Abstract
Background
Since the pandemic onset, deprivation has been seen as a significant determinant of COVID-19 incidence and mortality. This study explores outcomes of COVID-19 in the context of material deprivation across three pandemic waves in Ireland.Methods
Between 1st March 2020 and 13th May 2021, 252,637 PCR-confirmed COVID-19 cases were notified in Ireland. Cases were notified to the national Computerised Infectious Disease Reporting (CIDR) system. Each case was geo-referenced and assigned a deprivation category according to the Haase-Pratschke (HP) Deprivation Index. Regression modelling examined three outcomes: admission to hospital; admission to an intensive care unit (ICU) and death.Results
Deprivation increased the likelihood of contracting COVID-19 in all age groups and across all pandemic waves, except for the 20-39 age group. Deprivation, age, comorbidity and male gender carried increased risk of hospital admission. Deprivation was not a factor in predicting ICU admission or death, and diagnosis in wave 2 was associated with the lowest risk of all three outcomes.Conclusions
Our study suggests that COVID-19 spreads easily through all strata of society and particularly in the more deprived population; however this was not a consistent finding. Ireland is ethnically more homogenous than other countries reporting a larger deprivation gradient, and in such societies, structural racial differences may contribute more to poor COVID outcomes than elements of deprivation.Free full text

COVID-19 incidence and outcome by affluence/deprivation across three pandemic waves in Ireland: A retrospective cohort study using routinely collected data
Abstract
Background
Since the pandemic onset, deprivation has been seen as a significant determinant of COVID-19 incidence and mortality. This study explores outcomes of COVID-19 in the context of material deprivation across three pandemic waves in Ireland.
Methods
Between 1st March 2020 and 13th May 2021, 252,637 PCR-confirmed COVID-19 cases were notified in Ireland. Cases were notified to the national Computerised Infectious Disease Reporting (CIDR) system. Each case was geo-referenced and assigned a deprivation category according to the Haase-Pratschke (HP) Deprivation Index. Regression modelling examined three outcomes: admission to hospital; admission to an intensive care unit (ICU) and death.
Results
Deprivation increased the likelihood of contracting COVID-19 in all age groups and across all pandemic waves, except for the 20–39 age group. Deprivation, age, comorbidity and male gender carried increased risk of hospital admission. Deprivation was not a factor in predicting ICU admission or death, and diagnosis in wave 2 was associated with the lowest risk of all three outcomes.
Conclusions
Our study suggests that COVID-19 spreads easily through all strata of society and particularly in the more deprived population; however this was not a consistent finding. Ireland is ethnically more homogenous than other countries reporting a larger deprivation gradient, and in such societies, structural racial differences may contribute more to poor COVID outcomes than elements of deprivation.
Introduction
Initially called “The Great Leveller”, 1918’s influenza pandemic appeared to affect rich and poor equally. Subsequent research, however, showed a disproportionate impact on poorer populations [1]. More recently, seasonal influenza has mirrored this effect, but without yet revealing a causal relationship [2].
When the World Health Organisation declared a COVID-19 pandemic, a common refrain was that “we are all in this together” [3]. However, early reports showed greater mortality rates from COVID-19 in areas with lower income and higher unemployment [4]. In the United Kingdom, more deprived areas reported twice the mortality rate of affluent neighbourhoods [5] and similar patterns were observed in South America [6], and in the Asia-Pacific region [7].
Debate in the UK and US has focussed on ethnicity, rather than deprivation, as being the more critical determinant of COVID-19 in inequitable societies [8]. However, Ireland’s population does not display the same level of ethnic diversity as other, larger countries. Other factors linked with COVID-19 incidence and mortality have been proposed, including age [9], comorbidity [10], urban density [11] and health-seeking behaviour [12].
The objective of this paper is to explore COVID-19 incidence and outcomes across three pandemic waves in the context of affluence and deprivation in Ireland using the Pobal Haase-Pratschke (HP) Relative Index [13], given Ireland’s unique demographic structure.
Methods
This is a retrospective cohort study of routinely notified COVID-19 cases confirmed by polymerase chain reaction (PCR), recorded on a standardised notification form with a list of possible comorbidities and recorded in the Computerised Infectious Disease Reporting system (CIDR) [14] managed by the Health Protection Surveillance Centre (HPSC), Ireland [15]. All cases were notified between 1st March 2020 and 13th May 2021.
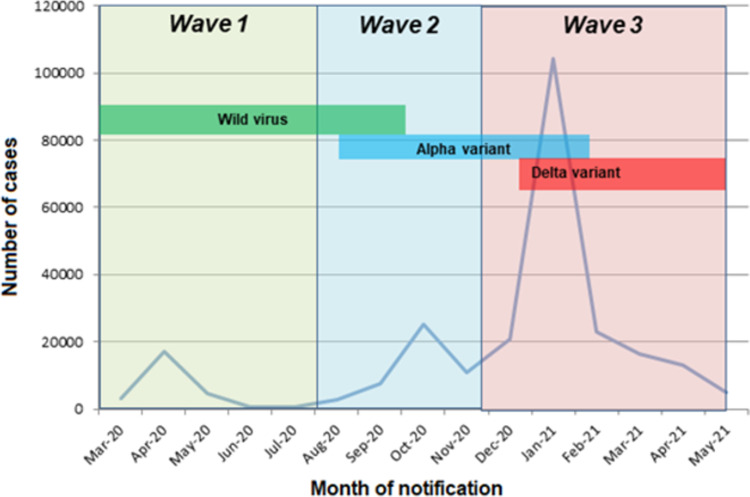
Pandemic timing was represented in waves, based on rising or falling trends in national incidence rates: wave 1 from 1st March 2020 to 1st August 2020; wave 2 from 2nd August 2020 to 21st November 2020; and wave 3 from 22nd November 2020 to the end of the study period of 13th May 2021 [16]. This period was chosen to reflect the period before COVID vaccination was well established within the Irish population.
The deprivation score was based upon the 2016 Pobal HP Relative Deprivation Index, applied to the Central Statistics Office (CSO) national 2016 Census. The HP Index measures deprivation at the CSO small area level (approximately 100 households) [17] and identifies three dimensions: demographic profile; social class composition; and labour market situation. Each dimension is determined using routinely-collected Census data and the HP Index is derived from factor analysis.
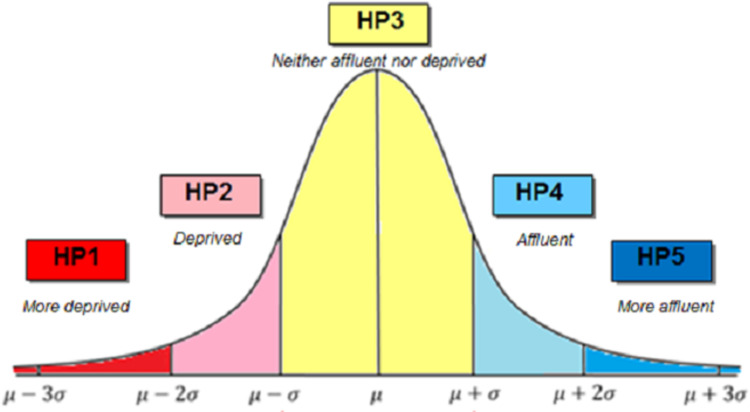
The relative HP Index score was used in this study and cases were categorised from most deprived (HP1) to most affluent (HP5) based on standard deviations from the mean as shown in Fig 1. The HP relative deprivation index, in which affluence or deprivation is determined on the basis of distance in standard deviations from the mean, is an internationally recognised measure of deprivation and is used extensively within the public sector in Ireland [13]. Due to relatively small numbers, categories HP1 and HP2, and HP4 and HP5 were combined in the analysis to produce three categories: deprived (HP1 & HP2); average (HP3); and affluent (HP4 & HP5).
The small area HP Index score for each case was derived from the residential address from the CIDR database, using Health Atlas Ireland, interfaced with An Post’s GeoDirectory [18] (www.healthatlasireland.ie). The automatic address matching process was supplemented by manual matching to achieve an overall small area geocoding rate of approximately 98%. Nursing home residents were classified by location of the home and occasionally the residence of occupational groups was recorded as the workplace. Where addresses were non-unique, a random neighbourhood match was used. This entailed linking the address to the closest identifiable address in the same geographic small area, meaning that the neighbourhood characteristics including deprivation score and population density can be applied to the non-unique address.
The outcome variables included: admission to hospital; admission to intensive care unit (ICU) and mortality as recorded on CIDR. COVID-19 mortality included cases with laboratory-confirmed COVID-19 infection; causality is not inferred [19].
Cases with an outbreak identifier were categorised as “outbreak-associated”, and “travel-related” if that was the “most likely transmission source”. “Comorbidity”, a binary Yes/No variable, was documented as “Yes” if one or more underlying condition was recorded, and “No” if recorded as such or if left blank.
Statistical methods included: Pearson’s chi-square test and particularly the G-test to determine Odds Ratios for multiple variables; Likelihood odds ratios with 95% C.I for multivariable analysis.; and Cuzick non-parametric test for trends across pandemic waves. For the purposes of modelling, variables were included which had been highlighted by other authors and which were significant at the p = 0.10 level during initial univariate analysis. A backward stepwise elimination process was followed and the model finally selected which returned the highest R2 value. The CSO Census 2016 projected to 2020 (M2F2) [20] available on Health Atlas Ireland was used as the background population denominator [21]. Calculation of R2 and effect size partial eta-squared (ƞ2p) were included in order to illustrate the overall contribution of each variable to the change in outcome status. COVID-19 incidence was calculated by age group and by deprivation category. A rate ratio was determined to compare the more affluent and more deprived categories against the average deprivation value.
Given the legal obligation and operational imperatives of the HSE to protect the health and wellbeing of the general public during the pandemic in Ireland, Research Ethics Committee (REC) approval was not sought for this study. In compliance with the General Data Protection Regulations (GDPR), and the Health Research Regulations (HRR), an anonymised dataset was used. Funding was not required, or sought, for this study.
Results
The first Irish case of COVID-19 in Ireland was notified on March 2nd 2020, with infections in wave 1 rising to a peak in April, falling to low levels during the summer (wave 2), rising again in autumn and into winter (wave 3) [22]. Wave 3 continued into the early summer of 2021.
By 13th May 2021, 252,637 PCR-confirmed cases were notified to HPSC. Of these, 14,420 (5.7%) were admitted to hospital; and of these admissions, 1,536 (0.6%) were admitted to ICU. There were 4,646 (1.8%) deaths among those diagnosed with COVID-19.
Descriptive analysis
Table 1 summarises the distribution of key parameters of the study population by each HP category. Of the total cases, 131,515 (52.1%) were female, and 121,021 (47.9%) were male. The age distribution of cases was as follows: 136,784 (54.1%) were aged under 40 years of age; 38,867 (15.4%) aged 40 to 49 years; 34,112 (13.5%) aged 50–59 years; 19,037 (7.5%) aged 60–69 years; 11,283 (4.5%) aged 70–79 years and 12,513 (5.0%) were aged 80 years and over.
Table 1
| HP1 Most deprived | HP2 More deprived | HP3 Average | HP4 More affluent | HP5 Most affluent | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | |
| Cases | 9,685 | 3.8 | 34,790 | 13.8 | 166,382 | 65.9 | 37,614 | 14.9 | 4,166 | 1.6 | 252,637 |
| Age group | Missing n = 30 | ||||||||||
| 0–39 | 5,326 | 55.0 | 17,908 | 51.5 | 88,055 | 52.9 | 22,575 | 60.0 | 2,920 | 70.1 | 136,784 |
| 40–49 | 1,363 | 14.1 | 4,963 | 14.3 | 26,099 | 15.7 | 5,838 | 15.5 | 604 | 14.5 | 38,867 |
| 50–59 | 1,119 | 11.6 | 4,445 | 12.8 | 23,475 | 14.1 | 4,724 | 12.6 | 349 | 8.4 | 34,112 |
| 60–69 | 798 | 8.2 | 2,949 | 8.5 | 12,803 | 7.7 | 2,334 | 6.2 | 153 | 3.7 | 19,037 |
| 70–79 | 646 | 6.7 | 2,214 | 6.4 | 7,392 | 4.4 | 958 | 2.5 | 73 | 1.8 | 11,283 |
| 80+ | 646 | 6.7 | 2,214 | 6.4 | 7,392 | 4.4 | 958 | 2.5 | 73 | 1.8 | 12,513 |
| Sex | Missing n = 105 | ||||||||||
| M | 4,423 | 45.7 | 16,401 | 47.2 | 79,764 | 48.0 | 18,410 | 49.0 | 2,023 | 48.6 | 121,021 |
| F | 5,260 | 54.3 | 18,378 | 52.8 | 86,559 | 52.0 | 19,179 | 51.0 | 2,139 | 51.4 | 131,515 |
| Comorbidity (if any) | Missing n = 23,082 | ||||||||||
| Yes | 1,934 | 21.8 | 6,860 | 21.6 | 27,342 | 18.0 | 5,136 | 15.0 | 534 | 14.3 | 41,806 |
| No | 6,918 | 78.2 | 24,848 | 78.4 | 124,653 | 82.0 | 29,030 | 85.0 | 3,204 | 85.7 | 188,653 |
| Healthcare worker | Missing n = 0 | ||||||||||
| Yes | 728 | 7.5 | 3,263 | 9.4 | 19,121 | 11.5 | 4,373 | 11.6 | 556 | 13.3 | 28,041 |
| Other* | 8,957 | 92.5 | 31,527 | 90.6 | 147,261 | 88.5 | 33,241 | 88.4 | 3,610 | 86.7 | 224,596 |
| Outbreak associated | Missing n = 0 | ||||||||||
| Yes | 3,508 | 36.2 | 12,545 | 36.1 | 53,698 | 32.3 | 9,538 | 25.4 | 866 | 20.8 | 80,155 |
| No | 6,177 | 63.8 | 22,245 | 63.9 | 112,684 | 67.7 | 28,076 | 74.6 | 3,300 | 79.2 | 172,482 |
| Travel related | Missing n = 0 | ||||||||||
| Yes | 80 | 0.8 | 298 | 0.9 | 2,269 | 1.4 | 811 | 2.2 | 173 | 4.2 | 3,631 |
| Other* | 9,605 | 99.2 | 34,492 | 99.1 | 164,113 | 98.6 | 36,803 | 97.8 | 3,993 | 95.8 | 249,006 |
| Wave of pandemic | Missing n = 0 | ||||||||||
| 1 | 659 | 6.8 | 3,134 | 9.0 | 16,812 | 10.1 | 4,074 | 10.8 | 468 | 11.2 | 25,147 |
| 2 | 1,798 | 18.6 | 5,904 | 17.0 | 28,796 | 17.3 | 6,363 | 16.9 | 699 | 16.8 | 43,560 |
| 3 | 7,228 | 74.6 | 25,752 | 74.0 | 120,774 | 72.6 | 27,177 | 72.3 | 2,999 | 72.0 | 183,930 |
| Hospital admission | Missing n = 0 | ||||||||||
| Yes | 737 | 7.6 | 2,618 | 7.5 | 9,326 | 5.6 | 1,633 | 4.3 | 106 | 2.5 | 14,420 |
| Other* | 8,948 | 92.4 | 32,172 | 92.5 | 157,056 | 94.4 | 35,981 | 95.7 | 4,060 | 97.5 | 238,217 |
| ICU admission | ,Missing n = 0 | ||||||||||
| Yes | 72 | 0.7 | 264 | 0.8 | 1,005 | 0.6 | 183 | 0.5 | 12 | 0.3 | 1,536 |
| Other* | 9,613 | 99.3 | 34,526 | 99.2 | 165,377 | 99.4 | 37,431 | 99.5 | 4,154 | 99.7 | 251,101 |
| Mortality | Missing n = 0 | ||||||||||
| Yes | 225 | 2.3 | 828 | 2.4 | 3,204 | 1.9 | 369 | 1.0 | 20 | 0.5 | 4,646 |
| No | 9,460 | 97.7 | 33,962 | 97.6 | 163,178 | 98.1 | 37,245 | 99.0 | 4,146 | 99.5 | 247,991 |
* Includes “No” or “Not stated”
Approximately 80,155 cases (31.7%) were outbreak-associated and 3,631 (1.4%) were related to travel. Healthcare workers accounted for 11.1% of cases (28,041). Where information was available, 41,806 cases (18.1%) had at least one underlying condition.
Over the study period 25,147 cases (10.0%) occurred during wave 1, 43,560 (17.2%) during wave 2 and 183,930 (72.8%) during wave 3. As shown in Fig 2, the epidemic curve showed peaks in April 2020, October 2020 and January 2021. Throughout wave 1, only the wild virus type was present, and by wave 3, the alpha variant was dominant [23].
Deprivation/affluence by incidence and risk ratio by COVID wave
As shown in Fig 3, the incidence of COVID-19 throughout the three pandemic waves was higher in the more deprived population, except among those aged 20 to 39 years, and this latter finding was consistent in each wave. In wave 1, possibly due to the “masking” effect of the 20 to 39 year cohort, there was no overall gradient of incidence with deprivation for all age groups combined.
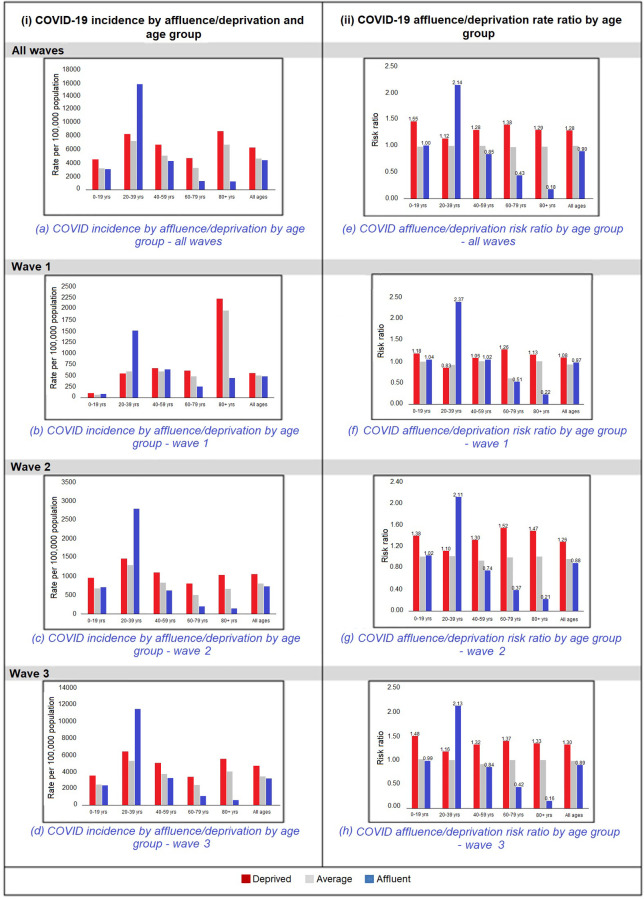
The affluent/deprived rate ratio was found to follow the above pattern with a higher risk of the deprived being a COVID-19 case across all waves of the pandemic, and in all age groups with the exception of the 20 to 29 year age group, which showed a reversed pattern as seen in Fig 3.
The above findings were found to be most marked in the older age groups, i.e. above 60 years of age.
Multivariable analysis
The following parameters were included in the multivariable analysis: HP category, age; gender; comorbidity; pandemic wave; outbreak-associated; and travel-related infection. Factors deemed to be significant at the 10% level (p< 0.10) in univariate analysis were included in building the final model. The method adopted was a backward elimination stepwise regression approach. Significant results are shown in Table 2 for the three outcomes: hospital admission, ICU admission and mortality. Variable interactions are included for age and deprivation.
Table 2
| Variable | Value | n | aOR | 95% CI | η2 Main | η2 Total | p-value |
|---|---|---|---|---|---|---|---|
| Hospital admission (n = 14,420) | |||||||
| Age | 0–39 | 2,454 | 1.00 | 0.590 | 0.730 | - | |
| 40–49 | 1,354 | 1.94 | (1.78, 2.10) | < 0.0001 | |||
| 50–59 | 1,902 | 3.07 | (2.84, 3.31) | < 0.0001 | |||
| 60–69 | 2,170 | 7.08 | (6.58, 7.62) | < 0.0001 | |||
| 70–79 | 3,029 | 19.44 | (18.05, 20.92) | < 0.0001 | |||
| 80+ | 3,693 | 23.93 | (22.30, 25.67) | < 0.0001 | |||
| Comorbidity | Yes | 7,689 | 3.27 | (3.11, 3.42) | 0.236 | 0.328 | 0.3319 |
| Wave of pandemic | 1 | 3,328 | 1.00 | (Ref) | 0.015 | 0.029 | - |
| 2 | 1,821 | 0.67 | (0.62, 0.72) | 0.0001 | |||
| 3 | 9,457 | 0.82 | (0.77, 0.88) | < 0.0001 | |||
| Outbreak-associated | Yes | 6,030 | 1.07 | (1.02, 0.12) | 0.002 | 0.004 | < 0.0078 |
| Deprivation | HP1 & HP2 | 3,355 | 1.22 | (1.15, 1.28) | < 0.0001 | ||
| HP3 (average) | 9,326 | 1.00 | (Ref) | 0.008 | 0.027 | - | |
| HP4 & HP5 | 1,739 | 0.97 | (0.91, 1.04) | 0.3918 | |||
| Sex | Male | 7,702 | 1.32 | (1.26, 1.38) | 0.012 | 0.021 | 0.5455 |
| Interaction Deprivation*age | HP1 & HP2 | 3,355 | 1.26 | (1.21, 1.32) | < .0001 | ||
| HP3 (average) | 9,326 | Ref | Ref | - | |||
| HP4 & HP5 | 1,739 | 0.95 | (0.90, 1.01) | 0.099 | |||
| Model | X2(13) = 16680.95 p< 0.0001; Model R2 value = 0.2109 | ||||||
| ICU admission (n = 1,536) | |||||||
| Comorbidity | Yes | 1,396 | 27.82 | (22.47, 34.46) | 0.416 | 0.697 | < 0.0001 |
| Age | 0–39 | 133 | 1.00 | 0.230 | 0.470 | - | |
| 40–49 | 205 | 5.38 | (4.1, 7.1) | < 0.0001 | |||
| 50–59 | 315 | 9.69 | (7.5, 12.5) | < 0.0001 | |||
| 60–69 | 453 | 26.70 | (26.7, 33.8) | < 0.0001 | |||
| 70–79 | 367 | 36.37 | (28.3, 46.7) | < 0.0001 | |||
| 80+ | 85 | 7.97 | (5.6, 11.3) | 0.0034 | |||
| Wave of pandemic | 1 | 438 | 1.00 | (Ref) | 0.013 | 0.036 | - |
| 2 | 183 | 0.63 | (0.50, 0.78) | < 0.0001 | |||
| 3 | 937 | 0.90 | (0.77, 1.05) | 0.2035 | |||
| Outbreak associated | Yes | 517 | 1.30 | (1.14, 1.49) | 0.01 | 0.029 | < 0.0001 |
| Travel associated | Yes | 32 | 0.69 | (0.47, 0.99) | 0.014 | 0.044 | 0.0481 |
| Deprivation | HP1 & HP2 | 336 | 1.07 | (0.92, 1.44) | 0.3703 | ||
| HP3 (average) | 1,005 | 1.00 | (Ref) | 0.009 | 0.025 | - | |
| HP4 & HP5 | 195 | 0.93 | (0.76, 1.13) | 0.4567 | |||
| Sex | Male | 1,008 | 0.54 | (0.48, 0.62) | 0.035 | 0.1 | 0.8931 |
| Interaction Deprivation*age | HP1 & HP2 | 336 | 1.10 | (0.94, 1.28) | 0.22 | ||
| HP3 (average) | 1,005 | 1 | |||||
| HP4 & HP5 | 195 | 0.97 | (0.81, 1.15) | 0.70 | |||
| Model | X2(13) = 3457.89; p< 0.0001; Model R2 value = 0.2609 | ||||||
| Mortality (n = 4,646) | |||||||
| Age | 0–39 | 30 | 1.00 | 0.370 | 0.750 | - | |
| 40–49 | 57 | 6.80 | (3.94, 11.77) | < 0.0001 | |||
| 50–59 | 152 | 21.19 | (13.00, 34.54) | < 0.0001 | |||
| 60–69 | 437 | 109.65 | (69.07, 174.08) | < 0.0001 | |||
| 70–79 | 1,121 | 403.70 | (255.68, 637.41) | < 0.0001 | |||
| 80+ | 2,886 | 932.53 | (593.96, 1464.07) | < 0.0001 | |||
| Comorbidity | Yes | 4,123 | 9.44 | (8.29, 10.76) | 0.149 | 0.445 | < 0.0001 |
| Wave of pandemic | 1 | 1,530 | 1.00 | (Ref) | 0.026 | 0.069 | - |
| 2 | 386 | 0.44 | (0.38, 0.51) | < 0.0001 | |||
| 3 | 2,767 | 0.89 | (0.81, 0.98) | 0.0237 | |||
| Outbreak associated | Yes | 3,083 | 1.73 | (1.58, 1.89) | 0.015 | 0.048 | < 0.0001 |
| Travel associated | Yes | 11 | 0.45 | (0.23, 0.85) | 0.030 | 0.109 | 0.0146 |
| Sex | Male | 2,486 | 0.83 | (0.74, 0.92) | 0.014 | 0.044 | 0.0006 |
| Deprivation | HP1 & HP2 | 1,053 | 0.93 | (0.85, 1.02) | 0.1382 | ||
| HP3 (average) | 3,204 | 1.07 | (Ref) | 0.005 | 0.015 | - | |
| HP4 & HP5 | 389 | 0.83 | (0.71, 0.97) | 0.0146 | |||
| Interaction Deprivation*age | HP1 & HP2 | 1,053 | 1.23 | (1.01, 1.51) | 0.05 | ||
| HP3 (average) | 3,204 | 1 | |||||
| HP4 & HP5 | 389 | 0.81 | (0.64, 1.04) | 0.10 | |||
| Model | Χ2(13) = 15933.05; p< 0.0001; Model R2 value = 0.4863 | ||||||
Admission to hospital was strongly associated with: increasing age (age 70–79 years aOR = 13.07; 95% CI 12.14–14.06; age 80+ years aOR = 13.26; 95% CI 12.31–14.27); and comorbidity (aOR = 3.27; 95% CI 3.11–3.42). Cases diagnosed in wave 2 or 3 of the pandemic were less likely to be admitted to hospital (wave 2 aOR = 0.67; 95% CI 0.62–0.72; wave 3 aOR = 0.82; 95% CI 0.77–0.88). Admission risk was also positively associated with increasing deprivation (HP1 & HP2 aOR = 1.22; 95% CI 1.15–1.28), but there was no association with increasing affluence (HP4 & HP5 aOR = 0.97; 95% 0.91–1.04). Male gender was associated with increased risk of hospital admission (aOR = 1.32; 95% CI 1.26–1.38). Overall, this model accounted for 21.1% of the variation (R2 = 0.2109) in the outcome (hospital admission).
The strongest predictor of ICU admission was comorbidity (aOR = 27.82, 95% CI 22.47–34.46), followed by increasing age up to the age of 80+ years (70–79 years aOR = 9.67; 95% CI 6.74–12.58). The next strongest predictor of ICU admission was whether the infection was associated with an outbreak (aOR = 1.30; 95% CI 1.14–1.49). Patients diagnosed in wave 2 of the pandemic were significantly less likely to be admitted to ICU (aOR = 0.63; 95% CI 0.50–0.78). Males were significantly less likely to be admitted to ICU (aOR = 0.54; 95% CI 0.48–0.62), as were cases with a history of travel (aOR = 0.69; 95% CI 0.47–0.99), although in the latter case, the effect size was not large. Deprivation was not associated with any difference in likelihood of ICU admission. Overall, this model accounted for 26.1% of the variation (R2 = 0.2609) in the outcome (ICU admission).
Case mortality showed a steadily increasing risk up to the age of 80+ (aOR = 494.6, 95% C.I. 305.56–800.59). Case mortality was strongly associated with the presence of one or more comorbidities (aOR = 9.44, 95% C.I. 8.29–10.76). Mortality was highest in wave 1 when compared to wave 2 (aOR = 0.44; 95% CI 0.38–0.51) or wave 3 (aOR = 0.89; 95% CI 0.81–0.98). Outbreak-associated cases also had a higher mortality (aOR = 1.73; 95% CI 1.58–1.89). Patients contracting COVID-19 as a result of travel were less likely to die (aOR = 0.45; 95% CI 0.23–0.85), as were males (aOR = 0.83; 95% CI 0.74–0.92), although in the latter case, the effect size was smaller. The risk of death was slightly less in the more affluent category (HP4 & HP5 aOR = 0.83; 95% CI 0.71–0.97) but there was no association in the more deprived category (HP1 & HP2 aOR = 0.93; 95% CI 0.85–1.02).
Because increasing age can be associated with increasing deprivation, the age term shown in Table 2 is corrected for interaction with deprivation. For purposes of space, the uncorrected term is not shown. While some interaction was observed between age and deprivation, this was only significant for the outcome of hospital admission.
For purposes of space, age is shown only as an interaction term with deprivation. This was done to try and consider whether the impact of age on all three outcomes could be related more to increasing deprivation in the older age groups. However, when compared directly, there was a certain interaction between age and deprivation, but this was only significant for hospital admission. Otherwise, age remains an independently high predictor of all three outcomes, and indeed is the greatest predictor for hospital admission and for mortality, and is second only to comorbidity for ICU admission.
Discussion
This study is the first in Ireland to explore COVID-19 by small geographic area as a function of affluence/deprivation using the HP index across three pandemic waves. Of the total number of cases, 98.4% were geo-referenced to a small area. Our study showed that the risk of being a confirmed case of COVID-19 is higher among the more deprived categories for all ages across all three pandemic waves, except for the 20–39 years age category, in whom the reverse pattern was found.
This latter finding may be associated with a high proportion (46%) of cases in the 20–39 age category being health care workers, who had both an increased occupational exposure to COVID-19 and a requirement for serial testing, leading to possible ascertainment bias. Ascertainment bias due to increased testing for travel purposes may also play a part, as the 20–39 age group is more associated with travel. This finding, however, has not been reported internationally. Typically, those professions either with greater public contact or for whom working from home was not an option were considered to be more at risk of COVID-19, in the pre-vaccine era. Those from more affluent professions, for whom social distancing and working from home were a realistic option, had a greater degree of protection during this time [24].
The main determinants of hospital admission were: increasing age, comorbidity, a positive COVID-19 diagnosis in an earlier wave of the pandemic, male sex and being resident in a more deprived small area. By contrast, the main determinants for ICU admission and death were: comorbidity, increasing age, association with an outbreak and COVID-19 diagnosis during an earlier wave of the pandemic. No convincing deprivation gradient was found for ICU admission, but mortality was slightly lower in the more affluent population. While there was some degree of variable interaction between age and deprivation, age remained, even after correction, as one of the strongest predictors of hospital admission, ICU admission and death from COVID.
Those patients diagnosed in later waves were likely to have benefitted from the experience and confidence that grew among healthcare providers. Additionally, diagnostic bias in the earliest wave may have resulted in less severe cases not being recognised or diagnosed. Along with the provision of supports for quarantining and isolation, it is likely that cases were able to be managed at lower levels of complexity and without hospital admission [25]. Additionally, with increasingly focussed testing criteria, a greater number of less severe cases are likely to have been identified later in the pandemic.
While deprivation is closely associated with poorer outcomes for many health conditions, that pattern was not observed consistently in our study. This finding is echoed in a recent study of trends in mortality rates over a five-month period in England, which found that deprivation and ethnic group were not associated with death among hospitalised patients once appropriately adjusted for risk. The authors note that their finding was consistent with other US studies which suggest that the widely reported effect of deprivation and ethnicity is most likely due to differences in exposure to the disease [26]. Ireland is ethnically more homogenous than the UK and the US and removing ethnicity as a factor in terms of COVID outcomes may have reduced the overall impact of deprivation in an Irish population.
Deprivation is a complex concept, and the Irish HP Index is a sensitive, multi-dimensional tool. Our findings suggest that once adjustment is carried out for factors that are confounders for deprivation–age and comorbidity in this case–the overall impact of deprivation is reduced. The lack of a clear deprivation gradient for COVID-19 outcomes suggests equitable access and provision of COVID-19 related health care across all strata of Irish society. Internationally, however low income [27] and overcrowding [28] have been associated with poor outcomes from COVID-19.
The apparent divergence of Irish findings may be explained by the presence of significant ethnic minorities in other countries, perhaps confounded by deeper inequities in these societies. Ethnicity is not included in the Irish HP Index, nor is it mentioned in other international measures of deprivation [29–31].
It must be noted, however, that recent Irish research has noted a link between COVID-19 incidence and deprivation [32]. In Europe, association between deprivation and COVID admission and mortality has been determined in Northern Ireland [33], England [34, 35], Scotland [36], France [37] and Spain [38], among others. However, it is not necessarily simple, or linear. Germany, for example, reported that earlier cases tended to be more affluent, followed by an increasing likelihood of illness in the more deprived [39]. This was also determined in early Irish observations [40] and suggests additional complexity that has not previously been considered. In addition, Italian researchers determined that the deprivation profile of cases changed after lockdown, and pointed out the differential impact of lockdown on people in different deprivation categories [41].
This study was one of secondary data analysis, that is, it analysed data that had been collected for another purpose, namely COVID-19 surveillance for Public Health protection and infection control. We used literature sources to identify those factors which were deemed to have the greatest impact on COVID outcome. As a result, our choice of variables was limited to those identified as being important for the purposes of surveillance. It is possible, therefore, that we may not have therefore been able to pick up on nuances that would otherwise be available to researchers collecting data in a less emergent setting.
Critically, this study was performed using data from the pre-vaccine phase of the COVID pandemic in Ireland. We believed that in order to determine whether any deprivation gradient existed in an Irish population with respect to coronavirus infection, we had to look at pre-immunisation data. The reason for this is that the COVID vaccine was made available to all individuals, free of charge, and on the basis of risk. It therefore sought to reduce any socio-demographic inequalities that may have existed. By including waves subsequent to the vaccine roll-out, we believe that any nuance or gradient that may have existed would have been nullified. We therefore wanted to know whether, and the extent to which, social deprivation would prove to be a risk factor in an Irish population for future pandemic planning.
Data quality overall, however, was a strength in this study, because it was derived from a single source, namely the Computerised Infectious Disease Reporting (CIDR) system governed by the Health Protection Surveillance Centre [15]. CIDR includes all hospital- and community-based, laboratory-confirmed COVID-19 cases in Ireland, and is the “source of truth” for all national COVID-19 reporting processes. Clinically suggestive but PCR-negative cases of COVID-19 were not included in our study, providing for a more specific but perhaps less inclusive case definition.
A number of potential causes of bias were considered. COVID-19 testing is free to all in Ireland and it is unlikely that test uptake was influenced by deprivation. Despite address quality issues, over 98% of cases were successfully geo-referenced, and was not considered a source of bias in deprivation categorisation.
Other studies have examined age as a continuous variable, whereas in our study, we used age categories. We are aware in doing so that we may have lost some power, but these age categories also strongly reflect decision making in a clinical setting in Ireland, and we wished our analysis to maintain the same clinical significance. In addition, when we included age as a continuous variable in the model, the resulting lower Odds Ratios made it difficult to represent fully the changing impact on outcome at different ages. In other words, we believe that there was a non-linear relationship between age and outcomes. Finally, when we included age as a continuous variable, the resulting R2 was lower than when it was modelled as a categorical variable.
The degree of asymptomatic carriage of COVID-19, or false-negative testing may have resulted in bias. These are difficult to determine and validations were not carried out for this study. Therefore the definition of a case as a person with laboratory-confirmed infection will not include persons who were ill and who returned a negative test, or those who were asymptomatic and did not seek a test.
Missing data for the “Comorbidity” variable must be balanced against the very high odds ratios we determined for all three of the outcomes in this study and the fact that international literature consistently cites comorbidity as a predictive factor in COVID-19 outcomes [10].
Conclusion
Our study found a deprivation gradient in COVID-19 incidence (with the exception of the 20–39 age group) and in hospital admission, but no similar trend in relation to ICU admission or mortality. Deprivation, however, is a multi-factorial concept, and removing some of these factors by statistical adjustment helps to illustrate which variables have the greatest impact. This study echoed international literature in determining that the principal determinants of all outcomes (hospital admission, ICU admission and mortality) were age, comorbidity and diagnosis at an early wave of the pandemic.
Our results suggest a “non-democratic” transmission of COVID-19 in the Irish population, but this did not translate into poorer outcomes. Demographic differences, particularly relating to ethnicity, may help to explain the disparity between Irish and international experience.
Our study suggests that COVID-19 spreads easily through all strata of society and particularly in the more deprived population. However, in terms of more serious outcomes from COVID-19 in Ireland, our study suggests that there would appear to be truth in the saying that “we are all in this together”.
Data Availability
Data cannot be shared publicly because of General Data Protection Regulations (GDPR) which have been introduced in Europe since 2018. Because the Health Protection Surveillance Centre are the designated Data Controllers under GDPR, any request for data would need to be made through them. Data requests can be sent to the director of HPSC, Dr. Greg Martin [email protected], or [email protected]
References
Decision Letter 0
17 Oct 2022
PONE-D-22-14948COVID-19 incidence and outcome by affluence/deprivation across three pandemic waves in Ireland: a retrospective cohort study using routinely collected dataPLOS ONE
Dear Dr. McKeown,
Thank you for submitting your manuscript to PLOS ONE. After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process. Please address these issues and others raised in the reviews.
The modeling could be made more clear and additional models considered.
Please address the selection process for the predictors included in the model? Were there other predictors available in the data source but not used in the model?
Why did you categorize age in the modeling? Why not just use splines and model the effect of age as a continuous predictor? There is the potential to really lose information by categorizing. Please justify why those categories were chosen.
Similarly, why was the HP index categorized? Are these standard categorizations or selected based on the data in this study? Could a continuous version, perhaps with splines, be used in the regression?
Please submit your revised manuscript by Dec 01 2022 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp. When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Please include the following items when submitting your revised manuscript:
A rebuttal letter that responds to each point raised by the academic editor and reviewer(s). You should upload this letter as a separate file labeled 'Response to Reviewers'.
A marked-up copy of your manuscript that highlights changes made to the original version. You should upload this as a separate file labeled 'Revised Manuscript with Track Changes'.
An unmarked version of your revised paper without tracked changes. You should upload this as a separate file labeled 'Manuscript'.
If you would like to make changes to your financial disclosure, please include your updated statement in your cover letter. Guidelines for resubmitting your figure files are available below the reviewer comments at the end of this letter.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: https://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols. Additionally, PLOS ONE offers an option for publishing peer-reviewed Lab Protocol articles, which describe protocols hosted on protocols.io. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols.
We look forward to receiving your revised manuscript.
Kind regards,
Edward Jay Trapido, ScD
Academic Editor
PLOS ONE
Journal requirements:
When submitting your revision, we need you to address these additional requirements.
1.Please ensure that your manuscript meets PLOS ONE's style requirements, including those for file naming. The PLOS ONE style templates can be found at
https://journals.plos.org/plosone/s/file?id=wjVg/PLOSOne_formatting_sample_main_body.pdf and
2. We note that you have indicated that data from this study are available upon request. PLOS only allows data to be available upon request if there are legal or ethical restrictions on sharing data publicly. For more information on unacceptable data access restrictions, please see http://journals.plos.org/plosone/s/data-availability#loc-unacceptable-data-access-restrictions.
In your revised cover letter, please address the following prompts:
a) If there are ethical or legal restrictions on sharing a de-identified data set, please explain them in detail (e.g., data contain potentially sensitive information, data are owned by a third-party organization, etc.) and who has imposed them (e.g., an ethics committee). Please also provide contact information for a data access committee, ethics committee, or other institutional body to which data requests may be sent.
b) If there are no restrictions, please upload the minimal anonymized data set necessary to replicate your study findings as either Supporting Information files or to a stable, public repository and provide us with the relevant URLs, DOIs, or accession numbers. For a list of acceptable repositories, please see http://journals.plos.org/plosone/s/data-availability#loc-recommended-repositories.
We will update your Data Availability statement on your behalf to reflect the information you provide.
3. Please upload a copy of Figure 4, to which you refer in your text on page 7. If the figure is no longer to be included as part of the submission please remove all reference to it within the text.
[Note: HTML markup is below. Please do not edit.]
Reviewers' comments:
Reviewer's Responses to Questions
Comments to the Author
1. Is the manuscript technically sound, and do the data support the conclusions?
The manuscript must describe a technically sound piece of scientific research with data that supports the conclusions. Experiments must have been conducted rigorously, with appropriate controls, replication, and sample sizes. The conclusions must be drawn appropriately based on the data presented.
Reviewer #1: Yes
Reviewer #2: Partly
**********
2. Has the statistical analysis been performed appropriately and rigorously?
Reviewer #1: Yes
Reviewer #2: Yes
**********
3. Have the authors made all data underlying the findings in their manuscript fully available?
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
Reviewer #1: Yes
Reviewer #2: Yes
**********
4. Is the manuscript presented in an intelligible fashion and written in standard English?
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please note any specific errors here.
Reviewer #1: Yes
Reviewer #2: Yes
**********
5. Review Comments to the Author
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Reviewer #1: The present study conducted two major analyses: (1) calculation of COVID-19 incidence per population by deprivation and age group, and (2) multivariate logistic regression of three outcome measures to detect the association with deprivation among COVID-19 cases.
The two-part composition is the uniqueness of this manuscript. Thus, revisions would be recommended to make it clear for readers.
Abstract
Page 2 lines 29-32: Additional explanation may be inserted into second paragraph, to mention about calculation of COVID-19 incidence.
Page 2 lines 37-43: Forth paragraph (discussion/conclusion) appears to be a bit long; last sentence might be omitted.
Introduction section is concise and condensed.
Methods
Page 3 lines 68-70: the rationales of pandemic timing may need elaboration. For example, is this based on the effective reproduction number (Rt) or other measures suggesting declining/increasing trends?
It would also be noted whether vaccination was launched in early 2021 (Wave 3) in Ireland. It may be relavant to some outcome measures such as admission to an ICU and death.
Page 4 lines 87-88: a brief explanation may be added to "a random neighbourhood match".
Page 4 lines 95-101: description of statistical methods would be restructured in order to indicate the aim (to examine XXX) of each procedure.
Results
Page 7 lines 139-148: it is not sure whether any test statistics are available to judge "higher" incidence and risk ratio.
Page 10 lines 170-178: criteria for the effect size would be declared in Methods section in addition to Table 2.
Discussion
Page 10 line 191-page 11 line 201: some speculation may be added regarding why high incidence was observed among the more deprived categories, such as occupation-associated risk or hygiene habits?
Page 11 lines 215-222: again, vaccination rollout would be discussed as it could reduce hospitalization/severe symptoms and mortality.
Conclusion
Page 13 lines 257-263: it is recommended that the conclusion section does not include citations. Second paragraph might be moved to Discussion section.
Reviewer #2: General Comments:
Overall an interesting manuscript that uses administrative data sources to examine the association of material deprivation with COVID-19 incidence and outcomes (hospital admission, ICU admission, mortality). Literature review is adequate. The sample size is large and population-based. The methodology appears appropriate. The conclusions drawn are reasonable.
Major Comments:
The modeling could be made more clear and additional models considered. First, I don’t see how there is any forward/stepwise regression done as all that is reported seems to include all of the terms. What there some sort of selection process for the predictors included in the model? Where there other predictors available in the data source but not used in the model? They authors need to explain a bit more about what predictors were available and which were ultimately chosen. I personally also like to see unadjusted (bivariable) results (i.e., regression with individual predictors) so that I can see how inclusion of all predictors has changed some of the estimates.
Age is a very important predictor of death and likely other outcomes. Why did the authors chose to categorize age in the modeling? Why not just use splines and model the effect of age as a continuous predictor? There is the potential to really lose information by categorizing. Also if categorizing, would have to justify why those categories were chosen. Similarly, why was the HP index categorized? Are these standard categorizations or selected based on the data in this study? Could a continuous version, perhaps with splines, be used in the regression?
I like Figure 3 very much and it seems to suggest important interactions between age and deprivation and perhaps age, deprivation, and wave. Were interactions considered in the model? It would seem that some should be given the exploratory data analysis.
Minor Comments:
1. How were the 3 waves defined?
2. HP Index: Does the HP index cover the entire population? Are there any subgroups that wouldn’t apply? There is a suggestion that perhaps people living in a continuing care setting may not be covered. More importantly, what are the properties of the deprivation index. The authors could do more to explain what the different dimensions mean and what the relationship to COVID-19 could be.
3. Is the unit of analysis the patient/individual? Is it possible to have more than one infections for a person added to the administrative data source?
4. How many hospitals are there in the study population? Is there variation in admission standards to hospital (and ICU)? If so, a random effect for hospital may need to be considered in the modeling to account for correlation in patients/outcomes in the same hospital.
5. The data collection process could be made more clear. Is there, say, a 1 page form for reporting? Are comorbidities just gleaned from that form or could comorbidities be determined from another data source? Is there data available about the type(s) of comorbidities? It is not clear how much missing and/or misclassification is present within the comorbidity variable.
6. The outcomes are not modeled jointly, so I would not refer to this as a multivariate regression but rather multivariable regression. A multivariate analysis is not really done in this manuscript.
7. Table 1. Seem to be missing a label under health worker: ie there are 2 yes and 2 no with that variable.
8. Figure 1 and think it would be good to actually show the distribution of HP index for the study population. Further, the same kind of graphic could be done for each of the 3 waves.
9. The justification for not requiring an Ethics Review is not clear to me.
**********
6. PLOS authors have the option to publish the peer review history of their article (what does this mean?). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy.
Reviewer #1: No
Reviewer #2: No
**********
[NOTE: If reviewer comments were submitted as an attachment file, they will be attached to this email and accessible via the submission site. Please log into your account, locate the manuscript record, and check for the action link "View Attachments". If this link does not appear, there are no attachment files.]
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com/. PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Registration is free. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email PLOS at gro.solp@serugif. Please note that Supporting Information files do not need this step.
Author response to Decision Letter 0
27 Mar 2023
Reviewer #1
The present study conducted two major analyses: (1) calculation of COVID-19 incidence per population by deprivation and age group, and (2) multivariate logistic regression of three outcome measures to detect the association with deprivation among COVID-19 cases. The two-part composition is the uniqueness of this manuscript. Thus, revisions would be recommended to make it clear for readers.
Abstract
Comment: Page 2 lines 29-32: Additional explanation may be inserted into second paragraph, to mention about calculation of COVID-19 incidence.
Response: Additional comment added about notification to the national Computerised Infectious Diseases Register (CIDR)
Comment: Page 2 lines 37-43: Fourth paragraph (discussion/conclusion) appears to be a bit long; last sentence might be omitted.
Response: Last sentence has been omitted.
Introduction
Comment: Section is concise and condensed.
Methods
Comment: Page 3 lines 68-70: the rationales of pandemic timing may need elaboration. For example, is this based on the effective reproduction number (Rt) or other measures suggesting declining/increasing trends?
Response: Agree – the timing is based on rising and falling crude incidence at a national level and agreed by the Health Protection Surveillance Centre. Clarification has been added to text.
Comment: It would also be noted whether vaccination was launched in early 2021 (Wave 3) in Ireland. It may be relevant to some outcome measures such as admission to an ICU and death.
Response: Vaccination in Ireland only became established towards the end of the third wave, and while it may have contributed to the slowing off of infections in the summer of 2021, uptake only rapidly took off after the end of the third wave. Vaccination onset acknowledged in the text.
Comment: Page 4 lines 87-88: a brief explanation may be added to "a random neighbourhood match".
Response: The Eircode (postal code) system in Ireland was not fully embedded before the COVID pandemic hit. Databases had not been fully updated to reflect the new postal codes, which meant that patient addresses had to be recorded in long form. Because of the large rural population, many addresses were by townland only, meaning that a number of them are non-unique. For our purposes, we did not require x,y coordinates, and instead, non-unique addresses were linked to the closest geographic Small Area (an SA comprising 50-100 households). The SA is the smallest boundary available and each has a calculated population density and deprivation index. Therefore, the analysis did not require precise x,y location, but rough neighbourhood matching allowed us to identify the SA in which the address should be located.
Comment: Page 4 lines 95-101: description of statistical methods would be restructured in order to indicate the aim (to examine XXX) of each procedure.
Response: This has been done
Results
Comment: Page 7 lines 139-148: it is not sure whether any test statistics are available to judge "higher" incidence and risk ratio.
Response: The analysis is made on the basis of the error bars shown underneath the OR in figures 2 (e), (f), (g) and (h).
Comment: Page 10 lines 170-178: criteria for the effect size would be declared in Methods section in addition to Table 2.
Response: Criteria have been included in the methods section.
Discussion
Comment: Page 10 line 191-page 11 line 201: some speculation may be added regarding why high incidence was observed among the more deprived categories, such as occupation-associated risk or hygiene habits?
Response: A brief explainer has been added, with reference.
Comment: Page 11 lines 215-222: again, vaccination rollout would be discussed as it could reduce hospitalization/severe symptoms and mortality.
Response: Very good point. A paragraph has been added to reflect this.
Conclusion
Comment: Page 13 lines 257-263: it is recommended that the conclusion section does not include citations. Second paragraph might be moved to Discussion section.
Response: Agree and done.
Reviewer #2:
General Comments:
Overall an interesting manuscript that uses administrative data sources to examine the association of material deprivation with COVID-19 incidence and outcomes (hospital admission, ICU admission, mortality). Literature review is adequate. The sample size is large and population-based. The methodology appears appropriate. The conclusions drawn are reasonable.
Major Comments:
Comment: The modeling could be made more clear and additional models considered. First, I don’t see how there is any forward/stepwise regression done as all that is reported seems to include all of the terms. What there some sort of selection process for the predictors included in the model? Where there other predictors available in the data source but not used in the model? They authors need to explain a bit more about what predictors were available and which were ultimately chosen. I personally also like to see unadjusted (bivariable) results (i.e., regression with individual predictors) so that I can see how inclusion of all predictors has changed some of the estimates.
Response: We adopted a backward elimination stepwise regression approach. If a variable was deemed to be significant at p<0.1 at the univariate level, it was included in the overall model. Therefore the variables included in the final model were those that were significant at this level in the univariate phase of analysis. This has been clarified in the most recent submitted manuscript.
The predictors available for the model were limited to the data captured. Given that the variables were derived from surveillance data, we were limited by what was deemed significant for data capture during a pandemic. As a result, certain nuances may have been lost which would otherwise be available to researchers collecting de novo data in a more elective setting. We decided to single out for specific attention variables which had been indicated as having clinical or statistical significance in other studies. This has been further clarified in the text.
Comment: Age is a very important predictor of death and likely other outcomes. Why did the authors choose to categorize age in the modeling? Why not just use splines and model the effect of age as a continuous predictor? There is the potential to really lose information by categorizing. Also if categorizing, would have to justify why those categories were chosen. Similarly, why was the HP index categorized? Are these standard categorizations or selected based on the data in this study? Could a continuous version, perhaps with splines, be used in the regression?
Response: We are aware that in categorising age, we may have lost some power. However in doing so, and in selecting the chosen categories, we believed that we were reflecting other Irish analysis that seemed to determine differences in outcome in those age groups. When we used age as a continuous variable, our R-square was lower, and we believe that this may cloud the clinical impact that age has had on the Irish population. Therefore, we believed that in determining odds or risk differences by one year age unit could conceal the very real clinical impact that COVID has had on an Irish population. We felt that it was easier, when communicating risk, to do so using the age group approach, rather than run the risk of diluting the message, which we felt was important to share with our clinical and Public Health colleagues, both in Ireland and overseas. The COVID pandemic has already suffered as a result of public disinformation, and we wanted our message to be as clear as possible.
The Pobal HP Deprivation Index was generated for the Irish population by Trutz Haase and Jonathan Pratschke. For the purposes of determining material deprivation, the relative HP index is used as a five-category measurement that is based on standard deviations from a mean. The HP Index is recalculated at every Census cycle. The categorisations are therefore standard and will appear as the same categories throughout much of the public sector publications addressing deprivation in Ireland. We believe that a continuous version, because of the relatively small numbers involved in Ireland in comparison with other countries, would not have had the sensitivity to show deprivation differences by the time sub-analyses were being performed, and particularly for the multivariate calculations.
Comment: I like Figure 3 very much and it seems to suggest important interactions between age and deprivation and perhaps age, deprivation, and wave. Were interactions considered in the model? It would seem that some should be given the exploratory data analysis.
Response: Interactions were considered in the model, particularly interaction between deprivation and age, and that is controlled for in the model.
Minor Comments:
Comment 1. How were the 3 waves defined?
Response: The three waves were defined on the basis of increasing and decreasing crude COVID-19 incidence rates in the national population. The timing of the waves have been standardised by the national Health Protection Surveillance Centre in Dublin and are used commonly throughout all Irish government agencies when describing the pandemic timing
Comment 2. HP Index: Does the HP index cover the entire population? Are there any subgroups that wouldn’t apply? There is a suggestion that perhaps people living in a continuing care setting may not be covered. More importantly, what are the properties of the deprivation index. The authors could do more to explain what the different dimensions mean and what the relationship to COVID-19 could be.
Response: We felt that a more in-depth description of the HP index was beyond the scope of this paper. However, the source that is cited in the reference section (Haase T. The 2016 Pobal HP Deprivation Index (SA), Reference 13) states that the HP Index uses factor analysis to model three domains, each with a number of indicators, as shown below.
(1) Demographic profile
Rural deprivation is often the result of agricultural underemployment and/or emigration (which has been a longstanding cultural phenomenon in Ireland; the population has still not regained its 1841 population of 8 million, following the disastrous famines and emigrations of the later 1840s). Emigration from deprived rural areas is often the result of a mismatch between education, skills levels and expectations on the one hand, and on the other, with available job opportunities. Emigration is also socially selective, meaning that those who are of working age with skills leave the area, resulting in those left behind being even more economically dependent. Erosion of local workforce means that an area is less attractive for economic investment
There are six indicators within the domain of demographic profile
• the percentage change in population over the previous five years (positive association);
• the percentage of population aged under 15 or over 64 years of age (negative association);
• the percentage of population with a primary school education only (negative association);
• the percentage of population with a third level education (positive association);
• the percentage of households with children aged under 15 years and headed by a single parent (positive association);
• the mean number of persons per room (positive association).
(2) Social class composition
Social Class Composition is of equal relevance to both urban and rural areas. Social class background has a considerable impact in many areas of life, including educational achievements, health, housing, crime and economic status. Furthermore, social class is relatively stable over time and constitutes a key factor in the inter-generational transmission of economic, cultural and social assets. Areas with a weak social class profile tend to have higher unemployment rates, are more vulnerable to the effects of economic restructuring and recession and are more likely to experience low pay, poor working conditions as well as poor housing and social environments.
Social Class Composition is measured by five indicators:
• the percentage of population with a primary school education only (negative association);
• the percentage of population with a third level education (positive association);
• the percentage of households headed by professionals or managerial and technical employees, including farmers with 100 acres or more (positive association);
• the percentage of households headed by semi-skilled or unskilled manual workers, including farmers with less than 30 acres (negative association);
• the mean number of persons per room (negative association).
(3) Labour market situation
Labour market situation is predominantly, but not exclusively, an urban measure. Unemployment and long-term unemployment remain the principal causes of disadvantage at national level and are responsible for the most concentrated forms of multiple disadvantage found in urban areas. In addition to the economic hardship that results from the lack of paid employment, young people living in areas with particularly high unemployment rates frequently lack positive role models. A further expression of social and economic hardship in urban unemployment blackspots is the large proportion of young families headed by a single parent.
Labour market situation is measured by three indicators:
• the percentage of households with children aged under 15 years and headed by a single parent (negative association)
• the male unemployment rate (negative association)
• the female unemployment rate (negative association)
The fact that the HP Index derives from Census data should mean that all groups are equally and comprehensively represented in the Index. For the purposes of our analysis, a HP Index is appended to each geographic Small Area from which the address of a notification has been made. Each SA has a value for HP Index calculated as above. Patients in nursing homes, instead of attracting the deprivation index of their home address, instead are represented in the HP Index accorded to the nursing home address. For this group, we believe that their age profile may have overpowered any deprivation gradient, although this is purely from anecdotal evidence. In other words, the gradient profile may be less marked within the older age groups anyway, and the major obstacle that they face in terms of risk factors is their age.
Comment 3. Is the unit of analysis the patient/individual? Is it possible to have more than one infection for a person added to the administrative data source?
Response: Patients have unique identifiers within the dataset, and while it is possible for a patient to be infected more than once, we believe (again anecdotally), that repeat infections have been more of an issue in Ireland following relaxation of social distancing, masking and other community control measures, which came about with possibly an inflated sense of security around the degree of protection afforded by vaccination.
Comment 4. How many hospitals are there in the study population? Is there variation in admission standards to hospital (and ICU)? If so, a random effect for hospital may need to be considered in the modeling to account for correlation in patients/outcomes in the same hospital.
Response: There are 42 acute hospitals in the Republic of Ireland. Critically, because we are a small country and the professional communities of practice are so well established through the National Clinical Programme system, there has been widespread agreement and cohesion around factors for admission to hospital and to ICU.
Comment 5. The data collection process could be made more clear. Is there, say, a 1 page form for reporting? Are comorbidities just gleaned from that form or could comorbidities be determined from another data source? Is there data available about the type(s) of comorbidities? It is not clear how much missing and/or misclassification is present within the comorbidity variable.
Response: There is a standardised form for reporting COVID-19 in Ireland. Specific comorbidities are included on the form for inclusion as appropriate. This clarification has been added to the Methods section.
Comment 6. The outcomes are not modeled jointly, so I would not refer to this as a multivariate regression but rather multivariable regression. A multivariate analysis is not really done in this manuscript.
Response: The language has been changed to reflect this.
Comment 7. Table 1. Seem to be missing a label under health worker: ie there are 2 yes and 2 no with that variable.
Response: When a patient was a health care worker, for example it was very easy to interpret a “Y” as “Yes”, or a “N” as “No”. However, all we could tell from a missing value is that it was unlikely that the patient was a health care worker, as very clear instructions were given to data collectors to identify these individuals. In such cases, all we can do is say for definite that the binary result should be “Yes/Other”, where “Other” may mean “No” or it may mean missing data. To be honest, a “Yes” result was more significant for the purposes of follow up and occupational health notification, rather than a “No”.
Comment 8. Figure 1 and think it would be good to actually show the distribution of HP index for the study population. Further, the same kind of graphic could be done for each of the 3 waves.
Response: We felt that such a graphic may end up being too “busy”, because of smaller numbers in the five categories once we had divided them over three pandemic waves. For the purposes of our research question, we wanted to summarise the experience over the full three waves, and in order to do that, we felt that we had to combine the “Deprived” and “Very deprived” categories, in the same way as we had to combine the “Affluent” and “Very affluent” categories. We felt that this best represented what we were trying to convey.
Comment 9. The justification for not requiring an Ethics Review is not clear to me.
Response: Under the EU General Data Protection Regulations (2018), those aspects of health services research (e.g. clinical audit, health needs analysis, evaluation and monitoring etc.) which are designed for health service improvement or patient safety, and which use anonymized data from routinely-collected sources do not require clearance from an Ethics Committee. The Irish Health Services Research Regulations (2018) echo this.
Additional Comments:
Comment: Please upload a copy of Figure 4, to which you refer in your text on page 7. If the figure is no longer to be included as part of the submission please remove all reference to it within the text.
Response: Apologies. This was added in error and text has been amended. The reference should have been to Figure 3 and not Figure 4. Many thanks for spotting it.
Comment: Please remove your figures/ from within your manuscript file, leaving only the individual TIFF/EPS image files. These will be automatically included in the reviewer’s PDF
Response: Done.
Comment: Thank you for providing the following data availability statement: "The data underlying the result presented in the study are available from Joan O'Donnell (co-author), who can be contacted at [email protected]"
Response: In response to the comments on data availability, I believe that I am not in a position to share the data directly at this point because of General Data Protection Regulations (GDPR) which have been introduced in Europe since 2018. Because the Health Protection Surveillance Centre are the designated Data Controllers under GDPR, any request for data would need to be made through them. We have HPSC co-authors on the paper, but I gather a nominated contact would have to be someone other than a co-author, therefore I can understand that my colleague, Dr. Joan O’Donnell, would not be eligible for nomination for such a role. However, I believe that the newly-appointed director of HPSC would be an ideal contact, as he would have clinical and information governance oversight of all of the work carried out by HPSC. His name is Dr. Greg Martin and his email address is [email protected], or [email protected].
Many thanks for your kind attention and for your ongoing feedback.
Formatted letter is attached separately to this submission
Attachment
Submitted filename: PLOS RESPONSE TO REVIEWERS 07032023.docx
Decision Letter 1
10 Apr 2023
PONE-D-22-14948R1COVID-19 incidence and outcome by affluence/deprivation across three pandemic waves in Ireland: a retrospective cohort study using routinely collected dataPLOS ONE
Dear Dr. McKeown,
Thank you for submitting your manuscript to PLOS ONE. After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process.
==============================
ACADEMIC EDITOR: I read with interest the revised version of your manuscript. I appreciated the detailed responses you provided to the reviewers. In its current form the manuscript could be accepted, but reviewer #2 with expertise in biostatistics has raised an important issue with one of your responses. Please address this issue and resubmit your manuscript.
==============================
Please submit your revised manuscript by May 25 2023 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp. When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Please include the following items when submitting your revised manuscript:
A rebuttal letter that responds to each point raised by the academic editor and reviewer(s). You should upload this letter as a separate file labeled 'Response to Reviewers'.
A marked-up copy of your manuscript that highlights changes made to the original version. You should upload this as a separate file labeled 'Revised Manuscript with Track Changes'.
An unmarked version of your revised paper without tracked changes. You should upload this as a separate file labeled 'Manuscript'.
If you would like to make changes to your financial disclosure, please include your updated statement in your cover letter. Guidelines for resubmitting your figure files are available below the reviewer comments at the end of this letter.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: https://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols. Additionally, PLOS ONE offers an option for publishing peer-reviewed Lab Protocol articles, which describe protocols hosted on protocols.io. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols.
We look forward to receiving your revised manuscript.
Kind regards,
Victor Daniel Miron
Academic Editor
PLOS ONE
Journal Requirements:
Please review your reference list to ensure that it is complete and correct. If you have cited papers that have been retracted, please include the rationale for doing so in the manuscript text, or remove these references and replace them with relevant current references. Any changes to the reference list should be mentioned in the rebuttal letter that accompanies your revised manuscript. If you need to cite a retracted article, indicate the article’s retracted status in the References list and also include a citation and full reference for the retraction notice.
[Note: HTML markup is below. Please do not edit.]
Reviewers' comments:
Reviewer's Responses to Questions
Comments to the Author
1. If the authors have adequately addressed your comments raised in a previous round of review and you feel that this manuscript is now acceptable for publication, you may indicate that here to bypass the “Comments to the Author” section, enter your conflict of interest statement in the “Confidential to Editor” section, and submit your "Accept" recommendation.
Reviewer #1: All comments have been addressed
Reviewer #2: (No Response)
**********
2. Is the manuscript technically sound, and do the data support the conclusions?
The manuscript must describe a technically sound piece of scientific research with data that supports the conclusions. Experiments must have been conducted rigorously, with appropriate controls, replication, and sample sizes. The conclusions must be drawn appropriately based on the data presented.
Reviewer #1: Yes
Reviewer #2: Yes
**********
3. Has the statistical analysis been performed appropriately and rigorously?
Reviewer #1: Yes
Reviewer #2: Yes
**********
4. Have the authors made all data underlying the findings in their manuscript fully available?
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
Reviewer #1: Yes
Reviewer #2: Yes
**********
5. Is the manuscript presented in an intelligible fashion and written in standard English?
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please note any specific errors here.
Reviewer #1: Yes
Reviewer #2: Yes
**********
6. Review Comments to the Author
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Reviewer #1: Thank you for the revision in response to my comments. I found all comments have been fully addressed. I have no further questions.
Reviewer #2: I was satisfied with all of the responses to my comments except one.
"Comment: I like Figure 3 very much and it seems to suggest important interactions between age and
deprivation and perhaps age, deprivation, and wave. Were interactions considered in the model? It
would seem that some should be given the exploratory data analysis.
Response: Interactions were considered in the model, particularly interaction between deprivation and age,
and that is controlled for in the model."
The authors state that they have considered interactions and that an interaction is controlled for in the model. Presumably that means that the model with the interaction is presented in the manuscript. The methods and results about the multivariable analysis do not mention interactions. Further, if the model included an interaction between deprivation and age then the aORs would be presented separately for the different combinations of age group and deprivation. That is, there should be an OR for age 40-49 HP1&HP2, age 40-40 HP4&HP5, etc. Having age group and deprivation entered as just main effects and not as an interaction means that there was no interaction in the model. As the authors state that there were interactions this statement and there methods and results presented are not in agreement and need further clarification.
**********
7. PLOS authors have the option to publish the peer review history of their article (what does this mean?). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy.
Reviewer #1: No
Reviewer #2: No
**********
[NOTE: If reviewer comments were submitted as an attachment file, they will be attached to this email and accessible via the submission site. Please log into your account, locate the manuscript record, and check for the action link "View Attachments". If this link does not appear, there are no attachment files.]
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com/. PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Registration is free. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email PLOS at gro.solp@serugif. Please note that Supporting Information files do not need this step.
Author response to Decision Letter 1
6 Jun 2023
Dear colleague
Very many thanks for your recent editorial advice. The only point outstanding was number 6, which I have included in full below:
6. Review Comments to the Author
Reviewer #1: Thank you for the revision in response to my comments. I found all comments have been fully addressed. I have no further questions.
Reviewer #2: I was satisfied with all of the responses to my comments except one.
"Comment: I like Figure 3 very much and it seems to suggest important interactions between age and deprivation and perhaps age, deprivation, and wave. Were interactions considered in the model? It would seem that some should be given the exploratory data analysis.
Response: Interactions were considered in the model, particularly interaction between deprivation and age, and that is controlled for in the model."
The authors state that they have considered interactions and that an interaction is controlled for in the model. Presumably that means that the model with the interaction is presented in the manuscript. The methods and results about the multivariable analysis do not mention interactions. Further, if the model included an interaction between deprivation and age then the aORs would be presented separately for the different combinations of age group and deprivation. That is, there should be an OR for age 40-49 HP1&HP2, age 40-40 HP4&HP5, etc. Having age group and deprivation entered as just main effects and not as an interaction means that there was no interaction in the model. As the authors state that there were interactions this statement and there methods and results presented are not in agreement and need further clarification.
I have had the opportunity to discuss this more closely with my colleagues and to seek additional epidemiological opinion on the issue of interaction. Because of the potential for interaction between age and deprivation, we agreed that we should include a specific interaction term including these two variable, and to determine the extent to which it may influence the overall model.
While we did observe some variable interaction, this only achieved significance for the hospital admission outcome. The overall impact on the three outcomes was substantively unchanged. In other words, age still had the greatest impact on hospital admission and mortality, and the second greatest impact on ICU admission after comorbidity, even after the model was corrected for the age*deprivation interaction.
I have amended Table 2 to include the interaction terms and have also amended the text (please see Track changes version) to draw readers’ attention to the interaction.
I hope that this helps. Very many thanks once again for the opportunity to explore this data in a more robust manner, as I believe it helps to reinforce the findings which we have observed.
Very best wishes and many thanks as ever for your ongoing interest and support.
Attachment
Submitted filename: PLOS ONE RESPONSE TO REVIEWERS 06062023.docx
Decision Letter 2
12 Jun 2023
COVID-19 incidence and outcome by affluence/deprivation across three pandemic waves in Ireland: a retrospective cohort study using routinely collected data
PONE-D-22-14948R2
Dear Dr. McKeown,
We’re pleased to inform you that your manuscript has been judged scientifically suitable for publication and will be formally accepted for publication once it meets all outstanding technical requirements.
Within one week, you’ll receive an e-mail detailing the required amendments. When these have been addressed, you’ll receive a formal acceptance letter and your manuscript will be scheduled for publication.
An invoice for payment will follow shortly after the formal acceptance. To ensure an efficient process, please log into Editorial Manager at http://www.editorialmanager.com/pone/, click the 'Update My Information' link at the top of the page, and double check that your user information is up-to-date. If you have any billing related questions, please contact our Author Billing department directly at gro.solp@gnillibrohtua.
If your institution or institutions have a press office, please notify them about your upcoming paper to help maximize its impact. If they’ll be preparing press materials, please inform our press team as soon as possible -- no later than 48 hours after receiving the formal acceptance. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information, please contact gro.solp@sserpeno.
Kind regards,
Victor Daniel Miron
Academic Editor
PLOS ONE
Acceptance letter
7 Jul 2023
PONE-D-22-14948R2
COVID-19 incidence and outcome by affluence/deprivation across three pandemic waves in Ireland: a retrospective cohort study using routinely collected data
Dear Dr. McKeown:
I'm pleased to inform you that your manuscript has been deemed suitable for publication in PLOS ONE. Congratulations! Your manuscript is now with our production department.
If your institution or institutions have a press office, please let them know about your upcoming paper now to help maximize its impact. If they'll be preparing press materials, please inform our press team within the next 48 hours. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information please contact gro.solp@sserpeno.
If we can help with anything else, please email us at gro.solp@enosolp.
Thank you for submitting your work to PLOS ONE and supporting open access.
Kind regards,
PLOS ONE Editorial Office Staff
on behalf of
Dr. Victor Daniel Miron
Academic Editor
PLOS ONE
Articles from PLOS ONE are provided here courtesy of PLOS
Full text links
Read article at publisher's site: https://doi.org/10.1371/journal.pone.0287636
Read article for free, from open access legal sources, via Unpaywall:
https://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0287636&type=printable
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/151949092
Article citations
The impact of the COVID-19 vaccination programme on symptomatic and severe SARS-CoV-2 infection during a period of Omicron variant dominance in Ireland, December 2021 to March 2023.
Euro Surveill, 29(28), 01 Jul 2024
Cited by: 0 articles | PMID: 38994604 | PMCID: PMC11241852
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A retrospective cohort study of outcomes in hospitalised COVID-19 patients during the first pandemic wave in Ireland.
Ir J Med Sci, 191(5):1973-1983, 19 Nov 2021
Cited by: 3 articles | PMID: 34796450 | PMCID: PMC8601868
Modelling COVID-19 severity in the Republic of Ireland using patient co-morbidities, socioeconomic profile and geographic location, February to November 2020.
Sci Rep, 11(1):18474, 16 Sep 2021
Cited by: 6 articles | PMID: 34531478 | PMCID: PMC8446039
Association of socio-economic deprivation with COVID-19 incidence and fatality during the first wave of the pandemic in Italy: lessons learned from a local register-based study.
Int J Health Geogr, 22(1):10, 04 May 2023
Cited by: 1 article | PMID: 37143110 | PMCID: PMC10157567
Area Deprivation and COVID-19 Incidence and Mortality in Bavaria, Germany: A Bayesian Geographical Analysis.
Front Public Health, 10:927658, 15 Jul 2022
Cited by: 6 articles | PMID: 35910894 | PMCID: PMC9334899

 #
1
,*
#
1
,*