Abstract
Background
Paragonimiasis, primarily caused by Paragonimus westermani and P. skrjabini in China, is a common food-borne parasitic zoonosis. However, the national distribution of Paragonimus spp. infection and its associated environmental determinants remain poorly understood. In this paper, we summarize the infection of P. westermani and P. skrjabini and describe key biogeographical characteristics of the endemic areas in China.Methods
Data on Paragonimus infection in humans and animal hosts were extracted from eight electronic databases, including CNKI, CWFD, Chongqing VIP, SinoMed, Medline, Embase, PubMed, and Web of Science. A random-effects meta-analysis model was used to estimate the pooled prevalence. All survey locations were georeferenced and plotted on China map, and scatter plots were used to illustrate the biogeographical characteristics of regions reporting Paragonimus infection.Results
A total of 28,948 cases of human paragonimiasis have been documented, with 2,401 cases reported after 2010. Among the 11,443 cases with reported ages, 88.05% were children or adolescents. The pooled prevalence of P. skrjabini is 0.45% (95% CI: 0.27-0.66%) in snails, 31.10% (95% CI: 24.77-37.80%) in the second intermediate host, and 20.31% (95% CI: 9.69-33.38%) in animal reservoirs. For P. westermani, the pooled prevalence is 0.06% (95% CI: 0.01-0.13%) in snails, 52.07% (95% CI: 43.56-60.52%) in the second intermediate host, and 21.40% (95% CI: 7.82-38.99%) in animal reservoirs. Paragonimus are primarily distributed in regions with low altitude, high temperature, and high precipitation. In northeastern China, only P. westermani infections have been documented, while in more southern areas, infections of both P. westermani and P. skrjabini have been reported.Conclusions
Paragonimiasis remains prevalent in China, particularly among children and adolescents. Variations exist in the intermediate hosts and geographical distribution of P. westermani and P. skrjabini. Additionally, altitude, temperature, and precipitation may influence the distribution of Paragonimus.Free full text

Infection and biogeographical characteristics of Paragonimus westermani and P. skrjabini in humans and animal hosts in China: A systematic review and meta-analysis
Abstract
Background
Paragonimiasis, primarily caused by Paragonimus westermani and P. skrjabini in China, is a common food-borne parasitic zoonosis. However, the national distribution of Paragonimus spp. infection and its associated environmental determinants remain poorly understood. In this paper, we summarize the infection of P. westermani and P. skrjabini and describe key biogeographical characteristics of the endemic areas in China.
Methods
Data on Paragonimus infection in humans and animal hosts were extracted from eight electronic databases, including CNKI, CWFD, Chongqing VIP, SinoMed, Medline, Embase, PubMed, and Web of Science. A random-effects meta-analysis model was used to estimate the pooled prevalence. All survey locations were georeferenced and plotted on China map, and scatter plots were used to illustrate the biogeographical characteristics of regions reporting Paragonimus infection.
Results
A total of 28,948 cases of human paragonimiasis have been documented, with 2,401 cases reported after 2010. Among the 11,443 cases with reported ages, 88.05% were children or adolescents. The pooled prevalence of P. skrjabini is 0.45% (95% CI: 0.27–0.66%) in snails, 31.10% (95% CI: 24.77–37.80%) in the second intermediate host, and 20.31% (95% CI: 9.69–33.38%) in animal reservoirs. For P. westermani, the pooled prevalence is 0.06% (95% CI: 0.01–0.13%) in snails, 52.07% (95% CI: 43.56–60.52%) in the second intermediate host, and 21.40% (95% CI: 7.82–38.99%) in animal reservoirs. Paragonimus are primarily distributed in regions with low altitude, high temperature, and high precipitation. In northeastern China, only P. westermani infections have been documented, while in more southern areas, infections of both P. westermani and P. skrjabini have been reported.
Conclusions
Paragonimiasis remains prevalent in China, particularly among children and adolescents. Variations exist in the intermediate hosts and geographical distribution of P. westermani and P. skrjabini. Additionally, altitude, temperature, and precipitation may influence the distribution of Paragonimus.
Author summary
Paragonimiasis, a foodborne zoonotic parasitic disease caused by lung flukes (Paragonimus spp.), remains a significant neglected public health threat in many Asian countries, including China. Human infection occurs through the ingestion of raw or undercooked freshwater crab or crayfish containing the metacercariae stage. Given the popularity of consuming raw or undercooked freshwater products in many areas of China, understanding the infection status and spatial distribution of Paragonimus spp. in humans and animal hosts is crucial for controlling paragonimiasis. Our study provides a comprehensive summary of the infection levels of the two most important zoonotic Paragonimus species, P. westermani and P. skrjabini, in humans and animal hosts in China, along with a description of the spatial distribution and environmental characteristics of their endemic areas. We observe a wide distribution of Paragonimus infection in China, with a significant prevalence found in freshwater crabs and crayfish. Our findings underscore the importance of avoiding the consumption of raw or undercooked freshwater products to prevent foodborne diseases, including paragonimiasis.
Introduction
Paragonimiasis is a food-borne zoonotic disease caused by several species of lung flukes belonging to genus Paragonimus spp. [1]. The infection occurs primarily in the lungs and pleura of humans and animals. When the parasite infects the lungs, it can cause a pulmonary disease resembling tuberculosis and lung cancer [2]. Misdiagnosis of paragonimiasis as pulmonary tuberculosis or lung cancer can lead to significant socioeconomic losses and impose a mental and physical burden on patients due to unnecessary hospitalization, laboratory tests, surgical procedures, and prolonged medication [3]. Human paragonimiasis is widely distributed in Asia, Americas, and Africa, and is still a significant neglected public health threat in China. The global estimate of infected individuals is approximately 20 million, with around 293 million individuals at risk [4]; however, these figures may have been underestimated. New endemic areas are continually being identified, such as in India [5]. It is worth noting that a significant number of paragonimiasis cases have been misdiagnosed as pneumonia, tuberculosis, or lung cancer [6,7]. An estimated 293.8 million individuals are at risk of Paragonimus spp. infection, with 195 million of them residing in China [8,9].
More than 30 Paragonimus species have been documented in China, among which P. westermani and P. skrjabini are the most important zoonotic species [2,10]. P. westermani (Japanese lung fluke or oriental lung fluke) is most commonly distributed in eastern Asia and in South America, and is the most common cause of human paragonimiasis. P. skrjabini is especially prevalent in China, with cases appearing in India and Vietnam as well [1,11]. P. westermani followed by P. skrjabini are the major pathogens for human paragonimiasis in China [9].
Parasites of Paragonimus spp. have a three-host life cycle, with aquatic snails serving as the first intermediate host, freshwater decapod crustaceans as the second intermediate host, while human and other mammals as the definitive host. Human infection is acquired by eating inadequately cooked or pickled freshwater crabs or cray fishes containing the infective forms called metacercariae [12,13]. Drinking untreated stream or river water is also considered to be a possible route of infection [14].
Given the three host nature of the parasite and the fact that consuming raw or undercooked freshwater products is still popular in many areas of China, the infection status of Paragonimus spp. in animal hosts is closely related to the epidemic of human paragonimiasis [15]. Therefore, comprehending the level of infection in animals will provide valuable insights for controlling human paragonimiasis. However, prevalence estimates of Paragonimus spp. infection in the literature vary greatly across different studies. To date, there has been no comprehensive estimation of Paragonimus spp. infection in humans and animal hosts. In addition, very few attempts at the spatial and environmental characteristics of Paragonimus spp. infection in China have been made. Consequently, the aims of the current study are to summarize the infection level of two most important zoonotic Paragonimus species, P. westermani and P. skrjabini, in humans and animal hosts in China, and to describe the spatial distribution and environmental characteristics of their endemic areas.
Method
Literature retrieval and selection
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines [16], and has been registered with PROSPERO under the identifier CRD42024474528.
A systematic literature search was conducted to identify all studies reporting Paragonimus infection in humans and animals from inception to January 1, 2024, using the following electronic databases: China National Knowledge Infrastructure (CNKI), Chinese Wanfang database (CWFD), Chongqing VIP, SinoMed, Medline, Embase, PubMed, and Web of Science. Full-text search was performed using the terms ‘paragonimiasis’, ‘Paragonimus’, ‘lung fluke’, ‘lung trematode’, in conjunction with ‘China’. The search was limited to English and Chinese languages.
After removing duplicates, two reviewers (KL and YC-S) independently reviewed all the titles and abstracts, with assistance of a third reviewer (RT-P) to reach a consensus in case of disagreement. Subsequently, the full texts were assessed for inclusion by the same reviewers. All studies included in the meta-analysis were published in English or Chinese, and were primary research articles, and epidemiological studies reporting prevalence of Paragonimus in humans and animal hosts. Studies were further excluded from meta-analysis if they were letters to the editor, non-epidemiological studies, or had a sample size of fewer than 20 [17]. Additionally, we collected case reports and case series of human infections to summarize the characteristics of cases of human paragonimiasis.
Data extraction and quality assessment
The following information was extracted from the included articles: title, first author, language, year of publication, year of investigation, study location, Paragonimus species, detection method, sample size, number of positive cases, prevalence, taxonomic category of animal hosts (include genus and family), and life style of animal hosts. For human studies, we also collected information on the gender and type of specimens. In population-based surveys, the participants first underwent immunological testing (usually skin testing), and those who tested positive further underwent etiological testing. In this case, the prevalence was calculated using the total number of participants as the denominator, with etiologically confirmed positives as the numerator.
Two reviewers (KL and YC-S) independently evaluated the quality of each included study using a standardized assessment tool developed by Hoy [18]. This tool provides ten items to access the risk of bias, with each item given a score of 0 or 1 for the absence or presence of bias. A summary score of 0–3 indicates a low risk of bias, 4–6 indicates a moderate risk of bias, and 7–10 indicates a high risk of bias.
Statistical analysis
Freeman-Tukey double arcsine transformation was used to normalize the prevalence and ensure the validity of subsequent analyses [19]. Heterogeneity across studies was assessed using Cochran’s Q test and I2 statistics, where I2 statistics quantified the percentage of variation across studies (with I2 values indicating low, moderate, and high heterogeneity at 25%, 50%, and 75%, respectively). If the heterogeneity is statistically significant, a random-effects model was used for meta-analysis; otherwise, a fixed-effects model was used [20,21]. The random-effects model and Peto method were ultimately used to estimate the pooled prevalence as well as their confidence interval (CI) in this study [22], following the results of the heterogeneity test. Additionally, subgroup analyses were employed to explore the potential source of heterogeneity across studies, conducted meta-regression analysis with moderators as independent variables and prevalence as the dependent variable to further assess the effects of moderators on the prevalence.
R2, QM, and QE statistics were utilized to interpret the results of subgroup and meta-regression analyses [17]. R2 represents the proportion of true heterogeneity that can be explained by the moderator; QM and its P-value determine the significance of the moderators in explaining heterogeneity; and QE and its P-value evaluate the significance of unexplained residual heterogeneity [23,24].
Funnel plots and Egger’s test were employed to assess potential publication bias. Sensitivity analyses were conducted to evaluate the robustness of the pooled estimate [25,26]. Initially, outlier analyses were performed using Baujat plots. Studies located in the top right quadrant of the Baujat plot, or with studentized residuals exceeding 2 in absolute value, were considered potential outliers. After removing identified outliers, the overall pooled prevalence estimates were recalculated and compared with the main findings. Furthermore, we examined whether excluding smaller-sample data points (i.e., data points with the lowest quintile of sample sizes) yielded findings similar to the main results.
All statistical analyses were performed using R4.2.1 software (Lucent Technologies, Jasmine Mountain, USA). For all tests, p values less than 0.05 were considered statistically significant.
Data collection on environmental factors and visualization of the spatial distribution and biogeographical characteristics
Baidu Map was used to determine the latitude and longitude coordinates of each study location. For human infection, all etiological confirmed paragonimiasis cases documented in population surveys, case reports, and case series were included in the spatial analyses. Environmental factors for each location, including annual mean temperature, annual precipitation, mean temperature of warmest quarter, precipitation of warmest quarter, mean temperature of coldest quarter, precipitation of coldest quarter were obtained from the WorldClim database (https://www.worldclim.org/) [27]. Altitude data was obtained from the Space Shuttle Radar Topography Mission (SRTM, http://www.gscloud.cn/) [28].
To visualize the spatial distribution of P. westermani and P. skrjabini infection, we georeferenced the etiologically definite human paragonimiasis cases and the prevalence of various animal hosts, and plotted them on a map of China using software ArcGIS10.7 (Environmental System Research Institute, Redlands, USA). The base layers of maps were downloaded from Resource and Environment Science Data Center of the Chinese Academy of Sciences (RESDC, http://www.resdc.cn) [29]. Additionally, scatter plots were used to illustrate the biogeographical characteristics of regions reporting P. westermani and P. skrjabini infection. T-tests were further conducted to explore the potential differences in biogeographical characteristics between the two Paragonimus species.
Results
Literature selection and quality assessment
Initially, 16,876 publications were identified through literature search. After removing duplicates, 10,642 articles were screened based on titles and abstracts, resulting in 1,880 articles for full-text assessment. Following full-text assessments, 38 studies were ultimately included in the meta-analysis for human infections, 107 for snail infections, 172 for infections in the second intermediate host, and 22 for infections in animal reservoirs (Fig 1). Additionally, we extracted human case report and case series from 965 publications (S1 Table). Among the collected literature, the earliest report of Paragonimus infection in humans or animals in China was in 1954 (S1–S5 Tables).
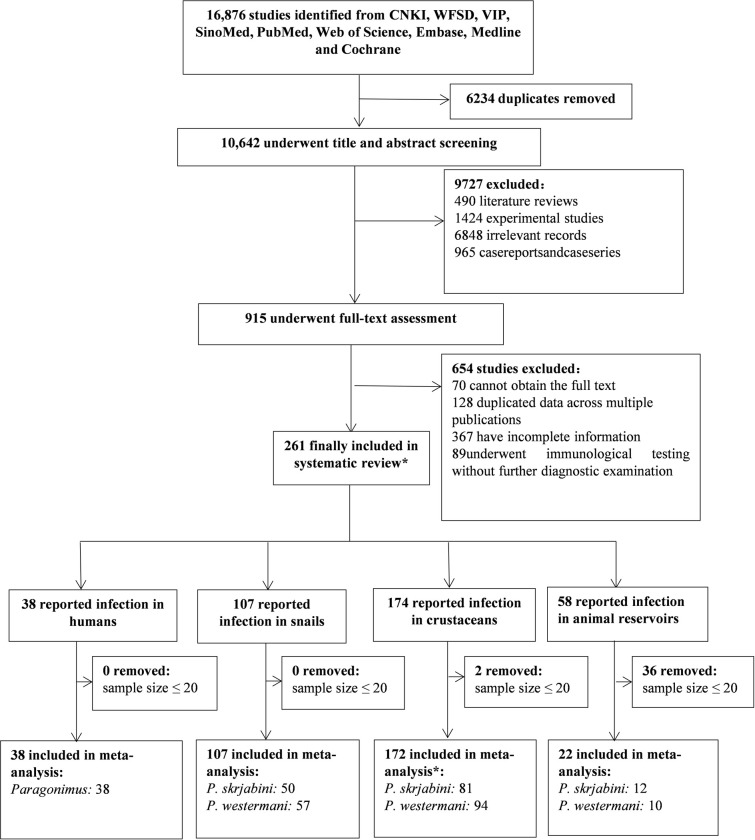
* Due to some studies simultaneously investigating humans, intermediate hosts, and reservoir hosts, the total number of literatures included in the systematic review (271) does not equal the sum of literature for different types of hosts. Similarly, several studies reported infection rates of both lung flukes in crustaceans, resulting in the total number of literature included in the study for the second intermediate host (172) being less than the sum of literature for P. westermani and P. skrjabini.
In the risk of bias assessment, all the studies were rated as having low to moderate bias (S2–S5 Tables). Specifically, 6 out of 38 publications for human infections, 81 out of 107 publications for snail infections, 143 out of 172 for the second intermediate hosts, and 20 out of 22 for animal reservoirs were rated as having low bias, the most common risk identified was the lack of random selection of the sample.
Infection of P. westermani and P. skrjabini in humans
A total of 28,948 cases of human paragonimiasis have been reported in the literature, of which 2,401 cases occurred after 2010, 14,654 cases were male, and 6,089 cases were from rural areas (see Table 1). Additionally, a total of 10,076 cases of infection in children or adolescents have been reported, with 8,695 cases reported before 2010 and 1,381 reported after 2010. However, the number of cases by gender, source, and age is obviously higher than those documented, given that a considerable number is unspecified. As shown in Fig 2, human infections of Paragonimus have been documented in all provinces except for Tibet, Qinghai, Gansu, Ningxia, Macao, and Hong Kong. The cases of human infection are mainly documented in provinces or municipalities in the Yangtze River Basin, including Chongqing (6,035), Zhejiang (5,324), Hubei (4,945), Sichuan (2,896), and Hunan (1,414), which together account for 71.21% of the total national cases (see Table 1). It is worth noting that after 2010, there are still a considerable number of reported cases in areas such as Chongqing (1,073) and Sichuan (595), and many other provinces and municipalities also continue to report cases.

The base layer of the map was downloaded from Resource and Environment Science Data Center of the Chinese Academy of Sciences (RESDC, http://www.resdc.cn).
Table 1
| 1954–1990 | 1990–1999 | 2000–2009 | 2010–present | Total | |
|---|---|---|---|---|---|
| Province | |||||
| Chongqing | 779 | 984 | 3199 | 1073 | 6035 |
| Zhejiang | 1879 | 1649 | 1595 | 201 | 5324 |
| Hubei | 2466 | 989 | 1440 | 50 | 4945 |
| Sichuan | 640 | 537 | 1124 | 595 | 2896 |
| Guizhou | 1202 | 295 | 197 | 149 | 1843 |
| Hunan | 755 | 546 | 106 | 7 | 1414 |
| Shaanxi | 489 | 311 | 161 | 14 | 975 |
| Liaoning | 261 | 604 | 33 | 3 | 901 |
| Fujian | 490 | 33 | 341 | 4 | 868 |
| Anhui | 636 | 95 | 2 | 0 | 733 |
| Shanghai | 182 | 193 | 215 | 26 | 616 |
| Heilongjiang | 542 | 13 | 3 | 0 | 558 |
| Jiangsu | 82 | 369 | 38 | 40 | 529 |
| Henan | 176 | 104 | 44 | 64 | 388 |
| Beijing | 99 | 24 | 12 | 67 | 202 |
| Shandong | 0 | 176 | 5 | 0 | 181 |
| Jiangxi | 157 | 9 | 6 | 0 | 172 |
| Yunnan | 17 | 1 | 31 | 89 | 138 |
| Jilin | 92 | 3 | 0 | 2 | 97 |
| Guangdong | 4 | 23 | 19 | 15 | 61 |
| Shanxi | 0 | 14 | 14 | 0 | 28 |
| Guangxi | 1 | 23 | 1 | 0 | 25 |
| Hebei | 9 | 1 | 1 | 1 | 12 |
| Hainan | 0 | 0 | 3 | 0 | 3 |
| Inner Mongoria | 0 | 0 | 1 | 0 | 1 |
| Taiwan | 0 | 0 | 0 | 1 | 1 |
| Tianjing | 1 | 0 | 0 | 0 | 1 |
| Xinjiang | 0 | 1 | 0 | 0 | 1 |
| Gansu | 0 | 0 | 0 | 0 | 0 |
| Ningxia | 0 | 0 | 0 | 0 | 0 |
| Qinghai | 0 | 0 | 0 | 0 | 0 |
| Tibet | 0 | 0 | 0 | 0 | 0 |
| Hong Kong | 0 | 0 | 0 | 0 | 0 |
| Macao | 0 | 0 | 0 | 0 | 0 |
| Age | |||||
| < 18 | 2613 | 2660 | 3422 | 1381 | 10076 |
| ≥ 18 | 386 | 454 | 298 | 229 | 1367 |
| Not specified | 7960 | 3883 | 4871 | 791 | 17505 |
| Gender | |||||
| Male | 4201 | 4227 | 4587 | 1639 | 14654 |
| Female | 1677 | 1932 | 2220 | 677 | 6506 |
| Not specified | 5081 | 838 | 1784 | 85 | 7788 |
| Source | |||||
| Urban | 540 | 263 | 327 | 52 | 1182 |
| Rural | 1462 | 1270 | 2992 | 365 | 6089 |
| Not specified | 8957 | 5464 | 5272 | 1984 | 21677 |
| Total | 10959 | 6997 | 8591 | 2401 | 28948 |
Only a few cases differentiated whether the infection was caused by P. westermani and P. skrjabini. Cases of P. westermani infection are widely distributed, while P. skrjabini infections are primarily concentrated in more southern regions (Fig 2).
A total of 38 studies, containing 662,003 participants, reported screening for Paragonimus infection in human populations (see S2 Table), with 253 confirmed cases being reported and pooled prevalence of 0.05% (95% CI: 0.00–0.12%). The heterogeneity across the studies was high (I2 = 93.3%, Table 2; forest plot shown in S1A Fig). Subgroup analysis and the meta-regression model indicated that none of the moderators could significantly explain the heterogeneity (see S6 Table).
Table 2
| No. of data points | Sample size | No. of positive | Pooled prevalence, % (95% CI) | I2, % | R2, % (QM P value) | QE P value | |
|---|---|---|---|---|---|---|---|
| Pathogen | 54 | 662003 | 253 | 0.05 (0.00; 0.12) | 93.3 | 0.00 (0.575) | < 0.0001 |
| P. westermani | 30 | 58811 | 127 | 0.07 (0.00; 0.19) | 90.1 | ||
| P. skrjabini | 9 | 10636 | 22 | 0.04 (0.00; 0.22) | 87.1 | ||
| Not specified | 15 | 592556 | 104 | 0.03 (0.00; 0.16) | 95.2 | ||
| Year of investigation | 4.36 (0.290) | < 0.0001 | |||||
| 1954–1990 | 36 | 57540 | 184 | 0.08 (0.01; 0.20) | 88.4 | ||
| 1990–1999 | 7 | 32729 | 35 | 0.08 (0.00; 0.33) | 91.6 | ||
| 2000–2010 | 5 | 81900 | 25 | 0.02 (0.00; 0.21) | 66.7 | ||
| 2010–present | 6 | 489834 | 9 | 0.00 (0.00; 0.06) | 33.0 | ||
| Gender | 4.17 (0.156) | < 0.0001 | |||||
| Man | 8 | 16340 | 1 | 0.00 (0.00; 0.11) | 0 | ||
| Woman | 7 | 5174 | 0 | 0.00 (0.00; 0.13) | 0 | ||
| Not specified | 39 | 640489 | 252 | 0.09 (0.02; 0.19) | 95.2 | ||
| Specimen | 0.00 (0.357) | < 0.0001 | |||||
| Sputum | 38 | 100117 | 187 | 0.04 (0.00; 0.12) | 90.3 | ||
| Stool | 14 | 492277 | 22 | 0.03 (0.00; 0.18) | 79.2 | ||
| Stool or sputum | 2 | 69609 | 44 | 0.45 (0.04; 1.27) | 98.5 |
R2 represents the proportion of true heterogeneity that can be explained by the moderator, the QE P value shows the significance of residual heterogeneity that is unaccounted for by the moderator, and the QM P value shows whether the moderator is statistically significant in explaining heterogeneity.
Infection of P. westermani and P. skrjabini in the first intermediate hosts
A total of 57 studies reported the presence of P. westermani infection in the first intermediate host (snails), with prevalence ranging from 0.00% to 6.72% (see S3 Table). The pooled prevalence of P. westermani in the first intermediate host was 0.11% (95% CI: 0.02–0.25%), and there was high heterogeneity across the studies (I2 = 93.6%, Table 3; forest plot is presented in S1B Fig). Semisulcospira spp. was identified as the most common vector of P. westermani, with a pooled prevalence of 0.12% (95% CI: 0.02–0.28%). Additionally, Tricula spp., Erhaiini spp., and Bythinella spp. were identified as potential vectors of P. westermani.
Table 3
| No. of data points | Sample size | No. of positive | Pooled prevalence, % (95% CI) | I2, % | R2, % (QM P value) | QE P value | |
|---|---|---|---|---|---|---|---|
| P. westermani | 61 | 263423 | 639 | 0.11 (0.02; 0.25) | 93.6 | ||
| Year of investigation | 3.35 (0.149) | < 0.0001 | |||||
| 1954–1990 | 32 | 120342 | 425 | 0.14 (0.01; 0.36) | 91.7 | ||
| 1990–1999 | 12 | 69290 | 52 | 0.04 (0.00; 0.27) | 89.4 | ||
| 2000–2009 | 11 | 58357 | 81 | 0.00 (0.00; 0.24) | 85.6 | ||
| 2010–present | 6 | 15434 | 81 | 0.62 (0.14; 1.39) | 97.3 | ||
| Genus of snail | 0.00 (0.637) | < 0.0001 | |||||
| Semisulcospira | 54 | 240158 | 599 | 0.12 (0.02; 0.28) | 94.1 | ||
| Tricula | 5 | 15918 | 11 | 0.04 (0.00; 0.41) | 62.5 | ||
| Erhaiini | 1 | 6227 | 26 | 0.42 (0.00; 2.49) | NE | ||
| Bythinella | 1 | 1120 | 3 | 0.27 (0.00; 2.27) | NE | ||
| P. skrjabini | 75 | 411797 | 1343 | 0.46 (0.27; 0.70) | 93.4 | ||
| Year of investigation | 0.00 (0.678) | < 0.0001 | |||||
| 1954–1990 | 22 | 112904 | 428 | 0.52 (0.18; 1.01) | 92.6 | ||
| 1990–1999 | 14 | 75143 | 247 | 0.34 (0.02; 0.90) | 93.9 | ||
| 2000–2009 | 24 | 193038 | 502 | 0.34 (0.07; 0.75) | 91.9 | ||
| 2010–present | 15 | 30712 | 166 | 0.74 (0.26; 1.43) | 95.5 | ||
| Genus of snail | 0.00 (0.830) | < 0.0001 | |||||
| Tricula | 36 | 253031 | 643 | 0.58 (0.28; 0.96) | 94.6 | ||
| Pseudobythinella | 11 | 64914 | 340 | 0.57 (0.11; 1.32) | 90.5 | ||
| Bythinella | 10 | 9481 | 79 | 0.41 (0.01; 1.19) | 84.7 | ||
| Semisulcospira | 9 | 63806 | 178 | 0.06 (0.00; 0.60) | 79.6 | ||
| Erhaiini | 3 | 5789 | 26 | 0.80 (0.00; 2.66) | 92.4 | ||
| Akiyoshia | 3 | 2575 | 20 | 0.71 (0.00; 2.48) | 89.5 | ||
| Oncomelania | 2 | 10925 | 57 | 0.20 (0.00; 2.28) | 0.0 | ||
| Assiminea | 1 | 1276 | 0 | 0.00 (0.00; 1.83) | NE |
NE: not estimated; R2 represents the proportion of true heterogeneity that can be explained by the moderator, the QE P value shows the significance of residual heterogeneity that is unaccounted for by the moderator, and the QM P value shows whether the moderator is statistically significant in explaining heterogeneity.
Fifty studies reported P. skrjabini infection in the first intermediate host, with prevalence varied from 0.00% to 14.80% (see S3 Table). The pooled prevalence of P. skrjabini in the first intermediate host was 0.46% (95%CI: 0.27–0.70%), and the heterogeneity across studies was high (I2 = 93.4%, Table 3; forest plot was shown in S1C Fig). The majority of infections in snails were reported in Tricula spp., with a pooled prevalence of 0.58% (95% CI: 0.28–0.96%). Additionally, Pseudobythinella spp., Bythinella spp., Semisulcospira spp., Oncomelania spp., Erhaiini spp., and Akiyoshia spp. were also potential vectors of P. skrjabini.
Spatial distribution of P. westermani and P. skrjabini infection in the first intermediate hosts is depicted in Figs Figs33 and and4.4. In the northeast area of China, Semisulcospira spp. serve as the primary transmission vectors of Paragonimus, and only P. westermani infection has been reported in this region. In more southern areas, Semisulcospira spp. are identified as the primary transmission vectors of P. westermani, while Tricula spp. are identified as the primary transmission vectors of P. skrjabini.
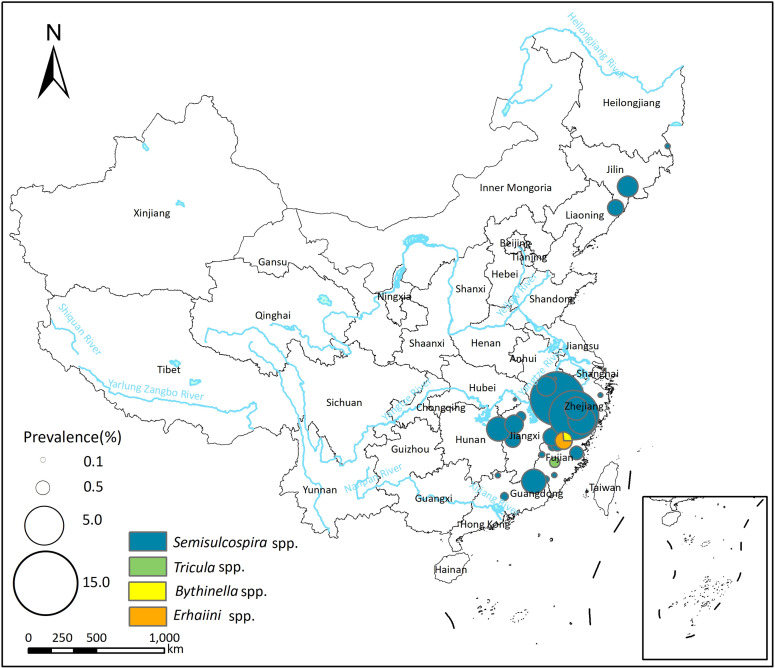
The base layer of the map was downloaded from Resource and Environment Science Data Center of the Chinese Academy of Sciences (RESDC, http://www.resdc.cn).
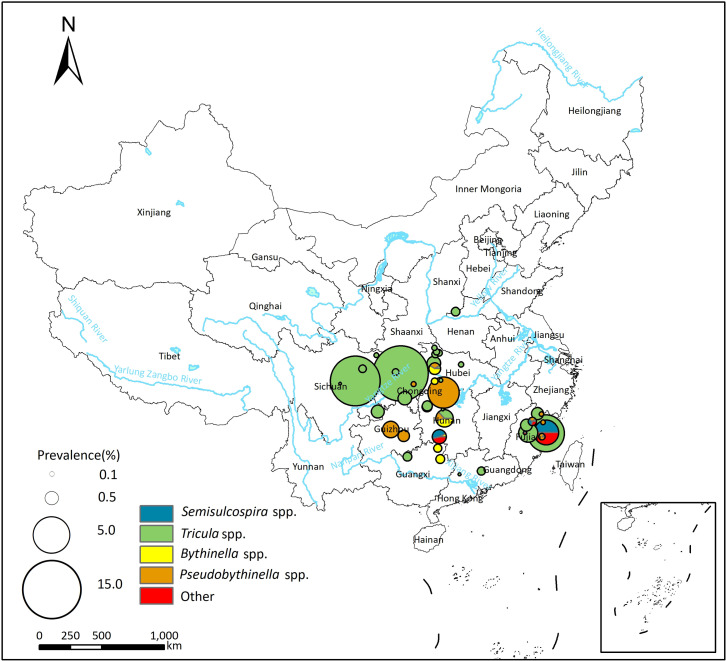
The base layer of the map was downloaded from Resource and Environment Science Data Center of the Chinese Academy of Sciences (RESDC, http://www.resdc.cn).
Subgroup analysis and the meta-regression model indicated that the prevalence of P. westermani and P. skrjabini in the first intermediate host did not exhibit significant differences across different snail genera and time periods (see Tables Tables33 and S7).
Infection of P. westermani and P. skrjabini in the second intermediate hosts
In total, 94 studies reported P. westermani infection in the second intermediate host (see S4 Table), with a pooled prevalence of 52.02% (95% CI: 44.35–59.64%) and high heterogeneity across studies (I2 = 99.6%, Table 4; forest plot presented in S1D Fig). Genus Cambaroides was identified as the primary second intermediate host for P. westermani in the northeastern areas of China (Fig 5), with a pooled prevalence of 59.79% (95% CI: 42.65–75.79%; Table 4). In other areas of China, Sinopotamon spp. were the primary second intermediate host, with a pooled prevalence of 52.86% (95% CI: 43.68–61.94%); other freshwater crabs such as Nanhaipotamon spp. and Huananpotamon spp. could also serve as the second intermediate host (see Tables Tables44 and S4).
Table 4
| No. of data points | Sample size | No. of positive | Pooled prevalence, % (95% CI) | I2, % | R2, % (QM P value) | QE P value | |
|---|---|---|---|---|---|---|---|
| P. westermani | 100 | 165276 | 40049 | 52.02 (44.35; 59.64) | 99.6 | ||
| Year of investigation | 3.27 (0.097) | < 0.0001 | |||||
| 1954–1990 | 44 | 86716 | 24212 | 62.67 (51.44; 73.25) | 99.5 | ||
| 1990–1999 | 15 | 66150 | 10488 | 43.24 (24.96; 62.51) | 99.8 | ||
| 2000–2009 | 22 | 6490 | 2762 | 41.69 (26.41; 57.82) | 98.8 | ||
| 2010–present | 19 | 5920 | 2587 | 45.80 (29.06; 63.03) | 99.5 | ||
| Genus of hosts | 0.00 (0.492) | < 0.0001 | |||||
| Sinopotamon | 70 | 157429 | 35929 | 52.86 (43.68; 61.94) | 99.7 | ||
| Nanhaipotamon | 3 | 175 | 53 | 26.02 (0.26; 69.67) | 95.0 | ||
| Huananpotamon | 2 | 1349 | 697 | 27.65 (0.00; 79.19) | 98.8 | ||
| Malayopotamon | 1 | 21 | 3 | 14.29 (0.00; 88.26) | NE | ||
| Lithodes | 1 | 72 | 61 | 84.72 (14.42; 100.00) | NE | ||
| Eriocheir | 1 | 85 | 16 | 18.82 (0.00; 88.53) | NE | ||
| Cambaroides | 20 | 5515 | 3110 | 59.79 (42.65; 75.79) | 99.4 | ||
| Macrobrachium | 2 | 630 | 180 | 28.57 (0.00; 79.70) | 0.00 | ||
| Detection method | 0.00 (0.501) | < 0.0001 | |||||
| Artificial digestion | 55 | 94460 | 26665 | 50.15 (39.81; 60.49) | 99.4 | ||
| Direct compression | 25 | 8923 | 4135 | 59.75 (44.42; 74.17) | 99.5 | ||
| Not specified | 20 | 61893 | 9249 | 47.42 (30.79; 64.34) | 99.7 | ||
| P. skrjabini | 109 | 198209 | 41426 | 30.37 (24.72; 36.34) | 99.8 | ||
| Year of investigation | 1.90 (0.184) | < 0.0001 | |||||
| 1954–1990 | 24 | 21578 | 4833 | 32.76 (19.69; 47.33) | 99.1 | ||
| 1990–1999 | 22 | 84633 | 6773 | 21.85 (11.35; 34.57) | 99.8 | ||
| 2000–2009 | 23 | 66211 | 23849 | 40.59 (27.31; 54.59) | 99.9 | ||
| 2010–present | 40 | 25787 | 5971 | 30.13 (18.95; 42.62) | 99.2 | ||
| Genus of hosts | 7.59 (0.065) | < 0.0001 | |||||
| Sinopotamon | 74 | 167883 | 37566 | 31.53 (24.92; 38.53) | 99.8 | ||
| Nanhaipotamon | 7 | 1318 | 480 | 34.30 (13.74; 58.42) | 80.5 | ||
| Potamon | 5 | 1911 | 458 | 26.03 (6.31; 53.05) | 99.1 | ||
| Tenuilapotamon | 3 | 3195 | 2182 | 27.96 (3.52; 63.48) | 99.6 | ||
| Aparapotamon | 3 | 1880 | 307 | 15.66 (0.00; 48.85) | 78.0 | ||
| Bottapotamon | 3 | 189 | 127 | 75.72 (39.60; 98.48) | 96.1 | ||
| Malayopotamon | 2 | 104 | 62 | 42.94 (5.44; 85.95) | 95.8 | ||
| Huananpotamon | 2 | 82 | 27 | 33.89 (1.83; 78.46) | 0.0 | ||
| Sinolapotamon | 1 | 3596 | 6 | 0.17 (0.00; 38.11) | NE | ||
| Tiwaripotamon | 1 | 3898 | 2 | 0.05 (0.00; 36.44) | NE | ||
| Neilupotamon | 1 | 116 | 6 | 5.17 (0.00; 58.20) | NE | ||
| Parvuspotamon | 1 | 223 | 73 | 32.74 (0.00; 89.38) | NE | ||
| Potamiscus | 1 | 24 | 23 | 95.83 (38.43; 100.00) | NE | ||
| Tenuipotamon | 1 | 141 | 38 | 26.95 (0.00; 85.44) | NE | ||
| Lithodes | 3 | 13627 | 69 | 9.41 (0.00; 39.93) | 97.0 | ||
| Somanniathelphusa | 1 | 22 | 0 | 0.00 (0.00; 47.47) | NE | ||
| Detection method | 1.75 (0.135) | < 0.0001 | |||||
| Artificial digestion | 68 | 172632 | 35089 | 26.01 (19.38; 33.23) | 99.9 | ||
| Direct compression | 28 | 17125 | 4220 | 36.91 (25.41; 49.19) | 98.3 | ||
| Not specified | 13 | 8452 | 2117 | 40.59 (23.73; 58.66) | 99.2 |
NE: not estimated; R2 represents the proportion of true heterogeneity that can be explained by the moderator, the QE P value shows the significance of residual heterogeneity that is unaccounted for by the moderator, and the QM P value shows whether the moderator is statistically significant in explaining heterogeneity.

The base layer of the map was downloaded from Resource and Environment Science Data Center of the Chinese Academy of Sciences (RESDC, http://www.resdc.cn).
Eighty-one studies reported P. skrjabini infection in the second intermediate host (see S4 Table), with a pooled prevalence of 30.37% (95% CI: 24.72–36.34%) and high heterogeneity across studies (I2 = 99.8%, Table 4; forest plot presented in S1E Fig). In the northeastern region of China, only P. westermani has been reported in the second intermediate host, with no reports of the existence of P. skrjabini (see Fig 6). The second intermediate hosts of P. skrjabini included crabs of the Potamidae, Lithodidae, and Parathelphusidae families. Crabs of the Potamidae family were the most common second intermediate host, with Sinopotamon spp. being the most significant, exhibiting a pooled prevalence of 31.53% (95% CI: 24.92% - 38.53%). Additionally, other freshwater crabs such as Nanhaipotamon spp., Potamon spp., and Tenuilapotamon spp. of the Potamidae family, Somanniathelphusa spp. of the Parathelphusidae family, and Malayopotamon spp. of the Lithodidae family can also serve as the second intermediate hosts for P. skrjabini (see Table 4).
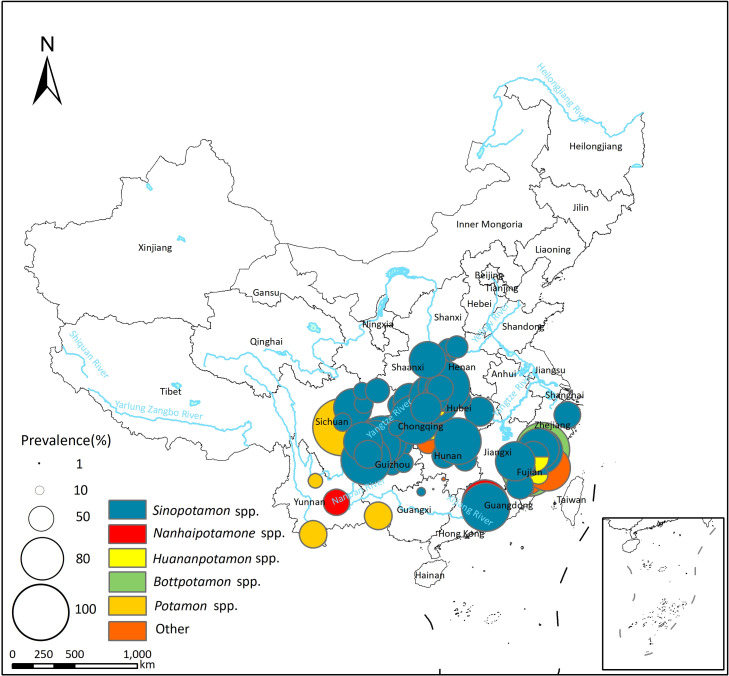
The base layer of the map was downloaded from Resource and Environment Science Data Center of the Chinese Academy of Sciences (RESDC, http://www.resdc.cn).
Subgroup analysis and the meta-regression model indicated that the prevalence of P. westermani and P. skrjabini in the second intermediate host did not exhibit significant differences among different crustacean genera, across different time periods, and with different detection methods (see Tables Tables44 and S8).
Infection of P. westermani and P. skrjabini in animal reservoirs
Overall, 10 studies reported P. westermani infection in animal reservoirs (see S5 Table), with a pooled prevalence of 21.40% (95% CI: 7.82–38.99%) and high heterogeneity across studies (I2 = 94.9%, Table 5; forest plot presented in S1F Fig). Cats (37.15% (95% CI: 9.61–69.92%)) and dogs (11.68% (95% CI: 0.00–36.56%)) were identified as the most common animal reservoirs for P. westermani.
Table 5
| No. of data points | Sample size | No. of positive | Pooled prevalence, % (95% CI) | I2, % | R2, % (QM P value) | QE P value | |
|---|---|---|---|---|---|---|---|
| P. westermani | 13 | 1353 | 307 | 21.40 (7.82; 38.99) | 94.9 | ||
| Year of investigation | 5.40 (0.266) | < 0.0001 | |||||
| 1954–1990 | 7 | 999 | 275 | 34.00 (12.82; 59.07) | 94.0 | ||
| 1990–1999 | 3 | 269 | 25 | 12.25 (0.00; 46.27) | 95.2 | ||
| 2010–present | 3 | 85 | 7 | 6.37 (0.00; 37.97) | 75.4 | ||
| Family of hosts | 0.00 (0.609) | < 0.0001 | |||||
| Canidae | 6 | 936 | 210 | 11.68 (0.00; 36.56) | 96.2 | ||
| Felidae | 5 | 299 | 74 | 37.15 (9.61; 69.92) | 96.1 | ||
| Viverridae | 1 | 66 | 13 | 19.70 (0.00; 85.85) | NE | ||
| Mustelidae | 1 | 52 | 10 | 19.23 (0.00; 85.87) | NE | ||
| Life style | 0.00 (0.702) | < 0.0001 | |||||
| Domestic | 10 | 1214 | 274 | 24.70 (4.93; 40.41) | 96.1 | ||
| Wild | 3 | 139 | 33 | 22.57 (1.18; 68.23) | 69.3 | ||
| Detection method | 0.00 (0.545) | < 0.0001 | |||||
| Sedimentation | 2 | 55 | 7 | 12.44 (0.00; 60.11) | 42.5 | ||
| Direct compression | 2 | 54 | 22 | 41.81 (2.41; 89.04) | 0.00 | ||
| Kato-Katz | 1 | 30 | 0 | 0.00 (0.00; 51.65) | NE | ||
| Not specified | 8 | 1214 | 278 | 23.59 (6.13; 47.41) | 96.6 | ||
| P. skrjabini | 20 | 1067 | 180 | 20.31 (9.69; 33.38) | 95.2 | ||
| Year of investigation | 10.34 (0.168) | <0.0001 | |||||
| 1954–1990 | 5 | 199 | 53 | 30.53 (8.60; 58.19) | 94.5 | ||
| 1990–1999 | 5 | 408 | 17 | 3.52 (0.00; 21.03) | 91.3 | ||
| 2000–2009 | 5 | 167 | 56 | 30.31 (8.36; 58.09) | 83.4 | ||
| 2010–present | 5 | 293 | 54 | 23.88 (4.94; 50.39) | 96.3 | ||
| Family of hosts | 26.53 (0.046) | < 0.0001 | |||||
| Felidae | 11 | 433 | 146 | 36.35 (20.74; 53.51) | 93.7 | ||
| Canidae | 5 | 319 | 20 | 5.79 (0.00; 23.03) | 79.5 | ||
| Muridae | 1 | 223 | 0 | 0.00 (0.00; 29.15) | NE | ||
| Viverridae | 1 | 43 | 8 | 18.60 (0.00; 72.12) | NE | ||
| Suidae | 1 | 21 | 0 | 0.00 (0.00; 39.21) | NE | ||
| Mustelidae | 1 | 28 | 6 | 21.43 (0.00; 76.78) | NE | ||
| Life style | 20.34 (0.018) | < 0.0001 | |||||
| Domestic | 11 | 480 | 130 | 33.12 (17.50; 50.78) | 95.3 | ||
| Wild | 9 | 587 | 50 | 8.09 (0.40; 22.06) | 92.3 | ||
| Detection method | 25.37 (0.029) | < 0.0001 | |||||
| Direct compression | 6 | 231 | 90 | 45.69 (23.38; 68.90) | 92.7 | ||
| Sedimentation | 9 | 542 | 65 | 13.88 (3.16; 29.65) | 94.9 | ||
| Kato-Katz | 2 | 194 | 5 | 1.28 (0.00; 24.62) | 70.6 | ||
| Not specified | 3 | 100 | 20 | 15.93 (0.05; 46.32) | 89.7 |
NE: not estimated; R2 represents the proportion of true heterogeneity that can be explained by the moderator, the QE P value shows the significance of residual heterogeneity that is unaccounted for by the moderator, and the QM P value shows whether the moderator is statistically significant in explaining heterogeneity.
Twelve studies reported P. skrjabini infection in animal reservoirs (see S5 Table), with a pooled prevalence of 20.31% (95% CI: 9.69–33.38%) and high heterogeneity across studies (I2 = 95.2%, Table 5; forest plot presented in S1G Fig). Similar to P. westermani, cats (36.35% (95% CI: 20.74–53.51%)) and dogs (5.79% (95% CI: 0.00–23.03%)) were identified the most common animal reservoirs for P. skrjabini.
Subgroup analysis and meta-regression models indicated that animal categories, lifestyle (wild or domestic), or detection methods could significantly explain the observed heterogeneity (see Tables Tables55 and S9).
Publish bias and sensitivity analysis
Asymmetry in the funnel plots and the results of Egger’s test indicated the presence of publication bias (see S2 Fig). The sensitivity analysis results demonstrated that the pooled prevalence estimate did not change significantly after the removal of outlier data points or data points with small sample sizes (95% CI overlapped; see S10 Table).
Biogeographical characteristics of P. westermani and P. skrjabini infections
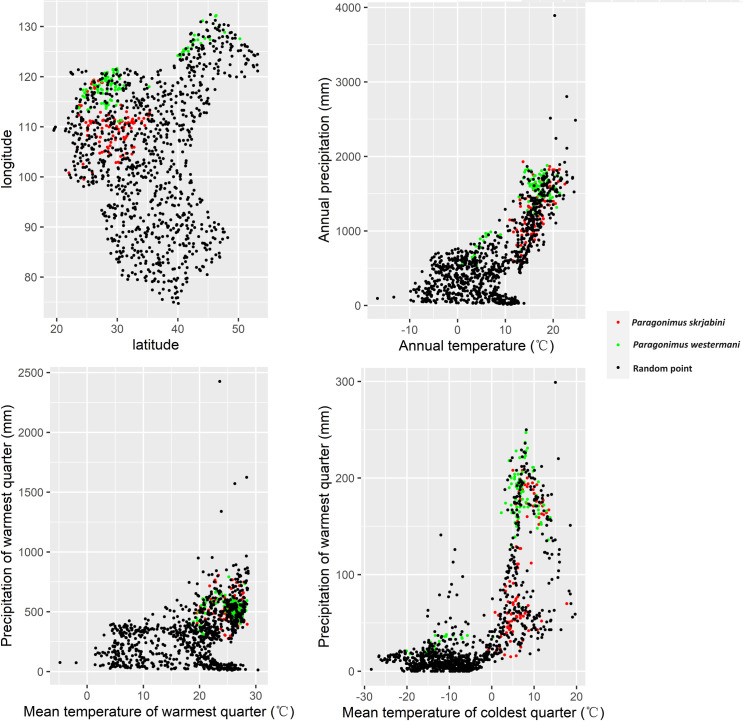
To investigate the biogeographical characteristics of Paragonimus occurrences, we created scatter plots using the climate features of P. westermani and P. skrjabini endemic sites and 1000 random points. The results indicate that, compared to random points, endemic sites of P. westermani and P. skrjabini are mainly distributed in regions with lower altitude and higher temperature and precipitation (see S11 Table and Fig 7). Specifically, endemic sites of P. westermani are predominantly distributed in areas with an altitude below 1166.0m, annual temperature above 1.0°C, annual precipitation above 541.0mm, mean temperature of the warmest quarter above 18.3°C, and precipitation of the warmest quarter above 304.0mm. On the other hand, endemic sites of P. skrjabini are distributed in areas with altitude below 2188.0m, annual temperature above 10.9°C, annual precipitation above 578.0mm, mean temperature of the warmest quarter above 19.5°C, and precipitation of the warmest quarter above 257.0mm. When comparing the two Paragonimus species, the endemic points of P. westermani have lower altitudes (below 1166.0m for P. westermani; 2188.0m for P. skrjabini) and lower mean temperature of the coldest quarter (above -20.1°C for P. westermani; -0.8°C for P. skrjabini).
Discussion
In this study, we summarized the infection status and geographical distribution of P. westermani and P. skrjabini in humans and animal hosts in China. Our findings indicate that Paragonimus infection is widely distributed and remains prevalent in China, with variations in the transmission vectors, second intermediate hosts, and geographical distribution between P. westermani and P. skrjabini. Furthermore, environmental factors such as temperature and precipitation may influence the distribution of Paragonimus.
After years of educational efforts, the reported number of human paragonimiasis cases has significantly decreased in most areas of China (see Table 1). However, it is noteworthy that after 2010, a considerable number of reported cases persist in areas such as Chongqing (1073) and Sichuan (595), with many other provinces and municipalities also continuing to report cases, highlighting the need for ongoing control efforts against paragonimiasis. The number of cases in males is significantly higher than in females. This disparity is attributed to differences in behavior and occupational exposure. There is a higher proportion of males among fishermen, and males are more likely than females to catch and consume freshwater crabs and crayfish [30]. Another notable issue is the significant involvement of children and adolescents in paragonimiasis cases, both before and after 2010 [31–33]. In certain endemic areas, particularly in rural or mountainous regions, practices such as local children drinking untreated water and consuming undercooked shrimp and crab are more common among children compared to adults [34,35], underscoring the necessity for enhanced health education on paragonimiasis in schools in key areas. Additionally, human infection may be more widespread and underestimated due to a lack of training of health workers to identify paragonimiasis and a deficient case-reporting system [36].
The distribution regions of P. westermani and P. skrjabini in China exhibit both differences and overlaps. In the northeastern areas of China, only P. westermani has been documented, while in the southern part of China, both species coexist. The difference in the distribution of the two Paragonimus species is likely due to variations in their second intermediate hosts. Specifically, Sinopotamon, primarily distributed in the southern part of China, serves as the main second intermediate host for both P. westermani and P. skrjabini [37,38]. On the other hand, Cambaroides, which inhabits the northeastern region of China, can only serve as the second intermediate host for P. westermani [39]. On the other hand, Cambaroides, which inhabits the northeastern region of China, can only serve as the second intermediate host for P. westermani [30]. It has been reported that P. westermani and P. skrjabini share some common intermediate hosts, such as Semisulcospira, Tricula, Erhaiini, and Bythinella in the first intermediate host, and Huananpotamon in the second intermediate host [40,41]. Additionally, the cercariae and metacercariae of P. westermani and P. skrjabini are morphologically similar [42]. Therefore, in areas where the two Paragonimus species overlap, there may be misclassification when detecting the infection in intermediate hosts. To accurately differentiate between the different Paragonimus species, nucleic acid detection is recommended to be conducted simultaneously in epidemiological surveys.
The prevalence of Paragonimus in intermediate hosts exhibits significant variation. In the first intermediate host, the prevalence of P. westermani ranged from 0.00% to 6.72%, while the prevalence of P. skrjabini ranged from 0.00% to 14.80% (see S3 Table). In the second intermediate host, the prevalence ranged from 0% to 100% (see S4 Table). None of the known moderators, including the taxonomic category of the intermediate host, year of survey, and detection methods, can significantly explain the heterogeneity across studies (see S7 and S8 Tables). Therefore, it is necessary to conduct random sampling surveys in different regions to further understand the factors that influence the prevalence of Paragonimus in intermediate hosts.
In many regions of China, it is common for residents to consume marinated or drunken crabs in their raw state [9,43]. However, the methods of salting and soaking in alcohol are not completely effective in killing the metacercariae [44,45]. Another prevalent practice is the consumption of freshwater crabs and crayfish through stir-frying, but inadequate heating may not fully eliminate the parasites [46,47]. Human infection occurs through the consumption of inadequately cooked freshwater crustaceans containing the infective metacercariae. Given the persistently high prevalence of Paragonimus in the second intermediate host (with a pooled prevalence of 52.02% (95% CI: 44.35–59.64%) for P. westermani and 30.37% (95% CI: 24.72–36.34%) for P. skrjabini; see Table 4), and the continued popularity of consuming raw or undercooked freshwater crustaceans in many areas of China, paragonimiasis remains a significant public health threat to the Chinese population.
The analysis of biogeographical characteristics revealed that temperature and precipitation might influence the distribution of Paragonimus (see Fig 7). Temperature may affect the distribution of Paragonimus by influencing the survival of the intermediate host (snails and crustaceans) or by affecting the development of Paragonimus. For example, research by Hu et al. indicates that the development of the eggs of P. heterotremus is closely related to the external temperature [48]. Development is slow or even halted at temperatures below 12°C, and does not occur at temperatures above 37°C. Chiu has found that the optimum temperature for the development of P. iloktsuenensis in Tricula chiui is 22 to 30°C [49]. Our study results indicate that, compared to P. skrjabini, P. westermani can survive in regions with lower temperatures, such as northeastern China (see Figs Figs33–6), suggesting that P. westermani exhibits great tolerance to low temperatures. Similarly, Fan and colleagues have found that metacercariae of P. westermani can still develop into mature worms in rats after storage at 4°C for up to 234 days [50].
Paragonimus infections have been predominantly documented in eastern China. This geographical distribution is closely associated with water supply, with precipitation playing a crucial role in the distribution of aquatic snails and crustaceans [51], both of which are integral to the Paragonimus life cycle. The higher levels of precipitation in eastern China create environments that are more conducive to the survival and proliferation of intermediate hosts, thereby increasing the risk of Paragonimus infections in these areas [52].
In this study, we pooled studies from numerous sites to achieve a relatively large sample size to summarize the prevalence of P. westermani and P. skrjabini in humans, intermediate hosts, and animal reservoirs. However, several limitations of our study should be considered. Firstly, the absence of literature reporting Paragonimus spp. infections in certain areas does not necessarily indicate that Paragonimus spp. infections do not exist there; it may be due to a lack of research in those areas or unpublished research findings. Secondly, significant heterogeneity was detected across studies, and most of the heterogeneity could not be explained by known moderators. Lastly, publication bias exists in this study, which may cause bias in the estimates of pooled prevalence. Therefore, the results of our study should be interpreted with caution. Despite these limitations, our study systematically summarizes the infection status of P. westermani and P. skrjabini in humans, intermediate hosts, and animal reservoirs in China, and elucidates their spatial distribution. The findings may provide valuable insights for the control of paragonimiasis in China. In the future, it is advisable to incorporate paragonimiasis into China’s National Notifiable Infectious Diseases Surveillance System to comprehensively monitor the incidence of the disease and identify high-risk populations more accurately. Furthermore, it is essential to systematically investigate the prevalence of Paragonimus spp. in various hosts in endemic areas and analyze the factors influencing these rates to enhance our understanding of the dynamics of Paragonimus spp. transmission.
Conclusions
Paragonimus infection remains widely distributed and prevalent in China, with children and adolescents at high risk in endemic areas. Variations exist in the intermediate hosts and geographical distribution of P. westermani and P. skrjabini infections in China. P. skrjabini infections are predominantly concentrated in more southern regions compared to P. westermani. Additionally, altitude, temperature, and precipitation may influence the distribution of P. westermani and P. skrjabini.
Supporting information
S1 Table
Cases of human paragonimiasis docummented in literatures.(XLSX)
S2 Table
Publications reporting Paragonimus prevalence in humans.(XLSX)
S3 Table
Publications reporting Paragonimus prevalence in the first intermediate hosts.(XLSX)
S4 Table
Publications reporting Paragonimus prevalence in the second intermediate hosts.(XLSX)
S5 Table
Publications reporting Paragonimus prevalence in animal reservoirs.(XLSX)
S6 Table
Multivariable meta-regression analyses for Paragonimus prevalence in humans.(XLSX)
S7 Table
Multivariable meta-regression analyses for Paragonimus prevalence in the first intermediate hosts.(XLSX)
S8 Table
Multivariable meta-regression analyses for Paragonimus prevalence in the second intermediate hosts.(XLSX)
S9 Table
Multivariable meta-regression analyses for Paragonimus prevalence in animal reservoirs.(XLSX)
S10 Table
Sensitivity analysis of the pooled prevalence of Paragonimus in humans, the first intermediate hosts, the second intermediate hosts, and animal reservoirs.(XLSX)
S11 Table
Environmental characteristics of areas with reported P. westermani and P. skrjabini infections in China.(XLSX)
S1 Fig
Forest plots of prevalence of Paragonimus species in humans, the first intermediate host, the second intermediate host, and animal reservoirs.(a) Paragonimus in humans; (b) P. westermani in the first intermediate host; (c) P. skrjabini in the first intermediate host; (d) P. westermani in the second intermediate host; (e) P. skrjabini in the second intermediate host; (f) P. westermani in animal reservoir; (g) P. skrjabini in animal reservoir.
(DOC)
S2 Fig
Funnel plot for assessing publication bias in studies reporting prevalence of Paragonimus species in humans, the first intermediate host, the second intermediate host, and animal reservoirs (a) Paragonimus in humans; (b) P. skrjabini in the first intermediate host; (c) P. westermani in the first intermediate host; (d) P. skrjabini in the second intermediate host; (e) P. westermani in the second intermediate host; (f) P. skrjabini in animal reservoir; (g) P. westermani in animal reservoir.
(DOC)
Funding Statement
This research was funded by the Shandong Provincial Natural Science Foundation (https://cloud.kjt.shandong.gov.cn/) (ZR2019MH093 to L-H L; ZR2023MH313 to F-Y S), the Shandong Provincial Youth Innovation Team Development Plan of Colleges and Universities (http://edu.shandong.gov.cn/) (2019-6-156 to F-Y S), the National Parasite Resource Bank (https://www.most.gov.cn/index.html) (NPRC-2019-194-30 to Y L), Three-Year Initiative Plan for Strengthening Public Health System Construction in Shanghai (2023-2025) Key Discipline Project (https://wsjkw.sh.gov.cn/) (No. GWVI-11.1-12 to Y L),the Quality Education Teaching Resources Project of Shandong Province and Weifang Medical University (http://edu.shandong.gov.cn/) (SDYAL2022152, 22YZSALK01, and 23YJSALK01 to L-H L), Joint Research Program of China National Center for Food Safety Risk Assessment (https://cfsa.net.cn/) (LH2022GG08 and LH2022GG02 to L-H L), and the National Natural Science Foundation of China (https://www.nsfc.gov.cn/) (81902095 to L-H L). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
References
Decision Letter 0
7 Jun 2024
Dear Dr. Li,
Thank you very much for submitting your manuscript "Infection and biogeographical characteristics of Paragonimus westermani and P. skrjabini in humans and animal hosts in China: a systematic review and meta-analysis" for consideration at PLOS Neglected Tropical Diseases. As with all papers reviewed by the journal, your manuscript was reviewed by members of the editorial board and by several independent reviewers. The reviewers appreciated the attention to an important topic. Based on the reviews, we are likely to accept this manuscript for publication, providing that you modify the manuscript according to the review recommendations.
All reviewers concur on the study's value and quality, offering only minor comments. Kindly be aware that Reviewer 3's comments have been included as annotations in the PDF file.
Please prepare and submit your revised manuscript within 30 days. If you anticipate any delay, please let us know the expected resubmission date by replying to this email.
When you are ready to resubmit, please upload the following:
[1] A letter containing a detailed list of your responses to all review comments, and a description of the changes you have made in the manuscript.
Please note while forming your response, if your article is accepted, you may have the opportunity to make the peer review history publicly available. The record will include editor decision letters (with reviews) and your responses to reviewer comments. If eligible, we will contact you to opt in or out
[2] Two versions of the revised manuscript: one with either highlights or tracked changes denoting where the text has been changed; the other a clean version (uploaded as the manuscript file).
Important additional instructions are given below your reviewer comments.
Thank you again for your submission to our journal. We hope that our editorial process has been constructive so far, and we welcome your feedback at any time. Please don't hesitate to contact us if you have any questions or comments.
Sincerely,
Qu Cheng, Ph.D.
Academic Editor
PLOS Neglected Tropical Diseases
jong-Yil Chai
Section Editor
PLOS Neglected Tropical Diseases
***********************
All reviewers concur on the study's value and quality, offering only minor comments. Kindly be aware that Reviewer 3's comments have been included as annotations in the PDF file.
Reviewer's Responses to Questions
Key Review Criteria Required for Acceptance?
As you describe the new analyses required for acceptance, please consider the following:
Methods
-Are the objectives of the study clearly articulated with a clear testable hypothesis stated?
-Is the study design appropriate to address the stated objectives?
-Is the population clearly described and appropriate for the hypothesis being tested?
-Is the sample size sufficient to ensure adequate power to address the hypothesis being tested?
-Were correct statistical analysis used to support conclusions?
-Are there concerns about ethical or regulatory requirements being met?
Reviewer #1: Methodology and study design week articulated and appropriate.
Reviewer #2: The work complies with the aspects mentioned above. The period covered by the study, although the end date is stated (it is understood from 2010 to the present), the beginning could be better specified (it is a very broad period to say before 1990)
Reviewer #3: All in the MS
--------------------
Results
-Does the analysis presented match the analysis plan?
-Are the results clearly and completely presented?
-Are the figures (Tables, Images) of sufficient quality for clarity?
Reviewer #1: The analysis presented do match the analysis plan and are well presented.
Reviewer #2: The work complies with the itms indicated above
Suggested details:
Line 202-203: The total number of publications is not clear (876 - 10642?)
Line 222-224: Table 1 does not show human cases in Hong Kong and Macau either.
Line 227: 71.12% or 71.21%
Fig 2: the reference system with parentheses is difficult to interpret
Line 219-222: it could be indicated that the cases by gender, source and age are obviously above those documented, explicitly consider that there is a significant number that is not specified
Fig 1: the value 261 is not clear? (38+107+174+58=377)
Linea 297: 94 estudios and line 307: 81 estudios = 175. En la linea 206 menciona 172 en second intermediate host
Reviewer #3: All in the MS
--------------------
Conclusions
-Are the conclusions supported by the data presented?
-Are the limitations of analysis clearly described?
-Do the authors discuss how these data can be helpful to advance our understanding of the topic under study?
-Is public health relevance addressed?
Reviewer #1: Conclusions well justified and limitations well noted.
Reviewer #2: The items indicated above are fully contemplated in the work.
Reviewer #3: All in the MS
--------------------
Editorial and Data Presentation Modifications?
Use this section for editorial suggestions as well as relatively minor modifications of existing data that would enhance clarity. If the only modifications needed are minor and/or editorial, you may wish to recommend “Minor Revision” or “Accept”.
Reviewer #1: No editorial suggestions other than what has been suggested to the authors below.
Reviewer #2: (No Response)
Reviewer #3: All in the MS
--------------------
Summary and General Comments
Use this section to provide overall comments, discuss strengths/weaknesses of the study, novelty, significance, general execution and scholarship. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. If requesting major revision, please articulate the new experiments that are needed.
Reviewer #1: This study provides a comprehensive meta-analysis for studies on the epidemiology paragonimiasis in China. I would suggest that the authors add in the Introduction some information about the possible socio-economic impacts of the disease in endemic areas. In the discussion: is there any explanation for the marked gender difference in the prevalence of the infection? Based on this extensive review I think it would be helpful to add any suggestions to standardize future surveys
Reviewer #2: The work is a valuable contribution by summarizing the situation of infection by P. westermani and P. skrjabini in their main hosts, as well as the geographical distribution. The figures that allow a visual-spatial representation of the infection situation in China stand out, being a fundamental pillar when establishing prevention-control measures.
Otros puntos:
When only Paragonimus is mentioned without specifying the species, it could be indicated as Paragonimus spp.
Line 87: P. westerman would be P. westermani
Line 87 - 91: would include some bibliographical reference
Line 446: ...prevalence of 52.02 % (95% CI 42.65 - 75.79%). The CI differs from Table 4
Line 458: P. iloktsuensis or P. iloktsuenensis?.
T. chiui: is it previously defined?
Reviewer #3: All in the MS
--------------------
PLOS authors have the option to publish the peer review history of their article (what does this mean?). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy.
Reviewer #1: Yes: Ahmed Awad Adeel
Reviewer #2: Yes: Zully Hernández
Reviewer #3: Yes: José Alberto Iannacone Oliver
Figure Files:
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com. PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email us at gro.solp@serugif.
Data Requirements:
Please note that, as a condition of publication, PLOS' data policy requires that you make available all data used to draw the conclusions outlined in your manuscript. Data must be deposited in an appropriate repository, included within the body of the manuscript, or uploaded as supporting information. This includes all numerical values that were used to generate graphs, histograms etc.. For an example see here: http://www.plosbiology.org/article/info%3Adoi%2F10.1371%2Fjournal.pbio.1001908#s5.
Reproducibility:
To enhance the reproducibility of your results, we recommend that you deposit your laboratory protocols in protocols.io, where a protocol can be assigned its own identifier (DOI) such that it can be cited independently in the future. Additionally, PLOS ONE offers an option to publish peer-reviewed clinical study protocols. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols
References
Please review your reference list to ensure that it is complete and correct. If you have cited papers that have been retracted, please include the rationale for doing so in the manuscript text, or remove these references and replace them with relevant current references. Any changes to the reference list should be mentioned in the rebuttal letter that accompanies your revised manuscript. If you need to cite a retracted article, indicate the article's retracted status in the References list and also include a citation and full reference for the retraction notice.
Author response to Decision Letter 0
2 Jul 2024
Decision Letter 1
9 Jul 2024
Dear Dr. Li,
We are pleased to inform you that your manuscript 'Infection and biogeographical characteristics of Paragonimus westermani and P. skrjabini in humans and animal hosts in China: a systematic review and meta-analysis' has been provisionally accepted for publication in PLOS Neglected Tropical Diseases.
Before your manuscript can be formally accepted you will need to complete some formatting changes, which you will receive in a follow up email. A member of our team will be in touch with a set of requests.
Please note that your manuscript will not be scheduled for publication until you have made the required changes, so a swift response is appreciated.
IMPORTANT: The editorial review process is now complete. PLOS will only permit corrections to spelling, formatting or significant scientific errors from this point onwards. Requests for major changes, or any which affect the scientific understanding of your work, will cause delays to the publication date of your manuscript.
Should you, your institution's press office or the journal office choose to press release your paper, you will automatically be opted out of early publication. We ask that you notify us now if you or your institution is planning to press release the article. All press must be co-ordinated with PLOS.
Thank you again for supporting Open Access publishing; we are looking forward to publishing your work in PLOS Neglected Tropical Diseases.
Best regards,
Qu Cheng, Ph.D.
Academic Editor
PLOS Neglected Tropical Diseases
Jong-Yil Chai
Section Editor
PLOS Neglected Tropical Diseases
***********************************************************
Acceptance letter
30 Jul 2024
Dear Dr. Li,
We are delighted to inform you that your manuscript, "Infection and biogeographical characteristics of <i>Paragonimus westermani<i> and <i>P. skrjabini<i> in humans and animal hosts in China: a systematic review and meta-analysis," has been formally accepted for publication in PLOS Neglected Tropical Diseases.
We have now passed your article onto the PLOS Production Department who will complete the rest of the publication process. All authors will receive a confirmation email upon publication.
The corresponding author will soon be receiving a typeset proof for review, to ensure errors have not been introduced during production. Please review the PDF proof of your manuscript carefully, as this is the last chance to correct any scientific or type-setting errors. Please note that major changes, or those which affect the scientific understanding of the work, will likely cause delays to the publication date of your manuscript. Note: Proofs for Front Matter articles (Editorial, Viewpoint, Symposium, Review, etc...) are generated on a different schedule and may not be made available as quickly.
Soon after your final files are uploaded, the early version of your manuscript will be published online unless you opted out of this process. The date of the early version will be your article's publication date. The final article will be published to the same URL, and all versions of the paper will be accessible to readers.
Thank you again for supporting open-access publishing; we are looking forward to publishing your work in PLOS Neglected Tropical Diseases.
Best regards,
Shaden Kamhawi
co-Editor-in-Chief
PLOS Neglected Tropical Diseases
Paul Brindley
co-Editor-in-Chief
PLOS Neglected Tropical Diseases
Articles from PLOS Neglected Tropical Diseases are provided here courtesy of PLOS
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Paragonimus and paragonimiasis in Vietnam: an update.
Korean J Parasitol, 51(6):621-627, 31 Dec 2013
Cited by: 15 articles | PMID: 24516264 | PMCID: PMC3916448
Review Free full text in Europe PMC
[Survey on the foci of Paragonimus in Youxi, Yongtai and Pinghe Counties of Fujian Province].
Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi, 28(6):406-410, 01 Dec 2010
Cited by: 2 articles | PMID: 21500525
Paragonimus and its hosts in China: An update.
Acta Trop, 223:106094, 11 Aug 2021
Cited by: 7 articles | PMID: 34389330
Review
Paragonimus and paragonimiasis in Asia: An update.
Acta Trop, 199:105074, 08 Jul 2019
Cited by: 40 articles | PMID: 31295431
Review
Funding
Funders who supported this work.
Joint Research Program of China National Center for Food Safety Risk Assessment (1)
Grant ID: LH2022GG08, LH2022GG02
Shandong Provincial Natural Science Foundation (2)
Grant ID: ZR2023MH313
Grant ID: ZR2019MH093
The National Natural Science Foundation of China (1)
Grant ID: 81902095
The National Parasite Resource Bank (1)
Grant ID: NPRC-2019-194-30
The Quality Education Teaching Resources Project of Shandong Province and Weifang Medical University (1)
Grant ID: SDYAL2022152, 22YZSALK01, 23YJSALK01
The Shandong Provincial Youth Innovation Team Development Plan of Colleges and Universities (1)
Grant ID: 2019-6-156
Three-Year Initiative Plan for Strengthening Public Health System Construction in Shanghai (1)
Grant ID: (2023-2025) Key Discipline Project (https://wsjkw.sh.gov.cn/) (No. GWVI-11.1-12

 1
,*
1
,*