Abstract
Introduction
Fatigue is a common symptom observed in post-cancer treatment, yet its underlying mechanisms remain poorly understood. Acupuncture has been employed to alleviate cancer-related fatigue (CRF); however, its effectiveness in addressing associated comorbidities that may influence fatigue is also poorly understood. This study represents the first investigation to use acupuncture as an intervention for fatigue in breast cancer survivors within a Norwegian cohort. The study will employ questionnaires to evaluate various facets of fatigue. As a pragmatic trial, it statistically assesses its clinical relevance, documents adverse events and evaluates the cost-effectiveness of the acupuncture treatment.Methods and analysis
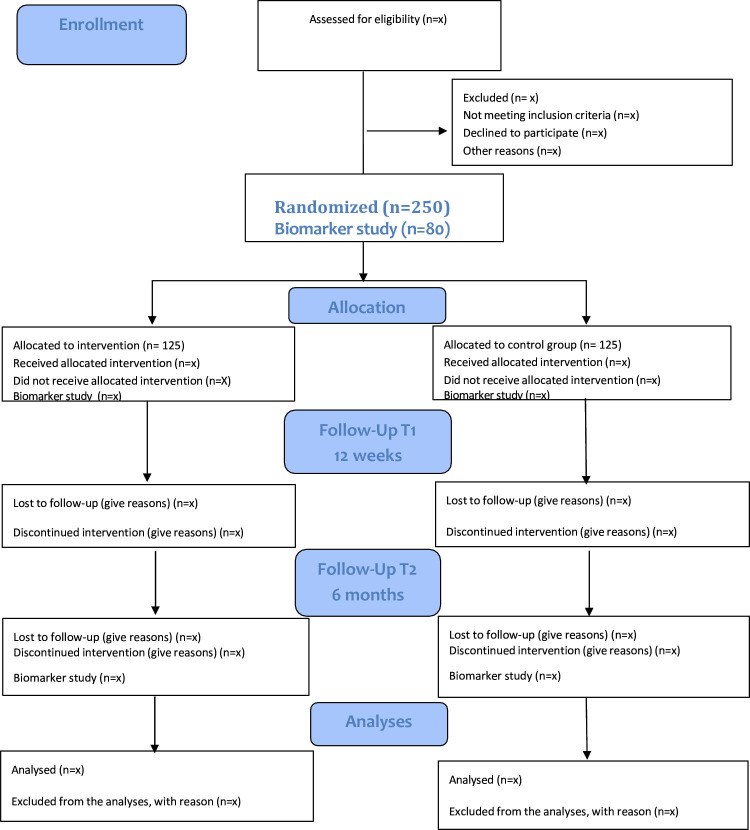
This assessor-blinded, pragmatic, randomised, mixed method, controlled trial with two parallel arms aims to evaluate the effectiveness, safety and cost-effectiveness of acupuncture. It will recruit 250 participants presented with CRF for 6 months or longer. Patients will be randomly allocated either to acupuncture and usual care (n=125) or to usual care alone (n=125). Acupuncture treatments (12 in total) are to be given within 12 weeks. The statistician who will analyse the data will be blinded to group allocation. The primary outcome will be changes in CRF measured by the Chalder fatigue scale. Measurements will be taken 12 weeks and 6 months after randomisation. The secondary outcomes include patient-reported outcomes of pain, anxiety, depression, hot flashes, insomnia and sleepiness. Health-related quality of life and economic evaluation will also be conducted 12 weeks and 6 months after randomisation. Nested within this randomised controlled trial are two qualitative studies and one sub-study measuring biomarkers (C-reactive protein, interleukin (IL)-1, IL-6, tumour necrosis factor alpha (TNF-α) and aPL in addition to the current genotype genes TNF-308 and IL-6-174) from blood samples (n=80). Such biomarkers can potentially address changes in CRF.Ethics and dissemination
Ethical approval of this study has been granted by the Regional Committees for Medical and Health Research Ethics (REC southeast ID number: 112285). Written informed consent will be obtained from all participants. The outcomes of the trial will be disseminated through peer-reviewed publications.Trial registration number
NCT04418115.Free full text

Acupuncture for fatigue in breast cancer survivors: a study protocol for a pragmatic, mixed method, randomised controlled trial
Associated Data
Abstract
Introduction
Fatigue is a common symptom observed in post-cancer treatment, yet its underlying mechanisms remain poorly understood. Acupuncture has been employed to alleviate cancer-related fatigue (CRF); however, its effectiveness in addressing associated comorbidities that may influence fatigue is also poorly understood. This study represents the first investigation to use acupuncture as an intervention for fatigue in breast cancer survivors within a Norwegian cohort. The study will employ questionnaires to evaluate various facets of fatigue. As a pragmatic trial, it statistically assesses its clinical relevance, documents adverse events and evaluates the cost-effectiveness of the acupuncture treatment.
Methods and analysis
This assessor-blinded, pragmatic, randomised, mixed method, controlled trial with two parallel arms aims to evaluate the effectiveness, safety and cost-effectiveness of acupuncture. It will recruit 250 participants presented with CRF for 6 months or longer. Patients will be randomly allocated either to acupuncture and usual care (n=125) or to usual care alone (n=125). Acupuncture treatments (12 in total) are to be given within 12
months or longer. Patients will be randomly allocated either to acupuncture and usual care (n=125) or to usual care alone (n=125). Acupuncture treatments (12 in total) are to be given within 12 weeks. The statistician who will analyse the data will be blinded to group allocation. The primary outcome will be changes in CRF measured by the Chalder fatigue scale. Measurements will be taken 12 weeks and 6 months after randomisation. The secondary outcomes include patient-reported outcomes of pain, anxiety, depression, hot flashes, insomnia and sleepiness. Health-related quality of life and economic evaluation will also be conducted 12 weeks and 6 months after randomisation. Nested within this randomised controlled trial are two qualitative studies and one sub-study measuring biomarkers (C-reactive protein, interleukin (IL)-1, IL-6, tumour necrosis factor alpha (TNF-α) and aPL in addition to the current genotype genes TNF-308 and IL-6–174) from blood samples (n=80). Such biomarkers can potentially address changes in CRF.
weeks. The statistician who will analyse the data will be blinded to group allocation. The primary outcome will be changes in CRF measured by the Chalder fatigue scale. Measurements will be taken 12 weeks and 6 months after randomisation. The secondary outcomes include patient-reported outcomes of pain, anxiety, depression, hot flashes, insomnia and sleepiness. Health-related quality of life and economic evaluation will also be conducted 12 weeks and 6 months after randomisation. Nested within this randomised controlled trial are two qualitative studies and one sub-study measuring biomarkers (C-reactive protein, interleukin (IL)-1, IL-6, tumour necrosis factor alpha (TNF-α) and aPL in addition to the current genotype genes TNF-308 and IL-6–174) from blood samples (n=80). Such biomarkers can potentially address changes in CRF.
Ethics and dissemination
Ethical approval of this study has been granted by the Regional Committees for Medical and Health Research Ethics (REC southeast ID number: 112285). Written informed consent will be obtained from all participants. The outcomes of the trial will be disseminated through peer-reviewed publications.
Trial registration number
NCT04418115.
Introduction
Background and rationale
According to the National Comprehensive Cancer Network, cancer-related fatigue (CRF) is defined as ‘a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness, related to cancer or cancer treatment, that is not proportional to recent activity and interferes with usual functioning’.1 CRF is a common late effect of cancer and its therapy and impairs the quality of life (QoL) of people with cancer and their relatives.2 3 The symptoms of CRF are underreported by cancer survivors and undertreated by clinicians, despite its prevalence and negative impact.4 There is evidence that unemployment is higher and work ability lower among breast cancer survivors than among similar healthy women.5 The prevalence of fatigue among cancer survivors varies by assessment method, cancer type and definitions, but the most commonly reported figures are between 19% and 38%.6 Notably, fatigue is the most common late effect across all cancer survivors.7 A recent publication reported fatigue among breast cancer survivors to be at 28%.8
Although CRF is a commonly experienced late effect, there are only a few evidence-based treatments available.9 Many attempts have been made to manage treatment-related late effects, including physical, psychological and vocational methods. These approaches, such as multidisciplinary oncological rehabilitation programmes, have led to a better QoL than just usual care.10 In several countries, acupuncture is part of this multidisciplinary approach.
Acupuncture and CRF
There is emerging evidence that acupuncture can help alleviate CRF.11 12 A recent meta-analysis examining the relationship between CRF and acupuncture yielded promising results. The authors’ recommendations for acupuncture treatment, while acknowledging some limitations of the included studies, provide valuable insights for guiding future research in this area: ‘first, among the 10 trials included in the meta-analysis, three trials had a sample of less than 30 subjects. Second, it is known that various symptoms, such as pain, sleep disturbance, nutrition, and activity level, among others, can influence CRF level; however, the included RCTs did not report variations in these factors that contribute to CRF, which may have significantly influenced study outcomes. Therefore, further studies with high-quality methodology, larger sample sizes, and a multi-centre design are needed’.13 The intention of this pragmatic randomised controlled trial (n=250) is to test acupuncture as a complete treatment package, that is, the overall effect of acupuncture care, which is highly relevant to breast cancer survivors experiencing CRF. Pragmatic trials also ensure that the sorts of interventions tested can be feasibly rolled out in clinical practice and that the outcomes used to assess effectiveness are valid and easy to understand. Unlike explanatory trials, pragmatic trials examine an intervention under conditions usually found in a clinical setting. This includes an understanding of acupuncture as a complex intervention, leaving the decision of which acupuncture points to use to the clinician based on the individual patient.14 Pragmatic trials are not designed to explore the benefit of placebos. By contrast, we will collect data on expectations and preferences, along with elements that are associated with placebo effects, and factor out their impact on the overall outcome by adding them as covariates to the analysis. For a pragmatic trial to be positive, the results must meet at least four quantitative challenges: statistical significance, clinical relevance, adequate safety and cost-effectiveness.15 Our primary outcome is changes in fatigue scores. However, in line with the limitations identified by Zhang, we will evaluate secondary outcomes that may impact fatigue, which includes anxiety, depression, sleep disturbances, hot flashes and pain. Furthermore, we will include a quality-of-life questionnaire used for cancer patients, including breast cancer survivors.
Principal research questions
Three main questions will be addressed in breast cancer survivors experiencing CRF.
Is acupuncture in combination with usual care more effective in treating fatigue than usual care alone?
What is the impact of using acupuncture on symptom management?
Is acupuncture cost-effective? (This will be measured in terms of cost per quality-adjusted life year (QALY) gained.)
Experiences from a pilot study
A research group (TA, HS, AS, HØ and SAL) ran a pragmatic randomised controlled pilot study on fatigue in breast cancer survivors with acupuncture as the intervention (n=20). The pilot study started in January 2018 and closed in January 2019. The Regional Committee for Medical and Health Research Ethics approved the pilot study (REC southVardesentereast A; reference 2017/2197). The pilot study examined the feasibility of the design and methodological approach, recruitment, randomisation, collaboration, assessment procedures and implementation of acupuncture. Based on the findings of the pilot study, we developed a fully powered multi-centre, pragmatic randomised controlled study outlined in this study protocol. The present study was modified to address challenges encountered in the pilot study including (1) the recruitment process was too slow, so changes have been applied (see study setting) and (2) travelling to the acupuncture treatment centre was too far for some of the pilot study participants. Hence, this present study has 10 participating acupuncturists to cover several boroughs in Oslo, Norway, for treatment availability.
Method and analysis
Study design
The current study is a pragmatic randomised, mixed method, controlled trial with two parallel arms. The arms are (1) acupuncture added to usual care and (2) usual care alone. The protocol is reported along the Standard Protocol Items for Clinical Trials guidelines (online supplemental file 1). Potential participants were initially invited to participate through the study’s homepage or via an information sheet available at local cancer hospitals or Vardesenter. However, during the COVID-19 pandemic, the recruitment process went very slowly and was halted altogether on 12 March 2020 when the Norwegian government imposed a national lockdown. After the society returned to normal, recruiting was still slow. We started recruiting participants via social media. Interest groups dedicated to breast cancer survivorship were contacted, and we were allowed to announce the AcuBreast study on their Facebook pages.
Anyone willing to take part in the study was sent information about the study and requested to sign and return an informed consent form. Randomisation occurs after participants are screened for eligibility and have completed the baseline questionnaires. A statistician from the University of Bergen, who is not involved in any other part of the study, will generate a computer-generated block randomisation list. We will then randomly allocate participants using a secure system that ensures allocation is unambiguously concealed. The randomisation list will be made available to one person at Kristiana University College, namely, the project coordinator doing the baseline registration. This person will telephone the person in charge of the randomisation list and receive the needed information, that is, the treatment or control group.
Study settings
The treatments will be conducted at 10 private acupuncture clinics and one established university-based clinical research centre (Kristiania University College, School of Health Sciences) in Oslo, Norway. The latter will take care of the inclusion procedures. In addition, blood samples will be taken and analysed there.
Eligibility criteria
All interested participants, women aged 18–80 who have finished their curative cancer treatments, will first register their fatigue on a visual analogue scale (VAS) score. If a participant’s score is above or equal to 4, she will be further assessed for eligibility by our trained project coordinator in accordance with the following inclusion and exclusion criteria in a face-to-face consultation.
Inclusion criteria
CRF for 6 months or more, after finishing their curative treatments.
Fatigue score on a VAS of ≥4
 will be eligible.
will be eligible.Women aged 18 years or older.
Participants who have freely agreed to take part in the study and have signed an informed consent form.
Adequate knowledge of Norwegian for communicating with the acupuncturist and for filling in and understanding the questionnaires within the study.
Exclusion criteria
Individuals presenting with clinically diagnosed conditions or pathologies known to contribute to fatigue.
Persons whose fatigue could be attributed to pre-existing conditions.
Ongoing acupuncture treatment for any condition.
Persons who are pregnant or become pregnant within the study period.
Any comorbidity with a bleeding disorder (figure 1).
Intervention group
The design of the trial is pragmatic in that it determines the effectiveness of an intervention under ‘real-world’ conditions. Hence, the participating acupuncturists provide their care as in normal practice. Those participants who have been randomised to acupuncture treatment will receive 12 treatments over 8 to 12 weeks. The number of participating acupuncturists will be 10 in total and should have at least a bachelor’s degree in acupuncture with more than 5 years of clinical experience. Further, they should be members of the Norwegian Association of Acupuncturists Akupunkturforeningen. The participating acupuncturists will also receive training from previously trained acupuncturists (TA, HS and AS) within our research team. These three received training from our collaborating partners in the USA. This training was targeted at treating breast cancer survivors with acupuncture.16 17 This will ensure that the treatment in Norway will be based on a mutual understanding between the acupuncturists taking part in the trial. The form of acupuncture to be used in this trial is framed within theories of Traditional Chinese Medicine (TCM) and will be reported according to the Standards for Reporting Interventions in Clinical Trials of Acupuncture guidelines.18 These guidelines, which have become an official extension to the Consolidated Standards of Reporting Trail Statement, were developed to improve the completeness and transparency of reporting in acupuncture research. Hence, they will allow readers to understand, interpret and evaluate the results of a clinical trial by providing clear, precise and comprehensive details about the acupuncture intervention. The initial TCM diagnosis, at thein first consultation, will be recorded. Any changes regarding initial acupuncture points and relevant TCM advice will be reported via the electronic journal. The journal will identify the participants only by their registration numbers.
Safety
Acupuncture is safe in the hands of a well-educated acupuncturist.19 20 However, when administering acupuncture to people with cancer, special precautions are needed.21 Insertion of needles into the arm is to be avoided in participants who have undergone axillary dissection; the same applies to any lymphoedematous limbs. Reporting of any adverse events will involve both participants and acupuncturist by using relevant instruments, that is, a listing of any events.
Control group
Our control group will receive ‘treatment as usual’, that is, they will continue with their usual care for their CRF. At the beginning and end of the study, the participants in the control group will fill in the same questionnaires as those in the acupuncture group. Any use of acupuncture during the study period is an exclusion criterion.
Qualitative studies
Participants from the acupuncture and control groups will be asked to write about their experiences. The former with acupuncture treatment and the latter in the control group. Further, focus group interviews with the participating acupuncturists will be conducted to get a sound description of their experiences and of the pros and cons of taking part in a study with acupuncture for CRF.
We want to explore how the lockdown of Norwegian society during COVID-19 might have influenced our participants and their CRF. In line with other researchers, we think that qualitative studies are the best method for capturing individual responses to this pandemic. As has been shown with other epidemics and upheavals, these methods allow the researcher to capture and understand how people find meaning and make sense of what is happening around them. Hence, our data will consist of free text answers. Systematic text condensation will be used for analysing the data.22
Biomarkers and CRF
CRF is a complex condition. Findings from a review of the literature may indicate that CRF is associated with immunological, inflammatory, metabolic, neuroendocrine and genetic biomarkers. Several genes and signalling molecules are associated with fatigue. Some studies show a change in IL1, IL6, tumour necrosis factor-alpha (TNF-α), C-reactive protein (CRP) and aPL in addition to the current genotype genes TNF-308 and IL-6–174. A higher CRP is significantly associated with worse fatigue in breast cancer survivors.23
The biomarkers mentioned will be measured in the project in addition to current genotype genes TNF-308 and IL-6–174. Cytokines exhibit significant circadian changes. TNF-α and IL-6 have higher levels observed during the early night in comparison to the early day. To mitigate the confounding effects of circadian changes on cytokine measurements, all blood samples for this study should be collected within a standardised time window, between 9 a.m. and 5
a.m. and 5 p.m. Furthermore, sleep deprivation can impact cytokine levels. To account for this, the measurement of sleep was included in the questionnaire. Data for both the treatment and control groups shall be obtained from questionnaires and kept alongside the blood samples. The first blood sample will be taken during their first appointment for both groups. Next, the blood sample will be taken after the end of treatments for the treatment group and 12 weeks after the first appointment for the control group. For both groups, a blood sample will be taken 12 weeks after the last one. A detailed approved Biomarker Protocol is available in an online supplemental file 2.
p.m. Furthermore, sleep deprivation can impact cytokine levels. To account for this, the measurement of sleep was included in the questionnaire. Data for both the treatment and control groups shall be obtained from questionnaires and kept alongside the blood samples. The first blood sample will be taken during their first appointment for both groups. Next, the blood sample will be taken after the end of treatments for the treatment group and 12 weeks after the first appointment for the control group. For both groups, a blood sample will be taken 12 weeks after the last one. A detailed approved Biomarker Protocol is available in an online supplemental file 2.
Effect measures
The degree of fatigue, as measured by the modified version of the Chalder Fatigue Questionnaire (FQ), will be taken for all participants at inclusion/baseline and after the acupuncture treatment for the intervention group, that is, 12 weeks after study inclusion. The control group will have their measurements 12 weeks after the inclusion. Follow-up measurements in both groups at 6 months after baseline evaluation will then be taken. The reason for using the FQ for fatigue is that it is used in the Norwegian Cancer Register for registering CRF in breast cancer, thus making possible comparisons between the registry and the study population.
Primary outcome measure
Our primary endpoint is changes in scores of CRF from baseline to 12 weeks as measured by a modified version of the Chalder FQ. The Chalder Fatigue Scale is a 14-item questionnaire. In FQ, three questions have been removed, leaving an 11-item questionnaire to measure the severity of physical and mental fatigue on two separate subscales. Items 1–7 represent physical fatigue, while 8–11 cover mental fatigue. It is brief and easy to administer, and it has been used in population studies and has normative data available for comparison with people with cancer.
Secondary outcome measures
The fatigue severity scale (FSS) consists of nine items and is one of the most often used instruments for measuring the impact of fatigue in patients with chronic illnesses. It has also been used in breast cancer survivors. The psychometric properties of the Norwegian version of the FSS have been evaluated.24 Furthermore, the FSS is used to compare sleepiness (Epworth) and fatigue.
Among cancer survivors with CRF, there are reports of depression. To evaluate this, we will use the hospital and anxiety depression scale (HADS). This scale is both reliable and valid in diverse groups of cancer patients and is the most frequently used questionnaire for measuring depression in relation to fatigue in cancer populations.25 The Norwegian version of the HADS is a relatively well-validated screening instrument for symptoms of psychological distress.26
Since there is a possible correlation between fatigue and different sleep patterns, we will measure sleep and sleepiness using the Bergen Insomnia Scale and Epworth. Both scales are accessible and have been validated in Norwegian.27 The Bergen Insomnia Scale has good psychometric properties. It is one of the few insomnia scales that provide normative data for comparisons and has been validated against subjective as well as polysomnographic data.28
Sleep in breast cancer survivors who had objectively documented hot flashes warrants attention because sleep disruptions may be related to post-treatment fatigue. Hot flashes are a significant problem for breast cancer survivors. Among survivors aged 40 or younger, 46% reported hot flashes. Hot flashes have also been associated with fatigue, worse physical health and pain.29 Hot flashes are persistent among women who are given Tamoxifen and/or Zoladex as a part of their post cancer treatment, ie, the women in the present study. Therefore, we will measure hot flashes by asking participants to report the frequency and severity of their flashes in a self-managed diary. Such approaches have been used in previous studies of hot flashes in cancer survivors.30
The instrument developed by the European Organisation for Research and Treatment of Cancer QOL Cancer Specific Version (EORTC QLQ-C30) will be used to measure QoL. It is a widely used, cancer-specific, self-administered questionnaire with strong validity. Added to this will be the EORTC QLQ-BR23, a questionnaire that is a complementary module to QLQ-C30 and is dedicated to patients with breast cancer. The most widely used EuroQol five-dimensional questionnaire (EQ-5D) will also be used as a measure of QoL. EQ-5D is a generic preference-based measure of health used across clinical, disease conditions and population surveys.31
Effect modifiers
To explore potential treatment effect modifiers, we will collect data at baseline on each participant’s age, education and employment, income and ethnicity as well as preferences, expectations and beliefs about the interventions.
Patient and public involvement
The user perspective is taken care of by a user representative with a special responsibility for ensuring that the research is in line with the preferences, values and needs of breast cancer survivors suffering from CRF. User involvement will primarily consist of patients and their next of kin. The primary aim is to ensure that the participants will benefit from our research project after it is completed. The Norwegian Cancer Society (NCS) will be contacted in due time before the study starts to plan for user involvement. In this project, the user will be a consultant who will participate throughout the entire research process from planning to conducting and implementing the results. Through this, the user representative can comment on the planning of the project and be a member of the research project’s steering group. A key person within the research team will be the user representative’s contact.
The user representative will receive training and follow-up to be able to assess the tasks thoroughly. The user representative must have a good command of English and Norwegian and adequate computer skills.
Cost-effectiveness
In both control and treatment arms, we will collect data on health service resource use, including visits to GPs, specialists, psychologists, physiotherapists and the use of medication at baseline and 6-month follow-up. Quantities of services used will be multiplied by national unit cost estimates to obtain cost profiles for participants in the trial. Costs related to acupuncture treatment are unique to the intervention group. We will also collect data on productivity-related costs, such as days off work due to fatigue. The cost of productivity changes will be presented separately in accordance with the National Institute for Health and Care Excellence (NICE) as a sensitivity analysis.
The intervention outcome will be measured by QALY. QALY combines change in the length of life and QoL due to the intervention into a single metric. We will use self-reported EQ-5D-5L questionnaires to calculate QALY. EQ-5D is the most widely used preference-based instrument to measure the ‘quality’ weight of QALY in economic evaluation.32 As a Norwegian value set for the EQ-5D-5L is not yet available, we will use a value set derived from the stated preference data of members of the Danish general public.33 In the absence of a national value set, the Norwegian Medicines Agency recommends the UK’s cross-walk value set for single technology assessments.34 Thus, in the scenario analysis, we will apply utility weights from the UK general population calculated using a cross-walk algorithm, ie, mapping the EQ-5D-5L descriptive system data onto the EQ-5D 3-level (EQ-5D-3L value set) from van Hout et al.35 We have health outcome data on the individual-level responses to the five dimensions of the EQ-5D questionnaire at baseline and follow-up for both the intervention and control groups.
By using QALY as an outcome measure, we can evaluate the incremental cost per QALY of the intervention, which is usually referred to as the incremental cost-effectiveness ratio. The base-case evaluation will combine intervention-related costs and wider NHS costs with changes in QALYs, derived from EQ-5D 5 L, to perform a cost-utility analysis.
L, to perform a cost-utility analysis.
Statistical power and sample size
For the primary outcome measure (FQ), we expect that the intervention group will exhibit a 10% decrease in the mean score (from 22 to 20), while the control group will have no change. We assume that the SD for change in FQ is 5 (based on numbers presented byHanevik et al.36 Hence, using a statistical significance of 0.05 (5%) and a power of 0.8 (80%), we calculate a sample size of 200 (100 in each group) will be needed to obtain a statistically significant difference. Considering the loss to follow-up and possible changes in the control group, our sample size has been increased to 250 (125 in each group). The sample size was calculated using the statistical package Stata (version 15.0, Texas, USA).
Data analysis
All analyses will be blinded to group allocation in the analyses of group differences. The primary analysis is intention-to-treat, comparing differences in mean changes from baseline to the end of treatment after 14 weeks in the two groups, using a two-sample t-test. In the presence of significant baseline differences, the analysis of covariance will be employed to adjust for confounding variables, when analysing the difference in change over time. It is anticipated that the data will be somewhat right-skewed; therefore, analyses for the normality distribution of residuals will be performed after appropriate data conversion (ie, log transformation). Non-parametric methods will be performed for data (or log-transformed data) that is not normally distributed. Secondary analyses will compare the changes at different time points. Secondary measures will be analysed using appropriate parametric or non-parametric methods. Subgroup analyses include per-protocol analyses to assess the efficacy of acupuncture care and a comparison of changes in fatigue scores among participants grouped by TCM syndromes. Due to missing data caused by drop-outs, analyses of covariance may be extended to mixed effects models. For continuous measures, linear mixed-effects models will be applied, while for dichotomous data, logistic mixed-effects models will be applied. In these analyses, missingness under the assumption of missing at random will be assumed and accounted for. The results from the linear mixed models will be equal to the analyses from the covariance analyses in the situation of no missingness. Analysis of the qualitative data will be accomplished by systematic text condensation22 following four major steps1: reading all the material to obtain an overall impression and bracketing previous preconceptions2; identifying units of meaning, representing different aspects of participants’ experiences, and coding for these3; condensing and summarising the contents of each of the coded groups; and4 generalising descriptions and concepts concerning experiences.
Data management
All data sheets will be made anonymous by removing participants’ names and addresses. The personal information and the index that links trial numbers with individual participants are to be kept under lock and key, with the key in the possession of our coordinator. The trial number alone will identify all computerised data. To prevent reporting bias, a study coordinator at Kristiania University College will administer all the participant evaluation forms. A person who is not involved in the study will perform data entry and coding. This will take place at the Norwegian National Network for Arthroplasty and Hip Fractures, and data material will be stored here. Data will be analysed without knowing group allocation. Data will be computed on laptops, which are not connected to the internet. Participants in either study group, who choose to use any therapist-provided care to relieve fatigue (eg, massage, homeopathy or any prescribed medication to relieve fatigue from any source) during the 12-week study period, are to be followed up with registration of events and included in the intention-to-treat analysis.
Data and safety monitoring
A Steering Group will be responsible for quality control. In addition, the Steering Group can be convened at short notice in an emergency to decide on appropriate action to prevent recurrence. Meetings will consider any reported adverse events, protocol violations, recruitment rate and any issues concerning local coordination and acupuncture practitioners as well as any issues raised by participants.
Ethics and dissemination
The study shall be performed according to the Helsinki Declaration and Good Clinical Practice requirements. The Regional Committee for Medical and Health Research Ethics (REC southeast) have approved the study (REC southeast ID number: 112285). The trial is registered with ClinicalTrials.gov (NCT 04418115).
We will use public outreach to boost the impact of the work in the research project. The project aims at generating new knowledge as well as making an impact on the treatment of CRF in Norway. The results will be submitted to peer-reviewed journals for publication, and the results will be presented at national and international scientific meetings. We will actively interact with the patient organisation within the NCS. An overview of the results and main experiences from the studies will be written in Norwegian and submitted to a relevant Norwegian medical journal.
supplementary material
online supplemental file 1
online supplemental file 2
Acknowledgements
The late Hugh MacPherson was a colleague and close friend to most of the authors of the present protocol. The first author (TA) invited Hugh to be a member of the AcuBreast research group. Hugh played an important role in the development of the protocol submitted to Pink Ribbon Foundation in 2019 and was successfully funded through this committee. We would like to pay tribute to his work by listing him as the last author.
Footnotes
Funding: The study is funded by a grant from Pink Ribbon, a collaboration between the Norwegian Cancer Society and the Breast Cancer Society (Contract nr 207697-2019). The Biomarker study is funded by Kristiania University College. The funders have no influence in any part of the study nor in the dissemination of the results.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2023-077514).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Consent obtained directly from patient(s).
Patient and public involvement: Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Methods section for further details.
References
Review Process File
Articles from BMJ Open are provided here courtesy of BMJ Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1136/bmjopen-2023-077514
Read article for free, from open access legal sources, via Unpaywall:
https://bmjopen.bmj.com/content/bmjopen/14/7/e077514.full.pdf
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT04418115
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A randomised, double-blind, placebo-controlled, pilot trial of intravenous plasma purified alpha-1 antitrypsin for SARS-CoV-2-induced Acute Respiratory Distress Syndrome: a structured summary of a study protocol for a randomised, controlled trial.
Trials, 22(1):288, 19 Apr 2021
Cited by: 9 articles | PMID: 33874981 | PMCID: PMC8054126
Implementing an evidence-based somatic acupressure intervention in breast cancer survivors with the symptom cluster of fatigue, sleep disturbance and depression: study protocol of a phase II randomised controlled trial.
BMJ Open, 12(1):e054597, 20 Jan 2022
Cited by: 0 articles | PMID: 35058263 | PMCID: PMC8783815
Effects of Qigong, Tai Chi, acupuncture, and Tuina on cancer-related fatigue for breast cancer patients: A protocol of systematic review and meta-analysis.
Medicine (Baltimore), 99(45):e23016, 01 Nov 2020
Cited by: 3 articles | PMID: 33157949 | PMCID: PMC7647542
Review Free full text in Europe PMC
Integrating acupuncture: are there positive health outcomes for women?
J Zhejiang Univ Sci B, 18(3):233-238, 01 Mar 2017
Cited by: 7 articles | PMID: 28271658 | PMCID: PMC5369247
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (1)
Grant ID: P30 CA008748
Pink Ribbon Foundation, The Norwegian Cancer Society and The Norwegian Breast Cancer Society (1)
Grant ID: Contract nr 207697-2019

 1,2
1,2