erythritol production. The double deletion of GCY1 or YPR1 with GRE3, ΔYPR1ΔGRE3 and ΔGCY1ΔGRE3, decreased erythritol synthesis to a similar level to ΔGRE3. Nevertheless, the combined deletion ΔYPR1ΔGCY1ΔGRE3 is the triple knockout exhibiting the highest decrease in erythritol production (0.21 ±
± 0.03
0.03 g/L), even more than the single ΔGRE3 mutant does. Therefore, YPR1, GCY1 and GRE3 could be the main genes involved in erythritol production. Finally, the combined deletion of the five AKR genes, called Δ5K, produced almost a 3‐fold reduction of the erythritol yield compared to the WT, although there was some production yet (Figure 2).
g/L), even more than the single ΔGRE3 mutant does. Therefore, YPR1, GCY1 and GRE3 could be the main genes involved in erythritol production. Finally, the combined deletion of the five AKR genes, called Δ5K, produced almost a 3‐fold reduction of the erythritol yield compared to the WT, although there was some production yet (Figure 2).
Analysis of the possible role of erythritol in osmotic and cold stress tolerances
The higher erythritol production observed in Saccharomyces yeast grown in media with high sugar concentrations indicates that erythritol synthesis could be related to a response to osmotic stress. To determine if erythritol could play a role in osmotic stress tolerance, strains T73, BMV58 (WT) and the BMV58 knockouts ΔGRE3, ΔYPR1ΔGCY1ΔGRE3 (from now called Δ3K) and ΔYPR1ΔGCY1ΔARA1ΔYJR096W (called Δ4K) were grown under different sorbitol concentrations (1, 2 and 3 M). As a control, the addition of 10
M). As a control, the addition of 10 mM glycerol was also tested because it is well‐known that glycerol is produced and accumulated in yeasts to counterbalance external osmotic pressure (Blomberg & Adler, 1989).
mM glycerol was also tested because it is well‐known that glycerol is produced and accumulated in yeasts to counterbalance external osmotic pressure (Blomberg & Adler, 1989).
A simple way to summarize the effect of the osmotic stress on the growth of the yeast is to observe significant changes in the maximum specific growth rate (μ
max), which is a key parameter calculated as the first derivative at the inflection point of the growth curve (Zwietering et al., 1990). Our results showed the effect of osmotic stress due to increasing concentrations of sorbitol in the growth media. At 1 M sorbitol, no effect was observed in the μ
max of T73, but the μ
max was decreased in strain BMV58 and all its knockout mutants (Figure 3A). At 2
M sorbitol, no effect was observed in the μ
max of T73, but the μ
max was decreased in strain BMV58 and all its knockout mutants (Figure 3A). At 2 M sorbitol, all the strains were significantly affected, especially the quadruple knockout Δ4K that was unable to grow (Figure S3). Finally, in the presence of 3
M sorbitol, all the strains were significantly affected, especially the quadruple knockout Δ4K that was unable to grow (Figure S3). Finally, in the presence of 3 M sorbitol, no growth was observed in any of the strains (data not shown). Focusing on 1
M sorbitol, no growth was observed in any of the strains (data not shown). Focusing on 1 M sorbitol on BMV58 it was observed that this stress significantly reduced the μ
max from 0.52 to 0.35. Nevertheless, with the addition of erythritol and glycerol, growth was recovered, being at the same level as without sorbitol stress with erythritol addition (0.45) and not significantly different with sorbitol when adding glycerol (0.4). In the case of ΔGRE3, the presence of 1
M sorbitol on BMV58 it was observed that this stress significantly reduced the μ
max from 0.52 to 0.35. Nevertheless, with the addition of erythritol and glycerol, growth was recovered, being at the same level as without sorbitol stress with erythritol addition (0.45) and not significantly different with sorbitol when adding glycerol (0.4). In the case of ΔGRE3, the presence of 1 M sorbitol significantly reduced growth. Although μ
max was recovered by adding erythritol and glycerol at the same level as without sorbitol stress. The same effect happened on Δ4K, although μ
max was lower, being 0.12 with 1
M sorbitol significantly reduced growth. Although μ
max was recovered by adding erythritol and glycerol at the same level as without sorbitol stress. The same effect happened on Δ4K, although μ
max was lower, being 0.12 with 1 M sorbitol stress and doubling this value without stress or with stress but adding erythritol or glycerol. In the case of Δ3K, the effect of the addition of erythritol with 1
M sorbitol stress and doubling this value without stress or with stress but adding erythritol or glycerol. In the case of Δ3K, the effect of the addition of erythritol with 1 M sorbitol stress, significantly increases μ
max (being 0.48, even higher than without any stress that was 0.33). Also, the addition of glycerol recovered growth until 0.41.
M sorbitol stress, significantly increases μ
max (being 0.48, even higher than without any stress that was 0.33). Also, the addition of glycerol recovered growth until 0.41.
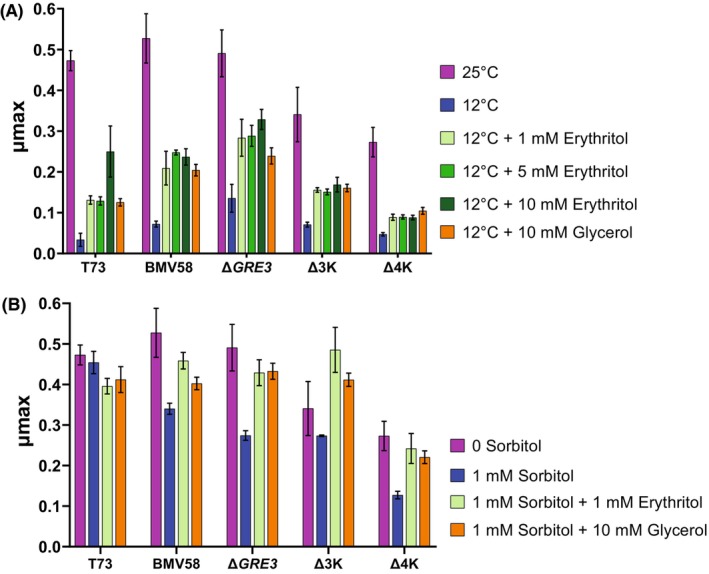
(A) μ max (log. OD/h) of Saccharomyces cerevisiae (T73), S. uvarum (BMV58), BMV58 mutants ΔGRE3, ΔYPR1ΔGCY1ΔGRE3 (Δ3K) and ΔYPR1ΔGCY1ΔARA1ΔYJR096W (Δ4K) in YNB at 25°C without sorbitol and with different concentrations of sorbitol, as well as sorbitol and the addition of erythritol or glycerol. Values represent media of biological triplicates and the standard deviation is shown. (B) μ max log. OD/h of S. cerevisiae (T73), S. uvarum (BMV58), BMV58 mutants ΔGRE3, ΔYPR1ΔGCY1ΔGRE3 (Δ3K) and ΔYPR1ΔGCY1ΔARA1ΔYJR096W (Δ4K) in YNB at 25 and 12°C as cold stress as well as 12°C with the addition of erythritol and glycerol. Values represent media of biological triplicates and the standard deviation is shown. The statistics were performed by anova and Tukey as a post‐hoc range test.
To study the role of erythritol in cold stress, we performed fermentation at low temperature (12°C) with the selected knockout strains ΔGRE3, Δ3K and Δ4K, and T73, BMV58 as WT, in the presence of erythritol at different concentrations: 1 mM, a low concentration similar to the amount that S. cerevisiae produces, 5
mM, a low concentration similar to the amount that S. cerevisiae produces, 5 mM as a medium concentration, similar to the highest concentration produced by S. uvarum, and 10
mM as a medium concentration, similar to the highest concentration produced by S. uvarum, and 10 mM as a high concentration for comparative purposes because fermentations at low temperature was also performed in the presence of 10
mM as a high concentration for comparative purposes because fermentations at low temperature was also performed in the presence of 10 mM glycerol, a concentration produced and accumulated in Saccharomyces species as a cryoprotectant (Oliveira et al., 2014).
mM glycerol, a concentration produced and accumulated in Saccharomyces species as a cryoprotectant (Oliveira et al., 2014).
As controls, fermentation in the absence of erythritol were performed at low (12°C) and medium (25°C) temperatures. A temperature of 12°C is stressing for yeast growth, which significantly decreased in all the strains (Figure 3B). The addition of glycerol and erythritol improved growth under cold stress in all strains, although μ
max values were not as high as the ones at 25°C. There were no significant differences between the addition of glycerol and erythritol except in T73, where it was observed that the effect of 10 mM glycerol is the same as 1 and 5
mM glycerol is the same as 1 and 5 mM erythritol, but the μ
max was higher when using 10
mM erythritol, but the μ
max was higher when using 10 mM erythritol. In the case of Δ4K, the μ
max is lower than the rest, being (0.27
mM erythritol. In the case of Δ4K, the μ
max is lower than the rest, being (0.27 ±
± 0.03) at 25°C and (0.04
0.03) at 25°C and (0.04 ±
± 0.004) at 12°C. In this strain, the addition of erythritol did not recover growth significantly, but glycerol did. Δ4K lacks GCY1, YPR1, ARA1 and YJR096W but GRE3 is present; therefore, this could be due to different responses to cold stress. To sum up, these results suggest that erythritol contributes to growth recovery when cold and osmotic stresses are present (Figure 3).
0.004) at 12°C. In this strain, the addition of erythritol did not recover growth significantly, but glycerol did. Δ4K lacks GCY1, YPR1, ARA1 and YJR096W but GRE3 is present; therefore, this could be due to different responses to cold stress. To sum up, these results suggest that erythritol contributes to growth recovery when cold and osmotic stresses are present (Figure 3).
Selected mutant performance during fermentation and analysis of the expression of the putative genes involved in erythritol synthesis
Fermentations at 25°C were conducted by the selected knockout strains ΔGRE3, Δ3K and Δ4K, and the WT strains BMV58 and T73 to study their performance. The production of important fermentative by‐products (ethanol, glycerol, succinate, etc.) was also quantified to assess the impact of the deleted genes on the central carbon metabolism during fermentation. Samples were taken at 0, 1, 4, 24, 48, 72, 96, 192 h, and at the end of the fermentation, to measure for each knockout mutant both the amount of metabolites produced as well as gene expression levels, described in the next section.
h, and at the end of the fermentation, to measure for each knockout mutant both the amount of metabolites produced as well as gene expression levels, described in the next section.
The differences in extracellular concentrations of the main products of fermentation are depicted in Figure 4 and Table S6. The gene expressions among the different knockout mutants and reference strains are shown in Figure 5. As expected, the fastest growth is exhibited by the S. cerevisiae T73 strain, followed by S. uvarum BMV58 and its mutant derivatives, which behave in the same manner, and, although deletant Δ4K shows a delayed growth, it finished at the same time (Figure S5).

Metabolic pathways of Saccharomyces including glycolysis and pentose phosphate pathway, which are involved in erythritol metabolism, are the main cofactors and the enzymes that could have an impact on erythritol formation. The graphics show the extracellular concentrations of the main products of fermentation on SM 240 g/L sugars and 300
g/L sugars and 300 YAN measured by HPLC produced by S. cerevisiae T73 (blue), S. uvarum BMV58 (red) and BMV58 mutants ΔGRE3 (green), ΔYPR1ΔGCY1ΔGRE3 (Δ3K, purple) and ΔYPR1ΔGCY1ΔARA1ΔYJR096W (Δ4K, orange). The values are presented as the mean and SDs from triplicate experiments. For more detail, the statistics of all metabolites can be found in Table S6.
YAN measured by HPLC produced by S. cerevisiae T73 (blue), S. uvarum BMV58 (red) and BMV58 mutants ΔGRE3 (green), ΔYPR1ΔGCY1ΔGRE3 (Δ3K, purple) and ΔYPR1ΔGCY1ΔARA1ΔYJR096W (Δ4K, orange). The values are presented as the mean and SDs from triplicate experiments. For more detail, the statistics of all metabolites can be found in Table S6.

Expression of the selected genes represented by the mRNA levels of the genes relative to ACT1 and 18S ribosomal RNA gene at 0, 1, 4, 24, 48, 72, 96 and 192 h of fermentation by Saccharomyces cerevisiae (T73), S. uvarum (BMV58) and BMV58 mutants ΔYPR1ΔGCY1ΔGRE3 (Δ3K) and ΔYPR1ΔGCY1ΔARA1ΔYJR096W (Δ4K). The values represent the median and standard deviations of biological and technical triplicates.
h of fermentation by Saccharomyces cerevisiae (T73), S. uvarum (BMV58) and BMV58 mutants ΔYPR1ΔGCY1ΔGRE3 (Δ3K) and ΔYPR1ΔGCY1ΔARA1ΔYJR096W (Δ4K). The values represent the median and standard deviations of biological and technical triplicates.
Concerning erythritol (Figure 4), the highest yield was observed in BMV58 (0.72 ±
± 0.02
0.02 g/L), which started to produce extracellular erythritol after 48
g/L), which started to produce extracellular erythritol after 48 h of fermentation increasing at a constant rate until the end of fermentation. Mutant Δ4K, in which GRE3 is not deleted, exhibited a similar but delayed pattern in which a slightly lower production started after 72
h of fermentation increasing at a constant rate until the end of fermentation. Mutant Δ4K, in which GRE3 is not deleted, exhibited a similar but delayed pattern in which a slightly lower production started after 72 h of fermentation but increased at a similar constant rate as BMV58 until the end of fermentation. In the case of T73 and ΔGRE3, they showed a similar pattern of erythritol production, but slightly higher in ΔGRE3 (0.4
h of fermentation but increased at a similar constant rate as BMV58 until the end of fermentation. In the case of T73 and ΔGRE3, they showed a similar pattern of erythritol production, but slightly higher in ΔGRE3 (0.4 ±
± 0.01
0.01 g/L). In them, production started after 72
g/L). In them, production started after 72 h at a similar level that in BMV58, but along the rest of fermentation only increased slightly at a very low rate. Finally, mutant Δ3K started to produce erythritol after 96
h at a similar level that in BMV58, but along the rest of fermentation only increased slightly at a very low rate. Finally, mutant Δ3K started to produce erythritol after 96 h of fermentation but after that, the concentration remained constant until the end of fermentation. At the end of the fermentation, strain BMV58 produced the highest erythritol contents; followed by mutant Δ4K (0.54
h of fermentation but after that, the concentration remained constant until the end of fermentation. At the end of the fermentation, strain BMV58 produced the highest erythritol contents; followed by mutant Δ4K (0.54 ±
± 0.02
0.02 g/L). Mutant Δ3K produced significantly less erythritol than the rest of the strains (0.21
g/L). Mutant Δ3K produced significantly less erythritol than the rest of the strains (0.21 ±
± 0.03
0.03 g/L).
g/L).
The effect of the genes knocked out in BMV58 also had an impact, in addition to erythritol, on other metabolites, such as ethanol, glycerol, 2,3‐butanediol, succinic acid and acetic acid.
The production of ethanol (Figure 4) follows a similar pattern, indicating that the kinetics and yield of ethanol in the knockouts ΔGRE3 and Δ3K were not affected compared to BMV58 WT (11.86% ±
± 0.68%), while the kinetics was delayed in Δ4K, ending up to 12.71
0.68%), while the kinetics was delayed in Δ4K, ending up to 12.71 ±
± 0.22%. In the case of glycerol production, as expected S. uvarum BMV58 is the highest producer (8.08
0.22%. In the case of glycerol production, as expected S. uvarum BMV58 is the highest producer (8.08 ±
± 0.65
0.65 g/L) but the three knockout mutants produced very similar yields. In terms of acetic acid, the highest production was observed in Δ4K (8.39
g/L) but the three knockout mutants produced very similar yields. In terms of acetic acid, the highest production was observed in Δ4K (8.39 ±
± 0.16
0.16 g/L), then BMV58 (0.62
g/L), then BMV58 (0.62 ±
± 0.04
0.04 g/L), the rest of the mutants, and T73 presented the lowest production of acetic acid (0.46
g/L), the rest of the mutants, and T73 presented the lowest production of acetic acid (0.46 ±
± 0.02). BMV58 produced 1.22
0.02). BMV58 produced 1.22 ±
± 0.05
0.05 g/L of 2–3 butanediol, ΔGRE3 produced 1.15
g/L of 2–3 butanediol, ΔGRE3 produced 1.15 ±
± 0.07
0.07 g/L, and Δ3K ended up with 1.12
g/L, and Δ3K ended up with 1.12 ±
± 0.05
0.05 g/L, values significantly higher than the 0.47
g/L, values significantly higher than the 0.47 ±
± 0.07
0.07 g/L produced by T73. Mutant Δ4K produced less 2–3 butanediol (0.9
g/L produced by T73. Mutant Δ4K produced less 2–3 butanediol (0.9 ±
± 0.04
0.04 g/L) than the rest. Δ4K produced less ethanol, 2–3 butanediol and succinic acid, but more acetic acid (Figure 4), compounds that have in common an acetaldehyde precursor. Ethanol and 2–3 butanediol produce NAD+, meanwhile during succinate and acetate production, NAD(P)+ is consumed. Therefore, this is a case of different flux pathways to balance the cofactors needed in other processes.
g/L) than the rest. Δ4K produced less ethanol, 2–3 butanediol and succinic acid, but more acetic acid (Figure 4), compounds that have in common an acetaldehyde precursor. Ethanol and 2–3 butanediol produce NAD+, meanwhile during succinate and acetate production, NAD(P)+ is consumed. Therefore, this is a case of different flux pathways to balance the cofactors needed in other processes.
In global, the results of the production of metabolites in all the mutants developed in the present study are presented in a heat map where the strains were grouped by hierarchical clustering (Figure S6). BMV58 exhibits a pattern similar to those observed for ΔYJR096W, ΔARA1, ΔGRE3, ΔYPR1 and ΔGCY1 (single mutants), characterized by higher production of erythritol, succinic acid and glycerol but lower lactic and acetic acids. On the contrary, T73 metabolite production was more similar to that exhibited by multiple deletants ΔYPR1ΔGCY1ΔARA, ΔYPR1ΔGCY1ΔYJR096W, Δ4K and Δ5K, which synthesize lower amounts of erythritol, 2,3‐butanediol, succinic and lactic acids, but higher of acetic acid and ethanol. All the mutants with a deletion of gene GRE3 decreased erythritol production and showed higher levels of succinic and lactic acids.
Analysis of the expression of the putative genes involved in erythritol synthesis during fermentation
The relative expression levels of the genes GRE3, ARA1, GCY1, YPR1 and YJR096W, potentially involved in the erythritol synthesis, were measured along the fermentation (Figure 5) to correlate their expressions with the kinetics of erythritol production. In all cases, expression levels were normalized to the expression of the constitutive genes ACT1 and RDN18‐1.
For comparative purposes, the expression of gene GPD1, involved in glycerol synthesis, was also quantified. Glycerol is produced from the beginning of wine fermentation as a response to osmotic stress reaching higher productions after 24 h (Figure 4). This pattern is observed in all strains and mutants but with lower values in T73. This pattern of production is related to the patterns of expression of the GPD1 gene (Figure 5), which shows a higher peak at the very beginning of fermentation, related to osmotic response and a second at 48
h (Figure 4). This pattern is observed in all strains and mutants but with lower values in T73. This pattern of production is related to the patterns of expression of the GPD1 gene (Figure 5), which shows a higher peak at the very beginning of fermentation, related to osmotic response and a second at 48 h of fermentation. This pattern is similar in BMV58 and their mutants but with lower levels in T73.
h of fermentation. This pattern is similar in BMV58 and their mutants but with lower levels in T73.
Of the genes potentially involved in erythritol synthesis, only GRE3 (present in T73, BMV58 and Δ4K) shows expression levels consistent with the erythritol production in BMV58 and Δ4K. Thus, GRE3 expression levels in these strains were lower than in T73 during the first 24 h of fermentation but significantly increased after 48
h of fermentation but significantly increased after 48 h to reach a maximum at 96
h to reach a maximum at 96 h, just before the beginning of the stationary phase. This peak of expression was not observed in T73, which maintained a low level of expression from 72
h, just before the beginning of the stationary phase. This peak of expression was not observed in T73, which maintained a low level of expression from 72 h to the end of fermentation. The peak of expression in BMV58 and Δ4K occurred when their erythritol production started to become higher than in the other strain. GRE3 expression in BMV58 and Δ4K was very similar, although slightly lower in the latter, which is also consistent with its lower production of erythritol (Figure 4). During fermentation, extracellular erythritol was detected after 48
h to the end of fermentation. The peak of expression in BMV58 and Δ4K occurred when their erythritol production started to become higher than in the other strain. GRE3 expression in BMV58 and Δ4K was very similar, although slightly lower in the latter, which is also consistent with its lower production of erythritol (Figure 4). During fermentation, extracellular erythritol was detected after 48 h of fermentation in BMV58 and after 72
h of fermentation in BMV58 and after 72 h in Δ4K, when the levels of expression were higher than in T73 and increased at a constant rate until the end of fermentation, during and after the peak of expression.
h in Δ4K, when the levels of expression were higher than in T73 and increased at a constant rate until the end of fermentation, during and after the peak of expression.
The paralogous genes GCY1 and YPR1 genes (present in T73, BMV58 and ΔGRE3) showed similar low expression levels, decreasing along the fermentation with no significant differences among strains. ARA1 (only absent in Δ4K) did not exhibit expression differences among strains at the beginning of fermentation. Since then, this gene in BMV58 and Δ4K exhibited a continuous decline in the level of expression until de the end of fermentation. T73 and ΔGRE3 presented picks of expression at 24 h, higher in T73, but also decreased until the end of fermentation. Finally, YJR096W (only absent in Δ4K) showed at the very beginning of fermentation a much higher expression in T73 than in the other strains, but in all cases, YJR096W expression was declining along the fermentation.
h, higher in T73, but also decreased until the end of fermentation. Finally, YJR096W (only absent in Δ4K) showed at the very beginning of fermentation a much higher expression in T73 than in the other strains, but in all cases, YJR096W expression was declining along the fermentation.
As a summary, GRE3 is the gene, of the five encoding AKRs, that most increased its expression in BMV58 along the fermentation, being predominant at time 96 h (Figure S4) and highly expressed at the stationary phase, indicating its putative function on redox balance and the production of erythritol at higher concentrations compared to strain T73.
h (Figure S4) and highly expressed at the stationary phase, indicating its putative function on redox balance and the production of erythritol at higher concentrations compared to strain T73.
DISCUSSION
The main goal of the present study was to determine the genes encoding the enzymes responsible for the erythritol synthesis pathway, focusing on ERs in S. cerevisiae and S. uvarum, and its putative function during fermentation. For this purpose, we first performed a phylogenetic analysis of genes of the Saccharomyces genus encoding enzymes belonging to the AKR superfamily, and GCY1, YPR1, GRE3, ARA1 and YJR096W were selected as possible candidates.
To determine which of these genes were involved in erythritol synthesis in S. uvarum, they were individually and jointly knocked out in the BMV58 strain. Then, the corresponding mutants were used to perform fermentation and measure the amount of erythritol produced. ΔGRE3 was the single mutant that most decreased erythritol levels. From the results of the analysis in double knockouts, it can be deduced that genes YJR096W, GRE3 and ARA1 are likely involved in erythrose reduction to erythritol, but the most important is GRE3. The level of reduction of the double and triple mutants in which GRE3 is present is not significantly different from that of the single mutant ΔGRE3. The triple knockout ΔYPR1ΔGCY1ΔGRE3 was the mutant that most reduce erythritol production. The explanation could be that two different pathways may be involved in erythritol synthesis, although the path in which the product of GRE3 is involved should be the main one because the deletion of GCY1 and YPR1 has no significant effect on erythritol production. However, when GRE3 is deleted, the other minor pathway, involving the closely related genes GCY1 and YPR1, can be responsible for the synthesis. It was shown that Gcy1p, in addition to its main glycerol dehydrogenase activity, can reduce both erythrose‐4‐P and erythrose (Ookura & Kasumi, 2007). Consequently, erythrose‐4‐phosphate could be reduced to erythritol‐4‐phosphate and then dephosphorylated to erythritol by GCY1, which would be very similar to the erythritol synthesis pathway present in bacteria (Veiga‐Da‐Cunha et al., 1993). Candidates for the subsequent dephosphorylation reaction of erythritol‐4‐P could be homologous to TM1254 phosphatase from Thermotoga maritima, such as those encoded by YKL033W, GPP1 and GPP2 involved in glycerol biosynthesis (Kuznetsova et al., 2005).
ARA1 and YJR096W could be on the same path as GRE3, although this gene is more expressed than the other two.
An alternative explanation is paralog compensation, a mechanism by which functional overlap between paralogous genes allows them to compensate for the loss of the other to maintain their functions despite environmental or genetic perturbations (Diss et al., 2014). In this case, the Gre3p function in erythritol synthesis could be compensated by paralogous AKRs exhibiting secondary or promiscuous erythritol reductase activities. This could also explain why our Δ5K mutant, with all putative ER genes deleted, still produced some amount of erythritol. Therefore, even if the AKRs described in this study appear to be the major contributors to erythritol synthesis, it is highly likely that other AKRs or enzymes can compensate for their absence. In that sense, in Y. lipolytica, in which erythritol metabolism has been explored more intensively than in S. cerevisiae, some ERs have been described, nevertheless, not all of them have been found yet (Cheng et al., 2018; Janek et al., 2017; Szczepańczyk et al., 2021). This corroborates that additional studies are required to completely decipher the erythritol pathway in yeasts.
Another important question that we wanted to address in this study is the biological role of erythritol synthesis during fermentation. A previous study by Carly and Fickers (2018) described that erythritol‐producing yeast can grow in the presence of high sugar and salt concentrations, concluding that erythritol could be involved in osmotic stress tolerance as glycerol does (Dihazi et al., 2004). Our results are in line with this assumption since we did not observe a significant difference when glycerol and erythritol were added to cells placed in a sorbitol medium mimicking hyperosmotic conditions. However, during alcoholic fermentation, the timing of glycerol and erythritol synthesis is different: while glycerol is rapidly produced as an early osmotic stress response to high‐sugar concentration present at the beginning of the fermentation (Pérez‐Torrado et al., 2016), we observed that erythritol is produced at the entry into the stationary phase as reported by other authors (Kobayashi et al., 2013; Minebois et al., 2020a, 2020b).
The mechanism by which erythritol activates the stress response has not been described yet. In Y. lipolytica, the AKR family proteins are induced by osmotic stress (Yang et al., 2015). In S. cerevisiae, it has also been suggested that AKRs are involved in stress response (Chang et al., 2007). Stress‐response elements (STRE) are generally located between 100 and 600 bps upstream of the regulated genes. We found that the GRE3 promoter contains an STRE sequence (AGGGG) at positions −134 and −143 in BMV58 and T73, respectively. GRE3 is negatively regulated by the cAMP–PKA transduction pathway and positively by the transcriptional factors Msn2p and Msn4p (Garay‐Arroyo & Covarrubias, 1999).
bps upstream of the regulated genes. We found that the GRE3 promoter contains an STRE sequence (AGGGG) at positions −134 and −143 in BMV58 and T73, respectively. GRE3 is negatively regulated by the cAMP–PKA transduction pathway and positively by the transcriptional factors Msn2p and Msn4p (Garay‐Arroyo & Covarrubias, 1999).
The expressions of the putative genes involved in erythritol synthesis were analysed during wine fermentation in S. uvarum BMV58 and its mutants, compared to S. cerevisiae T73. In BMV58, the expression of GRE3 was found to peak at 96 h, during the deceleration phase just before the start of the stationary phase. This is consistent with the observation that erythritol increases both extra‐ and intracellularly in BMV58 at the start of the stationary phase (Minebois et al., 2020a). This suggests that GRE3 plays a key role in the main synthesis of erythritol, produced at the start of the stationary phase.
h, during the deceleration phase just before the start of the stationary phase. This is consistent with the observation that erythritol increases both extra‐ and intracellularly in BMV58 at the start of the stationary phase (Minebois et al., 2020a). This suggests that GRE3 plays a key role in the main synthesis of erythritol, produced at the start of the stationary phase.
In the hypothesis of different pathways involved in the erythritol synthesis, the smaller amount of erythritol released before 72 h could be produced by other AKRs to contribute to the osmotic response. On the contrary, in strain S. cerevisiae T73 intracellular level of erythritol increased during the exponential growth phase perhaps contributing to the osmotic response and decreased in the stationary phase (Minebois et al., 2020a).
h could be produced by other AKRs to contribute to the osmotic response. On the contrary, in strain S. cerevisiae T73 intracellular level of erythritol increased during the exponential growth phase perhaps contributing to the osmotic response and decreased in the stationary phase (Minebois et al., 2020a).
Then, the higher expression of GRE3 during the stationary phase in S. uvarum results in higher production of erythritol, which could be related to the maintenance of the intracellular redox balance, as proposed by Minebois et al. (2020a). Thus, at the beginning of the stationary phase, when nitrogen sources are depleted, the flux from pyruvate to alpha‐ketoglutarate for the metabolism of amino acids is closed. This causes the diversion of the glycolysis carbon flux to the pentose phosphate pathway, increasing the expression of GRE3 to produce erythritol to compensate for the redox balance. The yeast cells can no longer use amino acids as a source of energy and instead must rely on other pathways to generate energy, such as the pentose phosphate pathway. This increase in the pentose phosphate pathway results in an accumulation of NADPH and an imbalance in the NADPH/NADP+ ratio. Erythritol production helps to balance this ratio by consuming NADPH during its synthesis, allowing the yeast cells to maintain redox homeostasis and survive during the stationary phase.
Also, erythrose‐4‐P and dihydroxyacetone phosphate (DHAP) could be produced by FBA1 from sedoheptulose 1,7‐bisphosphate (SBP). Therefore, the increase in erythrose‐4‐P substrate concentration could end up in more erythritol production (Figure 4). PYP1 has already been identified as a polyol phosphatase in yeasts and can dephosphorylate erythrose‐4‐P to erythrose (Xu et al., 2018).
Taken together, the higher production of erythritol in Saccharomyces with high sugar in the media is likely a response to maintaining redox balance when the cell arrives at the stationary phase. This is consistent with Gre3p as the main ER involved in redox balance as its expression increases during the stationary phase, where erythritol production is also increased.
CONCLUSIONS
This study is the first to investigate erythritol biosynthesis in two species of the Saccharomyces genus. Erythritol is produced in yeasts by a reduction reaction of erythrose to erythritol catalysed by erythritol reductases (ERs), which belong to the Aldo‐Keto Reductase (AKR) superfamily. The synthesis of this polyol in Saccharomyces, as well as in other yeasts, is higher when there is a high sugar concentration. The combination of knocking out GRE3, YPR1 and GCY1 has the highest impact on lowering erythritol production. The main ER involved in erythritol production in Saccharomyces uvarum was proved to be the enzyme coded by GRE3, which is homologous to ER25 from Yarrowia lipolytica. This study also shows that, even when GRE3 is deleted, some erythritol is still produced, indicating that more genes may be involved in its synthesis.
The addition of erythritol under stress presence helps to recover yeast growth, indicating that this metabolite can function as an osmoprotectant in Saccharomyces to tolerate stress. The combined deletion of all selected genes (GCY1, YPR1, ARA1, GRE3 and YJR096W) highly decreased the level of erythritol, although some amount remains present.
In S. uvarum, GRE3 expression is induced at the entry into the stationary phase, when erythritol production increases. This suggests that GRE3 plays its primary function to help yeast cells to maintain the redox balance during the stationary phase when nitrogen sources are depleted. This study contributes greatly to increasing the insight into erythritol in terms of its synthesis as well as its biological role and opens the door to more research to be explored.
AUTHOR CONTRIBUTIONS
Sonia Albillos‐Arenal: Formal analysis (equal); investigation (equal); methodology (equal); visualization (equal); writing – original draft (equal). Romain Minebois: Data curation (equal); investigation (equal); writing – review and editing (equal). Amparo Querol: Conceptualization (equal); funding acquisition (equal); methodology (equal); project administration (equal); resources (equal); supervision (equal); validation (equal); writing – review and editing (equal). Eladio Barrio: Conceptualization (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); project administration (equal); resources (equal); supervision (equal); validation (equal); visualization (equal); writing – review and editing (lead).
Supporting information
Appendix S1.
Figure S1.
ACKNOWLEDGEMENTS
The authors want to thank the entire research team of the Systems Biology of Yeasts of Biotechnological Interest group of the IATA‐CSIC. We also thank Dr. Eva Balsa‐Canto for her comments and suggestions to improve this article. SA was supported by an FPI contract from Ministerio de Ciencia, Innovación y Universidades (ref. PRE2019‐088621). This project has received funding from the Spanish Government and EU ERDF‐FEDER projects PID2021‐126380OB‐C31 and PID2021‐126380OB‐C33 to AQ and EB, respectively. Finally, IATA‐CSIC received funding from the Spanish Government, ref. MCIN/AEI/10.13039/501100011033, as a ‘Severo Ochoa’ Center of Excellence (CEX2021‐001189‐S), with AQ as PI.
Notes
Albillos‐Arenal, S. , Minebois, R. , Querol, A. & Barrio, E. (2023) Understanding the role of GRE3 in the erythritol biosynthesis pathway in Saccharomyces uvarum and its implication in osmoregulation and redox homeostasis. Microbial Biotechnology, 16, 1858–1871. Available from: 10.1111/1751-7915.14313 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
REFERENCES
- Blomberg, A. & Adler, L. (1989) Roles of glycerol and glycerol‐3‐phosphate dehydrogenase (NAD+) in acquired osmotolerance of Saccharomyces cerevisiae . Journal of Bacteriology, 171, 1087–1092. Available from: 10.1128/jb.171.2.1087-1092.1989 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Blomberg, A. & Adler, L. (1992) Physiology of osmotolerance in fungi. Advances in Microbial Physiology, 33, 145–212. Available from: 10.1016/s0065-2911(08)60217-9 [Abstract] [CrossRef] [Google Scholar]
- Brown, A.D. (1978) Compatible solutes and extreme water stress in eukaryotic microorganisms. Advances in Microbial Physiology, 17, 181–242. Available from: 10.1016/s0065-2911(08)60058-2 [Abstract] [CrossRef] [Google Scholar]
- Bukhman, Y.V. , DiPiazza, N.W. , Piotrowski, J. , Shao, J. , Halstead, A.G.W. , Bui, M.D. et al. (2015) Modeling microbial growth curves with GCAT. Bioenergy Research, 83(8), 1022–1030. [Google Scholar]
- Carly, F. & Fickers, P. (2018) Erythritol production by yeasts: a snapshot of current knowledge. Yeast, 35, 455–463. Available from: 10.1002/yea.3306 [Abstract] [CrossRef] [Google Scholar]
- Chang, Q. , Griest, T.A. , Harter, T.M. & Petrash, J.M. (2007) Functional studies of aldo‐keto reductases in Saccharomyces cerevisiae . Biochimica et Biophysica Acta ‐ Molecular Cell Research, 1773, 321–329. Available from: 10.1016/j.bbamcr.2006.10.009 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cheng, H. , Wang, S. , Bilal, M. , Ge, X. , Zhang, C. , Fickers, P. et al. (2018) Identification, characterization of two NADPH‐dependent erythrose reductases in the yeast Yarrowia lipolytica and improvement of erythritol productivity using metabolic engineering. Microbial Cell Factories, 17, 133. Available from: 10.1186/s12934-018-0982-z [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Darriba, D. , Taboada, G.L. , Doallo, R. & Posada, D. (2011) ProtTest 3: fast selection of best‐fit models of protein evolution. Bioinformatics, 27, 1164–1165. Available from: 10.1093/bioinformatics/btr088 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Dicarlo, J.E. , Norville, J.E. , Mali, P. , Rios, X. , Aach, J. & Church, G.M. (2013) Genome engineering in Saccharomyces cerevisiae using CRISPR‐Cas systems. Nucleic Acids Research, 41, 4336–4343. Available from: 10.1093/nar/gkt135 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Dihazi, H. , Kessler, R. & Eschrich, K. (2004) High osmolarity glycerol (HOG) pathway‐induced phosphorylation and activation of 6‐phosphofructo‐2‐kinase are essential for glycerol accumulation and yeast cell proliferation under hyperosmotic stress. Journal of Biological Chemistry, 279, 23961–23968. Available from: 10.1074/JBC.M312974200 [Abstract] [CrossRef] [Google Scholar]
- Diss, G. , Ascencio, D. , DeLuna, A. & Landry, C.R. (2014) Molecular mechanisms of paralogous compensation and the robustness of cellular networks. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 322, 488–499. Available from: 10.1002/jez.b.22555 [Abstract] [CrossRef] [Google Scholar]
- Edgar, R.C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797. Available from: 10.1093/nar/gkh340 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Garay‐Arroyo, A. & Covarrubias, A.A. (1999) Three genes whose expression is induced by stress in Saccharomyces cerevisiae . Yeast, 15, 879–892. Available from: 10.1002/(sici)1097-0061(199907)15:10a<879::aid-yea428>3.0.co;2-q [Abstract] [CrossRef] [Google Scholar]
- Generoso, W.C. , Gottardi, M. , Oreb, M. & Boles, E. (2016) Simplified CRISPR‐Cas genome editing for Saccharomyces cerevisiae . Journal of Microbiological Methods, 127, 203–205. Available from: 10.1016/j.mimet.2016.06.020 [Abstract] [CrossRef] [Google Scholar]
- Gietz, R.D. & Woods, R.A. (2006) Yeast transformation by the LiAc/SS carrier DNA/PEG method. Methods in Molecular Biology, 313, 107–120. Available from: 10.1385/1-59259-958-3:107 [Abstract] [CrossRef] [Google Scholar]
- Henriques, D. , Minebois, R. , Mendoza, S.N. , Macías, L.G. , Pérez‐Torrado, R. , Barrio, E. et al. (2021) A multiphase multiobjective dynamic genome‐scale model shows different redox balancing among yeast species of the Saccharomyces genus in fermentation. MSystems, 6, e00260–e00221. Available from: 10.1128/msystems.00260-21 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Janek, T. , Dobrowolski, A. , Biegalska, A. & Mirończuk, A.M. (2017) Characterization of erythrose reductase from Yarrowia lipolytica and its influence on erythritol synthesis. Microbial Cell Factories, 16, 118. Available from: 10.1186/s12934-017-0733-6 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jez, J.M. & Penning, T.M. (2001) The aldo‐keto reductase (AKR) superfamily: an update. Chemico‐Biological Interactions, 130‐132, 499–525. Available from: 10.1016/S0009-2797(00)00295-7 [Abstract] [CrossRef] [Google Scholar]
- Kobayashi, Y. , Yoshida, J. , Iwata, H. , Koyama, Y. , Kato, J. , Ogihara, O. et al. (2013) Gene expression and function involved in polyol biosynthesis of Trichosporonoides megachiliensis under hyper‐osmotic stress. Journal of Bioscience and Bioengineering, 115, 645–650. Available from: 10.1016/J.JBIOSC.2012.12.004 [Abstract] [CrossRef] [Google Scholar]
- Kuznetsova, E. , Proudfoot, M. , Sanders, S.A. , Reinking, J. , Savchenko, A. , Arrowsmith, C.H. et al. (2005) Enzyme genomics: application of general enzymatic screens to discover new enzymes. FEMS Microbiology Reviews, 29, 263–279. Available from: 10.1016/J.FMRRE.2004.12.006 [Abstract] [CrossRef] [Google Scholar]
- Le, S.Q. & Gascuel, O. (2008) An improved general amino acid replacement matrix. Molecular Biology and Evolution, 25, 1307–1320. Available from: 10.1093/molbev/msn067 [Abstract] [CrossRef] [Google Scholar]
- Lee, J.K. , Kim, S.Y. , Ryu, Y.W. , Seo, J.H. & Kim, J.H. (2003) Purification and characterization of a novel erythrose reductase from Candida magnoliae . Applied and Environmental Microbiology, 69, 3710–3718. Available from: 10.1128/AEM.69.7.3710-3718.2003 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lee, S. & Lee, D.K. (2018) What is the proper way to apply the multiple comparison test? Korean Journal of Anesthesiology, 71, 353–360. Available from: 10.4097/KJA.D.18.00242 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lõoke, M. , Kristjuhan, K. & Kristjuhan, A. (2011) Extraction of genomic DNA from yeasts for PCR‐based applications. BioTechniques, 50, 325–328. Available from: 10.2144/000113672 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Macías, L.G. , Flores, M.G. , Adam, A.C. , Rodríguez, M.E. , Querol, A. , Barrio, E. et al. (2021) Convergent adaptation of Saccharomyces uvarum to sulfite, an antimicrobial preservative widely used in human‐driven fermentations. PLoS Genetics, 17, e1009872. Available from: 10.1371/JOURNAL.PGEN.1009872 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Minebois, R. , Pérez‐Torrado, R. & Querol, A. (2020a) A time course metabolism comparison among Saccharomyces cerevisiae, S. uvarum and S. kudriavzevii species in wine fermentation. Food Microbiology, 90, 103484. Available from: 10.1016/j.fm.2020.103484 [Abstract] [CrossRef] [Google Scholar]
- Minebois, R. , Pérez‐Torrado, R. & Querol, A. (2020b) Metabolome segregation of four strains of Saccharomyces cerevisiae, Saccharomyces uvarum and Saccharomyces kudriavzevii conducted under low‐temperature oenological conditions. Environmental Microbiology, 22, 3700–3721. Available from: 10.1111/1462-2920.15135 [Abstract] [CrossRef] [Google Scholar]
- Moon, H.J. , Jeya, M. , Kim, I.W. & Lee, J.K. (2010) Biotechnological production of erythritol and its applications. Applied Microbiology and Biotechnology, 86, 1017–1025. Available from: 10.1007/s00253-010-2496-4 [Abstract] [CrossRef] [Google Scholar]
- Morard, M. , Macías, L. G. , Adam, A. C. , Lairón‐Peris, M. , Pérez‐Torrado, R. , Toft, C. & Barrio, E. (2019) Aneuploidy and ethanol tolerance in Saccharomyces cerevisiae. Frontiers in Genetics 10: 82. Available from: 10.3389/fgene.2019.00082 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Oliveira, B.M. , Barrio, E. , Querol, A. & Pérez‐Torrado, R. (2014) Enhanced enzymatic activity of glycerol‐3‐phosphate dehydrogenase from the cryophilic Saccharomyces kudriavzevii . PLoS One, 9, e87290. Available from: 10.1371/journal.pone.0087290 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ookura, T. & Kasumi, T. (2007) Yeast GCY1p reduces erythrose and erythrose‐4‐phosphate. Reports of the Natural Food Resources Institute, 71, 57–60. [Google Scholar]
- Pérez, D. , Denat, M. , Minebois, R. , Heras, J.M. , Guillamón, M.J. , Ferreira, V. et al. (2022) Modulation of aroma and chemical composition of Albariño semi‐synthetic wines by non‐wine Saccharomyces yeasts and bottle aging. Food Microbiology, 104, 103981. Available from: 10.1016/j.fm.2022.103981 [Abstract] [CrossRef] [Google Scholar]
- Pérez‐Torrado, R. , Barrio, E. & Querol, A. (2018) Alternative yeasts for winemaking: Saccharomyces non‐cerevisiae and its hybrids. Critical Reviews in Food Sciences and Nutrition, 58, 1780–1790. Available from: 10.1080/10408398.2017.1285751 [Abstract] [CrossRef] [Google Scholar]
- Pérez‐Torrado, R. , Oliveira, B.M. , Zemančíková, J. , Sychrova, H. & Querol, A. (2016) Alternative glycerol balance strategies among Saccharomyces species in response to winemaking stress. Frontiers in Microbiology, 7, 435. Available from: 10.3389/fmicb.2016.00435 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Querol, A. , Pérez‐Torrado, R. , Alonso‐del‐Real, J. , Minebois, R. , Stribny, J. , Oliveira, B.M. et al. (2018) New trends in the uses of yeasts in oenology. Advances in Food and Nutrition Research, 85, 177–210. Available from: 10.1016/bs.afnr.2018.03.002 [Abstract] [CrossRef] [Google Scholar]
- Rossignol, T. , Dulau, L. , Julien, A. & Blondin, B. (2003) Genome‐wide monitoring of wine yeast gene expression during alcoholic fermentation. Yeast, 20, 1369–1385. Available from: 10.1002/yea.1046 [Abstract] [CrossRef] [Google Scholar]
- Rzechonek, D.A. , Szczepańczyk, M. , Wang, G. , Borodina, I. & Mirończuk, A.M. (2020) HOG‐independent osmoprotection by erythritol in yeast Yarrowia lipolytica . Genes, 11, 1424. Available from: 10.3390/genes11121424 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Starovoytova, A.N. , Sorokin, M.I. , Sokolov, S.S. , Severin, F.F. & Knorre, D.A. (2013) Mitochondrial signaling in Saccharomyces cerevisiae pseudohyphae formation induced by butanol. FEMS Yeast Research, 13, 367–374. Available from: 10.1111/1567-1364.12039 [Abstract] [CrossRef] [Google Scholar]
- Stovicek, V. , Holkenbrink, C. & Borodina, I. (2017) CRISPR/Cas system for yeast genome engineering: advances and applications. FEMS Yeast Research, 17, fox030. Available from: 10.1093/femsyr/fox030 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Szczepańczyk, M. , Rzechonek, D.A. , Dobrowolski, A. & Mirończuk, A.M. (2021) Overexpression of YALI0B07117g encoding erythrose reductase homolog results in enhanced erythritol synthesis from glycerol by the yeast. Molecules, 26, 7549. Available from: 10.3390/molecules26247549 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tamura, K. , Stecher, G. & Kumar, S. (2021) MEGA11: molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution, 38(7), 3022–3027. Available from: 10.1093/molbev/msab120 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Veiga‐Da‐Cunha, M. , Santos, H. & Van Schaftingen, E. (1993) Pathway and regulation of erythritol formation in Leuconostoc oenos . Journal of Bacteriology, 175, 3941–3948. Available from: 10.1128/jb.175.13.3941-3948.1993 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wolfe, K.H. (2015) Origin of the yeast whole‐genome duplication. PLoS Biology, 13, e1002221. Available from: 10.1371/journal.pbio.1002221 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Xu, Y.F. , Lu, W. , Chen, J.C. , Johnson, S.A. , Gibney, P.A. , Thomas, D.G. et al. (2018) Discovery and functional characterization of a yeast sugar alcohol phosphatase. ACS Chemical Biology, 13, 3011–3020. Available from: 10.1021/acschembio.8b00804 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Yang, L.B. , Dai, X.M. , Zheng, Z.Y. , Zhu, L. , Zhan, X.B. & Lin, C.C. (2015) Proteomic analysis of erythritol‐producing Yarrowia lipolytica from glycerol in response to osmotic pressure. Journal of Microbiology and Biotechnology, 25, 1056–1069. Available from: 10.4014/jmb.1412.12026 [Abstract] [CrossRef] [Google Scholar]
- Zwietering, M.H. , Jongenburger, I. , Rombouts, F.M. & Van't Riet, K. (1990) Modeling of the bacterial growth curve. Applied and Environmental Microbiology, 56, 1875–1881. Available from: 10.1128/AEM.56.6.1875-1881.1990 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Articles from Microbial Biotechnology are provided here courtesy of Wiley
Full text links
Read article at publisher's site: https://doi.org/10.1111/1751-7915.14313
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/1751-7915.14313
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/151739873
Article citations
Understanding the role of GRE3 in the erythritol biosynthesis pathway in Saccharomyces uvarum and its implication in osmoregulation and redox homeostasis.
Microb Biotechnol, 16(9):1858-1871, 14 Jul 2023
Cited by: 1 article | PMID: 37449952 | PMCID: PMC10443344
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A time course metabolism comparison among Saccharomyces cerevisiae, S. uvarum and S. kudriavzevii species in wine fermentation.
Food Microbiol, 90:103484, 12 Mar 2020
Cited by: 16 articles | PMID: 32336360
Putative xylose and arabinose reductases in Saccharomyces cerevisiae.
Yeast, 19(14):1233-1241, 01 Oct 2002
Cited by: 51 articles | PMID: 12271459
Gene expression and function involved in polyol biosynthesis of Trichosporonoides megachiliensis under hyper-osmotic stress.
J Biosci Bioeng, 115(6):645-650, 05 Jan 2013
Cited by: 11 articles | PMID: 23294575
Recent advances in biosynthesis mechanisms and yield enhancement strategies of erythritol.
Crit Rev Food Sci Nutr, 1-21, 04 Oct 2023
Cited by: 0 articles | PMID: 37791716
Review
Funding
Funders who supported this work.
Ministerio de Ciencia, Innovación y Universidades (1)
Grant ID: PRE2019‐088621

 1
,
2
1
,
2