Abstract
Free full text

Cardioprotective effects of early versus late initiated antiretroviral treatment in adolescents with perinatal HIV-1 infection
Abstract
Whether, and how, cardioprotective effects of antiretroviral treatment (ART) in adolescents with perinatal HIV infection (APHIV) vary with age at treatment initiation is unknown. We used magnetic resonance imaging to compare cardiac status between APHIV initiated on ART at <
< 5 years of age (early ART, n
5 years of age (early ART, n =
= 37) and
37) and ≥
≥ 5 years of age (delayed ART, n
5 years of age (delayed ART, n =
= 34) versus HIV-uninfected peers (n
34) versus HIV-uninfected peers (n =
= 21), reporting z-score mean differences adjusted for confounders. Relative to HIV-uninfected adolescents, APHIV with early ART had higher left ventricular (LV) global circumferential strain (GCS) [adjusted mean (95%CI) z-score: 0.53 (0.13, 0.92)] and maximum indexed left atrium volume (LAVi) [adjusted z-score: 0.55 (0.08, 1.02)]. In contrast, APHIV with delayed ART had greater indexed LV end-diastolic volume (LVEDVi) [adjusted z-score: 0.47 (0.09, 0.86)] and extracellular volume fraction [adjusted z-score: 0.79 (0.20, 1.37)], but lower GCS [adjusted z-score: −0.51 (−0.91, −0.10)] than HIV-uninfected peers. APHIV had distinct albeit subclinical cardiac phenotypes depending on ART initiation age. Changes in early ART suggested comparatively worse diastology with preserved systolic function while delayed ART was associated with comparatively increased diffuse fibrosis and LV dilatation with reduced systolic function. The long-term clinical significance of these changes remains to be determined.
21), reporting z-score mean differences adjusted for confounders. Relative to HIV-uninfected adolescents, APHIV with early ART had higher left ventricular (LV) global circumferential strain (GCS) [adjusted mean (95%CI) z-score: 0.53 (0.13, 0.92)] and maximum indexed left atrium volume (LAVi) [adjusted z-score: 0.55 (0.08, 1.02)]. In contrast, APHIV with delayed ART had greater indexed LV end-diastolic volume (LVEDVi) [adjusted z-score: 0.47 (0.09, 0.86)] and extracellular volume fraction [adjusted z-score: 0.79 (0.20, 1.37)], but lower GCS [adjusted z-score: −0.51 (−0.91, −0.10)] than HIV-uninfected peers. APHIV had distinct albeit subclinical cardiac phenotypes depending on ART initiation age. Changes in early ART suggested comparatively worse diastology with preserved systolic function while delayed ART was associated with comparatively increased diffuse fibrosis and LV dilatation with reduced systolic function. The long-term clinical significance of these changes remains to be determined.
Introduction
Cardiovascular morbidity and mortality were common among adolescents with perinatal HIV infection (APHIV) prior to wide availability of antiretroviral therapy (ART)1,2. Successful treatment of HIV infection has been associated with significant improvements in cardiovascular status of these young people, especially those in high-income countries (HICs), confirming the cardioprotective effects of ART3. In contrast, subclinical cardiac abnormalities remain prevalent in their peers in sub-Saharan Africa (SSA)4–6 where 90% of global pediatric HIV patients reside7. Here, pediatric ART coverage is neither universal (54% in 2020)8 nor is it started early (median age at initiation 7.9 years (2018))7,8 before substantial immunosuppression and cardiac damage set in3. The concern is that these cardiac abnormalities in early life will likely be a substrate for symptomatic cardiovascular dysfunction in adulthood, particularly as maturing APHIV are cumulatively exposed to traditional cardiovascular risk factors9. Compounding this is the reality that perinatal HIV transmission in SSA is not expected to be eliminated before 203010 meaning that even modestly increased relative risks of cardiovascular disease will multiply into a large absolute case burden as more APHIV survive into adulthood.
However, our knowledge of the phenotypes and epidemiology of these perinatal HIV infection (PHIV) related cardiac forms remains incomplete, especially regarding the timing of ART. This, in turn, curtails our capacity to intervene preventively, let alone selectively. One major drawback to cardiovascular research in APHIV in SSA has been its almost exclusive reliance on echocardiography4–6 notwithstanding the modality’s limitations11. Cardiovascular magnetic resonance (CMR), with its multi-parametric capabilities, is the gold standard for cardiac volumetric and functional assessment11. Because of its capabilities to characterize myocardial tissue, CMR has been likened to a “virtual biopsy”12 and is thus well suited to the challenge at hand. Unfortunately, CMR is inaccessible in the typical adolescent HIV care setting in SSA13. In response to these gaps, we conducted a CMR study to comprehensively characterize residual cardiac abnormalities in ART-experienced APHIV in Cape Town, South Africa. Our specific objective was to identify and quantify domain specific, i.e., geometry, systolic and diastolic function, mechanical deformation, and tissue composition, cardio-protective effects of early versus delayed ART in a high PHIV burden African setting.
Results
Characteristics of study sample
We enrolled 103 [mean (SD) age 15.1 (1.7) years; male sex 49 (48%)] adolescents to undergo CMR and blood collection from the 422 CTAAC participants [335 (79%) PHIV] in active follow-up at visit 9. Of these, 37 were HIV uninfected, 45 were APHIV with early ART initiation and 21 APHIV with delayed ART initiation (Table (Table1).1). Both absolute (Table (Table1)1) and age-standardized (Fig. 1A–C), weight, height and BMI were largely comparable across the three groups. However, stunting was more common among APHIV with delayed (24%) and early ART (18%) than among HIV uninfected (8%) counterparts. Overweight/obesity was, on the other hand, more frequently observed among HIV uninfected participants (38%) than among APHIV with either early (16%) or delayed (11%) ART initiation (Table (Table1).1). Other than systolic BP, which was higher among HIV uninfected participants [median (IQR): 108 (103, 113) mmHg] than APHIV with delayed ART [101 (98, 110) mmHg; p =
= 0.040], there were no significant group differences either diastolic BP or MAP (Fig. 1D–F).
0.040], there were no significant group differences either diastolic BP or MAP (Fig. 1D–F).
Table 1
Participants' baseline characteristics according to perinatal HIV infection and antiretroviral treatment status.
| Characteristic | PHIV and ART Status | |||
|---|---|---|---|---|
| HIV uninfected | APHIV | |||
| Early ART initiation | Delayed ART initiation | Total | ||
| Number | 37 | 45 | 21 | 66 |
| Sociodemographic | ||||
| Mean age (years) | 15.0 (1.8) | 15.0 (14.0, 16.0) | 15.3 (1.6) | 15.3 (1.6) |
| Male sex | 15 (41%) | 23 (51%) | 11 (52%) | 34 (52%) |
| Anthropometrica | ||||
| BSA(m2) | 1.6 (0.2) | 1.5 (0.2) | 1.5 (0.2) | 1.5 (0.2) |
| Height (cm) | 159 (8) | 157 (9) | 159 (10) | 158 (9) |
| Weight (kg) | 60.7 (17.2) | 50.8 (12.9) | 55.1 (15.0) | 52.2 (13.6) |
| BMI (kg/m2) | 23.9 (6.6) | 20.6 (4.6) | 21.8 (5.7) | 20.9 (5.0) |
| Stunted | 3 (8%) | 8 (18%) | 5 (24%) | 13 (20%) |
| Underweight | 1 (3%) | 5 (11%) | 3 (14%) | 8 (12%) |
| Wasted | 0 (0%) | 3 (7%) | 0 (0%) | 3 (5%) |
| Overweight/obese | 14 (38%) | 7 (16%) | 2 (11%) | 9 (15%) |
Values are reported as mean (SD) or number (%).
aStunted =
= height-for-age z score
height-for-age z score <
< −2; underweight
−2; underweight =
= weight-for-age z score
weight-for-age z score <
< −2; wasted
−2; wasted =
= BMI-for-age z score
BMI-for-age z score <
< −2; overweight/obese
−2; overweight/obese =
= BMI-for-age z score
BMI-for-age z score >
> 1.
1.
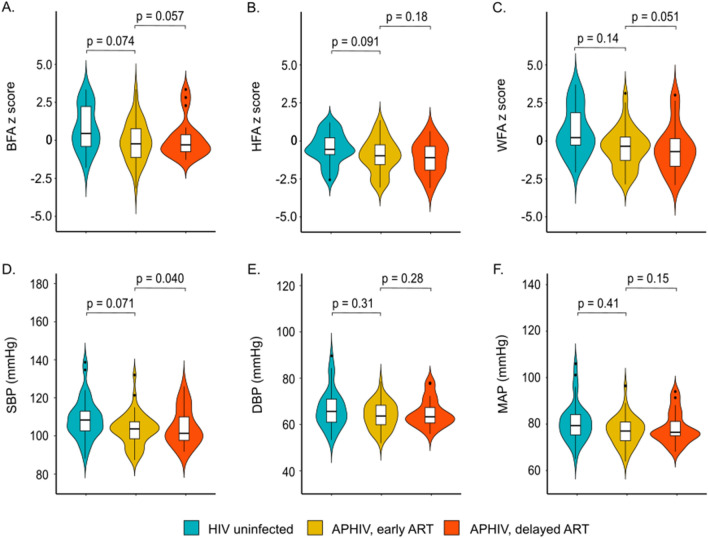
Distribution of (A–C) age-standardized anthropometric measures and (D–F) blood pressure according to perinatal HIV infection and antiretroviral treatment status. P-values are estimated from unadjusted median quintile regression models referenced to HIV uninfected group. BFA
 BMI-for-age, HFA
BMI-for-age, HFA height-for-age, WFA
height-for-age, WFA weight-for-age, MAP
weight-for-age, MAP mean arterial blood pressure, SBP
mean arterial blood pressure, SBP systolic blood pressure, DBP
systolic blood pressure, DBP
 diastolic blood pressure.
diastolic blood pressure.
HIV-related characteristics
Median (IQR) age at ART initiation was 2.4 (0.9, 3.4) years for APHIV with early ART versus 7.2 (6.7, 8.2) years (p <
< 0.001) for those with delayed ART (Table (Table2).2). Although treatment with PIs (yes/no) was equally frequent in early (51%) and delayed ART (48%; p
0.001) for those with delayed ART (Table (Table2).2). Although treatment with PIs (yes/no) was equally frequent in early (51%) and delayed ART (48%; p =
= 0.74) APHIV at time of CMR, those with early ART had considerably longer cumulative PI exposure [5.1 (0.0, 10.3) years] than those with delayed ART [0.1 (0.0, 4.9) years; p
0.74) APHIV at time of CMR, those with early ART had considerably longer cumulative PI exposure [5.1 (0.0, 10.3) years] than those with delayed ART [0.1 (0.0, 4.9) years; p <
< 0.001]. There were no significant group differences with respect to current ART regimen, which included a backbone of two nucleoside reverse transcriptase inhibitors (NRTIs) plus either one PI or one NNRTI agent. However, compared to early ART initiation, delayed ART was associated with a greater degree of immunosuppression [nadir CD4+
0.001]. There were no significant group differences with respect to current ART regimen, which included a backbone of two nucleoside reverse transcriptase inhibitors (NRTIs) plus either one PI or one NNRTI agent. However, compared to early ART initiation, delayed ART was associated with a greater degree of immunosuppression [nadir CD4+ cell count: 457 (368, 720) vs. 547 (422, 835) cells/mL; p
cell count: 457 (368, 720) vs. 547 (422, 835) cells/mL; p =
= 0.025], and a lower degree of immune reconstitution [current CD4+
0.025], and a lower degree of immune reconstitution [current CD4+ count: 592 (402, 795) vs. 753 (558, 928) cells/mL; p
count: 592 (402, 795) vs. 753 (558, 928) cells/mL; p =
= 0.011].
0.011].
Table 2
HIV disease markers stratified by antiretroviral treatment status among participants with perinatal HIV infection.
| Characteristic | APHIV with | P value | |
|---|---|---|---|
| Early ART initiation | Delayed ART initiation | ||
| Number | 45 | 21 | |
| Age at ART initiation (years) | 2.4 (0.9, 3.4) | 7.2 (6.7, 8.2) |  < < 0.001 0.001 |
| Overall ART duration (years) | 12.8 (11.3, 13.8) | 8.1 (7.8, 9.7) |  < < 0.001 0.001 |
| Cumulative NRTI exposure (years) | 12.8 (11.3, 13.8) | 8.1 (7.8, 9.7) |  < < 0.001 0.001 |
| Cumulative PI exposure (years) | 5.1 (0.0, 10.3) | 0.1 (0.0, 4.9) | 0.015 |
| Cumulative NNRTI exposure (years) | 7.0 (3.4, 10.6) | 6.4 (3.7, 8.1) | 0.49 |
| Cumulative INSTRI exposure (years)a | 0 | 0 | 0.92 |
| Current ART regimen | |||
2 different NRTIs + + 1 NNRTI 1 NNRTI | 21 (47%) | 11 (52%) | 0.74 |
2 different NRTIs + + 1 PI 1 PI | 23 (51%) | 10 (48%) | |
| Otherb | 1 (2%) | 0 (0%) | |
| Undetectable HIV RNA viral loadc | 28 (64%) | 12 (60%) | 0.78 |
Current CD4+ cell count (cells/mL) cell count (cells/mL) | 753 (558, 928) | 592 (402, 795) | 0.011 |
 < < 350 350 | 5 (12%) | 3 (14%) | 0.76 |
Nadir CD4+ cell count, (cells/mL) cell count, (cells/mL) | 574 (422, 835) | 457 (368, 720) | 0.025 |
 < < 350 350 | 7 (16%) | 5 (24%) | 0.44 |
| History of AIDS-related illness | 12 (29%) | 4 (21%) | 0.54 |
Values are reported as median (25th, 75th percentile) or number (%).
PI protease inhibitors, NNRTI
protease inhibitors, NNRTI nonnucleoside reverse transcriptase inhibitors, NRTI
nonnucleoside reverse transcriptase inhibitors, NRTI nucleoside reverse transcriptase inhibitors, INSTI
nucleoside reverse transcriptase inhibitors, INSTI integrase strand transfer inhibitor.
integrase strand transfer inhibitor.
aOnly one participant exposed to an integrase strand transfer inhibitor for total duration of 1.7 years.
bParticipant receiving 1 NRTI +
+ 1 PI
1 PI +
+ 1 INSTI.
1 INSTI.
cDetection limit ≥
≥ 40 viral copies/mL.
40 viral copies/mL.
Left ventricular structure and function
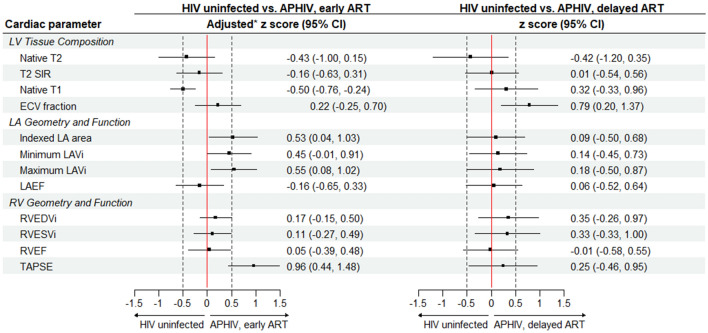
Table Table33 summarizes absolute mean values of left ventricular (LV) indices while Figs. 2 and and33 report the corresponding z score measures. Compared to HIV uninfected participants, APHIV with early ART initiation had reduced LVMi [adjusted mean (95% CI) z score: −0.36 (−0.61, −0.11); p =
= 0.042] but similar LV volumes, e.g., LVEDVi [adjusted z score: 0.19 (−0.13, 0.51); p
0.042] but similar LV volumes, e.g., LVEDVi [adjusted z score: 0.19 (−0.13, 0.51); p =
= 0.25] and RWT [adjusted z score: 0.01 (−0.44, 0.47); p
0.25] and RWT [adjusted z score: 0.01 (−0.44, 0.47); p =
= 0.96]. They also had comparatively better LV systolic function when assessed by mechanical deformation indices like GCS [z score: 0.53 (0.0.13, 0.92); p
0.96]. They also had comparatively better LV systolic function when assessed by mechanical deformation indices like GCS [z score: 0.53 (0.0.13, 0.92); p =
= 0.023] and time to peak GCS [adjusted z score: −0.45 (−0.81, −0.08); p
0.023] and time to peak GCS [adjusted z score: −0.45 (−0.81, −0.08); p =
= 0.041]. However, the two groups had similar LV diastolic function measured by either diastolic strain rate or diastolic velocity. (Fig. 2).
0.041]. However, the two groups had similar LV diastolic function measured by either diastolic strain rate or diastolic velocity. (Fig. 2).
Table 3
Participants' absolute mean values of cardiac parameters stratified by perinatal HIV infection and antiretroviral treatment status.
| Cardiac parameter | PHIV and ART status | ||||
|---|---|---|---|---|---|
| HIV uninfected | APHIV with early ART initiation | APHIV with delayed ART initiation | |||
| Mean (95% CI) | Mean (95% CI) | P valuea | Mean (95% CI) | P valueb | |
| Number | 37 | 45 | 21 | ||
| LV geometry | |||||
| LVMi (g/m2) | 58.5 (54.6, 62.5) | 54.9 (52.1, 57.8) | 0.22 | 59.1 (55.5, 62.7) | 0.83 |
| LVEDVi (mL/m2) | 77.5 (73.4, 83.5) | 81.3 (77.7, 85.0) | 0.12 | 86.7 (78.9, 94.5) | 0.028 |
| LVESVi (mL/m2) | 28.9 (26.6, 31.2) | 30.5 (28.1, 32.8) | 0.26 | 34.5 (30.7, 38.2) | 0.019 |
| Relative wall thickness | 0.34 (0.32, 0.36) | 0.34 (0.33, 0.35) | 0.95 | 0.36 (0.33, 0.38) | 0.19 |
| LV systolic function | |||||
| LVEF (%) | 63.0 (61.5, 64.6) | 63.0 (60.9, 65.0) | 0.83 | 60.2 (58.5, 62.0) | 0.18 |
| Peak systolic strain (%) | |||||
| Circumferential | −20.1 (−21.1, −19.1) | −21.5 (−22.4, −20.6) | 0.020 | −20.0 (−22.2, −19.8) | 0.23 |
| Longitudinal | −15.7 (−16.3, −15.1) | −16.9 (−17.6, −16.2) | 0.041 | −16.1 (−17.2, −14.9) | 0.72 |
| Radial | 33.2 (31.3, 35.1) | 34.3 (32.4, 36.3) | 0.35 | 32.5 (30.1, 34.9) | 0.52 |
| Time to peak systolic strain (ms) | |||||
| Circumferential | 247.4 (185.0, 309.8) | 205.3 (158.9, 251.7) | 0.56 | 227.8 (142.5, 313.1) | 0.88 |
| Longitudinal | 347.6 (333.0, 362.2) | 316.3 (293.0, 339.7) | 0.034 | 343.0 (328.6, 357.3) | 0.91 |
| LV diastolic function | |||||
| Peak diastolic strain rate (s−1) | |||||
| Circumferential | 1.41 (1.34, 1.48) | 1.40 (1.34, 1.47) | 0.86 | 1.33 (1.23, 1.44) | 0.20 |
| Longitudinal | 1.09 (1.00, 1.18) | 1.12 (1.02, 1.22) | 0.67 | 1.05 (0.93, 1.17) | 0.63 |
| Radial | −2.60 (−2.79, −2.42) | −2.58 (−2.74, −2.43) | 0.85 | −2.28 (−2.47, −2.10) | 0.029 |
| Peak diastolic velocity (cm/s) | |||||
| Circumferential | 11.7 (5.4, 17.9) | 16.1 (12.8, 19.4) | 0.17 | 14.8 (9.1, 20.4) | 0.44 |
| Longitudinal | 28.8 (25.9, 31.7) | 27.1 (24.7, 29.4) | 0.37 | 27.5 (23.1, 32.0) | 0.60 |
| Radial | 38.5 (36.5, 40.5) | 37.9 (36.1, 39.6) | 0.65 | 36.8 (33.5, 40.0) | 0.31 |
| LV tissue composition | |||||
| Native T2 (ms) | 38.8 (38.3, 39.3) | 38.4 (37.7, 39.1) | 0.16 | 38.5 (37.4, 39.5) | 0.58 |
| T2 SIR | 1.59 (1.49, 1.69) | 1.53 (1.44, 1.62) | 0.69 | 1.59 (1.47, 1.70) | 0.77 |
| Native T1 (ms) | 1,235 (1,222, 1,247) | 1,208 (1,197, 1,219) | 0.045 | 1,239 (1,222, 1,257) | 0.95 |
| ECV fraction (%) | 28.1 (27.0, 29.1) | 28.7 (27.7, 29.7) | 0.13 | 29.5 (28.0, 31.0) | 0.044 |
| Presence of LGE (%) | 57.1 (38.2, 76.1) | 52.6 (36.3, 69.0) | 0.72 | 47.1 (22.2, 71.9) | 0.52 |
| LA geometry ad function | |||||
| Indexed LA area (cm2/m2) | 11.2 (10.6, 11.9) | 12.4 (11.8, 13.0) | 0.016 | 11.5 (10.3, 12.7) | 0.67 |
| Minimum LAVi (mL/m2) | 33.4 (30.7, 36.0) | 36.9 (32.3, 41.4) | 0.16 | 38.2 (35.6, 40.8) | 0.029 |
| Maximum LAVi (mL/m2) | 13.8 (12.0, 15.6) | 14.8 (12.3, 17.3) | 0.56 | 16.0 (14.5, 17.5) | 0.056 |
| LAEF (%) | 59.1 (56.0, 62.2) | 58.2 (55.6, 60.8) | 0.63 | 60.3 (56.9, 63.6) | 0.61 |
| RV geometry and function | |||||
| RVEDVi (mL/m2) | 77.0 (71.4, 82.5) | 83.0 (78.9, 87.0) | 0.099 | 87.0 (78.1, 95.8) | 0.029 |
| RVESVi (mL/m2) | 33.3 (29.8, 36.80 | 35.4 (33.0, 37.7) | 0.34 | 37.7 (32.5, 42.9) | 0.11 |
| RVEF (%) | 57.4 (55.0, 59.8) | 57.3 (55.4, 59.3) | 0.98 | 57.0 (54.3, 59.7) | 0.84 |
| TAPSE (cm) | 1.4 (1.3, 1.6) | 1.8 (1.6. 2.0) | 0.005 | 1.5 (1.2, 1.8) | 0.89 |
LV left ventricle, LVM
left ventricle, LVM LV mass, LVEDV
LV mass, LVEDV LV end diastolic volume, LVESV
LV end diastolic volume, LVESV LV end systolic volume, LVEF
LV end systolic volume, LVEF LV ejection fraction, RWT
LV ejection fraction, RWT relative wall thickness, GCS
relative wall thickness, GCS peak global circumferential strain, GLS
peak global circumferential strain, GLS peak global longitudinal strain, GRS
peak global longitudinal strain, GRS peak global radial strain, DSR
peak global radial strain, DSR peak diastolic strain rate, SIR
peak diastolic strain rate, SIR signal intensity ratio, ECV
signal intensity ratio, ECV extracellular volume, LGE
extracellular volume, LGE late gadolinium enhancement, LA
late gadolinium enhancement, LA left atrium, LAV
left atrium, LAV LA volume, LAEF
LA volume, LAEF LA ejection fraction, RV
LA ejection fraction, RV right ventricle, RVEDV
right ventricle, RVEDV RV end diastolic volume, RVESV
RV end diastolic volume, RVESV RV end systolic volume, RVEF
RV end systolic volume, RVEF RV ejection fraction, Postscript (i)
RV ejection fraction, Postscript (i)
 indexed to body surface area.
indexed to body surface area.
aP value for differences in mean (or proportionc) between HIV uninfected versus PHIV with early ART initiation.
bP value for differences in mean (or proportionc) between HIV uninfected versus PHIV with delayed ART initiation.

z scores of left ventricular parameters according to antiretroviral treatment status among participants with perinatal HIV infection. Asterisk: adjusted for age, sex, mean arterial pressure (MAP) and body mass index (BMI). Abbreviations as elsewhere defined. Postscript (i)
 indexed to body surface area.
indexed to body surface area.
z scores of left and right ventricular and left atrial parameters according antiretroviral treatment status among participants with perinatal HIV infection. Asterisk: adjusted for age, sex, mean arterial pressure (MAP) and body mass index (BMI). Abbreviations as elsewhere defined. Postscript (i) indexed to body surface area.
indexed to body surface area.
On the other hand, preserved LVMi [adjusted z score: 0.11 (−0.30, 0.53); p =
= 0.59] and RWT [adjusted z score: 0.32 (−0.45, 1.09); p
0.59] and RWT [adjusted z score: 0.32 (−0.45, 1.09); p =
= 0.34] in APHIV with delayed ART were accompanied by a relatively dilated LV chamber [LVEDVi adjusted z score: 0.47 (0.0.09, 0.86); p
0.34] in APHIV with delayed ART were accompanied by a relatively dilated LV chamber [LVEDVi adjusted z score: 0.47 (0.0.09, 0.86); p =
= 0.014]. Although individually not achieving statistical significance (except GCS [adjusted z score: −0.51 (−0.91, −0.10); p
0.014]. Although individually not achieving statistical significance (except GCS [adjusted z score: −0.51 (−0.91, −0.10); p =
= 0.020]), all study measures of LV systolic function (ejection fraction, systolic strain and time to peak systolic strain) were collectively suggestive of comparatively lower function (albeit within normal range) in APHIV with delayed ART relative to their HIV uninfected peers. However, LV diastolic function was preserved as determined by measures like longitudinal [adjusted z score: −0.04 (−0.45, 0.73); p
0.020]), all study measures of LV systolic function (ejection fraction, systolic strain and time to peak systolic strain) were collectively suggestive of comparatively lower function (albeit within normal range) in APHIV with delayed ART relative to their HIV uninfected peers. However, LV diastolic function was preserved as determined by measures like longitudinal [adjusted z score: −0.04 (−0.45, 0.73); p =
= 0.98] and radial [adjusted z score: 0.20 (−0.34, 0.57); p
0.98] and radial [adjusted z score: 0.20 (−0.34, 0.57); p =
= 0.61] diastolic strain rate (Fig. 2). The distribution of absolute means (Table (Table3)3) was largely consistent with the adjusted z score based results. Of note, all the observed PHIV/ART related LV differences were of small-to-moderate size based on the z scores.
0.61] diastolic strain rate (Fig. 2). The distribution of absolute means (Table (Table3)3) was largely consistent with the adjusted z score based results. Of note, all the observed PHIV/ART related LV differences were of small-to-moderate size based on the z scores.
Left atrial structure and function
Maximum LAVi was 13.8 (12.0, 15.6) mL/m2 for HIV uninfected controls compared to 14.8 (12.3, 17.3) mL/m2 (p =
= 0.56) for APHIV with early ART and 16.0 (14.5, 17.5) mL/m2 (p
0.56) for APHIV with early ART and 16.0 (14.5, 17.5) mL/m2 (p =
= 0.056) for APHIV with delayed ART (Table (Table3).3). After adjustment, early ART [maximum LAVi adjusted z score: 0.55 (0.08, 1.02); p
0.056) for APHIV with delayed ART (Table (Table3).3). After adjustment, early ART [maximum LAVi adjusted z score: 0.55 (0.08, 1.02); p =
= 0.032], and not delayed ART [maximum LAVI adjusted z score: 0.28 (−0.40, 0.97); p
0.032], and not delayed ART [maximum LAVI adjusted z score: 0.28 (−0.40, 0.97); p =
= 0.36], was associated with larger LA chambers (Fig. 3). The magnitude of differences in LA chamber dimension associated with early ART were of moderate size. There were no significant differences in LAEF with or without adjustment for either early ART [adjusted z score: −0.16 (−0.65, 0.33); p
0.36], was associated with larger LA chambers (Fig. 3). The magnitude of differences in LA chamber dimension associated with early ART were of moderate size. There were no significant differences in LAEF with or without adjustment for either early ART [adjusted z score: −0.16 (−0.65, 0.33); p =
= 0.47] or delayed ART [LAEF adjusted z score: 0.06 (−0.52, 0.64; p
0.47] or delayed ART [LAEF adjusted z score: 0.06 (−0.52, 0.64; p =
= 0.82].
0.82].
Right ventricular structure and function
The RVEDVi was 77.0 (71.4, 82.5) mL/m2 for HIV uninfected controls compared to 83.0 (78.9, 87.0) mL/m2 (p =
= 0.099) for APHIV with early ART and 87.0 (78.1, 95.8) mL/m2 (p
0.099) for APHIV with early ART and 87.0 (78.1, 95.8) mL/m2 (p =
= 0.029) for APHIV with delayed ART (Table (Table3).3). These corresponded to adjusted z scores of 0.27 (-0.05, 0.60) for APHIV with early ART and 0.35 (−0.26, 0.97) for APHIV with delayed ART. Thus, while there were no statistically significant PHIV/ART-related differences in RV chamber size or RVEF, APHIV with early ART had considerably improved RV systolic function if assessed by TAPSE [adjusted z score: 0.96 (0.44, 1.48); p
0.029) for APHIV with delayed ART (Table (Table3).3). These corresponded to adjusted z scores of 0.27 (-0.05, 0.60) for APHIV with early ART and 0.35 (−0.26, 0.97) for APHIV with delayed ART. Thus, while there were no statistically significant PHIV/ART-related differences in RV chamber size or RVEF, APHIV with early ART had considerably improved RV systolic function if assessed by TAPSE [adjusted z score: 0.96 (0.44, 1.48); p <
< 0.001].
0.001].
Tissue characteristics
There were no significant differences in T2 mapping and T2-STIR signal intensity ratios between HIV uninfected participants and APHIV with early or delayed ART, whether measured in absolute values (Table (Table3)3) or as adjusted z scores (Fig. 3). However, APHIV with delayed ART had a greater ECV fraction [29.5 (28.0, 31.0) %; p =
= 0.044] than, while APHIV with early ART had a comparable ECV fraction [28.7 (27.7, 29.7) %; p
0.044] than, while APHIV with early ART had a comparable ECV fraction [28.7 (27.7, 29.7) %; p =
= 0.13] to HIV uninfected controls [28.1 (27.0, 29.1) %]. This translated to adjusted z scores of 0.79 (0.20, 1.37) (p
0.13] to HIV uninfected controls [28.1 (27.0, 29.1) %]. This translated to adjusted z scores of 0.79 (0.20, 1.37) (p =
= 0.010) for delayed ART and 0.32 (−0.15, 0.80) (p
0.010) for delayed ART and 0.32 (−0.15, 0.80) (p =
= 0.17) for early ART (Fig. 3).
0.17) for early ART (Fig. 3).
Discussion
In this CMR study, we identified distinct albeit subclinical cardiac phenotypes associated with early (< 5 years of age) and delayed (≥
5 years of age) and delayed (≥ 5 years of age) ART initiation among APHIV. Changes seen with early ART included relatively low LV mass, improved LV systolic function, and LA enlargement notwithstanding preserved LV diastolic mechanical deformation. On the other hand, delayed ART was associated with comparative LV chamber enlargement, reduced systolic function and greater interstitial fibrosis. These differences may be rooted in differing degrees of systemic inflammatory activation and immunosuppression correlated with ART timing in PHIV and, in turn, the extent and type of cardiac damage sustained in early life. Whether and how these difference might impact clinical cardiovascular trajectories of APHIV initiating ART at different ages remains to be determined.
5 years of age) ART initiation among APHIV. Changes seen with early ART included relatively low LV mass, improved LV systolic function, and LA enlargement notwithstanding preserved LV diastolic mechanical deformation. On the other hand, delayed ART was associated with comparative LV chamber enlargement, reduced systolic function and greater interstitial fibrosis. These differences may be rooted in differing degrees of systemic inflammatory activation and immunosuppression correlated with ART timing in PHIV and, in turn, the extent and type of cardiac damage sustained in early life. Whether and how these difference might impact clinical cardiovascular trajectories of APHIV initiating ART at different ages remains to be determined.
Our results, while consistent with previous reports of cardioprotection with ART in APHIV, also highlight the potential heterogeneity of these effects. This may have far-reaching clinical implications in the setting of SSA`s pediatric HIV epidemic. Currently available studies3,5,6,14 of ART and the heart in Africa`s young persons with PHIV have almost exclusively included participants with late HIV diagnoses, delayed ART initiation, and/or short duration of ART exposure. These studies found not infrequent cardiac functional and morphological abnormalities including LV hypertrophy (67% prevalence15), dilatation (8% prevalence1), systolic (5.5–33% prevalence6,15) and diastolic (36% prevalence16) dysfunction, and RV dilatation (29%15), and often accompanied by symptoms of exertional dyspnea, leg swelling and cough, among others. In contrast, Mahtab et al. (2020) 5 reported very low prevalence of echocardiographic abnormalities (6.7% LV hypertrophy, 7.6% diastolic dysfunction, 0.2% systolic dysfunction) in a South African APHIV cohort. This is possibly the only African report to date of a cohort with early ART initiation and prolonged ART exposure. Their results are in keeping with studies in HICs where pediatric ART is both universal and early3,17,18.
Our study did not enumerate cardiac abnormalities, and thus we cannot compare their prevalence with prior reports. However, there are notable differences in cardiac phenotype observed with either early or delayed ART in our study compared to previous reports. For example, delayed ART in our cohort was phenotypically marked by relative LV chamber enlargement, reduced systolic function and greater interstitial fibrosis. LV diastolic function was preserved as were LA and RV function and morphology. In other studies, LV enlargement and reduced LV systolic function with delayed ART were reported along with other abnormalities including LV hypertrophy, impaired LV diastolic function, RV dilatation and increased pulmonary artery systolic pressure1,4,6,14,15. Further, the cardiac changes seen with delayed ART initiation in our study were not associated with any symptoms and were, at best, of moderate size. This contrasts with other reports, mostly from SSA, where cardiac abnormalities in delayed ART frequently occurred with symptoms of heart failure. It is noteworthy that ART duration (median 8.1 years) with delayed ART in our cohort was much longer than in comparable studies.
Similarly, studies from HICs invariably found no clinically significant cardiac differences between those with PHIV and early ART initiation and their HIV uninfected counterparts3,17,18. The subclinical differences that have been described, at times contradictory, entail LV systolic strain, LV fractional shortening, and LV mass. Our observation of reduced LV mass with early ART echoes the results of a US study of children and adolescents early exposed to ART19. The comparatively greater peak systolic strain and shorter times to peak strain seen in APHIV with early ART are suggestive of hyperdynamic LV function. This has been previously noted in a similarly aged (mean age 15.3 years) cohort of APHIV undergoing echocardiographic examination in Kenya20. However, the notable difference is that the latter had late ART initiation (mean age 8.1 years) and high rates (53%) of uncontrolled HIV infection. Previously unreported, however, was our finding of relative LA enlargement. LA enlargement is often a marker of chronically elevated LA pressure, and signifies increased LV stiffness and diastolic dysfunction. However, formal LV diastolic functional assessment requires measurement of flow and velocities at the mitral annulus, mitral inflow and pulmonary veins21—which we did not evaluate—together with LA dimensions.
Nonetheless, the cardiac phenotype we observed in APHIV with early ART, i.e., preserved systolic function with likely impaired diastology, may mirror the shifts in epidemiology of HIV cardiomyopathy that have been observed in adult HIV care in HICs22,23. LV diastolic dysfunction, often with preserved systolic function, is now described in one in two (43–50%)22,23 asymptomatic HIV infected adults on long-term suppressive ART. This in turn warrants concern about future heart failure (HF) risks, especially HF with preserved ejection (HFpEF). HFpEF is often preceded by LV diastolic dysfunction with normal systolic function and is the predominant manifestation of HF in adults with HIV. It is noteworthy that uptake of early infant diagnosis of HIV and linkage to pediatric ART are increasing across SS24. Thus, the prevalence and incidence of the cardiac phenotype associated with APHIV with early ART are likely to increase. Follow-up studies mapping the evolution and long-term outcomes of this cardiac phenotype are, therefore, urgently required.
Strengths and limitations
We believe that our study is among the first to deploy CMR in adolescents with PHIV in SSA. Previous studies have invariably used echocardiography, which despite its lower cost, ready availability, and ease of use, has well documented limitations in accuracy and reproducibility. CMR, in contrast, has superior spatial resolution, excellent inter- and intra-study reproducibility, no acoustic window dependency, and makes no geometric assumptions. A drawback, however, was lack of repeat imaging precluding us from knowing whether cardiac status was static, improving, or deteriorating. Similarly, the study`s cross-sectional design prevents causal inferences. Our small sample size was another limitation as it prevented examination of important subgroups like sex, and the estimation of precise results. Lastly, our HIV uninfected participants may not have been the ideal reference group given their comparatively high rates of overweight/obesity, and the potential for confounding. However, this limitation will continue to plague adolescent cardiac imaging research in Africa until normative CMR ranges are published.
Conclusions
We found residual albeit subclinical cardiac changes in South African APHIV on successful antiretroviral therapy suggesting that ART is cardioprotective. However, these cardioprotective effects are heterogenous and may depend on age ART initiation. Their long-term clinical significance remains to be determined, especially considering growing numbers of APHIV who are surviving into adulthood in Africa.
Methods
We followed the guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) in the conduct and reporting of our analyses25.
Study population and setting
This was an observational cross-sectional study of participants enrolled in the ongoing prospective Cape Town Adolescent Antiretroviral Cohort (CTAAC). The CTAAC, described fully elsewhere5, enrolled young persons, then aged 9–14 years old (2012–2013), with PHIV and established on ART and a comparison group of similar age and sex HIV uninfected peers. Cohort members with PHIV and their HIV uninfected controls are all drawn from similar communities in Cape Town. CTAAC`s overall aim is to track the development of chronic diseases in children and adolescents with PHIV. For the current cross-sectional analysis, we approached and enrolled cohort members consecutively presenting for a scheduled study visit (Visit #9, 2018–2019) to complete a comprehensive CMR examination. Participants were eligible to participate if they were aged ≥
≥ 13 years, had no history of clinical heart disease, no active systemic infections, no current use of anti-inflammatory therapies, and no known contraindications to CMR [e.g., gadolinium sensitivity or reduced glomerular filtration rate (≤
13 years, had no history of clinical heart disease, no active systemic infections, no current use of anti-inflammatory therapies, and no known contraindications to CMR [e.g., gadolinium sensitivity or reduced glomerular filtration rate (≤ 30 ml/min/1.73 m2)].
30 ml/min/1.73 m2)].
Ethics
The investigation conforms with the principles outlined in the Declaration of Helsinki. The Human Research Ethics Committee of the Faculty of Health Sciences, University of Cape Town, approved all study activities. Participants’ and parents’ informed assent and consent were obtained in accordance with South African practice.
Data collection
Sociodemographic data, past medical history, age at ART initiation, WHO HIV clinical staging (I–IV) and CD4+ cell count at time of HIV diagnosis (presumed nadir CD4+) and ART exposure up to the time of CMR imaging were collected under the parent CTAAC protocol. Seated brachial blood pressure (BP) and anthropometrics were measured. The last two of three same arm BP readings were averaged to give final systolic (SBP), diastolic (DBP), and mean arterial (MAP) blood pressure. Height, weight, and body mass index (BMI) were referenced to age and sex according to WHO growth reference data26, to obtain height-for-age (HFA), weight-for-age (WFA), and BMI-for-age (BFA) z scores. Stunting (HFA z score
cell count at time of HIV diagnosis (presumed nadir CD4+) and ART exposure up to the time of CMR imaging were collected under the parent CTAAC protocol. Seated brachial blood pressure (BP) and anthropometrics were measured. The last two of three same arm BP readings were averaged to give final systolic (SBP), diastolic (DBP), and mean arterial (MAP) blood pressure. Height, weight, and body mass index (BMI) were referenced to age and sex according to WHO growth reference data26, to obtain height-for-age (HFA), weight-for-age (WFA), and BMI-for-age (BFA) z scores. Stunting (HFA z score <
< −2), underweight (WFA z score
−2), underweight (WFA z score <
< −2), wasting (BFA z score
−2), wasting (BFA z score <
< −2) and overweight/obesity (BFA z score
−2) and overweight/obesity (BFA z score >
> 1) were also determined.
1) were also determined.
Venous blood was collected from APHIV for measurement of current CD4+ cell count (Beckman Coulter FC500 MPL analyzers, USA) and HIV viral load (lower limit of detection 40 copies/mL; Roche Cobas AmpliPrep/TaqMan). We classified HIV-related markers into the following ordinal categories: age at ART initiation (early:
cell count (Beckman Coulter FC500 MPL analyzers, USA) and HIV viral load (lower limit of detection 40 copies/mL; Roche Cobas AmpliPrep/TaqMan). We classified HIV-related markers into the following ordinal categories: age at ART initiation (early: <
< 5 years old, and delayed:
5 years old, and delayed: ≥
≥ 5 years old); nadir and current CD4+
5 years old); nadir and current CD4+ (<
(< 350 vs.
350 vs. ≥
≥ 350 cells/mL); HIV viral load (viremia:
350 cells/mL); HIV viral load (viremia: ≥
≥ 40 vs. aviremia:
40 vs. aviremia: <
< 40 copies/mL); AIDS history (HIV clinical stage IV vs. stages I–III); and ART history (protease inhibitor (PI) exposure vs. none; and non-nucleoside reverse transcriptase inhibitor (NNRTI) exposure vs. none). ART exposure duration was measured from initiation to time of CMR examination.
40 copies/mL); AIDS history (HIV clinical stage IV vs. stages I–III); and ART history (protease inhibitor (PI) exposure vs. none; and non-nucleoside reverse transcriptase inhibitor (NNRTI) exposure vs. none). ART exposure duration was measured from initiation to time of CMR examination.
Cardiovascular magnetic resonance (CMR) procedures
Image acquisition
As previously described, protocol-directed CMR was performed at 3 Tesla (Siemens, Magneton Skyra, Erlangen, Germany)27. The protocol included balanced steady-state free precession (bSSFP) for volumetrics and function, T1 mapping by Modified Look-Locker Inversion Recovery (MoLLI) sequence, T2 mapping using fast-low-angle-shot (FLASH) sequence, and late gadolinium enhancement (LGE) using a phase sensitive inversion recovery (PSIR) sequence 10–15 min after gadolinium contrast administration. Imaging parameters for the sequences used were maintained between participants and are summarized in Supplementary Table S1. CMR image analysis was done offline, blind to PHIV/ART status, using proprietary software (CVI42, Circle Cardiovascular Imaging, Calgary, Canada)28.
Image analysis
LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), LV ejection fraction (LVEF) and LV mass (LVM) were automatically derived from SAX views. Relative wall thickness (RWT) was calculated as twice inferolateral wall thickness divided by LV end-diastolic diameter, whereby both inferolateral wall thickness and LV end-diastolic dimension were measured in end-diastole from a SAX frame immediately basal to the tip of papillary muscle tips28. We assessed the tricuspid annular plane systolic excursion (TAPSE) in the LAX four-chamber view by measuring the distance travelled by the tricuspid annulus from end-diastole to end-systole. Basal, mid-ventricle, and apical SAX slices were manually contoured to outline the endocardium and epicardium, and generate maps for native T1, T2 and extracellular volume (ECV) estimation. We calculated the T2 signal intensity (SI) ratio as previously described28, and visually assessed the presence or absence of LGE. LV circumferential, radial, and longitudinal deformation parameters (global systolic strain, time to peak systolic strain, peak diastolic strain rate, and diastolic velocity) were analyzed by semi-automated feature tracking during diastole and systole from long axis (LAX) and SAX cine images.
Statistical analyses
A formal sample size calculation was not performed due to the exploratory nature of the study. Missingness in the key study variables was less than 2%, and thus we proceeded with complete case analysis. Where relevant, cardiovascular parameters were indexed to Mosteller body surface area (BSA)29 to account for somatic growth, and are indicated by the postscript (i). Ninety-five percent confidence intervals (95%CI) were calculated based on bootstrap replications. Summary statistics are presented as mean (standard deviation, SD) or median (interquartile range, IQR) for continuous variables and number (percent) for categorical variables. Our primary exposure groups were HIV uninfected, APHIV with early ART, and APHIV with delayed ART. Comparisons by exposure were made using linear and quintile regression modeling, as appropriate. Differences in the distribution of blood pressure and standardized anthropometric measures were visually displayed.
There are currently no published reference standards for CMR-derived parameters for children and adolescents (< 20 years old) in SSA. We, therefore, derived z scores of cardiac variables for APHIV by referencing their HIV uninfected peers as normative. For a given variable, its z score was calculated by: (meanaphiv − meanhiv uninfected)/(SDhiv uninfected). A z-score measures in SD units the distance of a raw score from the mean. Thus, a z score of 0.5 (or -0.5), for example, means that APHIV have a cardiac parameter that is 0.5 SDs above (or below) the HIV uninfected (i.e., control) group’s mean. To quantify group differences in cardiac parameters, we classified the z scores as small (<
20 years old) in SSA. We, therefore, derived z scores of cardiac variables for APHIV by referencing their HIV uninfected peers as normative. For a given variable, its z score was calculated by: (meanaphiv − meanhiv uninfected)/(SDhiv uninfected). A z-score measures in SD units the distance of a raw score from the mean. Thus, a z score of 0.5 (or -0.5), for example, means that APHIV have a cardiac parameter that is 0.5 SDs above (or below) the HIV uninfected (i.e., control) group’s mean. To quantify group differences in cardiac parameters, we classified the z scores as small (< 0.5), medium (≥
0.5), medium (≥ 0.5 and
0.5 and <
< 0.8), or large (≥
0.8), or large (≥ 0.8) using Cohen’s criteria30. We fitted linear regression models to evaluate the association between PHIV/ART status and cardiac parameters (measured as z scores) after adjustment for age, sex, MAP and BMI. All statistical analyses were performed using Stata version 17.0 (StataCorp, College Station, TX) and R, version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
0.8) using Cohen’s criteria30. We fitted linear regression models to evaluate the association between PHIV/ART status and cardiac parameters (measured as z scores) after adjustment for age, sex, MAP and BMI. All statistical analyses were performed using Stata version 17.0 (StataCorp, College Station, TX) and R, version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
Acknowledgements
In addition to thanking all study participants and their families, the authors would also like to acknowledge the support of the following people who made the study possible: Sana Mahtab, Nana Akua Asafu-Agyei, Landiwe Daka, Sharon Wakefield, Shanaaz Davids, Nikelwa Mvango-Njemla, Nomawethu Jele, Nomandla Udogwu (posthumously), Pumeza Nazo, Njemia Nikelwa, Keyola George, Akhona Mazingi, Tafadzwa Mautsa, Mothabisi Nyathi, Faith Qeja, Luyanda Nkondlwana, Mazwi Maishi, Mariaan Jaftha, Daniel Doetz, Liezl Julius, Michael Wheeler, Shaheed Sorathia, Nabeal Kaskar, Dave Nshuntishema, Okeyo, Patricie Niyitegeka, and Ben Jann.
Author contributions
IMM, NABN conceptualization; IMM, CEGC, SJ, PS and BC data curation; IMM, CEGC, and BC formal analysis and visualization; IMM, CEGC, BC, SJ, PS, LM, HZ, JJ, MN, MJS, NABN investigation, methodology and data interpretation; IMM and AA project administration; IMM writing-original draft; and IMM, CEGC, BC, SJ, PS, LM, HZ, JJ, MN, MJS, NABN writing-review & editing. All authors have read and approved the manuscript before submission.
Funding
Itai Magodoro was supported by a training award from the Fogarty International Center and National Institute of Mental Health of the National Institutes of Health (D43 TW010543) and the AIDS Healthcare Foundation, Los Angeles, USA. Heather Zar is supported by the South African Medical Research Council (SA MRC). CTAAC was funded by the NIH (R01HD074051; PI Heather Zar). Ntobeko Ntusi gratefully acknowledges funding from the National Research Foundation, South African Medical Research Council, US National Institutes of Health, Medical Research Council (UK), and the Lily and Ernst Hausmann Trust.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Itai M. Magodoro, Email: [email protected].
Ntobeko A. B. Ntusi, Email: [email protected].
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-65119-9.
References
Articles from Scientific Reports are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1038/s41598-024-65119-9
Read article for free, from open access legal sources, via Unpaywall:
https://www.nature.com/articles/s41598-024-65119-9.pdf
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/164727617
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Echocardiographic Findings in a Cohort of Perinatally HIV-Infected Adolescents Compared with Uninfected Peers from the Cape Town Adolescent Antiretroviral Cohort.
J Am Soc Echocardiogr, 33(5):604-611, 05 Mar 2020
Cited by: 6 articles | PMID: 32147093
Long-Term Effects of In Utero Antiretroviral Exposure: Systolic and Diastolic Function in HIV-Exposed Uninfected Youth.
AIDS Res Hum Retroviruses, 32(7):621-627, 09 May 2016
Cited by: 10 articles | PMID: 26794032 | PMCID: PMC4931731
Effectiveness of antiretroviral therapy in HIV-infected children under 2 years of age.
Cochrane Database Syst Rev, (7):CD004772, 11 Jul 2012
Cited by: 17 articles | PMID: 22786492
Review
Funding
Funders who supported this work.
AIDS Healthcare Foundation, Los Angeles, USA
FIC NIH HHS (1)
Grant ID: D43 TW010543
Fogarty International Center (1)
Grant ID: D43 TW010543
Lily and Ernst Hausmann Trust
NICHD NIH HHS (1)
Grant ID: R01 HD074051
NIH HHS (1)
Grant ID: R01HD074051
National Institutes of Health (1)
Grant ID: R01HD074051

 1
1 


