Abstract
Background
It has been suggested that chronic hypoxia underlies the higher prevalence of microalbuminuria in high-altitude residents than in sea-level dwellers. This study explored the risk factors for microalbuminuria in Tibetans with high-altitude pulmonary hypertension (HAPH).Methods
This retrospective cross-sectional study included adult patients with HAPH admitted to the People's Hospital of Tibet Autonomous Region between November 2018 and August 2019.Results
One hundred and twenty patients with HAPH were included in this study, and 69 patients (57.5%) had microalbuminuria. Compared with the patients without microalbuminuria, the microalbuminuria group had significantly higher values for age, pulmonary arterial systolic pressure (PASP), systolic blood pressure, diastolic blood pressure, blood hemoglobin concentration, glycated hemoglobin, serum creatinine, and serum uric acid, significantly lower values for heart rate, peripheral oxygen saturation (SpO2), estimated glomerular filtration rate, and 6-min walking distance, and poorer New York Heart Association functional class (P<0.05 for all variables). PASP [odds ratio (OR): 1.55; 95% CI: 1.19-2.00; P=0.001] and SpO2 (OR = 0.78; 95% CI: 0.63-0.97; P=0.02) were independently associated with microalbuminuria.Conclusions
Higher PASP and lower SpO2 were independently associated with microalbuminuria in adult Tibetan patients with HAPH.Free full text

Risk factors for microalbuminuria in adult Tibetan patients with high-altitude pulmonary hypertension: a cross-sectional study
Associated Data
Abstract
Background
It has been suggested that chronic hypoxia underlies the higher prevalence of microalbuminuria in high-altitude residents than in sea-level dwellers. This study explored the risk factors for microalbuminuria in Tibetans with high-altitude pulmonary hypertension (HAPH).
Methods
This retrospective cross-sectional study included adult patients with HAPH admitted to the People’s Hospital of Tibet Autonomous Region between November 2018 and August 2019.
Results
One hundred and twenty patients with HAPH were included in this study, and 69 patients (57.5%) had microalbuminuria. Compared with the patients without microalbuminuria, the microalbuminuria group had significantly higher values for age, pulmonary arterial systolic pressure (PASP), systolic blood pressure, diastolic blood pressure, blood hemoglobin concentration, glycated hemoglobin, serum creatinine, and serum uric acid, significantly lower values for heart rate, peripheral oxygen saturation (SpO2), estimated glomerular filtration rate, and 6-min walking distance, and poorer New York Heart Association functional class (P<0.05 for all variables). PASP [odds ratio (OR): 1.55; 95% CI: 1.19–2.00; P=0.001] and SpO2 (OR = 0.78; 95% CI: 0.63–0.97; P=0.02) were independently associated with microalbuminuria.
Conclusions
Higher PASP and lower SpO2 were independently associated with microalbuminuria in adult Tibetan patients with HAPH.
Introduction
Background
Pulmonary hypertension, defined as a resting mean pulmonary artery pressure (PAPm) ≥20 mmHg, encompasses a wide range of disorders that lead to an increase in blood pressure within the pulmonary circulation (1). Pulmonary hypertension most commonly arises secondary to idiopathic pulmonary arterial hypertension (IPAH) (2), but it can also develop in people exposed to high altitude. High-altitude pulmonary hypertension (HAPH) affects people who live at altitudes above 2,500 m (3) and has been reported to occur with a prevalence of 3.2% in native Tibetans in China (4). The pathogenesis of HAPH is complex and is thought to involve hypoxic pulmonary vasoconstriction, increased pulmonary vascular resistance, and vascular remodeling (3). HAPH typically presents exertional dyspnea, chest tightness, cough, hemoptysis, dizziness, syncope, and lower limb edema (5). The main treatment for HAPH involves descending to a lower altitude, although pharmacologic agents such as endothelin receptor antagonists (ERA), phosphodiesterase type-5 inhibitors (PDE5i), guanylate cyclase stimulator (GCS), and oral prostacyclin receptor agonist (PRA) have also been used (5). We present the following article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-385/rc).
Rationale and knowledge gap
Chronic hypoxia at high altitudes can also lead to detrimental effects on the kidneys, manifesting as polycythemia, systemic hypertension, hyperuricemia, and/or microalbuminuria (6-8). The amount of protein excreted in the urine increases with higher altitudes (9). High-altitude residents have a higher prevalence of proteinuria than sea-level residents (10), and it has been reported that 16–22% of high-altitude dwellers have microalbuminuria/proteinuria (11,12). Although the pathogenesis of microalbuminuria at high altitudes is not entirely clear, it is thought to be related to tissue hypoxia within the kidney parenchyma, glomerular capillary hypertension, blood hyperviscosity, and an increase in right heart pressure (6). Nickel et al. (13) reported that low-grade albuminuria is prevalent in pulmonary arterial hypertension patients and is associated with systemic inflammation and insulin resistance. Although HAPH and microalbuminuria both occur secondary to chronic hypoxia and vascular changes, little is known about the prevalence of microalbuminuria and risk factors for microalbuminuria in patients with HAPH. Tibetans have lived at very high altitudes for thousands of years and exhibit various adaptations to their hypoxic environment (14).
Objective
Therefore, this retrospective observational study aimed to determine the prevalence of microalbuminuria in adult Tibetan patients with HAPH and identify risk factors for microalbuminuria.
Methods
Study design and participants
This retrospective observational study included patients with HAPH admitted to the heart care unit of the People’s Hospital of Tibet Autonomous Region between November 2018 and August 2019. Some patients in this study cohort, but not all, were included in a previous study on heart rate variability in HAPH (15).
The inclusion criteria were (I) aged ≥18 years old, (II) PAPm ≥20 mmHg at rest as assessed by echocardiography or right heart catheterization (1,16), and (III) ethnic Tibetans who had lived in Lhasa, Tibet (which has an average elevation of 4,380 m) for at least 5 years. The exclusion criteria were (I) disorders that are known causes of secondary pulmonary hypertension, such as congenital heart disease, immune system disease, restrictive/obstructive pulmonary disease, chronic thromboembolic pulmonary hypertension, malignant tumors, portal hypertension, or renal insufficiency, (II) essential hypertension or diabetes mellitus, (III) unable to complete the 6-min walk test, (IV) history of mental illness, (V) received or receiving drugs to treat pulmonary hypertension, (VI) bleeding disorders, (VII) serious heart disease including right ventricular outflow tract obstruction, acute coronary syndrome (unstable angina or myocardial infarction), severe arrhythmia, or right ventricular insufficiency, (VIII) cerebrovascular events (transient cerebral ischemia or stroke) during the past 3 months, (IX) pregnant or breastfeeding, or (X) clinical evaluation not performed within 24 h of admission.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of the People’s Hospital of Tibet Autonomous Region, China (No. ME-TBH-19-01). The requirement for informed consent was waived because of the retrospective study design.
Data collection and definitions
Ethnic group, sex, age, residential altitude, smoking status, time since HAPH diagnosis, average heart rate, pulmonary arterial systolic pressure (PASP), systolic blood pressure (SBP), diastolic blood pressure (DBP), peripheral oxygen saturation (SpO2), blood hemoglobin concentration (Hb), fasting blood glucose level (FBG), glycated hemoglobin level (HbA1c), plasma total cholesterol level (TC), plasma triglyceride level (TG), plasma high-density lipoprotein cholesterol level (HDLC), plasma low-density lipoprotein cholesterol level (LDLC), serum creatinine level (SCr), serum uric acid level (SUA), blood N-terminal pro-B-type natriuretic peptide level (NT-proBNP), urine albumin level, urine creatinine level, estimated glomerular filtration rate (eGFR), 6-min walk distance (6MWD) (17), and New York Heart Association (NYHA) heart function classification were collected from the charts for each patient. The eGFR was calculated using the Cockcroft-Gault (CG) and Modification of Diet in Renal Disease (MDRD) formula (18). PASP was estimated from peak tricuspid regurgitation velocity measurements obtained by continuous Doppler ultrasonography using a simplified Bernoulli equation (19). Microalbuminuria was defined as 300 mg/g > urine albumin-to-creatinine ratio ≥30 mg/g in accordance with the 2012 criteria published by the Kidney Disease: Improving Global Outcomes (KDIGO) workgroup (20). SpO2 was routinely measured with a hand-held oximeter (TuffSat; GE Healthcare, London, UK) on a cleaned index finger. Systolic blood pressure (SBD) and DBP were measured using an electronic sphygmomanometer (OMRON Healthcare, Kyoto, Japan); the normal values were 90–140 mmHg for SDP and 60–90 mmHg for DBP. Hb was measured by the SLS-hemoglobin method; the normal reference value was 130–175 g/L for males and 110–150 g/L for females. FBG was measured by the hexokinase method; the normal reference value was 3.30–6.10 mmol/L. HbA1c was measured by high-performance liquid chromatography; the reference value was 4–6%. TC was measured by the cholesterol oxidase method; the normal reference value was 2.9–6.2 mmol/L. TG was measured by an enzymatic method; the normal reference value was 0.45–1.70 mmol/L.
Statistical analysis
SPSS 25.0 for Windows (IBM, Armonk, NY, USA) was used for all analyses. All quantitative data were tested for normality with the Kolmogorov-Smirnov test. Continuous data with a normal distribution were expressed as the mean ± standard deviation (SD) and analyzed using Student’s t-test for independent samples. Continuous data with a skewed distribution were presented as median (interquartile range) and analyzed using the Mann-Whitney U-test. Categorial data were expressed as n (%) and analyzed using the chi-square test. Factors associated with microalbuminuria were identified using multivariable logistic regression analysis (forward stepwise method based on maximum likelihood estimation). Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated. A two-sided P value <0.05 was taken to indicate a statistically significant difference.
Results
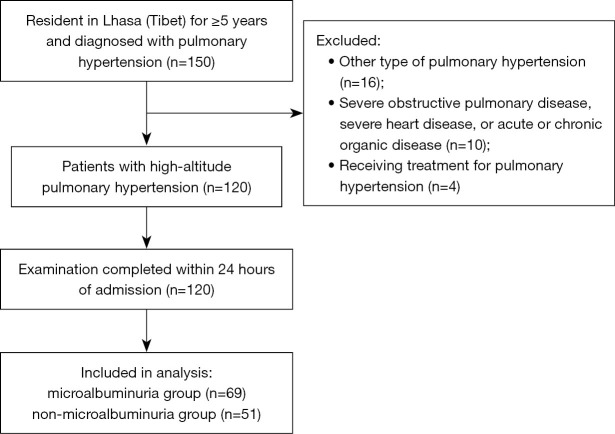
The final analysis included 120 adult patients with HAPH (Figure 1): 63 males (52.5%) aged 63.95±12.49 (range, 22–84) years old and 57 females (47.5%) aged 63.19±9.87 (range, 28–84) years old. The incidence of microalbuminuria was 57.5% (69/120). The baseline clinical characteristics for each group of patients are shown in Table 1.
Table 1
| Characteristic | Non-microalbuminuria group (n=51) | Microalbuminuria group (n=69) | P |
|---|---|---|---|
| Male | 24 (47.1%) | 39 (56.5%) | 0.36 |
| Age (years) | 60.75±12.28 | 65.70±10.06 | 0.02 |
| Residential altitude (m) | 3,768.86±437.38 | 3,819.54±516.10 | 0.57 |
| Smoking status | 0.31 | ||
| Never | 9 (17.6%) | 15 (21.7%) | |
| Current | 36 (70.6%) | 51 (73.9%) | |
| Former | 6 (11.8%) | 3 (4.3%) | |
| Time since HAPH diagnosis (years) | 3.57±1.06 | 3.62±1.10 | 0.79 |
| Average heart rate (beats/min) | 64.82±7.68 | 60.41±5.28 | <0.001 |
| PASP (mmHg) | 40.00±6.12 | 65.40±8.37 | <0.001 |
| SpO2 (%) | 69.04±5.22 | 58.49±6.93 | <0.001 |
| SBP (mm Hg) | 133.37±4.39 | 152.75±13.21 | <0.001 |
| DBP (mm Hg) | 77.22±9.37 | 90.41±8.98 | <0.001 |
| NYHA functional class | 0.02 | ||
| I or II | 8 (15.7%) | 8 (11.6%) | |
| III | 24 (47.1%) | 49 (71.0%) | |
| IV | 19 (37.3%) | 12 (17.4%) | |
| 6MWD (m) | 327.45±16.52 | 305.70±11.78 | <0.001 |
| Biochemical parameters | |||
| Hemoglobin (g/L) | 190.08±22.75 | 198.75±13.96 | 0.02 |
| Fasting blood glucose (mmol/L) | 11.82±3.65 | 12.04±3.50 | 0.75 |
| Glycated hemoglobin (%) | 6.96±0.56 | 9.47±2.41 | <0.001 |
| Total cholesterol (mmol/L) | 4.00±1.08 | 4.14±1.31 | 0.52 |
| Triglycerides (mmol/L) | 1.63±1.14 | 1.86±1.34 | 0.32 |
| HDLC (mmol/L) | 1.01±0.24 | 0.98±0.23 | 0.46 |
| LDLC (mmol/L) | 2.27±0.82 | 2.37±0.97 | 0.58 |
| Serum creatinine (mmol/L) | 90.53±5.63 | 113.00±17.58 | <0.001 |
| eGFR (mL/min/1.73 m2) | 70.62±10.33 | 48.20±7.87 | <0.001 |
| Serum uric acid (mmol/L) | 460.0 (433.0–499.0) | 585.0 (537.1–712.5) | <0.001 |
| Serum NT-proBNP (ng/L) | 287.0 (215.2–591.4) | 250.0 (145.8–574.9) | 0.22 |
Data are presented as mean ± standard deviation, median (interquartile range) or n (%). HAPH, high-altitude pulmonary hypertension; PASP, pulmonary arterial systolic pressure; SpO2, peripheral saturation; SBP, systolic blood pressure; DBP, diastolic blood pressure; NYHA, New York Heart Association; 6MWD, 6-minute walk distance; HDLC, high-density lipoprotein cholesterol; LDLC, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Compared with the non-microalbuminuria group, the microalbuminuria group had significantly higher values for age, PASP, SBP, DBP, Hb, HbA1c, SCr, and SUA, significantly lower values for heart rate, SpO2, eGFR, and 6MWD, and a smaller proportion of patients with NYHA functional class I/II (P<0.05 for all variables; Table 1). There were no significant differences between groups in sex distribution, residential altitude, smoking status, time since HAPH diagnosis, FBG, TC, TG, HDLC, LDLC, or NT-proBNP (Table 1).
Univariable logistic regression analyses showed that age, heart rate, PASP, DBP, SpO2, Hb, HbA1c, SCr, SUA, eGFR, and 6MWD were significantly associated with microalbuminuria (Table 2). The multivariable logistic regression analysis revealed that PASP (OR, 1.55; 95% CI: 1.19–2.00; P=0.001) and SpO2 (OR, 0.78; 95% CI: 0.63–0.97; P=0.02) were independent risk factors for microalbuminuria (Table 2).
Table 2
| Variables | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Age (years) | 1.04 | 1.01–1.08 | 0.02 | ||||
| Heart rate (beats/min) | 0.90 | 0.84–0.96 | 0.001 | ||||
| PASP (mmHg) | 1.49 | 1.25–1.78 | <0.001 | 1.55 | 1.19–2.00 | 0.001 | |
| Systolic BP (mmHg) | 145,270.22 | 0.00–∞ | 0.98 | ||||
| Diastolic BP (mmHg) | 1.16 | 1.10–1.22 | <0.001 | ||||
| SpO2 (%) | 0.75 | 0.67–0.83 | <0.001 | 0.78 | 0.63–0.97 | 0.02 | |
| NYHA functional class | 0.76 | 0.44–1.32 | 0.33 | ||||
| 6-minute walk distance (m) | 0.88 | 0.83–0.92 | <0.001 | ||||
| Hemoglobin (g/L) | 1.03 | 1.01–1.05 | <0.001 | ||||
| eGFR (mL/min/1.73 m2) | 0.75 | 0.66–0.84 | <0.001 | ||||
| Glycated hemoglobin (%) | 2.08 | 1.57–2.77 | <0.001 | ||||
| Serum creatinine (mmol/L) | 1.34 | 1.19–1.50 | <0.001 | ||||
| Serum uric acid (mmol/L) | 1.04 | 1.03–1.06 | <0.001 | ||||
OR, odds ratio; PASP, pulmonary arterial systolic pressure; BP, blood pressure; SpO2, peripheral saturation; NYHA, New York Heart Association; eGFR, estimated glomerular filtration rate.
Discussion
Key findings
A notable finding of the present study was that 57.5% of Tibetan patients with HAPH had microalbuminuria. Moreover, a higher PASP and a lower SpO2 were independent risk factors for microalbuminuria.
Strengths and limitations
A strength of this study was the involvement of a genetically homogeneous population. Still, this study has some limitations. First, the findings might be prone to selection or information bias because the analysis was retrospective. Second, it is unknown whether the results are generalizable since the participants were enrolled at a single center. Third, the sample size was quite small, so the analysis might have been underpowered to detect some real differences between groups. Fourth, most patients lived at an altitude of about 3,600 m, so it was impossible to explore whether residential altitude affected the prevalence of microalbuminuria in patients with HAPH. Fifth, the patients were not followed-up to examine the effects of microalbuminuria and treatment on adverse cardiovascular outcomes such as re-admission due to heart failure, stroke, and mortality. Sixth, the study excluded patients with right heart insufficiency; hence the risk factors for microalbuminuria in patients with HAPH and right heart dysfunction remain unknown. Seventh, pulmonary arterial pressure was measured using echocardiography rather than right heart catheterization since the latter ‘gold-standard’ method is far more invasive. Eighth, the patients were Tibetans with clear differences with sea-level dwellers regarding the physiological adaptation consequent to the genetic adaptation and phenotypic plasticity (i.e., acclimatization) to high altitude (21-24); these differences cannot be distinguished here since sea-level dwellers were not included. Finally, Wilkins et al. (25) reported that the α1-A680T sGC variant of GUCY1A3 conferred protection from pulmonary hypertension in Kyrgyz highlanders. Unfortunately, genetic analyses were not performed in the present study.
Comparisons with similar researches
The prevalence of microalbuminuria in the present study (57.5%) was much higher than that reported for the general population living at sea level in China (9.0%) (26). It should be noted that the exclusion criteria for the present study excluded common diseases that cause microalbuminuria, such as systemic hypertension and diabetes mellitus.
Our logistic regression analysis revealed that PASP and SpO2 were independent risk factors for microalbuminuria in patients with HAPH. This finding suggests that maintenance of an adequately high SpO2 might prevent the development of microalbuminuria. An elevation of pulmonary artery pressure is the first clinical feature that manifests in patients with HAPH. The pathophysiological mechanism of pulmonary hypertension is complex and involves environmental and genetic factors (1,27,28). Furthermore, the development of HAPH is complex and involves biochemical, immunological, and mechanical factors, as well as vascular endothelial damage caused by hypoxia (29). We speculate that microalbuminuria may be a biomarker of vascular endothelial damage in patients with HAPH and, thus, potentially could be used as a simple screening tool to identify those patients with HAPH who are at risk of vascular injury. This possibility will need to be explored in a future study.
Explanations of the findings
The pathogenesis of HAPH is thought to involve pulmonary arterial vasoconstriction and pulmonary vascular remodeling in response to chronic hypoxia, increased blood viscosity, and hemodynamic changes (3). The vascular changes that underlie HAPH are believed to be due, in part, to the overactivation of the sympathetic nervous system as well as inflammation and dysfunction of the endothelium, which leads to defective nitric oxide synthesis and exaggerated endothelin production (30). Consistent with the above proposal, a previous study reported hypoxia-induced inflammation in patients with pulmonary hypertension (31). Microalbuminuria is considered a marker of systemic vascular endothelial cell damage, dysfunction, and inflammation (32,33). Microalbuminuria is more prevalent in patients with pulmonary arterial hypertension and is associated with systemic inflammation (elevated C-reactive protein level), insulin resistance (elevated HbA1c level), and poorer outcomes (13). Furthermore, it has been suggested that endothelial injury is the common factor underlying the link between enhanced urinary albumin excretion and pulmonary hypertension in patients with sickle cell disease (34). Based on the above, we hypothesized that microalbuminuria would be prevalent in patients with HAPH and might reflect the degree of endothelial dysfunction and disease severity. Therefore, the high prevalence of microalbuminuria in Tibetan patients with HAPH was likely due to vasculitis that was secondary to vascular endothelial damage.
In accordance with the guidelines of the European Society of Cardiology and European Respiratory Society (35), the patients in the present study underwent a thorough clinical assessment. The results showed that patients in the microalbuminuria group were older and had poorer hemodynamic status, lower oxygen saturation, poorer cardiopulmonary function, and higher NYHA functional grade. Patients with pulmonary hypertension who have 700 ng/L > NT-proBNP ≥300 ng/L, 330 m < 6MWD <440 m, and 50 mmHg > PASP ≥40 mmHg can be classified as being at moderate risk. In this study, the microalbuminuria group had an NT-proBNP level of 1,626.66±142.41 ng/L, a 6MWD of 306.76±1.57 m, and a PASP of 67.01±1.35 mmHg, which would place these patients in the intermediate risk category. We speculate that patients with HAPH might begin to develop vascular damage when they reach the intermediate risk stage. Our previous heart rate variability study in the same intermediate-risk patients confirmed that sympathetic nerve activation and vagus nerve suppression caused increases in heart rate and cardiac output that manifested as an overload of left ventricular systolic function and an elevation of renal blood flow, which would promote the loss of albumin into the urine through an increase in glomerular filtration pressure (15). Vascular, neuronal, and endocrine changes occur before HAPH has progressed to right ventricular hypertrophy (36). This stage would be characterized by an increase in pulmonary artery pressure and vascular endothelial damage, which would also promote the excretion of albumin into the urine.
Implications and actions needed
Higher PASP and lower SpO2 were independent risk factors for microalbuminuria in adult Tibetan patients with HAPH. These findings suggested that chronic hypoxia and elevated right heart pressure (secondary to pulmonary hypertension) might promote the development of microalbuminuria in patients with HAPH. Further studies are needed to establish whether microalbuminuria might be a useful biomarker for vascular endothelial dysfunction and HAPH severity in high-altitude residents.
Conclusions
Higher PASP and lower SpO2 were independently associated with microalbuminuria in adult Tibetan patients with HAPH. The closely arterial blood gas analysis and echocardiography is needed and recommended.
Acknowledgments
We want to thank the services of MedSci for help in polishing our paper.
Funding: This work was supported by the project of Natural Science Foundation of Tibet Autonomous Region (No. XZ2019ZRG-121).
Notes
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Helsinki Declaration (as revised in 2013). The study was approved by the Medical Ethics Committee of the People’s Hospital of Tibet Autonomous Region, China (No. ME-TBH-19-01). The requirement for informed consent was waived because of the retrospective study design.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-385/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-385/dss
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-385/coif). The authors have no conflicts of interest to declare.
References
Articles from Cardiovascular Diagnosis and Therapy are provided here courtesy of AME Publications
Full text links
Read article at publisher's site: https://doi.org/10.21037/cdt-22-385
Read article for free, from open access legal sources, via Unpaywall:
https://cdt.amegroups.com/article/viewFile/112205/pdf
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The Prevalence and Risk Factors of High-Altitude Pulmonary Hypertension Among Native Tibetans in Sichuan Province, China.
High Alt Med Biol, 21(4):327-335, 30 Jun 2020
Cited by: 10 articles | PMID: 32614250
Cerebral oxygenation in highlanders with and without high-altitude pulmonary hypertension.
Exp Physiol, 100(8):905-914, 05 Jul 2015
Cited by: 4 articles | PMID: 26011291
Clinical and Predictive Value of Computed Tomography Angiography in High-Altitude Pulmonary Hypertension.
JACC Asia, 2(7):803-815, 13 Dec 2022
Cited by: 2 articles | PMID: 36713752 | PMCID: PMC9877215
High-altitude pulmonary hypertension.
Eur Respir Rev, 18(111):13-17, 01 Mar 2009
Cited by: 39 articles | PMID: 20956117
Review

 #
#
#
#