Abstract
Objective
In November 2022, teplizumab-mzwv became the first drug approved to delay the onset of stage 3 type 1 diabetes in adults and children age ≥8 years with stage 2 type 1 diabetes on the basis of data from the pivotal study TN-10.Research design and methods
To provide confirmatory evidence of the effects of teplizumab on preserving endogenous insulin production, an integrated analysis of C-peptide data from 609 patients (n = 375 patients receiving teplizumab and n = 234 control patients) from five clinical trials in stage 3 type 1 diabetes was conducted.Results
The primary outcome of the integrated analysis, change from baseline in stimulated C-peptide, was significantly improved at years 1 (average increase 0.08 nmol/L; P < 0.0001) and 2 (average increase 0.12 nmol/L; P < 0.0001) after one or two courses of teplizumab. An analysis of exogenous insulin use was also conducted, showing overall reductions of 0.08 (P = 0.0001) and 0.10 units/kg/day (P < 0.0001) at years 1 and 2, respectively. An integrated safety analysis of five clinical trials that enrolled 1,018 patients with stage 2 or 3 type 1 diabetes (∼1,500 patient-years of follow-up for teplizumab-treated patients) was conducted.Conclusions
These data confirm consistency in the preservation of β-cell function, as measured by C-peptide, across multiple clinical trials. This analysis showed that the most common adverse events included lymphopenia, rash, and headache, a majority of which occurred during and after the first few weeks of teplizumab administration and generally resolved without intervention, consistent with a safety profile characterized by self-limited adverse events after one or two courses of teplizumab treatment.Free full text

Teplizumab: A Disease-Modifying Therapy for Type 1 Diabetes That Preserves β-Cell Function
Abstract
OBJECTIVE
In November 2022, teplizumab-mzwv became the first drug approved to delay the onset of stage 3 type 1 diabetes in adults and children age ≥8 years with stage 2 type 1 diabetes on the basis of data from the pivotal study TN-10.
RESEARCH DESIGN AND METHODS
To provide confirmatory evidence of the effects of teplizumab on preserving endogenous insulin production, an integrated analysis of C-peptide data from 609 patients (n = 375 patients receiving teplizumab and n = 234 control patients) from five clinical trials in stage 3 type 1 diabetes was conducted.
RESULTS
The primary outcome of the integrated analysis, change from baseline in stimulated C-peptide, was significantly improved at years 1 (average increase 0.08 nmol/L; P < 0.0001) and 2 (average increase 0.12 nmol/L; P < 0.0001) after one or two courses of teplizumab. An analysis of exogenous insulin use was also conducted, showing overall reductions of 0.08 (P = 0.0001) and 0.10 units/kg/day (P < 0.0001) at years 1 and 2, respectively. An integrated safety analysis of five clinical trials that enrolled 1,018 patients with stage 2 or 3 type 1 diabetes (~1,500 patient-years of follow-up for teplizumab-treated patients) was conducted.
CONCLUSIONS
These data confirm consistency in the preservation of β-cell function, as measured by C-peptide, across multiple clinical trials. This analysis showed that the most common adverse events included lymphopenia, rash, and headache, a majority of which occurred during and after the first few weeks of teplizumab administration and generally resolved without intervention, consistent with a safety profile characterized by self-limited adverse events after one or two courses of teplizumab treatment.
Introduction
In November 2022, teplizumab-mzwv (TZIELD; Provention Bio, Inc., a Sanofi Company, Red Bank, NJ) became the first drug approved to change the progression of autoimmunity in type 1 diabetes (i.e., to delay the onset of stage 3 type 1 diabetes in adults and children age ≥8 years with stage 2 disease). The approval of teplizumab represents the first drug approval for the delay of any autoimmune disease in patients before clinical onset. Decades of studies preceded the approval of teplizumab, beginning with preclinical studies followed by six clinical trials, including five in patients after clinical diagnosis (stage 3) and one in patients before clinical diagnosis (stage 2) who had two or more pancreatic islet autoantibodies and dysglycemia. These studies were used as support in the U.S. Food and Drug Administration Biologics Licenses Application submission and design of the PROTECT (Phase 3 Trial Evaluating Teplizumab in Patients With Recent-Onset Type 1 Diabetes) study (clinical trial reg. no. NCT03875729, ClinicalTrials.gov).
Teplizumab is a humanized immunoglobulin G1 monoclonal antibody that binds with high affinity to the ε chain of CD3. Its complementarity-determining region is derived from ortho kung T3 (OKT3), the first monoclonal antibody licensed for human use for acute solid graft rejection. OKT3 was humanized to minimize immunogenicity. Two Leu→Ala substitutions in the Fc region were introduced to minimize Fc-receptor binding, resulting in 100- to 1,000-fold reduction in T-cell activation, T-cell proliferation, and cytokine release in human peripheral blood mononuclear cell cultures compared with OKT3 (1–5). Early studies of its mechanism showed that partial agonism led to effects on CD8+ T cells integral to autoimmune-mediated destruction of pancreatic β-cells (6–10).
Teplizumab was initially investigated for the treatment of acute transplant rejection and psoriatic arthritis (11–14). Simultaneously, experiments in spontaneous and chemically induced diabetic mouse models provided evidence for reversal or prevention of autoimmune diabetes and immune tolerance, and continuous administration, a requirement for previously studied immune therapies, was not required (15–17).
The first randomized clinical trial in stage 3 type 1 diabetes was a phase 2 clinical study (Study 1) that evaluated the safety, tolerability, and efficacy of teplizumab administered as a single 12- or 14-day course versus standard of care in patients with newly diagnosed stage 3 disease (18). Efficacy was evaluated based on preservation of β-cell function by assessing C-peptide responses to mixed-meal tolerance tests (MMTTs). The success of this trial led to a randomized phase 2 trial (AbATE [Autoimmunity-Blocking Antibody for Tolerance in Recently Diagnosed Type 1 Diabetes]; clinical trial reg. no. NCT00129259), in which a second course of teplizumab was administered after 1 year to prolong duration of response (18–20). Subsequently, two phase 3 trials (Protégé and Encore; clinical trial reg. nos. NCT00385697 and NCT00920582, respectively) tested three dosing regimens of teplizumab, administered over two courses 6 months apart. These trials used a novel and unvalidated composite primary end point of exogenous insulin use and HbA1c. In an interim analysis, the Protégé study did not meet its primary end point, which led to termination and discontinuation of further clinical development (21). At the time these two studies were terminated, another phase 2 trial (Delay; clinical trial reg. no. NCT00378508) evaluated the effect of teplizumab in patients recruited 4 to 12 months after type 1 diabetes diagnosis who had clinically significant levels of stimulated C-peptide (e.g., >0.2 nmol/L) (22). All five clinical studies showed promising C-peptide preservation.
The stage 2 study, TN-10 (clinical trial reg. no. NCT01030861), was sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases and conducted by Type 1 Diabetes TrialNet to evaluate whether a single course of teplizumab would delay or prevent clinical diagnosis of type 1 diabetes in patients with early-stage type 1 diabetes (stage 2) (23). The study enrolled relatives of patients with type 1 diabetes between ages 8 and 49 years who had two or more islet autoantibodies and dysglycemia. Results showed a statistically significant delay in median time to onset of stage 3 clinical type 1 diabetes of ~2 years compared with patients receiving placebo, indicating stabilization of the decline in β-cells, leading to preservation of β-cell function, with an updated analysis reporting a delay in progression extending to 2.7 years (23,24).
Although results of the clinical studies support the preservation of β-cell function as evaluated by C-peptide, a measure of endogenous insulin production, individual studies have had different protocol designs, including heterogeneous eligibility criteria and natures of control groups, which may have affected the overall interpretation of results (25).
Therefore, the primary aim of this integrated analysis was to confirm the consistency of the effects of teplizumab on β-cell function as measured by stimulated C-peptide levels across the five clinical trials in patients with stage 3 clinical type 1 diabetes. A secondary efficacy analysis of exogenous insulin use was conducted. A comprehensive review of the safety experience with teplizumab in patients with stage 2 or 3 type 1 diabetes was also performed.
Research Design and Methods
Clinical Trials
The designs of the six clinical trials have been previously published (Supplementary Table 1) (19,20,22,23). Dosing regimens evolved over time to accommodate children with diabetes based on the safety experience from a 14-day dosing regimen (Study 1: 4 days of escalation, 10 days of full dosing based on body weight) to 12-day (Study 1: 2 days of escalation, 10 days of full dosing based on body surface area [BSA]) to 14-day (AbATE, Delay, Protégé, and Encore: 4 days of escalation, 10 days of full dosing based on BSA). All trial patients received standard-of-care management of type 1 diabetes consistent with current guidelines, with insulin treatment and similar metabolic monitoring and follow-up evaluations. Investigators were advised to treat to target blood glucose based on recommendations from the American Diabetes Association. Specific algorithms for insulin administration were not given.
A majority of patients received the full 14-day regimen via intravenous infusion of teplizumab, including 51 μg/m2 on day 1, 103 μg/m2 on day 2, 206 μg/m2 on day 3, 413 μg/m2 on day 4, and 826 μg/m2 on days 5 to 14, for a cumulative dose of 9,034 μg/m2. Patients were randomly assigned to receive two treatment courses in Protégé, Encore (at randomization and month 6), and AbATE (at randomization and month 12) and a single course of treatment in Study 1 and Delay, with the option of a second course (open label) in the Delay study. The Protégé and Encore studies also investigated lower cumulative dose regimens (6-day [2,426 μg/m2] or one-third dose 14-day regimen [2,985 μg/m2]). Lower dosing regimens in Protégé and the second course in Delay were not included in the efficacy analysis but were included in the safety analysis.
Efficacy Analyses
Analysis of C-Peptide Responses
All five studies included in the integrated efficacy analyses were conducted in patients with stage 3 type 1 diabetes who received the full 14-day regimen and had a randomized, placebo, or standard-of-care fixed–time frame design (Supplementary Table 1). Patients in these studies were enrolled within 6, 8, 12, and 12 weeks of type 1 diabetes diagnosis in Study 1, AbATE, Protégé, and Encore, respectively, or 4 to 12 months from type 1 diabetes diagnosis in Delay. All patients had detectable levels of stimulated C-peptide. Study 1 and AbATE were open-label studies with random assignment to study drug or standard of care, whereas Delay, Protégé, and Encore were double-blind randomized placebo-controlled trials. Study 1, AbATE, and Delay were conducted by academic investigators at sites in the U.S., whereas Protégé and Encore were conducted by MacroGenics at sites located in the U.S., Eastern Europe, and India.
A one-stage integrated intention-to-treat (ITT) analysis was conducted using individual patient data from all five studies that included 1-year follow-up and three studies (Study 1, AbATE, and Protégé) that included 2-year follow-up. The ITT population included all patients who had been randomly assigned to the 12- and 14-day teplizumab regimens in Study 1 and the full 14-day teplizumab regimen in AbATE, Protégé, Encore, and Delay, as well as patients randomly assigned to standard of care or placebo (i.e., control group) in all five studies (Supplementary Table 2).
The primary end point was the change from baseline in C-peptide area under the curve (AUC). Baseline was defined as the C-peptide measurement obtained before the initial dose of teplizumab. The AUC in the 4-h MMTT was divided by 240 or the last measured time for C-peptide and transformed (ln[AUC + 1]). C-peptide was measured by radioimmunoassay (Study 1), Tosoh Bioscience assay (AbATE and Delay), and chemiluminescent assay on an Immulite 2000 (Protégé and Encore).
Two analyses were performed on both observed data and imputed missing data via multiple imputation methods under “missing at random” and “missing not at random” assumptions. Change from baseline in C-peptide was analyzed using ANCOVA. ANCOVA included treatment as a main effect, with age, baseline C-peptide, study, and study-by-treatment interaction as covariates. The study-by-treatment interaction term was included to assess whether there were differences in effect depending on individual study features and adjust for these differences. Least squares means (LSMs) of the change from baseline in C-peptide AUC with different treatments along with 95% CIs were estimated using results from ANCOVA. The number of treatment courses and their timings were determined by the study and therefore were not included as separate covariates in ANCOVA. Parameter estimates and 95% CIs were generated for imputed data sets by averaging estimated values for the 100 imputations conducted. All parameter estimates of differences in treatment LSMs and respective 95% CIs are presented in Forest plots.
Analysis of Exogenous Insulin Use
A second integrated analysis evaluated exogenous daily insulin use (units/kg/day) in the same studies. Average total daily insulin dose (all insulins) was used for this analysis. Insulin use data from individual patients were included from all five studies with 1 year of follow-up and from Study 1, AbATE, and Protégé where 2-year data were available. ANCOVA was used to assess treatment differences in insulin use with covariates including baseline insulin use, treatment, age, study, and study-by-treatment interaction. Analyses were performed on both observed and imputed data.
Safety Analysis
All five studies included in the integrated safety analysis were conducted in stage 2 (TN-10) or stage 3 (AbATE, Protégé, Encore, and Delay) clinical trials using BSA-based dosing regimens (Supplementary Tables 1 and 3). Because of the inclusion of weight-based dosing in Study 1, safety data from this study were not included. Safety assessments included analyses of adverse events (AEs), mechanism-related AEs that were considered AEs of special interest, and serious AEs (SAEs). The safety population comprised all patients in the three dosing regimens (full 14-day, 6-day, and one-third dose 14-day regimens) who received at least one dose of teplizumab or who were randomly assigned to placebo or standard of care (i.e., control group). Data are described using descriptive statistics. All analyses were performed using SAS software (version 9.4). Additional methods used for imputation are provided in the Supplementary Material.
Results
Efficacy Analyses
Analysis of C-Peptide Responses
A total of 609 patients from five clinical trials were included in the ITT population for the efficacy analysis, 375 in the teplizumab group and 234 in the control group. In the teplizumab group, 88% of patients completed their respective study. Three (0.8%) patients in the teplizumab group withdrew early because of an AE, and 17 (4.5%) were lost to follow-up. No participants in the control group withdrew early because of an AE, and six (2.6%) were lost to follow-up.
Baseline demographic and clinical characteristics were similar between treatment groups, but there were a number of differences between study cohorts (Supplementary Table 2). Protégé and Encore patients were older and had a longer time from diagnosis than patients in Study 1 and AbATE. Patients in Delay, by design, had a duration of 4 to 12 months since diagnosis.
Forest plots of LSM differences and corresponding 95% CIs for changes from baseline in C-peptide from the integrated analysis of the five studies are presented in Fig. 1A. These data show a highly statistically significant (P < 0.0001) preservation of C-peptide with teplizumab treatment compared with placebo or standard of care for both the 1- and 2-year data (observed and imputed). For observed data, the difference (95% CI) in treatment effect between the teplizumab and control groups was 0.08 nmol/L (0.04, 0.12) at year 1 and 0.12 nmol/L (0.07, 0.17) at year 2. For imputed data, the difference (95% CI) in treatment effect between teplizumab and control groups was 0.09 nmol/L (0.05, 0.13) at year 1 and 0.10 nmol/L (0.05, 0.16) at year 2.

Forest plot of LSM differences in the change from baseline in C-peptide AUC between teplizumab and control groups. A: Analysis includes the five clinical trials with 1-year data. Analysis is with and without the imputed data for missing values. B: Analysis includes the three clinical trials for which 1- and 2-year data were available. Analysis is with and without the imputed data for missing values.
Analyses by individual study and year are shown in Fig. 1B. There was a significant improvement in C-peptide in each of the studies except Encore, where there was a large amount of missing data as a result of the early termination. In each of the three studies with available 2-year data, teplizumab treatment significantly preserved C-peptide levels compared with placebo.
C-peptide plots for 1- and 2-year data are shown in Fig. 2A and B, respectively. C-peptide AUC levels at the 6-month time point showed an increase in the teplizumab group (an average increase of 5% and 8% in 1- and 2-year data, respectively), whereas they decreased in the control group. C-peptide AUC decline from baseline at 1 year was 8% and 27% in the teplizumab and control groups, respectively (P < 0.0001). For studies with 2-year data, the decline from baseline levels was 34% and 51% in the teplizumab and control groups, respectively (P < 0.0001).

Mean (SE) observed C-peptide and insulin use from the clinical trials. A: C-peptide AUC levels at 1 year from the five studies with 1-year data. P value at 1 year was derived from the ANCOVA model for the 1-year integrated observed data. B: C-peptide AUC levels at 2 years from the three studies with 2-year data. P value at 2 years was derived from ANCOVA for the observed 2-year integrated data. C: Average insulin use at 1 year. P value at 1 year was derived from ANCOVA for the observed 1-year integrated data. D: Average insulin use at 2 years. P value at 2 years was derived from ANCOVA for the observed 2-year integrated data.
Integrated Analysis of Exogenous Insulin Use
Each clinical trial used a treat-to-target approach to diabetes management, and therefore, any effect of teplizumab treatment on endogenous β-cell function would be reflected by changes in requirements for exogenous insulin.
Forest plots of the integrated analysis of insulin use showed statistically significantly lower insulin use at 1 (−0.08 units/kg/day; 95% CI −0.12, −0.05; P < 0.0001) and 2 years (−0.10 units/kg/day; −0.15, −0.05; P = 0.0001) for teplizumab-treated patients compared with those in control groups (Fig. 3A and B, respectively). In individual studies, Study 1 and AbATE showed statistically significantly lower insulin use for teplizumab-treated patients compared with control patients (P < 0.0001 at 1 and 2 years for both studies). However, point estimates across all studies favored lower insulin use in teplizumab-treated patients.
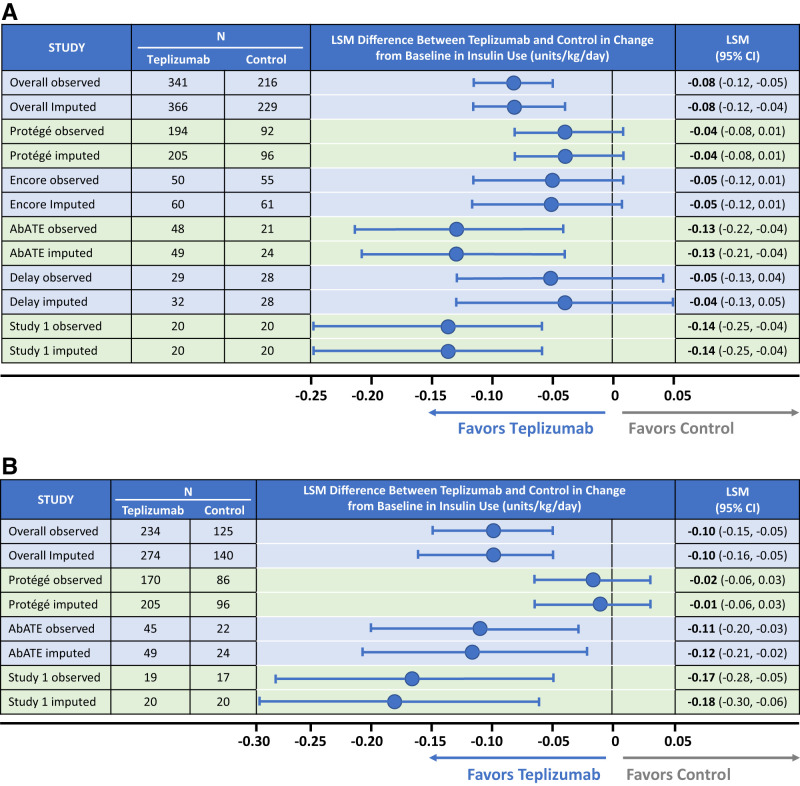
Forest plot of LSM differences in the change from baseline in insulin use between teplizumab and control groups. A: Analysis includes the five clinical trials with 1-year data. Analysis is with and without the imputed data for missing values. B: Analysis includes the three clinical trials for which 1- and 2-year data were available. Analysis is with and without the imputed data for missing values.
Insulin use over time is presented graphically in Fig. 2C and D. Baseline levels were similar in the two groups. Significantly lower insulin use was observed at 1 year (0.58 vs. 0.65 units/kg/day; P < 0.0001) and for the three studies (AbATE, Protégé, and Encore) with 2-year data (0.66 vs. 0.74 units/kg/day; P = 0.0001). Insulin discontinuation data are available only for Protégé, in which patients were not to discontinue insulin unless instructed to do so by the investigator. Despite being blinded to treatment, at 1 year, 5.3% (11 of 207) of patients in the full-dose 14-day teplizumab group were not taking insulin compared with 0% (zero of 98) of control patients (P = 0.02). At year 2, three of these 11 patients remained off insulin, whereas all control patients were still taking insulin (P < 0.05).
Safety Analysis
Integrated Analysis of Safety
A total of 1,018 patients with stage 2 or 3 type 1 diabetes were included in the integrated safety analysis, 773 randomly assigned to teplizumab treatment and 245 randomly assigned to control (Supplementary Table 3). There were ~1,500 patient-years of follow-up for patients treated with teplizumab. Baseline demographic and clinical characteristics of this expanded group were similar between treatment groups.
A summary of AEs is shown in Supplementary Table 4. Almost all patients in either treatment group experienced an AE, most of which were grade 1 or 2 in severity and typically occurred during or immediately after the dosing period. A majority of AEs resolved without intervention. AEs leading to permanent study drug discontinuation were reported in 14.3% and 3.7% of patients in the teplizumab and control groups, respectively. The most common reasons for discontinuation in the teplizumab group were laboratory investigations (7.7%; mainly liver enzyme elevations that met protocol-defined threshold for discontinuation) and blood and lymphatic disorders (3.2%; including neutropenia, thrombocytopenia, lymphopenia, and anemia).
SAEs were reported in 12.4% and 8.2% of patients in the teplizumab and control groups, respectively, but most (76.7%) were considered not related to treatment. The most common SAEs were related to the underlying type 1 diabetes pathophysiology, with the most notable being diabetic ketoacidosis (teplizumab n = 18 [2.3%]; control n = 1 [0.4%]) and ketoacidosis (teplizumab n = 1 [0.1%]; control n = 0). These events were reported at an average of 10 months after the end of teplizumab treatment, with a mean HbA1c level of 11.9% near the time of the event, suggesting underlying poor glycemic control. Hypoglycemic SAEs were observed only in patients with stage 3 disease, and none occurred in those with stage 2 disease. The rates of hypoglycemic unconsciousness and hypoglycemic coma in the two groups were comparable.
The most common AEs reported in ≥10% of patients in either group are shown in Table 1. AEs reported at a higher frequency in the teplizumab group versus the control group were lymphopenia, leukopenia, neutropenia, decreased blood bicarbonate, and rash, all of which occurred during the dosing period and typically resolved within 4 weeks after the initiation of dosing. Lymphopenia was observed in ~80% of teplizumab-treated patients, with the nadir on day 5 of treatment, and was resolved while dosing was continued, consistent with a margination of lymphocytes and not a depletion of the cells.
Table 1
AEs occurring in >10% of patients
| System organ class: preferred term | Teplizumab (n = 791) | Control (n = 245) |
|---|---|---|
| Patients with at least one AE | 787 (99.5) | 233 (95.1) |
| Lymphopenia | 632 (79.9) | 41 (16.7) |
| Leukopenia | 501 (63.3) | 65 (26.5) |
| Neutropenia | 313 (39.6) | 53 (21.6) |
| Blood bicarbonate decreased | 303 (38.3) | 67 (27.3) |
| Rash | 273 (34.5) | 25 (10.2) |
| Hemoglobin decreased | 228 (28.8) | 55 (22.4) |
| AST increased | 222 (28.1) | 50 (20.4) |
| Headache | 215 (27.2) | 61 (24.9) |
| ALT increased | 210 (26.5) | 28 (11.4) |
| Pyrexia | 188 (23.8) | 41 (16.7) |
| Hyponatremia | 170 (21.5) | 49 (20.0) |
| Thrombocytopenia | 172 (21.7) | 24 (9.8) |
| Nausea | 155 (19.6) | 34 (13.9) |
| Upper respiratory tract infection | 150 (19.0) | 43 (17.6) |
| Hypocalcemia | 137 (17.3) | 36 (14.7) |
| Blood sodium decreased | 129 (16.3) | 36 (14.7) |
| Pruritus | 118 (14.9) | 14 (5.7) |
| Vomiting | 110 (13.9) | 25 (10.2) |
| Blood alkaline phosphatase increased | 107 (13.5) | 34 (13.9) |
| Blood calcium decreased | 100 (12.6) | 20 (8.2) |
| Nasopharyngitis | 88 (11.1) | 23 (9.4) |
| Fatigue | 80 (10.1) | 14 (5.7) |
Data are presented as n (%).
Cytokine release syndrome (CRS) was reported in 5.8% (46 of 791) and 1.2% (three of 245) of teplizumab-treated and control patients, respectively. CRS typically occurred during the first 3 to 5 days of dosing and resolved within 2 to 3 days of onset. Most (88%) events were grade 1 or 2 in severity and were treated with nonprescription medications.
Infection rates were similar between teplizumab-treated (53.0%) and control patients (52.7%), with serious infections occurring in 3.5% and 2%, respectively. New Epstein-Barr virus (EBV) infections were reported in 18 (2.3%) and 10 (4.1%) teplizumab-treated and control patients, respectively. EBV reactivation was reported in 40 (5.1%) teplizumab-treated and six (2.4%) control patients. EBV viremia was detected in 25 (3.2%) and three (1.2%) teplizumab-treated and control patients, respectively, 2 to 3 weeks after dosing; it was generally asymptomatic and resolved spontaneously without antiviral medications. New cytomegalovirus infection was detected in five (0.6%) patients treated with teplizumab and two (0.8%) in the control group. Reactivation of cytomegalovirus occurred in four (0.5%) teplizumab-treated patients; none of these cases were associated with symptoms, and all cases resolved spontaneously without antiviral treatment.
Hypersensitivity reactions, including anaphylaxis (0.1%), angioedema (0.3%), peripheral edema (1.6%), and urticaria (1.9%), were reported in teplizumab-treated patients; rash was observed in 48% and 15% of teplizumab-treated and control patients, respectively.
Conclusions
For the first time, a disease-modifying therapy, teplizumab-mzwv, has been approved to delay the onset of clinical stage 3 type 1 diabetes. Teplizumab is thought to modify disease progression from stage 2 to 3 type 1 diabetes by preserving β-cell function. Over the last 20 years, five clinical trials of teplizumab in patients with stage 3 type 1 diabetes have demonstrated the preservation of β-cell function, as measured by C-peptide levels. To confirm the consistency of β-cell preservation, an integrated analysis of C-peptide data from five clinical trials was conducted and supported by an analysis of exogenous insulin use. The comprehensive safety experience was reviewed by integrating data from 791 teplizumab-treated patients representing ~1,500 patient-years of follow-up (18–23). This represents the most extensive data in type 1 diabetes disease modification with a single agent to date.
The integrated efficacy analysis showed C-peptide levels were preserved to a significantly greater extent in patients treated with teplizumab than in control patients at 1 and 2 years posttreatment. Higher C-peptide levels, a measure of endogenous insulin production, were supported by reduced use of exogenous insulin.
Notably, patients were heterogeneous in terms of age (a possible determinant of the rate of decline in C-peptide), location (North America, India, and Eastern Europe), clinical practices, and other patient characteristics such as pubertal status that may affect insulin sensitivity (26). It is also likely that there were biologic differences in the autoimmune processes of patients, because some patients were treated shortly after diagnosis, whereas others were treated up to 1 year after diagnosis. Furthermore, different assays for C-peptide measurement were used. However, the same protocol for MMTTs was followed in all studies, and the analyses involved changes in β-cell function from baseline. An exploratory analysis of data from Protégé showed that the reduction in the decline in C-peptide response with teplizumab was greater in magnitude in patients diagnosed <6 weeks before random assignment and in patients from the U.S., whose metabolic parameters at baseline suggested less advanced disease, than in those from other regions (21). In Delay, which investigated teplizumab in patients diagnosed with stage 3 type 1 diabetes within the previous 4 to 12 months, time from diagnosis did not significantly modify the treatment effect on C-peptide response (22). However, the magnitude of treatment effect between teplizumab-treated and control patients on C-peptide response was lower in that study than in studies in which patients were treated with teplizumab within weeks of diagnosis. Therefore, treatment soon after or even before the diagnosis of clinical disease may be required for optimal responses to the drug. In Protégé and Delay, teplizumab was effective across all age groups, but younger patients treated with teplizumab had better C-peptide response than older patients. Data from these trials were used in the design of the PROTECT study (27), which is evaluating teplizumab in patients age 8 to 17 years with newly diagnosed stage 3 type 1 diabetes who have evidence of residual β-cell function. The results of the integrated analysis illustrate a significant difference in C-peptide preservation across multiple studies, supporting the consistency of the effects of teplizumab treatment across and within studies, suggesting that its biologic effects are robust, reproducible, and not unique to a specific study or patient characteristic.
The approved label for TZIELD includes warnings and precautions for CRS, serious infections, lymphopenia, hypersensitivity reactions, and vaccinations. The safety profile of teplizumab was characterized by mild to moderate AEs that were self-limited. CRS was observed in 5.8% of patients and is likely attributable to the partial agonistic effect of teplizumab (1,28). Higher rates of serious infections (teplizumab 3.5% vs. control 2%) were observed, although overall rates of infections were similar between treatment groups. A majority of teplizumab-treated patients developed lymphopenia (~80%), which resolved even while dosing continued and without treatment interruption (29,30). Importantly, the development of transient lymphopenia did not seem to be associated with an overall increased risk of infection. Although not included in the integrated analysis, 7-year follow-up of patients in the AbATE study showed no increased risk of infections and no malignancies (31). Previous preclinical studies and analyses of cells from teplizumab-treated patients have suggested that this partial agonist signal, which leads to changes in the composition of differentiation and activation within circulating T cells (7), may lead to partial exhaustion of CD8+ T cells, which has been associated with reduced cytokine production after activation (24). Indeed, investigations of patients from the AbATE and TN-10 studies showed that the CD8+TIGIT+KLRG1+ memory T cells from teplizumab-treated patients showed signs of exhaustion and produced lower levels of the inflammatory cytokines tumor necrosis factor-α and interferon-γ, which are associated with T cell–mediated β-cell death (24). Because teplizumab targets activated effector cells and spares regulatory T cells and memory T cells against previously encountered pathogens, the preservation of β-cells is achieved without a long-term impact on immune competence (31).
The integrated C-peptide analysis was unable to address whether the benefits of a second course of teplizumab are greater than those of a single course, so future trials may be helpful to address this limitation. The treat-to-target blood glucose study designs limited the ability to evaluate the effects of treatment on HbA1c levels. Nonetheless, in Study 1 and AbATE, improvement in HbA1c levels at some time points was observed.
In conclusion, the integrated efficacy analyses provide robust evidence for the statistically significant preservation of β-cell function in patients with stage 3 type 1 diabetes, which was associated with the clinical benefit of reduced exogenous insulin requirement. These data provide the biologic foundation of a disease-modifying therapy for type 1 diabetes and further support teplizumab as a promising treatment that addresses the underlying autoimmune pathogenesis of type 1 diabetes. The recent approval of teplizumab for delay in the onset of stage 3 type 1 diabetes in adults and children age ≥8 years with stage 2 type 1 diabetes, built on the data presented herein, represents the first advance toward achieving the goal of developing disease-modifying therapies.
Article Information
Duality of Interest. This analysis was funded by Provention Bio, Inc., a Sanofi company (Red Bank, NJ). Editorial support was provided by ClinicalMind, LLC (New York, NY), and PharmaWrite, LLC (Princeton, NJ), and was funded by Provention Bio, Inc. K.C.H. has served as a consultant to Provention Bio, Inc., and is an inventor on a patent for the use of teplizumab for the delay of stage 3 type 1 diabetes but has declined royalties. S.E.G. has been a paid investigator for clinical trials sponsored by Provention Bio, Inc. P.A.G. has received funding from and served as a consultant to Provention Bio, Inc. No authors have been financially compensated for this manuscript. L.A.K., R.R., and E.L.R. are employees of Provention Bio, Inc., a Sanofi Company. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. K.C.H., S.E.G., and P.A.G. were responsible for the study concept and design and provided study materials or patients. K.C.H., S.E.G., P.A.G., L.A.K., R.R., and E.L.R. collected, analyzed, and interpreted the data; wrote the manuscript; were involved in critically revising the manuscript; and reviewed the final manuscript and gave approval for submission. K.C.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.23802516.
This article is featured in a podcast available at diabetesjournals.org/journals/pages/diabetes-core-update-podcasts.
References
Articles from Diabetes Care are provided here courtesy of American Diabetes Association
Full text links
Read article at publisher's site: https://doi.org/10.2337/dc23-0675
Read article for free, from open access legal sources, via Unpaywall:
https://diabetesjournals.figshare.com/articles/figure/_i_b_Teplizumab_b_i_i_b_A_Disease-Modifying_Therapy_for_Type_1_Diabetes_That_b_i_i_b_Preserves_Beta_Cell_Function_b_i_/23802516/1/files/41748990.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/153231950
Article citations
Therapy concepts in type 1 diabetes mellitus treatment: disease modifying versus curative approaches.
J Mol Med (Berl), 18 Oct 2024
Cited by: 0 articles | PMID: 39420138
Review
Evolving Concepts in Pathophysiology, Screening, and Prevention of Type 1 Diabetes: Report of Diabetes Mellitus Interagency Coordinating Committee Workshop.
Diabetes, 73(11):1780-1790, 01 Nov 2024
Cited by: 0 articles | PMID: 39167668
Review
TGF-β-mediated crosstalk between TIGIT<sup>+</sup> Tregs and CD226<sup>+</sup>CD8<sup>+</sup> T cells in the progression and remission of type 1 diabetes.
Nat Commun, 15(1):8894, 15 Oct 2024
Cited by: 0 articles | PMID: 39406740 | PMCID: PMC11480485
The Type 1 Diabetes T Cell Receptor and B Cell Receptor Repository in the AIRR Data Commons: a practical guide for access, use and contributions through the Type 1 Diabetes AIRR Consortium.
Diabetologia, 29 Oct 2024
Cited by: 0 articles | PMID: 39467874
Comment on the role of interferons in the pathology of beta cell destruction in type 1 diabetes.
Diabetologia, 67(11):2598-2599, 07 Sep 2024
Cited by: 0 articles | PMID: 39243307 | PMCID: PMC11519303
Go to all (18) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (Showing 6 of 6)
- (1 citation) ClinicalTrials.gov - NCT01030861
- (1 citation) ClinicalTrials.gov - NCT00385697
- (1 citation) ClinicalTrials.gov - NCT00129259
- (1 citation) ClinicalTrials.gov - NCT03875729
- (1 citation) ClinicalTrials.gov - NCT00920582
- (1 citation) ClinicalTrials.gov - NCT00378508
Show less
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Teplizumab and β-Cell Function in Newly Diagnosed Type 1 Diabetes.
N Engl J Med, 389(23):2151-2161, 18 Oct 2023
Cited by: 24 articles | PMID: 37861217
Role of Teplizumab, a Humanized Anti-CD3 Monoclonal Antibody, in Managing Newly Diagnosed Type 1 Diabetes: An Updated Systematic Review and Meta-Analysis.
Endocr Pract, 30(5):431-440, 20 Mar 2024
Cited by: 2 articles | PMID: 38519028
Review
Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial.
Lancet, 378(9790):487-497, 28 Jun 2011
Cited by: 291 articles | PMID: 21719095 | PMCID: PMC3191495
Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders.
Diabetes, 62(11):3766-3774, 08 Jul 2013
Cited by: 220 articles | PMID: 23835333 | PMCID: PMC3806618
Funding
Funders who supported this work.
NCATS NIH HHS (1)
Grant ID: UL1 TR001863

 4
4




