Abstract
Background
Despite advances in primary ciliary dyskinesia (PCD) research, many questions remain; diagnosis is complex and no disease-specific therapies exist. Using a mixed-methods approach, we aimed to identify priorities for clinical and epidemiological research and explore barriers to research.Methods
To obtain rich, relevant, diverse data, we performed in-depth semi-structured interviews with PCD specialists selected using purposive sampling. We transcribed, coded and analysed interview data using thematic analysis. Based on interview themes that we identified, we developed an anonymous survey and circulated it widely through the BEAT-PCD network.Results
We interviewed 28 participants from 15 countries across different disciplines and expertise levels. The main themes identified as priorities for PCD research were improving diagnosis; understanding prevalence and disease course; phenotypic variability; disease monitoring; treatment strategies; clinical trial end-points; and poorly researched areas. In total, 136 participants (49% paediatric pulmonologists) from 36 countries completed the survey. Most commonly reported barriers for research were low awareness about PCD and difficulties securing funding - in more than one-third of cases, participants reported undertaking predominantly unfunded research. Research questions ranked highest included priorities related to further improving diagnosis, treating PCD, managing upper and lower airway problems, and studying clinical variability and disease prognosis.Conclusion
We need to overcome barriers of limited funding and low awareness and promote collaborations between centres, disciplines, experts and patients to address identified PCD priorities effectively. Our results contribute to the ongoing efforts of guiding the use of existing limited research resources and setting up a roadmap for future research activities.Free full text

Priorities and barriers for research related to primary ciliary dyskinesia
Associated Data
Abstract
Background
Despite advances in primary ciliary dyskinesia (PCD) research, many questions remain; diagnosis is complex and no disease-specific therapies exist. Using a mixed-methods approach, we aimed to identify priorities for clinical and epidemiological research and explore barriers to research.
Methods
To obtain rich, relevant, diverse data, we performed in-depth semi-structured interviews with PCD specialists selected using purposive sampling. We transcribed, coded and analysed interview data using thematic analysis. Based on interview themes that we identified, we developed an anonymous survey and circulated it widely through the BEAT-PCD network.
Results
We interviewed 28 participants from 15 countries across different disciplines and expertise levels. The main themes identified as priorities for PCD research were improving diagnosis; understanding prevalence and disease course; phenotypic variability; disease monitoring; treatment strategies; clinical trial end-points; and poorly researched areas. In total, 136 participants (49% paediatric pulmonologists) from 36 countries completed the survey. Most commonly reported barriers for research were low awareness about PCD and difficulties securing funding – in more than one-third of cases, participants reported undertaking predominantly unfunded research. Research questions ranked highest included priorities related to further improving diagnosis, treating PCD, managing upper and lower airway problems, and studying clinical variability and disease prognosis.
Conclusion
We need to overcome barriers of limited funding and low awareness and promote collaborations between centres, disciplines, experts and patients to address identified PCD priorities effectively. Our results contribute to the ongoing efforts of guiding the use of existing limited research resources and setting up a roadmap for future research activities.
Shareable abstract
This study defines PCD research priorities including improving diagnosis, treatments, managing upper and lower airway disease, and understanding prognosis. Key barriers identified include low disease awareness and limited funding opportunities. https://bit.ly/4duXUCh
Introduction
Many factors hinder clinical and epidemiological research about rare diseases [1]. Low patient numbers, even in centres that provide specialised services for patients with primary ciliary dyskinesia (PCD), low awareness among clinicians and the public and limited funding are among rare disease research barriers. In recent years, rare diseases became a health priority in Europe with initiatives such as the International Rare Diseases Consortium Initiative and the European Reference Networks (ERNs) [2, 3]. Supported by recent policies, rare lung disease research experienced unprecedented growth through collaborative efforts in Europe and abroad. ERN-LUNG was established in 2017 and focuses on several rare lung diseases [4, 5]. At the same time, the European Respiratory Society (ERS) supports several clinical research collaborations for developing large specific rare lung disease networks, including children's interstitial lung disease, α-1 antitrypsin deficiency and PCD [6–8].
In the field of PCD specifically, several research collaborations between clinicians and scientists have led to great advances understanding PCD better and improving patient care [9–12]. BEAT-PCD (Better Experimental Approaches to Treat PCD) is a large international network set up initially in 2014 as a European Cooperation in Science and Technology (COST) Action (BM1407), which expanded in 2020 into an ERS-supported clinical research collaboration [8, 13]. The BEAT-PCD network provides an excellent opportunity to advance basic, clinical and epidemiologic research and develop collaborative projects by building upon existing knowledge and utilising existing data resources [14–19]. Despite advances from recent years, many questions about PCD remain unanswered. PCD diagnosis improved but algorithms followed are still complex and vary significantly between and within countries [20–23]. Use of genetic testing increased but not uniformly, although it is the main reference test to establish diagnosis, together with electron microscopy. Other tests like immunofluorescence and high-speed video microscopy are used complementarily but need standardisation and more data on accuracy. Since limited high-quality evidence supports development of PCD-specific guidelines, follow-up and management remain extrapolated from other diseases, such as cystic fibrosis or bronchiectasis [20–22, 24, 25]. However, in recent years, there has been significant interest in the development of gene therapeutics for PCD, and several companies have initiated such programmes [26]. We have a better understanding about the phenotypic variability of PCD and specific mutations and corresponding ultrastructural defects have been linked to more severe disease, but we need more studies on disease course especially across the lifespan and on clinical predictors [27–29]. Identifying research priorities, as well as potential challenges in performing research on PCD, supports the work of collaborative initiatives and research teams worldwide and guides the use of limited existing resources. Within the BEAT-PCD framework, our mixed-method study aimed to identify research gaps and priorities in clinical and epidemiological research in the field of PCD and explore barriers in research.
Methods
Our mixed-method study consisted of two parts: 1) a series of in-depth, semi-structured interviews with purposive selected healthcare professionals and researchers involved in PCD research and clinical care; and 2) an anonymous electronic survey informed by interview findings, circulated widely through the BEAT-PCD network. The study received approval by the Faculty of Medicine Ethics Committee of the University of Southampton (ERGO 47010.A1).
In-depth interviews
To obtain rich, relevant, diverse data, we performed in-depth, semi-structured interviews with specialists involved in PCD research and clinical care selected using purposive sampling [30]. We invited and included participants from different countries, diverse research backgrounds and various research experience levels (both senior and early career researchers). We aimed to interview participants from countries (participating in the BEAT-PCD network) with extensive, average and limited research resources. Based on our aim, we mainly selected researchers with clinical management or clinical or epidemiological research experience; however, we also recruited basic scientists and diagnostic scientists to capture their opinions. All participants provided informed consent.
All interviews were conducted in English in-person or online between February 2019 and June 2021. Interviews followed a non-prescriptive guide developed in the beginning of the project and followed interviewee-raised issues opportunistically to ensure depth of information (supplementary material). We derived interview guide questions from existing literature and feedback from collaborators. We tested them during the first three to four interviews, then adjusted the guide accordingly, adding prompts about issues raised by the first interviewees. The interviewer was the lead author (M. Goutaki) who is a full-time researcher in the field of PCD research and received in-depth interview and qualitative data analysis training for the project. With participant consent, interviews were audio-recorded and later transcribed verbatim by the lead (M. Goutaki) or second author (Y. T. Lam), in which case they were carefully validated by M. Goutaki. Interview transcripts used an identification code to ensure and maintain interviewee anonymity; we removed identifying information from transcripts. We offered participants the opportunity to review the full transcript, upon request.
The planned sample size for the interviews was pragmatic; we based our estimations of a sufficient number for information power to reach breadth of information and collect rich, relevant, detailed data [31]. As with other rare diseases, the field of PCD research is limited and numbers of eligible participants restricted. Our objective involved collecting data to develop the survey by capturing different opinions about PCD research priorities, not achieving data saturation. M. Goutaki coded and analysed data using an inductive thematic analysis approach [32, 33]. We grouped interview data into the subsequent coding steps until we identified common themes. We used NVivo software (1.5.2) to transcribe and analyse data; we followed the consolidated criteria for reporting qualitative research (COREQ) 32-item checklist for interviews and focus groups [34].
Survey
Based on themes we identified from our interview data analysis, we developed a 21-question survey taking on average 15 min to complete and including questions about: 1) general demographics, general participant PCD involvement and specific PCD research; 2) research funding for PCD projects and barriers for research; and 3) research priority rankings grouped by main topics (diagnosis, presentation/prognosis and follow-up, treatments, and other priorities) and overall. A multidisciplinary group of experts contributed to refining the survey questions by providing input on: i) general question content, questions related to acquiring funding, and barriers for research; and ii) wording and structuring of research priority questions. We developed the survey in English and programmed it in a Research Electronic Data Capture (REDCap) database hosted at the University of Bern (supplementary material) [35]. We circulated study information about the survey via e-mail to the BEAT-PCD network together with an invitation to participate. The BEAT-PCD mailing list includes >500 e-mail addresses worldwide of individuals generally interested in PCD and our activities. We asked interested healthcare professionals and researchers to contact the study team and consent to participate, then they received a link to complete the survey, which remained open between 1 June and 31 July 2023. During this period, we sent two study reminders to the network. Survey participation was anonymous.
We presented survey results using descriptive statistics. For the overall priority ranking of research questions, we used a reciprocal ranking scoring system; each question received points based on its first (1 point), second (1/2 points) or third (1/3 points) overall research priority rank and 0 points if it was not ranked among the top three. Based on the final score, each question ranged from 0 to 1 point, with a higher score indicating a higher priority. We prioritised questions from highest to lowest mean score. We analysed the survey data using STATA version 15.1.
Results
In-depth interviews
We interviewed 28 participants from 15 countries, six from outside Europe. Participants included 15 paediatric pulmonologists (several also cared for adult patients), three adult pulmonologists, three ear-nose-throat (ENT) specialists, one specialist nurse, one physiotherapist, one epidemiologist and four diagnostic scientists. All participants were involved in PCD care or research; not all were employed in specialist centres. Mean interview duration was 42 min and ranged from 18 to 77 min. 13 interviews took place face-to-face and 15 occurred online, using teleconferencing software.
During interviews, participants discussed their experiences with PCD-related research, barriers to successfully obtaining funding, other factors that hinder or facilitate research on PCD in their institution and country, and the importance of collaborations and patient involvement in research. They also discussed their personal research interests in the field and expanded upon existing research gaps and priorities for future research. Although some participants mainly discussed high-level priorities, most expressed their preferences for specific questions addressed in the near future (supplementary table S1).
From interviews, the main themes we identified as important focal areas for clinical and epidemiological PCD research included: 1) improving diagnosis; 2) prevalence and disease course; 3) phenotypic variability; 4) improving disease monitoring; 5) treatment strategies; 6) end-points for clinical trials and research; 7) neglected areas, such as ENT and fertility problems and mental health issues; and 8) research in other ciliopathies and specific patient groups (figure 1). We grouped priorities not directly linked to themes as other, more general priorities. We include representative quotes from interviews in table 1.
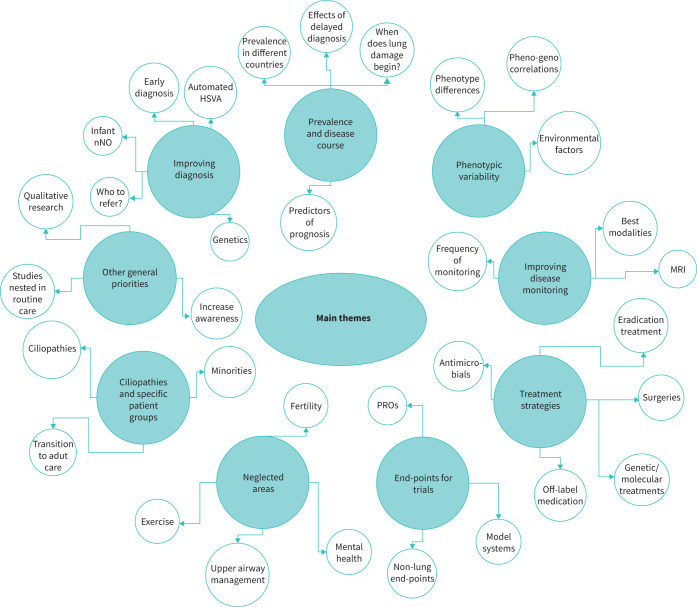
Main themes identified during in-depth interview by participants as important focus areas for clinical and epidemiological primary ciliary dyskinesia (PCD) research. MRI: magnetic resonance imaging; PROs: patient-reported outcomes; HSVA: high-speed video analysis; nNO: nasal nitric oxide.
TABLE 1
Representative quotes related to primary ciliary dyskinesia (PCD) research from in-depth interviews with healthcare professionals and researchers
| Topic | Quote |
|---|---|
| Challenges in PCD research | “I think the first challenge is just getting enough patients for [an] effective study. The second challenge is to fund, have enough funds for [a] coordinator, who can keep track of who is where and how to get in touch with them, get them in for new studies, so just the infrastructure. And then because of that you need to have to network with other centres. So […] just finding other groups who share the same passion. I think everybody who does PCD research is probably under resourced and it's what their passion is, [laughs] that's why they do it, so you have to find other people who have the same passion. They are not in it for the money; that's for sure.” |
| Lack of disease awareness | “[…] even [among] the health professionals there is no awareness and there is even ignorance many times, while there might be a clear suspicion for the diagnosis and it is clear that the next step towards the one or the other direction must be made it doesn't happen because simply, they do not have the knowledge.” |
| Lack of awareness and interest from clinical community | “In general, physicians they have like one, maybe two, maybe three PCD patients. So, it's difficult for them you know to be involved to think it's important.” |
| Prioritising research topics | “Because just doing research for the sake of research is not as important as doing things that specifically people will find useful.” |
| Research related to adult patients | “[We need more research] on adult PCD patients. We need to understand what this disease becomes; we need to understand the adult issues with complicated lung infections, fertility, things like that. It's been too paediatric based thus far, that's it.” |
| Research about neglected areas | “I think fertility is a main issue for adult patients. I've seen so many young men and women and see that it was very difficult for them to find a good fertility expert. It takes time […] So sometimes they miss the point, and they are not able to have children […].” |
| Research about treatments | “I would like to [be able to] say […] when I see a new patient where we could make the diagnosis […] that yes, the evolution will be that […] and then to say that there are a few treatments, validated by studies.” |
| Integrated research approach | “I think historically, clinical researchers focus on a single area, you know, so they come at these diseases from a microbiological perspective, or they come at these diseases from an immunology perspective. And I think all of those are […] that approach is quite flawed because these diseases are highly complex. So if you're only interested in diagnostics, or if you're only interested in microbiology, you are always I think, hitting up against the barriers of the questions you can answer.” |
| Research collaborations | “I think the only solution would be collaboration because […] nobody can get money for everything. So I think there must be a focus. Some research groups have to have different focuses, so that it might be more possible to get funding for this. Because when everybody wants to have funding for the same things, it could be difficult. I think you need to collaborate and as we did in the BEAT-PCD also, you have to get in groups and then maybe funding is more possible than if you were just playing alone.” |
| Patient involvement in research | “Rather than just recruiting patients into a study, we should much more have equal partnerships with people who have the disease, already from the start of a study, for designing a study, for designing questionnaires, for designing approaches, how to inform people with PCD about the study and how to participate, how to interpret results.” |
We edited quotes for brevity, clarity and correctness; we present quotes as mostly verbatim, while also ensuring anonymity.
Survey: barriers for PCD research
We excluded four (three incomplete; one duplicate) of 140 filled-in questionnaires. Participants represented 36 countries and 63% were female (table 2). Most were paediatric pulmonologists (49%), followed by ENT specialists (10%), diagnostic scientists (9%), adult pulmonologists (7%), paediatricians (7%), other healthcare professionals (6%), namely specialist nurses and physiotherapists, epidemiologists, or data scientists (5%), and other (7%) (table 2). Of survey respondents, 81% reported involvement with care or diagnosis of patients with PCD and 70% involvement in research currently; overall 82% reported personal involvement in PCD research. Almost half (47%) reported experience with PCD >10 years; 31% 5–10 years; and 22% <5 years (table 2).
TABLE 2
Characteristics of participants in the online survey about priorities and barriers for PCD research (n=136)
| Female sex | 86 (63) |
| Country of residence | |
 UK UK | 18 (13) |
 Switzerland Switzerland | 14 (11) |
 Turkey Turkey | 13 (10) |
 Spain Spain | 11 (8) |
 Germany Germany | 8 (6) |
 France France | 7 (5) |
 Israel Israel | 6 (4) |
 Italy Italy | 6 (4) |
 USA USA | 6 (4) |
 Other European countries Other European countries | 28 (21) |
 Other non-European countries Other non-European countries | 19 (14) |
| Involvement with PCD | |
 Research and diagnosis or care Research and diagnosis or care | 76 (56) |
 Diagnosis or care Diagnosis or care | 34 (25) |
 Research Research | 19 (14) |
 Other Other | 7 (5) |
| Occupation | |
 Paediatric pulmonologist Paediatric pulmonologist | 69 (51) |
 Adult pulmonologist Adult pulmonologist | 9 (7) |
 ENT specialist ENT specialist | 14 (10) |
 Diagnostic scientist Diagnostic scientist | 12 (9) |
 Paediatrician Paediatrician | 9 (7) |
 Non-physician healthcare professional Non-physician healthcare professional | 8 (6) |
 Epidemiologist or data scientist Epidemiologist or data scientist | 7 (5) |
 Other Other | 8 (6) |
| Work setting | |
 Academic hospital Academic hospital | 102 (75) |
 Academic research institution Academic research institution | 21 (15) |
 Non-academic hospital Non-academic hospital | 9 (7) |
 Other Other | 4 (3) |
| Years of involvement in PCD research or care | |
 >10 >10 | 64 (47) |
 5–10 5–10 | 42 (31) |
 <5 <5 | 30 (22) |
| Participated in PCD research during the past 15 years | 112 (82) |
Characteristics are presented as n (%); due to rounding, numbers do not always add up to 100%. PCD: primary ciliary dyskinesia; ENT: ear, nose and throat.
Survey respondents reported PCD research as most usually funded by competitive research grants, institutions or governments, and smaller foundations (table 3) or as unfunded (35%). Nearly half (49%) previously applied for PCD research funding and over half (51%) considered funding for PCD more difficult to acquire than for other diseases. Respondents reported the most important barriers for acquiring funding included low awareness about PCD, high competition for funding, lack of commercial application and rarity of the disease. Other factors they reported as hindering PCD research involved lack of dedicated research time (68%), small numbers of patients in each centre (63%), inactive or non-existent patient support groups (63%), disease heterogeneity (58%), few colleagues with expertise in PCD locally (57%), and lack of needed resources such as specialised equipment or databases (46%) (table 3). Participants strongly agreed about the need for national and international multidisciplinary research collaborations (82%), the importance of registries and cohort studies as research tools (89%), the importance of patient support groups (76%) and actively involving patients in different stages of research (78%), as well as the need for standardised care and information collection to improve future data quality (85%).
TABLE 3
Barriers and factors facilitating clinical and epidemiological research related to primary ciliary dyskinesia (PCD) according to the online survey participants (n=136)
| Main source of funding for PCD research# | |
 Institutional/governmental funding Institutional/governmental funding | 56 (41) |
 Competitive grants Competitive grants | 57 (42) |
 Funding from smaller foundations Funding from smaller foundations | 41 (30) |
 Funding from collaborative research Funding from collaborative research | 24 (18) |
 Unfunded Unfunded | 48 (35) |
| Compared to other diseases in the field, obtaining funding for PCD is | |
 More difficult More difficult | 69 (51) |
 Easier Easier | 1 (1) |
 No difference No difference | 29 (21) |
 I do not know I do not know | 37 (27) |
| Barriers in acquiring funding for PCD clinical and epidemiological research ¶ | |
 Low awareness about PCD Low awareness about PCD | 113 (83) |
 High competition for funding High competition for funding | 102 (75) |
 Lack of commercial application Lack of commercial application | 89 (65) |
 Rarity of disease Rarity of disease | 80 (59) |
 Low mortality rate/not considered severe Low mortality rate/not considered severe | 58 (43) |
 Lack of supporting evidence/existing research framework Lack of supporting evidence/existing research framework | 57 (42) |
 Lack of local support in preparing a funding application Lack of local support in preparing a funding application | 54 (40) |
 Lack of expertise of research team/limited publication record Lack of expertise of research team/limited publication record | 44 (32) |
 Higher interest in basic research projects Higher interest in basic research projects | 41 (30) |
| Other factors hindering PCD research ¶ | |
 Lack of dedicated research time Lack of dedicated research time | 93 (68) |
 Small numbers of patients Small numbers of patients | 86 (63) |
 No/inactive patient support group No/inactive patient support group | 86 (63) |
 Disease heterogeneity Disease heterogeneity | 79 (58) |
 Few colleagues locally with expertise in PCD Few colleagues locally with expertise in PCD | 78 (57) |
 Lack of needed resources Lack of needed resources | 64 (47) |
 Lack of good local or extended collaborative network Lack of good local or extended collaborative network | 38 (28) |
 Lack of interest about PCD from most colleagues Lack of interest about PCD from most colleagues | 38 (28) |
 Lack of motivation to participate from patients Lack of motivation to participate from patients | 31 (23) |
| Factors facilitating PCD research ¶ | |
 National and international registries and cohort studies National and international registries and cohort studies | 121 (89) |
 National and international multidisciplinary collaborations National and international multidisciplinary collaborations | 112 (82) |
 Standardisation of care and collected information to improve data quality Standardisation of care and collected information to improve data quality | 116 (85) |
 Active involvement of patients in research Active involvement of patients in research | 106 (78) |
 Patient support groups Patient support groups | 103 (76) |
Characteristics are presented as n (%). #: multiple answers possible; ¶: 5-point Likert scale responses here presented are agree or strongly agree.
Survey: priorities for PCD research
Nearly all participants (135 out of 136) responded to the research priority ranking questions. Among the three questions related to PCD diagnosis, nearly half of participants (49%) chose “How to improve the accuracy, speed, and cost-effectiveness of diagnostic testing in different age groups and healthcare settings?” (figure 2) as the most important question. In the questions related to PCD presentation, prognosis and follow-up, most participants selected “What is the clinical variability and natural course of upper and lower respiratory disease in PCD and which factors affect disease prognosis?” (41%) and “Which health-related behaviours or everyday interventions can have a positive role in improving symptoms or quality of life in people with PCD?” (27%) as the most important questions. The most important questions for participants regarding PCD treatments asked, “Are there any genetic or molecular treatments in the pipeline that could help restoring ciliary function?” (33%) and “Which of the already available and currently used medication and other management approaches for upper and lower airways are suitable for PCD patients?” (29%), respectively. Among other research priorities, increasing awareness and engagement of clinicians and patients in PCD research (54%) was reported as most important.
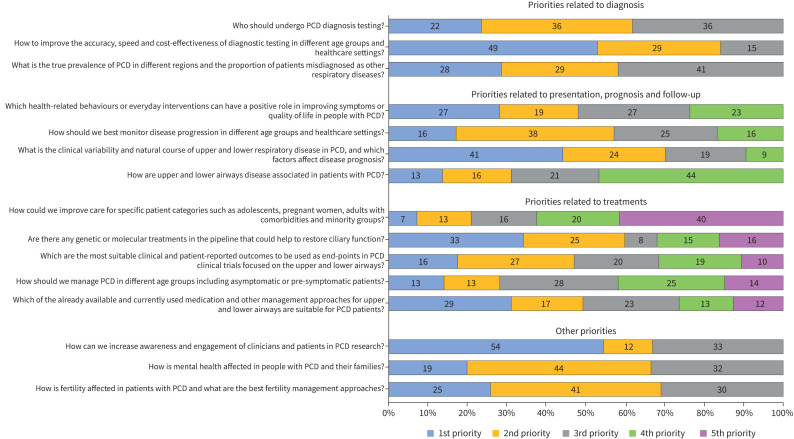
Ranked research priority questions by survey participants grouped by main topic. PCD: primary ciliary dyskinesia.
Ranked survey participant opinions on overall top priorities – across all topics related to PCD clinical and epidemiological research – varied with scores ranging from 0.02 to 0.31 (table 4).
TABLE 4
Top priorities across all topics related to primary ciliary dyskinesia (PCD) clinical and epidemiological research as ranked by survey participants
| Rank | Top priorities across all topics related to primary ciliary dyskinesia (PCD) clinical and epidemiological research | Mean score |
|---|---|---|
| 1 | How to improve the accuracy, speed and cost-effectiveness of diagnostic testing in different age groups and healthcare settings? | 0.312 |
| 2 | Are there any genetic or molecular treatments in the pipeline that could help restore ciliary function? | 0.273 |
| 3 | What is the clinical variability and natural course of upper and lower respiratory disease in PCD, and which factors affect disease prognosis? | 0.199 |
| 4 | Which of the already available and currently used medication and other management approaches for upper and lower airways are suitable for PCD patients? | 0.169 |
| 5 | Who should undergo diagnostic testing? | 0.168 |
| 6 | What is the true prevalence of PCD in different regions and the proportion of patients misdiagnosed as other respiratory diseases? | 0.121 |
| 7 | Which are the most suitable clinical and patient-reported outcomes to be used as end-points in PCD clinical trials focused on the upper and lower airways? | 0.115 |
| 8 | Which health-related behaviours or everyday interventions can have a positive role in improving symptoms or quality of life in people with PCD? | 0.114 |
| 9 | How should we best monitor disease progression in different age groups and healthcare settings? | 0.101 |
| 10 | How can we increase awareness and engagement of clinicians and patients in PCD research? | 0.077 |
| 11 | How should we manage PCD in different age groups including asymptomatic or pre-symptomatic patients? | 0.070 |
| 12 | How is the mental health affected in people with PCD and their families? | 0.028 |
| 13 | How is fertility affected in patients with PCD and what are the best fertility management approaches? | 0.025 |
| 14 | How could we improve care for specific patient categories such as adolescents, pregnant women, adults with comorbidities and minority groups? | 0.023 |
| 15 | How are upper and lower airways disease associated in patients with PCD? | 0.021 |
Questions ranked from most to least important based on the mean of a reciprocal ranking score (0–1); each question ranked either first (1 point); second (1/2 points); third (1/3 points) or not ranked (0 points) among the top three priorities.
The top three ranked questions asked are:
“How to improve the accuracy, speed, and cost-effectiveness of diagnostic testing in different age groups and healthcare settings?” (ranked first)
“Are there any genetic or molecular treatments in the pipeline that could help restoring ciliary function?” (ranked second)
“What is the clinical variability and natural course of upper and lower respiratory disease in PCD, and which factors affect disease prognosis?” (ranked third)
All three questions related to PCD diagnosis ranked in the top six of the overall priorities list. Questions related to relatively neglected areas from a research perspective, such as upper airways, mental health, fertility and care for specific patient groups and minorities, ranked lowest among participants (table 4). When comparing rankings between paediatric pulmonologists (largest groups) and other specialties, top priorities remained the same; however, results showed differences among lower ranked priorities (supplementary table S2).
Discussion
Using a mixed-method approach, our study identified main priorities and explored opinions about barriers for clinical and epidemiological research related to PCD as perceived by PCD professionals and researchers. Research in rare diseases, such as PCD, often faces different and additional challenges compared with more common conditions [36, 37]. Identification of specific barriers and factors for facilitating PCD-related research supports researchers and strengthens efforts to address these difficulties. Furthermore, when developing future research agendas, our results provide a roadmap for BEAT-PCD and the PCD community overall.
Most commonly reported barriers for PCD research were low awareness about the disease, which has already been previously reported, and difficulties in securing funding [38, 39]. Half of participants considered obtaining funding for PCD more difficult when compared with other diseases in the field; in more than one-third of cases, research was mostly performed without funding. In addition to high competition for research funding in general, difficulty in acquiring funding for PCD was mainly attributed to lack of commercial applications of research findings and to disease rarity. The engagement of specialists managing paediatric and adult patients across disciplines and promoting national and international collaborations, which include all relevant stakeholders such as patients and industry partners, could support efforts of acquiring funding. Such initiatives follow on from ongoing efforts from past years – promoting collaborations, exchanging expertise and sharing resources, such as setting up registries and multicentre cohort studies – and are all steps in the right direction to address these barriers.
Highlighted by relatively low mean ranking scores even for top-ranked priorities, we found variability in research question priority ranking. The finding emphasises several enduring important research gaps, instead of a clear consensus on just a handful of major priorities for PCD research. Participants from different disciplines possess different interests, which possibly reflects ranking. Top-ranked priorities were related to further improving diagnosis; treating PCD and managing upper and lower airway problems; and studying clinical variability and disease prognosis – all questions considered unlinked to specific disciplines. The overall minimum global prevalence of PCD was calculated recently to be at least one in 7500 individuals, much higher than previously considered [40]. PCD remains, however, underdiagnosed, particularly in adult patients who might be managed due to their bronchiectasis [41]. Our findings become more meaningful in the light of ongoing efforts to develop joint updated diagnostic guidelines for PCD. Furthermore, the limited evidence base for symptomatic and PCD-specific therapies, along with the new potential molecular treatments in the pipeline and the development of the PCD-specific clinical trials network, underline questions related to PCD treatment as high priorities [42, 43]. Notably, other topics strongly impacting the lives of people with PCD and their families, such as fertility or mental health, were ranked lower by experts, although they appeared in the priority list. In a study of bronchiectasis research priorities, in addition to topics included in the expert consensus, 42% of patient participants outlined additional topics, including research related to mental health [44]. For α1-antitrypsin deficiency, patients and caregivers included development of other aspects of integral care, such as caregiver support and psychological care, as their most important research areas, while respiratory specialists did not [45]. To ensure a common direction for the PCD research community with patient support groups and affected individuals, comparing priorities of people affected by PCD is an important next step [46].
Our study's main strengths include the mixed-methods design and far reach of the BEAT-PCD network [47]. Employing qualitative methods allowed us to gain rich information about healthcare professional and researcher perspectives, from participants from various backgrounds, areas and time of expertise, which we then used as a basis to develop a widely circulated survey [48–50]. Our approach ensured we noticed important aspects and allowed discussions of perspectives from purposefully selected participants in more detail than from questionnaires alone. Since most interviewees were prior acquaintances of M. Goutaki through the BEAT-PCD network, it created an environment of trust and comfort for open discussions. Yet, the familiarity possibly influenced participant answers during the interviews [51]. Through the BEAT-PCD mailing list we widely distributed information about the study and invitations for survey participation. However, the mailing list included people generally interested in PCD and the BEAT-PCD activities, who were not all eligible to participate in this study, e.g. patient representatives or healthcare professionals with very limited experience in the field. Therefore, it was not possible to calculate a survey response rate.
The main limitation of the study involves survey respondents closely representing the distributions of country, discipline and experience level with PCD in the BEAT-PCD network. We accomplished representation of experts from many countries with organised PCD care and research activities and high participation numbers among paediatric pulmonologists. Our participants included fewer participants from other specialties, such as adult pulmonologists, ENT specialists and other healthcare professionals, which highlights a need to increase multidisciplinary collaborations and awareness in other fields. Since paediatric pulmonologists represented only half of survey participants, it is noteworthy that our results include perspectives from other disciplines. Our study deliberately focused on healthcare professionals and researchers; we did not include people with PCD or parents of affected children; a separate, dedicated study focusing on patient and family perspectives regarding PCD research is ongoing [52]. Another limitation is that only one person coded all interviews. Although we followed an inductive approach, thematic analysis often relies on researcher judgement, possibly introducing biases from their own interpretations [51]. M. Goutaki is a female clinical epidemiologist with extensive experience in the field of epidemiological and clinical PCD research; she currently co-chairs the BEAT-PCD network. She coded and analysed the interview data under this lens. Some of the interviews were completed before the COVID-19 pandemic, others during the pandemic; however, analysis did not show any evident difference in themes.
Our study is the first assessing priorities and barriers for PCD research; it combines rich and detailed perspectives from in-depth interviews and representative high-level information from the PCD research community. We need to overcome barriers of limited funding and low disease awareness and promote collaborations between centres, disciplines, experts and patients to address priorities effectively. Our results contribute to ongoing efforts to guide the use of existing, limited research resources and set up a roadmap for future research activities to improve and streamline research in the field.
Acknowledgements
We thank all healthcare professionals and researchers who participated in the in-depth interviews or completed the survey. We thank Corine M. Driessens, Mary E. Barker and Polly Hardy-Johnson (University of Southampton) for their advice and support developing the interview guide and thematic analysis, and Kristin Marie Bivens (University of Bern) for her editorial assistance.
Provenance: Submitted article, peer reviewed.
Ethics statement: The study received approval by the Faculty of Medicine Ethics Committee of the University of Southampton (ERGO 47010.A1).
Author contributions: M. Goutaki and J.S. Lucas developed the concept and designed the study. M. Goutaki, J.S. Lucas and L. Behan developed the interview guide. M. Goutaki conducted in-depth interviews, and M. Goutaki and Y.T. Lam transcribed them. M. Goutaki performed thematic analysis, developed the survey, cleaned the dataset, performed the statistical analyses and drafted the manuscript. All authors commented and revised the survey and manuscript. M. Goutaki takes final responsibility for content.
Conflict of interest: M. Goutaki reports grants from the Johanna Dürmüller-Bol Foundation outside the submitted work and is Co-Chair of the BEAT-PCD clinical research collaboration.
Conflict of interest: Y.T. Lam is Work Package Co-Lead in clinical research collaboration BEAT-PCD funded by the European Respiratory Society.
Conflict of interest: J.D. Chalmers reports grants from AstraZeneca, GlaxoSmithKline, Grifols, Gilead Sciences, Boehringer Ingelheim, Trudell, Insmed and Genentech; and consulting fees from AstraZeneca, GlaxoSmithKline, Grifols, Boehringer Ingelheim, Trudell, Insmed, Genentech, Pfizer, Antabio and Zambon, all outside the submitted work; and is an associate editor of this journal.
Conflict of interest: J-F. Papon reports grants and consulting fees from Sanofi, GSK, Medtronic, AstraZeneca and ALK; lecture honoraria from Sanofi, GSK and Medtronic; and travel support from Sanofi and GSK, all outside the submitted work.
Conflict of interest: J.S. Lucas reports grants from the Medical Research Council, Life Arc, NHS England, NIHR Southampton Biomedical Research Centre, AAIR charity, NIHR Research for Patient Benefit and Wessex Medical Research, outside the submitted work.
Conflict of interest: All other authors have nothing to disclose.
Support statement: We acknowledge the support of the European Respiratory Society (ERS) Short-Term Research fellowship number 201804-00367. The study was developed as a collaboration between the Universities of Southampton and Bern within the framework of the BEAT-PCD COST Action BM1407 and completed in the BEAT-PCD clinical research collaboration framework, supported by ERS. M Goutaki is supported by a Swiss National Science Foundation Ambizione fellowship (PZ00P3_185923) and the Johanna Dürmüller-Bol Foundation. Several authors participate in ERN-LUNG (PCD core). Funding information for this article has been deposited with the Crossref Funder Registry.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary tables 00026-2024.SUPPLEMENT
Interview guide 00026-2024.SUPPLEMENT2
References
Articles from ERJ Open Research are provided here courtesy of European Respiratory Society
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/166783253
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The Swiss Primary Ciliary Dyskinesia registry: objectives, methods and first results
Swiss Med Wkly, 149:w20004, 13 Jan 2019
Cited by: 11 articles | PMID: 30691261
Infertility and pregnancy outcomes among adults with primary ciliary dyskinesia.
Hum Reprod Open, 2024(3):hoae039, 18 Jun 2024
Cited by: 0 articles | PMID: 38962571 | PMCID: PMC11219480
Primary Ciliary Dyskinesia: An Update on Clinical Aspects, Genetics, Diagnosis, and Future Treatment Strategies.
Front Pediatr, 5:135, 09 Jun 2017
Cited by: 84 articles | PMID: 28649564 | PMCID: PMC5465251
Review Free full text in Europe PMC
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Cochrane Database Syst Rev, 2(2022), 01 Feb 2022
Cited by: 12 articles | PMID: 36321557 | PMCID: PMC8805585
Review Free full text in Europe PMC
Funding
Funders who supported this work.
European Respiratory Society (1)
Grant ID: Short-Term Research fellowship Nr 201804-00367
Swiss National Science Foundation (1)
Grant ID: PZ00P3_185923

 1,2
1,2 

