Abstract
Aim
To investigate the efficacy and safety of HAIC combined with programmed cell death protein-1 (PD1) inhibitors in MVI-positive advanced hepatocellular carcinoma(HCC).Methods
From September 2017 to May 2019, we retrospectively collected the clinical data from three medical centers in China pertaining to patients diagnosed with BCLC C stage HCC with MVI and receiving treatment with a combination of HAIC and PD-1 inhibitors treatment or HAIC alone, and we compared the efficacy of HAIC combined with PD-1 inhibitors and HAIC monotherapy. Propensity score matching(PSM) was utilized to adjust for baseline differences between groups. Survival outcomes and tumor response rate were used to assess survival benefits, while the incidence of adverse events was used to evaluate safety.Results
After screening for eligibility, 489 patients diagnosed with HCC and concomitant MVI were enrolled. Of these, 173 patients received treatment combining HAIC with PD-1 inhibitors, while 316 patients underwent HAIC monotherapy. After PSM adjustment, the combination therapy group demonstrate superior survival outcomes. Median overall survival(OS) and progression free survival(PFS) were 31.8 months and 10.8 months, respectively, significantly higher than those in the monotherapy group (OS: 10.0 months; PFS: 6.1 months; both P<0.0001). Moreover, ORR and DCR remained significantly elevated in the combination therapy group (ORR: 44.3% vs 20.4%, P<0.0001; DCR: 89.8% vs 82.0%, P=0.041). Safety profiles indicated no significant differences in adverse event rates between the two treatment groups, encompassing both overall and grade-specific assessments.Conclusion
Compared to HAIC alone, the combination of HAIC with PD-1 inhibitors represents a more promising and effective approach for patients with HCC complicated by macrovascular invasion.Free full text

Efficacy and Safety of Hepatic Arterial Infusion Chemotherapy(HAIC) Combined with PD-1 Inhibitors for Advanced Hepatocellular Carcinoma with Macrovascular Invasion: A Multicenter Propensity Score Matching Analysis
Abstract
Aim
To investigate the efficacy and safety of HAIC combined with programmed cell death protein-1 (PD1) inhibitors in MVI-positive advanced hepatocellular carcinoma(HCC).
Methods
From September 2017 to May 2019, we retrospectively collected the clinical data from three medical centers in China pertaining to patients diagnosed with BCLC C stage HCC with MVI and receiving treatment with a combination of HAIC and PD-1 inhibitors treatment or HAIC alone, and we compared the efficacy of HAIC combined with PD-1 inhibitors and HAIC monotherapy. Propensity score matching(PSM) was utilized to adjust for baseline differences between groups. Survival outcomes and tumor response rate were used to assess survival benefits, while the incidence of adverse events was used to evaluate safety.
Results
After screening for eligibility, 489 patients diagnosed with HCC and concomitant MVI were enrolled. Of these, 173 patients received treatment combining HAIC with PD-1 inhibitors, while 316 patients underwent HAIC monotherapy. After PSM adjustment, the combination therapy group demonstrate superior survival outcomes. Median overall survival(OS) and progression free survival(PFS) were 31.8 months and 10.8 months, respectively, significantly higher than those in the monotherapy group (OS: 10.0 months; PFS: 6.1 months; both P<0.0001). Moreover, ORR and DCR remained significantly elevated in the combination therapy group (ORR: 44.3% vs 20.4%, P<0.0001; DCR: 89.8% vs 82.0%, P=0.041). Safety profiles indicated no significant differences in adverse event rates between the two treatment groups, encompassing both overall and grade-specific assessments.
Conclusion
Compared to HAIC alone, the combination of HAIC with PD-1 inhibitors represents a more promising and effective approach for patients with HCC complicated by macrovascular invasion.
Introduction
As the third leading cause of cancer-related deaths globally, hepatocellular carcinoma(HCC) exhibits significant heterogeneity based on its biological characteristics, leading to varying sensitivities to different treatment approaches.1–3 Surgical resection, ablation, and liver transplantation are the only curative treatment modalities available. Regrettably, a considerable proportion of patients receive their diagnosis at an intermediate to advanced disease stage, thereby forfeiting the prospect of undergoing curative interventions. On the other hand, the majority of patients exhibit macrovascular invasion (MVI) at the time of initial diagnosis,4,5 involving the formation of tumor thrombi in the portal vein, hepatic vein, and inferior vena cava, either independently or along with extra-hepatic metastasis. Nevertheless, such a particular subset of patients with MVI and/or metastasis is also not considered suitable candidates for curative treatment approaches, and they face a significantly grim prognosis coupled with the intricate challenge of limited therapeutic alternatives.
Prior studies suggest that sorafenib can delay time to progression (TTP) and extend survival, and Tyrosine kinase inhibitor represented by sorafenib was recommended as the standard treatment for any MVI-associated HCC.6,7 However, Subsequent studies manifested that MVI remains a negative prognostic factor for advanced HCC patients undergoing sorafenib therapy with limited prognosis. The median OS with sorafenib monotherapy for HCC with MVI ranges only from 3.1 to 8.1 months.8,9 Hepatic arterial infusion chemotherapy(HAIC) is a form of locoregional chemotherapy performed through macrocatheters. By super-selectively inserting macrocatheters into the main arterial supply of the tumor, chemotherapy drugs are directly infused, leading to the formation of a high local concentration of chemotherapy while reducing systemic adverse reactions. Furthermore, the infusion of chemotherapy drugs via the hepatic artery bypasses the first-pass effect of the liver, further enhancing the anti-tumor effect of chemotherapy. Recent studies indicate that HCC with MVI may benefit from either standalone local therapy or a combination of local and systemic treatments over sorafenib.10–12 A large-scale multicenter study conducted by Kazuomi Ueshima has demonstrated that HAIC yields superior efficacy compared to standard sorafenib treatment for MVI-positive HCC without extrahepatic metastasis. In another a multicenter retrospective study conducted by Kodama K, survival benefits in MVI-positive HCC patients were compared after receiving either HAIC or sorafenib treatment. The study showed a median OS time of 13.0 months with HAIC, compared to only 6.0 months with standard sorafenib therapy, indicating a significant statistical difference. Therefore, HAIC may be a more preferable and promising option for MVI-positive patients.
PD-1 is a major immune checkpoint on the surface of T cells, which, when bound to the overexpressed PD-L1 on malignant tumor cells, inhibits T cell proliferation and activation, leading to T cell inactivation and immune escape, ultimately resulting in treatment failure. PD-1 inhibitors, by blocking the PD-1/PD-L1 pathway, have achieved breakthroughs in cancer immunotherapy.13,14 Based on earlier KEYNOTE-224 and CheckMate-040 trials, the FDA has approved nivolumab and pembrolizumab for second-line treatment of advanced HCC, marking the era of immunotherapy for HCC. While single-agent PD-1 inhibitor immunotherapy reportedly has an efficacy rate of less than 20% in solid tumors, several studies have shown that PD-1 inhibitors in combination with HAIC exhibit good efficacy and tolerable adverse effects in BCLC stage C HCC patients.15–17 It is noteworthy that these studies included a population with a high proportion of vascular involvement. Therefore, the combination of locoregional chemotherapy with HAIC and PD-1 inhibitors may represent a promising combined therapeutic approach for MVI-positive HCC.
As of now, the safety and efficacy of combining HAIC with immunotherapy for advanced HCC with concomitant MVI positivity remain an area unexplored. Therefore, we conducted this multicenter retrospective study aimed at further investigating whether the combination therapy of HAIC plus PD-1 inhibitors could offer potential survival benefits for patients with MVI-positive HCC.
Methods
Study Population
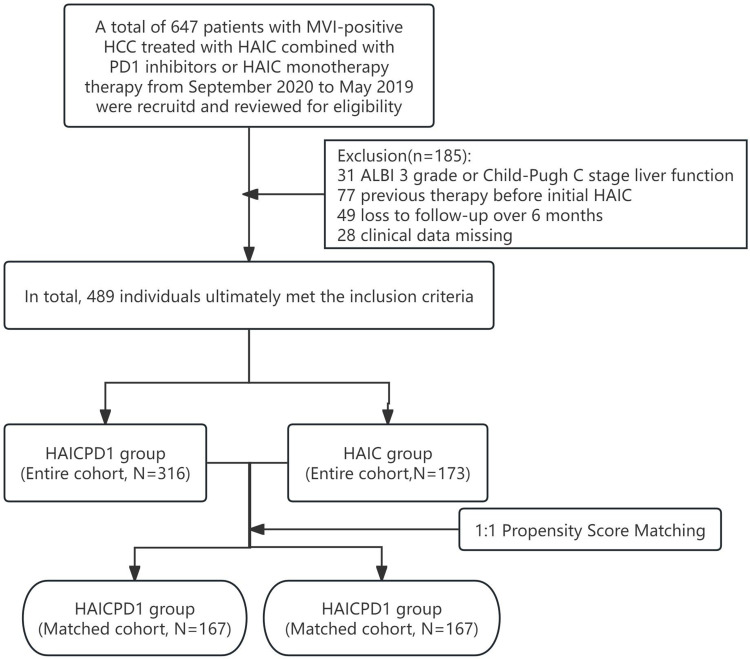
From September 2017 to May 2019, we retrospectively collected data from a total of 647 patients with MVI-positive HCC who underwent either HAIC or a combination of HAIC and PD-1 inhibitors across three medical centers. After a thorough eligibility review, a final cohort of 489 patients (316 in the HAIC group and 173 in the HAICPD1 group) was ultimately included.
This study obtained ethical approval from the Ethics Committees of Huazhong University of Science and Technology Union Shenzhen Hospital (Shenzhen Nanshan People’s Hospital), Zhuhai People’s Hospital (Zhuhai Clinical Medical College of Jinan University) and The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, and adhered to the principles of the Declaration of Helsinki. Due to the retrospective nature of this study, patients are not required to agree to the review of medical records. Additionally, the general information, clinical data, and imaging data collected in this study were obtained from previous clinical diagnosis and treatment, and the research project does not involve personal privacy or commercial interests; Not utilizing medical records and specimens that patients have clearly refused to use before; Exemption from informed consent will not have adverse effects on the rights and health of the subjects; The study also does not require further follow-up to obtain participant information. At the same time, we declare that the privacy and personal identification information of the subjects are protected. Treatment regimens were determined following recommendations from a multidisciplinary tumor board, which assessed factors including patient performance status, liver function, and tumor characteristics to tailor individualized strategies.
The diagnosis of HCC was confirmed either pathologically or through intravenous contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) following the criteria set by the European Association for the Study of the Liver.
The inclusion criteria were as follows: (a) age ranging from 18 to 80 years; (b) Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; (c) presence of at least one measurable intra-hepatic lesion according to modified Response Evaluation Criteria in Solid Tumors (mRECIST); and (d) preserved liver function classified as Child-Pugh A or B grade. Exclusion criteria included: (a) prior treatments before initial HAIC; (b) concurrent malignancies with HCC; (c) missing clinical data; and (d) loss to follow-up exceeding 6 months.
Treatment Procedures and Follow-Up Schedules
All HAIC treatment procedures were initially performed by two or more experienced interventional radiologists with the assistance of the Digital Subtraction Angiography (DSA, Siemens Artis Zeego, SIMENS) system to achieve successful hepatic artery catheterization. The process is as follows: Following local anesthesia with 5mL of 2% lidocaine at the femoral artery puncture site, femoral artery puncture is carried out using the modified Seldinger technique, and upon successful completion, a 5F sheath is inserted. Subsequently, a 5-Fr Yashiro catheter (Terumo, Tokyo, Japan) is advanced into the celiac trunk and superior mesenteric artery to evaluate the feeding hepatic artery. Finally, a 2.7-Fr macro-catheter (ASAHI INTECC, Tokyo, Japan) is utilized to selectively or super-selectively insert into the main artery supplying the tumor as feasible.
The chemotherapy protocol utilized was mFOLFOX6, comprising Oxaliplatin administered intravenously at a dose of 85mg/ m² within the initial 2 hours on day 1; Calcium folinate injected intravenously at a dosage of 200mg/ m² within the first 2 hours on day 1; and Fluorouracil at a dosage of 400mg/ m², followed by continuous intravenous infusion totaling 2400mg/m² with a period of 46 hours. Following HAIC, the combination therapy group persisted in receiving PD1 inhibitors for a duration of 2 days. All specific types and dosages of PD1 inhibitors were outlined in Table 1. During the treatment process, Oxaliplatin dosage could be appropriately reduced based on specific adverse reactions, while PD1 inhibitor discontinuation was required without allowance for dose reduction. Adverse reactions can recover after symptomatic relief measures were implemented. HAIC produce was scheduled for repetition every 3 weeks, encompassing a total of 4–6 cycles. Subsequent to each HAIC cycle, a comprehensive CT and/or MRI follow-up was scheduled one month subsequent to the first session, and subsequently at three-month intervals.
Table 1
Category and Dosage of PD-1 Inhibitors in the HAICPD1 Group
| Category | Dose (mg) | HAICPD1 (n=173) |
|---|---|---|
| Nivolumab (Bristol-Myers Squibb Holdings Pharma, Ltd.Liability Company) | 240 | 66(38.2) |
| Keytruda | 200 | 51(29.5) |
| (Carlow, Merck Sharp & Dohme Ireland Corp) | ||
| Toripalimab | 240 | 32(18.5) |
| (Suzhou, hezhong pharmaceutical Co.Ltd) | ||
| Sintilimab | 200 | 24(13.9) |
| (Suzhou, xinda pharmaceutical Co.Ltd)) |
Note: Data represent as n(%).
Abbreviations: PD1, programmed cell death protein 1; HAICPD1, Hepatic artery infusion chemotherapy plus PD1 inhibitors.
Clinical Data Collection
The clinical data encompassed age categories (≤65 years, >65 years) and gender (male, female), ECOG status (0, 1), comorbidities (hypertension and/or diabetes), ascites, liver disease etiology (HBV; others), largest tumor size (≤ 7cm; > 7cm), tumor count (≤ 3; > 3) and extrahepatic metastasis. Laboratory results comprised AFP, serum albumin [ALB], alanine aminotransferase [ALT], aspartate aminotransferase [AST], total bilirubin [TB], platelet count, prothrombin time [PT], international normalized ratio [INR], aspartate alanine, creatinine, neutrophil count, and lymphocyte count. ALBI grades were utilized for their objectivity instead of CTP grades. Prior to treatment, the ALBI score was determined based on specific clinical parameters, with ALBI grade defined as: (log10 bilirubin mmol/L×0.66) + (albumin g/L×−0.085), corresponding to grades 1, 2, and 3 as ≤ −2.60, > −2.60 to −1.39, and > −1.39, respectively.
Survival Outcomes
The primary endpoint of the study was focused on evaluating overall survival (OS), which was calculated from the time of the initial definitive diagnosis of HCC until either the last follow-up or the occurrence of death. Regarding the secondary endpoint, the study examined progression-free survival (PFS), which was computed from the onset of the first confirmed diagnosis of HCC to the assessment of disease progression based on the Modified Response Evaluation Criteria in Solid Tumors 1.1 mRECIST 1.1 criteria.18 Furthermore, treatment efficacy was assessed using the mRECIST 1.1 criteria, categorizing responses into complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Independent radiologists with extensive experience conducted radiographic assessments of tumor response as part of the endpoint evaluations. The third endpoint encompassed the objective response rate (ORR), representing the percentage of patients who achieved a tumor response classified as CR and PR, while the disease control rate (DCR) was defined as the percentage of patients achieving a tumor response categorized as CR, PR, and SD. Adverse events were further analyzed in accordance with the Common Terminology Criteria for Adverse Events version 5.0.
Propensity Score Matching(PSM)
We included covariates such as age, gender, ECOG PS, comorbidities, ascites, liver disease etiology, Liver function: ALBI grade, largest tumor size, tumor count, and extrahepatic metastasis into baseline balance. After adjusting for a matching tolerance of 0.02, a 1:1 propensity score matching (PSM) was performed to balance the baseline differences between groups, resulting in the acquisition of a matched cohort.
Statistical Analysis
All Statistical analyses were performed utilizing R software (version 4.2.3, R Studio) and SPSS software (version 26.0, IBM, USA). Normally distributed count data was presented as mean ± standard deviation and analyzed using Student’s t-test. For skewed distributions, the median was employed for representation, with analysis conducted using the Mann–Whitney U-test. Percentage representation was utilized for count data, and analysis was carried out employing either the Chi-square test or Fisher’s exact test. Survival analysis was executed via the Kaplan-Meier method to evaluate disparities in OS and PFS between groups, both before and after adjusting for PSM. Cox proportional hazards regression models were established for both univariate and multivariate analyses to discern prognostic risk factors.
A significance level of P<0.05 was applied to denote statistical significance.
Results
Baseline Characteristics
Through eligibility screening, 316 patients with MVI-positive HCC who underwent sole HAIC treatment and 173 patients who received combined HAIC and PD-1 inhibitor therapy were included. The screening process is detailed in Figure 1, and baseline differences between the entire cohort and the PSM-matched cohort are summarized in Table 2. The HAIC group comprised 293 male patients (92.7%), while the HAICPD-1 group included 154 male patients (89.0%). Median ages in the two groups were 50.0 years (IQR, 50.5–58.0) and 52.0 years (IQR, 43.0–60.0) respectively. Most patients in both groups had HBV infection, a maximum tumor diameter >7cm, and extrahepatic metastasis. Initial liver function in the sole treatment group was poorer compared to the combined treatment group in the overall cohort (P=0.014). Subsequently, 334 matched cases were obtained through 1:1 PSM, effectively balancing the inter-group differences.
Table 2
Baseline Characteristics Differences Before and After PSM Adjustment
| Entire Cohort | PSM Cohort | |||||
|---|---|---|---|---|---|---|
| HAICPD1 Group (n=173) | HAIC Group (n=316) | P value | HAICPD1 Group (n=167) | HAIC Group (n=167) | P value | |
| Gender | 0.348 | 0.850 | ||||
 Male Male | 154(89.0) | 293(92.7) | 151(90.4) | 152(91.0) | ||
 Female Female | 19(11.0) | 23(7.3) | 16(9.6) | 15(9.0) | ||
| Age(Median, IQR) | 52.0(43.0-60.0) | 50.0(40.5-58.0) | 0. 429 | 51.5±10.7 | 51.7±10.4 | 1.000 |
 ≤65y ≤65y | 159(91.9) | 282(89.2) | 154(92.2) | 154(92.2) | ||
 >65y >65y | 14(8.1) | 34(10.8) | 13(7.8) | 13(7.8) | ||
| ECOG | 0.797 | 0.875 | ||||
 0 0 | 148(85.5) | 273(86.4) | 144(86.2) | 143(85.6) | ||
 1 1 | 25(14.5) | 43(13.6) | 23(13.8) | 24(14.4) | ||
| Comorbidities | 0.404 | 0.862 | ||||
 Presence Presence | 20 (11.6) | 45(14.2) | 18(10.8) | 19(11.4) | ||
 Absence Absence | 153(88.4) | 271(85.8) | 149(89.2) | 148(88.6) | ||
| HBV | 0.735 | 0.479 | ||||
 Presence Presence | 163(94.2) | 300(94.9) | 159(95.2) | 156(93.3) | ||
 Absence Absence | 10(5.8) | 16(5.1) | 8(4.8) | 11(6.7) | ||
| Ascites | 0.780 | |||||
 Presence Presence | 30 (17.3) | 58(18.4) | 28(16.8) | 25(15.0) | 0.653 | |
 Absence Absence | 143 (82.7) | 258(81.6) | 139(83.2) | 142(85.0) | ||
| ALBI grade | 0.014 | 0.742 | ||||
 1 1 | 95(54.9) | 137(43.4) | 91(54.5) | 88(52.7) | ||
 2 2 | 78(45.1) | 179 (56.6) | 76(45.5) | 79(47.3) | ||
| AFP | 0.921 | 0.654 | ||||
 ≤400ng/L ≤400ng/L | 66(38.2) | 122(38.6) | 64(38.3) | 68(40.7) | ||
 >400ng/L >400ng/L | 107(61.8) | 194(61.4) | 103(61.7) | 99(59.3) | ||
| BCLC stage | 1.000 | 1.000 | ||||
 C C | 173(100) | 167(100) | 167 (100) | 167 (100) | ||
| Tumor size | 0.101 | 0.783 | ||||
 <7cm <7cm | 33(19.1) | 81(25.6) | 32(19.2) | 34(20.4) | ||
 ≥7cm ≥7cm | 140(80.9) | 235(74.4) | 135 (80.8) | 133(79.6) | ||
| Tumor number | 0.435 | 0.910 | ||||
 1-3 1-3 | 66(38.2) | 132(41.8) | 63(37.7) | 64(38.3) | ||
 >3 >3 | 107(61.8) | 184(58.2) | 104(62.3) | 103(61.7) | ||
| Metastasis | 0.872 | 0.112 | ||||
 Presence Presence | 71(41.0) | 132(41.8) | 69(41.3) | 54(32.3) | ||
 Absence Absence | 102(59.0) | 184(58.2) | 98(58.7) | 113(67.7) | ||
| Follow-up treatment | 0.138 | 0.284 | ||||
| Surgical resection | 13(7.5%) | 32(10.1%) | 12(7.2%) | 15(9.0%) | ||
| Ablation | 69(39.9%) | 96(30.4%) | 68(40.7%) | 65(38.9%) | ||
| Radiotherapy | 46(26.6%) | 74(23.4%) | 42(25.1%) | 44(26.3%) | ||
| TKI | 15(8.7%) | 41(13.0%) | 14(8.4%) | 21(12.5%) | ||
Note: Data represent as n(%).
Abbreviations: PSM, Propensity Score Matching; ECOG, Eastern Cooperative Oncology Group score; HBV, Hepatitis B virus; ALBI, Albumin‐bilirubin; BCLC, Barcelona Clinic Liver Cancer; AFP, α-fetoprotein; HAIC, Hepatic artery infusion chemotherapy; HAICPD1, Hepatic artery infusion chemotherapy plus PD1 inhibitors.
Comparison of Survival Outcomes
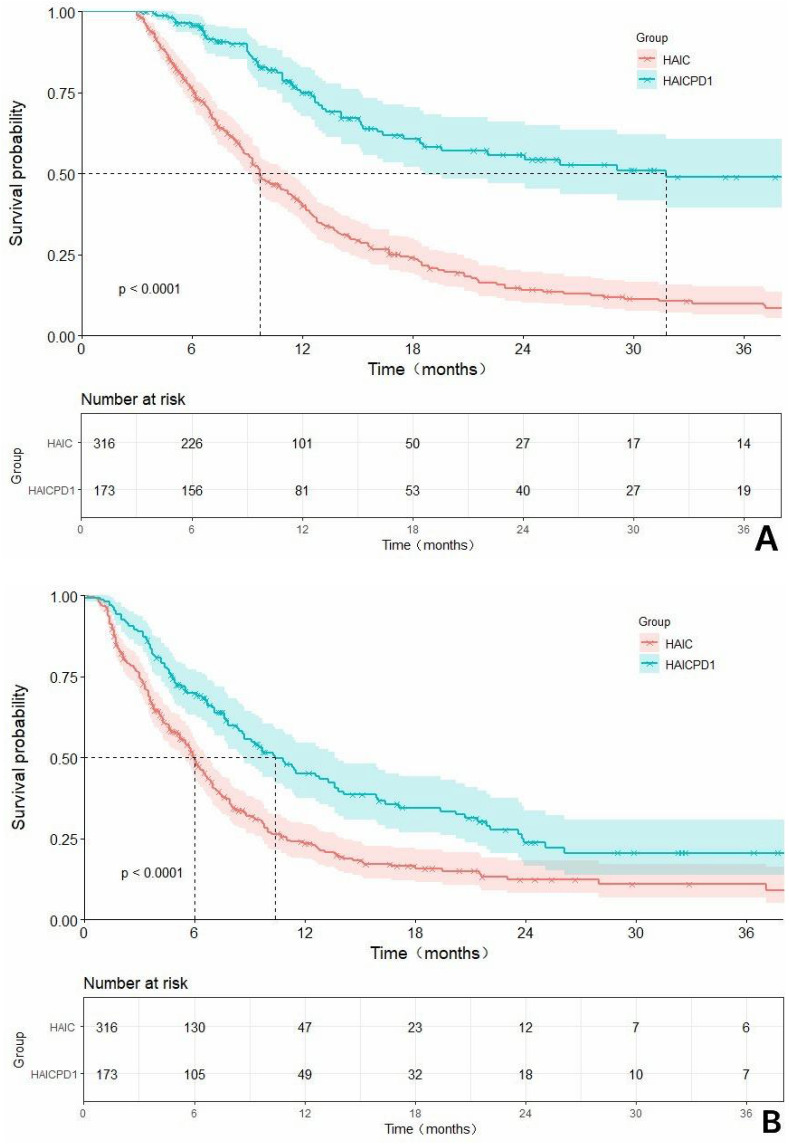
With a median follow-up of 35.1 months, the HAICPD1 group in the overall cohort showcased significantly prolonged median OS(mOS) of 31.8 months (HR: 0.36, 95% CI: 0.29–0.45) and median PFS(mPFS) of 10.8 months (HR: 0.58, 95% CI: 0.46–0.72) compared to the HAIC group with 9.70 months (HR: 2.78, 95% CI: 2.20–3.50) and 6.0 months (HR: 1.73, 95% CI: 1.39–2.16). These disparities were statistically significant (P<0.0001). The 6-month, 1-year, and 2-year survival rates for the HAICPD1 group and HAIC group were 90.2%, 46.8%, 23.1% and 71.5%, 32.0%, 8.5%, respectively, demonstrating statistical variability. In the cohort adjusted by PSM, the HAICPD1 group also displayed equally promising survival benefits. The median OS for the combination therapy and monotherapy groups were 31.8 months (HR: 0.32, 95% CI: 0.24–0.43) and 10.0 months (HR: 3.11, 95% CI: 2.31–4.20), while the PFS were 10.8 months (HR: 0.58, 95% CI: 0.45–0.76) and 6.1 months (HR: 1.71, 95% CI: 1.32–2.22), respectively. The 6-month, 1-year, and 2-year survival rates for these groups were 89.8%, 47.9%, 24.0% and 71.9%, 35.3%, 8.4%, all with P values < 0.0001, which further underscoring the superiority of the combination treatment approach. The survival curves related to OS and PFS before and after PSM adjustment are illustrated in Figure 2.
Figure 2
Continued.
Figure 2
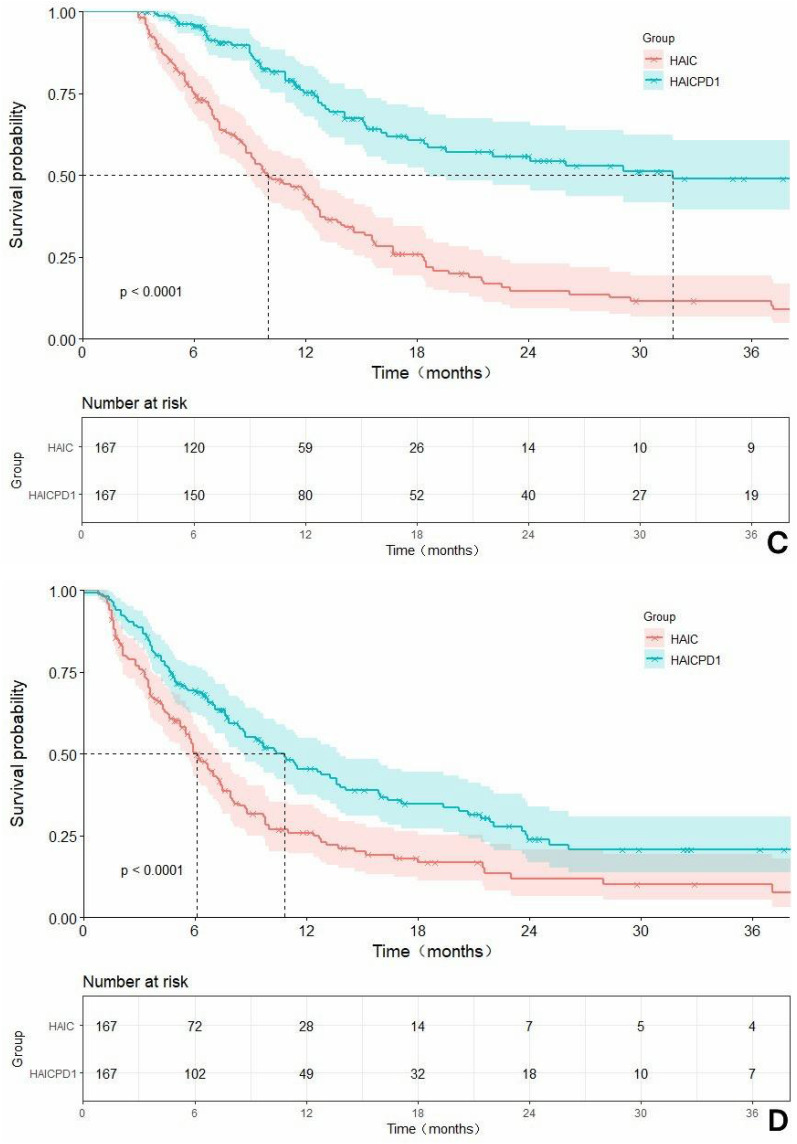
The Kaplan-Meier survival curves related to overall survival (OS) and progression-free survival (PFS) before and after PSM are compared in the context of: (A) a rough comparison of OS between the combination of HAIC with PD1 inhibitors and HAIC monotherapy; (B) a rough comparison of PFS between the combination of HAIC with PD1 inhibitors and HAIC monotherapy; (C). a matched adjusted comparison of OS between the combination of HAIC with PD1 inhibitors and HAIC monotherapy; (D) a matched adjusted comparison of PFS between the combination of HAIC with PD1 inhibitors and HAIC monotherapy.
Tumor Response
The tumor response status before and after matching are shown in Table 3. According to the mRECIST criteria, prior to matching, the HAICPD1 group exhibited 1 case of CR, 79 cases of PR, 76 cases of SD, and 17 cases of PD. In contrast, the HAIC group had 2 cases of CR, 64 cases of PR, 194 cases of SD, and 56 cases of PD, showing a marked superiority in overall response in the former group over the latter. Moreover, the combination therapy group demonstrated a significantly superior response compared to the monotherapy group, with an ORR of 46.2% (P<0.0001) and a DCR of 90.2% (P=0.019) versus 20.9% and 82.3% achieved by the monotherapy group. Following matching adjustment, the ORR for the combination therapy and monotherapy groups was 44.3% and 20.4%, respectively (P<0.0001), while the DCR was 89.8% and 82.0% (P=0.041). Overall response, ORR, and DCR remain significantly higher in the combination therapy group compared to the monotherapy group.
Table 3
Best Tumor Response Before and After the Adjustment of PSM in the Overall Group and Subgroup
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| HAICPD1 group | HAIC group | P value | HAICPD1 group | HAIC group | P value | |
| Overall | (n=173) | (n=316) | (n=167) | (n=167) | ||
| Best Response | < 0.0001 | < 0.0001 | ||||
 CR CR | 1(0.5) | 2(0.6) | 1(0.6) | 1(0.6) | ||
 PR PR | 79(45.7) | 64 (20.3) | 73(43.7) | 33(19.8) | ||
 SD SD | 76 (31.9) | 194(61.4) | 76(45.5) | 103(61.7) | ||
 PD PD | 17(9.8) | 56(17.7) | 17(10.2) | 30(18.0) | ||
 ORR ORR | 46.2%(80/173) | 20.9%(66/316) | < 0.0001 | 44.3%(74/167) | 20.4%(34/167) | < 0.0001 |
 DCR DCR | 90.2%(156/173) | 82.3%(260/316) | 0.019 | 89.8%(150/167) | 82.0%(137/167) | 0.041 |
| Subgroup | ||||||
| Metastasis(-) | n=102 | n=184 | < 0.0001 | n=98 | n=113 | < 0.0001 |
 CR CR | 1(1.0) | 1(0.5) | 1(1.0) | 0(0.0) | ||
 PR PR | 58(56.9) | 47(25.5) | 54(55.1) | 28(24.8) | ||
 SD SD | 36(35.3) | 104(56.5) | 36(36.7) | 66(58.4) | ||
 PD PD | 7(6.9) | 32(17.4) | 7(7.1) | 19(16.8) | ||
 ORR ORR | 57.8(59/102) | (48/184) | < 0.0001 | 56.1(55/98) | 24.8(28/113) | < 0.0001 |
 DCR DCR | 93.1(95/102) | (152/184) | 0.013 | 87.8(86/98) | 80.6(79/98) | 0.017 |
| Metastasis(+) | n=71 | n=132 | < 0.0001 | n=69 | n=54 | < 0.0001 |
 CR CR | 0(0.0) | 1(0.8) | 0(0.0) | 1(1.9) | ||
 PR PR | 21(29.6) | 17(12.9) | 19(27.5) | 5(9.3) | ||
 SD SD | 40(56.3) | 90(68.2) | 40(58.0) | 37(68.5) | ||
 PD PD | 10(14.1) | 24(18.2) | 10(14.5) | 11(20.4) | ||
 ORR ORR | 29.6(21/71) | 13.6(18/132) | < 0.0001 | 27.5(19/69) | 11.1(6/54) | < 0.0001 |
 DCR DCR | 85.9(61/71) | 81.8(108/132) | 0.456 | 85.5(59/69) | 79.6(43/54) | 0.390 |
Note: Data are presented as n (%).
Abbreviations: PSM, Propensity Score Matching; HAIC, Hepatic artery infusion chemotherapy; HAICPD1, Hepatic artery infusion chemotherapy plus PD1 inhibitors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
Independent Risk Factors for Survival Outcomes
The univariate and multivariate analyses of prognostic factors influencing OS and PFS are documented in Table 4. Univariate analysis was initially conducted, and covariates with P<0.1 were collectively included in the multivariate analysis. Ultimately, MVI-positive HCC patients without concurrent HBV infection, absence of extrahepatic metastasis, and receiving combined HAIC and PD-1 inhibitor therapy were identified as independent risk factors for extended OS. Furthermore, absence of concurrent HBV infection, AFP≤400ng/L, absence of extrahepatic metastasis, and receiving HAIC were identified as independent risk factors associated with favorable PFS.
Table 4
Risk Factors for Overall Survival and Progression-Free Survival Based on Uni- and Multivariate Analysis
| Factors | Overall survival | Progression-free survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis P value | Multivariate Analysis | Univariate Analysis P value | Multivariate Analysis | |||||
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Gender | 0.830 | - | - | - | 0.344 | - | - | - |
 Male Male | ||||||||
 Female Female | ||||||||
| Age | 0.580 | - | - | - | 0.760 | - | - | - |
 ≤65y ≤65y | ||||||||
 >65y >65y | ||||||||
| ECOG | 0.541 | - | - | - | 0.729 | - | - | - |
 0 0 | ||||||||
 1 1 | ||||||||
Comorbidities Presence Presence Absence Absence | 0.137 | 0.741 | ||||||
| HBV | 0.043 | 2.032 | 1.003–4.114 | 0.049 | 0.018 | 2.357 | 1.166–4.764 | 0.017 |
 Presence Presence | ||||||||
 Absence Absence | ||||||||
| Ascites | 0.805 | - | - | - | 0.320 | - | - | - |
 Presence Presence | - | - | - | |||||
 Absence Absence | - | - | - | |||||
| ALBI grade | 0.194 | - | - | - | 0.788 | - | - | - |
 1 1 | ||||||||
 2 2 | ||||||||
| AFP | 0.228 | - | - | - | 0.089 | 1.297- | 1.022–1.646 | 0.032 |
 ≤400ng/L ≤400ng/L | ||||||||
 >400ng/L >400ng/L | ||||||||
| Tumor size | 0.893 | - | - | - | 0.652 | - | - | - |
 ≤7cm ≤7cm | ||||||||
 >7cm >7cm | ||||||||
| Tumor number | 0.509 | - | - | - | - | - | - | - |
 1–3 1–3 | ||||||||
 >3 >3 | ||||||||
| Metastasis | 0.023 | 1.313 | 1.085–1.718 | 0.008 | 0.007 | 1.446 | 1.149–1.820 | 0.002 |
 Presence Presence | ||||||||
 Absence Absence | ||||||||
| Treatment regime | <0.0001 | 0.292 | 0.218-0.0391 | <0.0001 | <0.0001 | 0.290 | 0.216–0.387 | <0.0001 |
 HAICPD1 HAICPD1 | ||||||||
 HAIC HAIC | ||||||||
Note: Bold indicates statistical significance level at p-value < 0.05.
Abbreviations: HR, hazard ratios; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group score; HBV, hepatitis B virus; AFP, α-fetoprotein; HAIC, Hepatic artery infusion chemotherapy; HAICPD1, Hepatic artery infusion chemotherapy plus PD1 inhibitors.
Subgroup Analysis
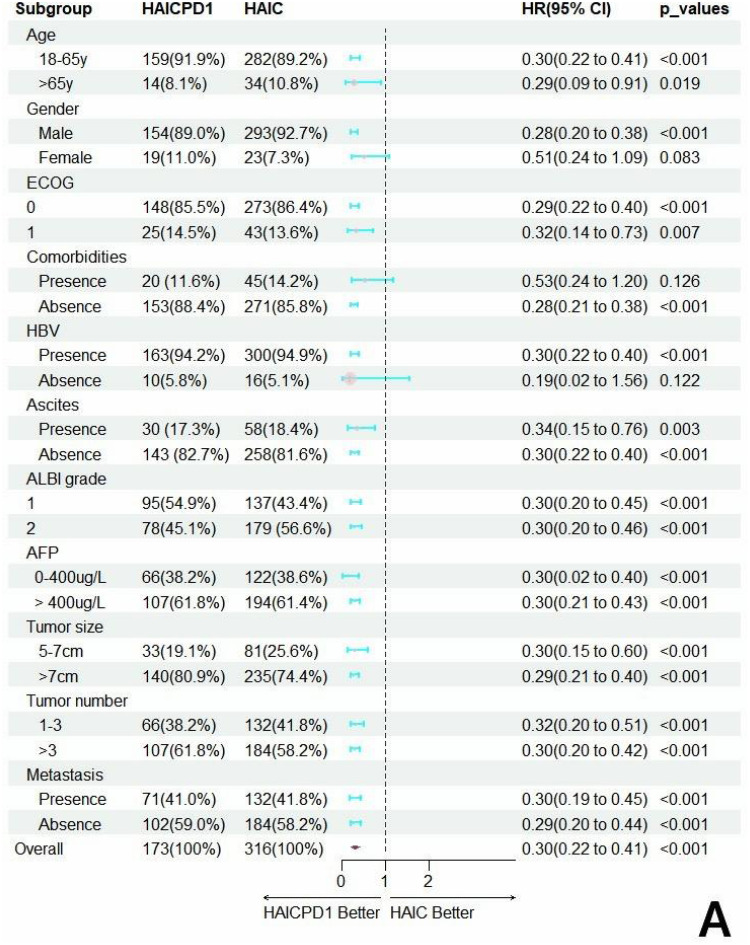
Forest plots were utilized to demonstrate the differences in survival among various subgroups as depicted in Figure 3. Among the subset of females, patients with comorbidities, and those without concurrent HBV infection, no treatment-related differences were observed. However, in all other subgroups, MVI-positive HCC patients receiving combined HAIC and PD-1 therapy exhibited superior survival benefits compared to those receiving HAIC alone.
Figure 3
Continued.
Figure 3
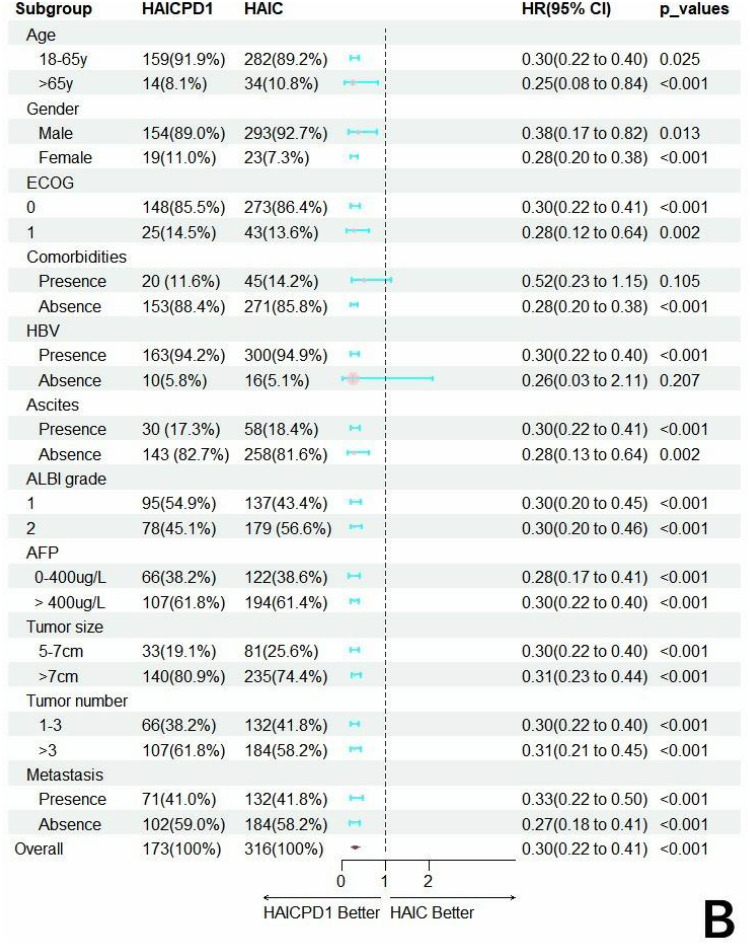
Forest plots of the hazard factors associated with overall survival (A) and progression-free survival (B) based on subgroup analysis.
In addition, we conducted further subgroup survival analysis for patients with or without combined liver metastasis, as shown in Figure 4. After matching, these differences became more pronounced. In the MVI-negative subgroup, the combined treatment group demonstrated mOS and mPFS of 40.4 months and 11.4 months respectively, while the monotherapy group had mOS of 10.9 months and mPFS of 7.0 months. In the MVI-positive subgroup, the combined treatment group had mOS of 26.0 months and mPFS of 8.4 months, while the monotherapy group had mOS of 7.3 months and mPFS of 5.5 months. In terms of tumor response, the response rates in the metastasis-negative or -positive subgroups are presented in Table 3. In the metastasis-negative subgroup, the ORR and DCR of the HAIC-PD1 group were 56.1% and 87.8%, respectively, significantly higher than those of the HAIC group, which were 87.8% and 80.6%, respectively. In the metastasis-positive subgroup, the ORR of the HAIC-PD1 group was 27.5%, significantly higher than that of the HAIC group, which was 11.1%. Similarly, the DCR of the HAIC-PD1 group was 85.5%, and that of the HAIC group was 79.6%, showing no significant statistical difference.
Figure 4
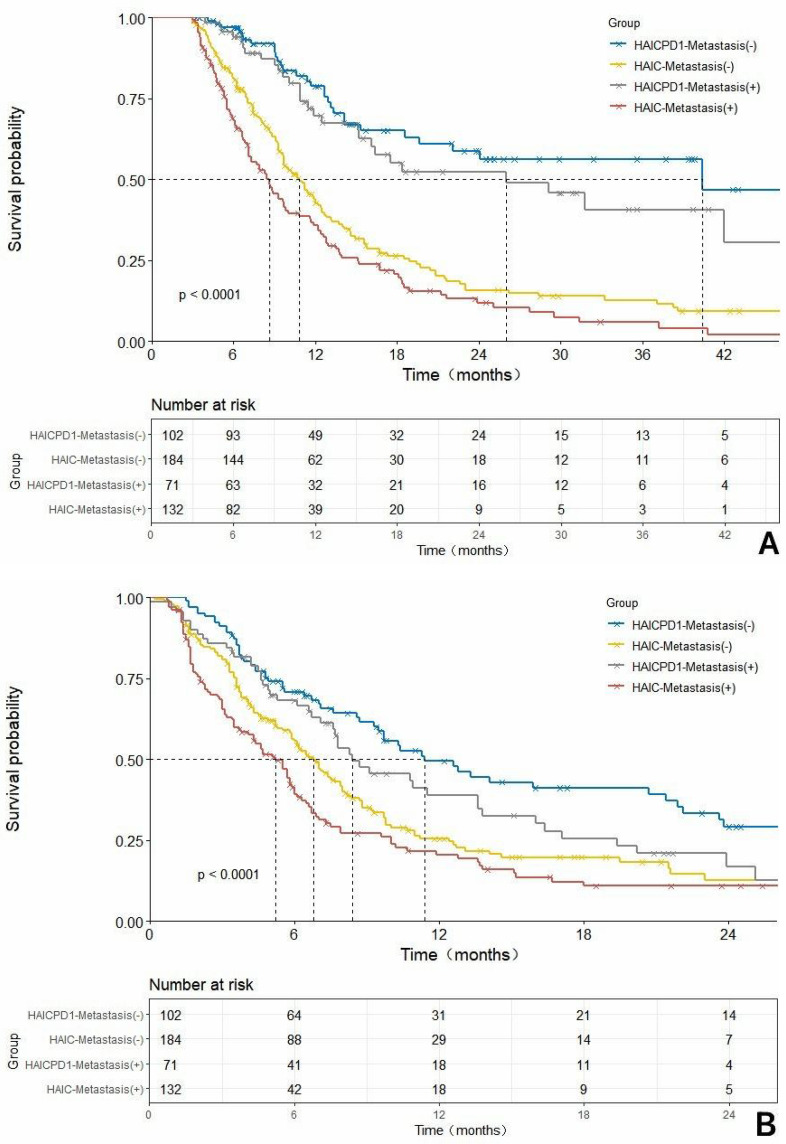
Continued.
Figure 4
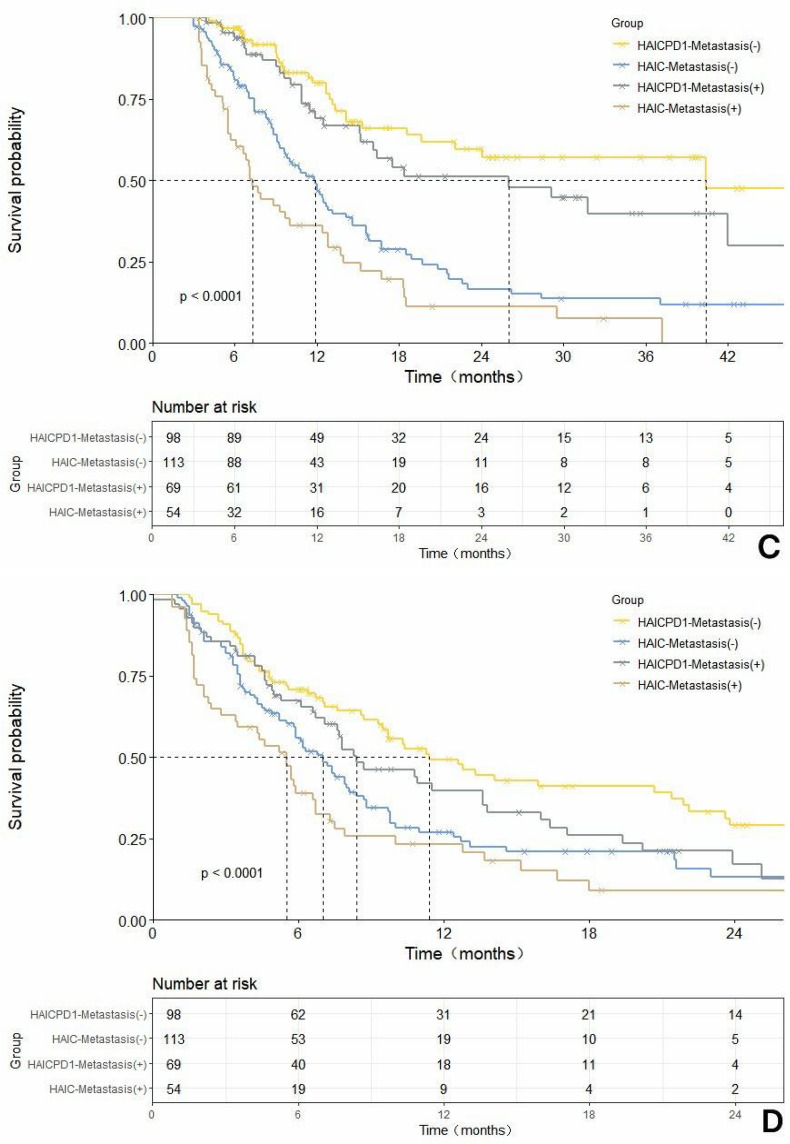
Comparison of overall survival (OS) and progression-free survival (PFS) before and after propensity score matching (PSM) adjustment based on the HAIC-PD1 group and HAIC group with or without merging hepatic extrahepatic spread subgroups. (A). Rough comparison of OS between the HAIC combined with PD1 inhibitor and HAIC monotherapy in the non-metastatic subgroup before matching adjustment.(B). Rough comparison of PFSS between the HAIC combined with PD1 inhibitor and HAIC monotherapy in the non-metastatic subgroup before matching adjustment.(C). Comparison of OS in the metastatic subgroup between the HAIC combined with PD1 inhibitor and HAIC monotherapy after matching adjustment. (D). Comparison of PFSS in the metastatic subgroup between the HAIC combined with PD1 inhibitor and HAIC monotherapy after matching adjustment.
Safety
The overall incidence of adverse events and the occurrence of treatment-related discontinuations and adverse reactions were comparable between the combination therapy group and the monotherapy group, with no statistically significant differences observed. All adverse events details are provided in Table 5. Throughout the treatment process, 18 cases and 21 cases of adverse events leading to the interruption or reduction of HAIC in the HAICPD1 group and HAIC group respectively, while no instances of HAIC discontinuation were observed. In the combined therapy group, a total of 29 cases experienced temporary interruption of PD1, followed by resumption of treatment after the resolution of adverse reactions. Nausea, elevated transaminases, and abdominal pain emerged as the most prevalent adverse reactions in both groups. Additionally, the incidence of adverse reactions in the combined therapy group was generally higher compared to the monotherapy group. Nevertheless, there were no statistically significant differences in the occurrence of grade 1–2 and grade 3–4 adverse reactions between the groups.
Table 5
Treatment-Related Adverse Events
| Adverse events | Grade 1/2 | Grade 3/4 | ||||
|---|---|---|---|---|---|---|
| HAICPD1 (n=173) | HAIC (n=316) | P value | HAICPD1 (n=173) | HAIC (n=316) | P value | |
| Any advance events | 121(69.9) | 209(66.1) | 0.391 | 43(24.9) | 63(19.9) | 0.207 |
| AEs-related treatment interruption or dose reduction | ||||||
 HAIC discontinuation HAIC discontinuation | 0(0) | 0(0) | - | 0(0) | 0(0) | - |
 HAIC interruption and dose reduction HAIC interruption and dose reduction | 3(1.7) | 0(0) | - | 15(8.7) | 21(6.6) | - |
 PD1 inhibitors interruption PD1 inhibitors interruption | 2(1.2) | NA | - | 27(15.6) | NA | - |
| Treatment-related AEs | ||||||
 Fever Fever | 32(18.5) | 41(13.0) | 0.101 | 2(1.2) | 0(0) | 0.240 |
 Abdominal pain Abdominal pain | 85(49.1) | 161(50.9) | 0.701 | 4(2.3) | 2(0.6) | 0.096 |
 Rash Rash | 18(10.4) | 24(7.6) | 0.289 | 3(1.7) | 1(0.3) | 0.255 |
 Diarrhea Diarrhea | 37(21.4) | 65(20.6) | 0.831 | 3(1.7) | 5(1.6) | 0.899 |
 Vomiting Vomiting | 66(38.2) | 101(32.0) | 0.168 | 2(1.2) | 7(2.2) | 0.405 |
 Nausea Nausea | 90(52.0) | 151(47.8) | 0.370 | 5(2.8) | 4(1.2) | 0.201 |
 Cough Cough | 16(9.2) | 21(6.7) | 0.298 | 2(1.2) | 0(0) | 0.240 |
 Neurologic toxicity Neurologic toxicity | 29(16.8) | 44(13.9) | 0.400 | 0(0) | 4(1.2) | 0.137 |
| Laboratory-related AEs | ||||||
 Anemia Anemia | 61(35.2) | 95(30.1) | 0.248 | 5(2.9) | 3(0.9) | 0.106 |
 Leukopenia Leukopenia | 23(13.3) | 26(8.2) | 0.074 | 3(1.7) | 2(0.6) | 0.247 |
 Neutropenia Neutropenia | 25(14.5) | 30(9.5) | 0.097 | 4(2.3) | 2(0.6) | 0.107 |
 Thrombocytopenia Thrombocytopenia | 38(22.0) | 51(16.1) | 0.110 | 3(1.7) | 3(0.9) | 0.451 |
 Hypoalbuminemia Hypoalbuminemia | 64(37.0) | 107(33.9) | 0.487 | 7(4.0) | 5(1.6) | 0.092 |
 Elevated ALT Elevated ALT | 73(42.2) | 111(35.1) | 0.123 | 16(9.2) | 18(5.7) | 0.140 |
 Elevated AST Elevated AST | 77(44.5) | 126(39.9) | 0.320 | 18(10.4) | 26(8.2) | 0.421 |
 Gamma-glutamyltransferase increased Gamma-glutamyltransferase increased | 33(19.1) | 51(16.1) | 0.411 | 3(1.7) | 7(2.2) | 0.719 |
 Hyperbilirubinemia Hyperbilirubinemia | 35(20.2) | 67(21.2) | 0.800 | 7(3.2) | 6(1.9) | 0.158 |
 Elevated creatinine Elevated creatinine | 20(11.6) | 35(11.1) | 0.871 | 4(2.3) | 3(0.9) | 0.225 |
Note: Data represent as: n(%).
Abbreviations: AEs, adverse events; HAIC, Hepatic artery infusion chemotherapy, HAICPD1, Hepatic artery infusion chemotherapy plus PD1 inhibitors.
Discussion
Despite the widespread endorsement of HAIC as a standard treatment modality for advanced HCC with macrovascular involvement with or without extra-hepatic spread in many regions, yielding survival benefits surpassing those of the standard sorafenib regimen, the efficacy of HAIC monotherapy remains challenging in patients with high tumor burden. Presently, immunotherapy targeting the PD-1/PD-L1 pathway for HCC has achieved unprecedented success,14,19 however, it also confronts substantial hurdles. The low response rates and moderate remission rates associated with monotherapy necessitate resolution, as the mere blockade of the PD-1/PD-L1 pathway fails to elicit a robust anti-tumor immune response and faces evident resistance. The combined efficacy of local chemotherapy with immunotherapy has been validated in advanced HCC.20–22 Consequently, we embarked on an exploration of the safety and efficacy of HAIC in conjunction with PD1 inhibitors for the treatment of HCC patients with MVI, also delving into a more profound analysis of the prognostic outcomes for the subgroup with and without extrahepatic metastases.
In this study, we have demonstrated the long-term survival, high response rates, and manageable adverse reactions presented by the chemo-immunotherapy regimen combining HAIC with PD1 inhibitors, and these advantages remain unaffected by extrahepatic spread. Employing the PSM method, we effectively mitigated the influence of baseline differences. Following baseline equilibrium, the HAIC combined with PD1 inhibitors regimen resulted in a median overall mOS of 31.8 months and a mPFS of 10.8 months. The ORR and DCR achieved notable levels at 44.3% and 89.8%, respectively. Compared to monotherapy, this combination significantly prolonged and enhanced corresponding survival times and response rates, underscoring the ability of PD-1 inhibitors to optimize both the management of high burdened tumors with HAIC and tumor shrinkage capabilities simultaneously. These benefits may stem from several aspects: firstly, the immunogenic cell death effect generated by the combination of oxaliplatin, 5-Fluorouracil, and antigens on tumor cell membranes, which improves the tumor immune microenvironment and reverses tumor resistance to immunotherapy.23,24 Secondly, the chemotherapy drugs enhance tumor antigenicity, thereby reducing “non-targeted” immune suppression in the tumor microenvironment and increasing tumor cell sensitivity to immune checkpoint inhibitors for escape blockade.25 Furthermore, the combination of PD-1 inhibitors amplifies the secretion of inflammatory cytokines, further inhibiting HCC growth and TGF secretion.26
Based on preliminary research findings, the combination of HAIC and immune suppressants demonstrates significant tumor reduction and anti-tumor effects in advanced HCC. A large-scale, multicenter retrospective study revealed promising outcomes in BCLC stage C HCC patients, the majority of whom presented with MVI (83.3%), receiving treatment combining HAIC with PD-1 inhibitors. The median OS and median PFS reached impressive 40.4 months and 22.1 months respectively, with an ORR of 42.5%,22 consistent with our conclusions across multiple study endpoints. A retrospective study conducted at another center in China has documented that the combination of HAIC and anti-PD1 therapy offers extended OS and PFS, along with improved DCR, for advanced HCC patients. However, subsequent subgroup analysis revealed that similar OS benefits were predominantly observed in PVTT Vp1-3 subgroups, whereas PVTT Vp4 subgroup did not significantly benefit from the combination treatment. This discrepancy may be linked to insufficient data in the positive subgroup, necessitating further large-scale studies to validate whether the PVTT Vp4 subgroup could indeed benefit from combination therapy.21 Conversely, an another a single-center study from Taiwan compared the efficacy of HAIC combined with immunotherapy against HAIC monotherapy. Reported findings indicate that the mOS with HAIC combined with immunotherapy was 18.2 months, which exceeded the 10.3 months mOS observed with HAIC alone,27 but did not reach statistical significance. The ORR reached 50.0%, significantly higher than that achieved with HAIC monotherapy, consistent with the outcomes of our study. In such effective tumor shrinkage, the primary endpoint of comparability is largely attributed to the markedly insufficient enrollment data in the combination therapy group and the absence of long-term follow-up data.
In subgroup analyses, Female patients, individuals with comorbidities, and those without concurrent HBV infection have shown superior survival benefits when receiving combined HAIC and PD-1 therapy compared to those receiving HAIC treatment alone, in MVI-positive HCC patients. Moreover, enhanced survival benefits persisted in the subgroup with concomitant extrahepatic metastases, where the mOS with HAIC combined immunotherapy reached a remarkable 26.0 months.21 This underscores the continued significance of PD-1 inhibitors in very late-stage HCC with high tumor burden. In contrast to a retrospective study by Mei Jie et al indicating limitations in the effectiveness of HAIC combined immunotherapy in managing extrahepatic metastases, this discrepancy can be attributed to the inadequate number of patients in the metastatic subgroup analyzed in their study, which was insufficient to substantiate the aforementioned conclusions. Although no significant differences were observed in DCR within the metastatic subgroup, the profound heterogeneity of advanced-stage HCC necessitates addressing inherent disparities and potential impacts on varying immune therapy sensitivities through widespread adoption of precision medicine. In terms of safety, the addition of PD-1 resulted in a mild increase in grade 3–4 adverse reactions, but there was no statistical difference compared to the group receiving monotherapy. This indicates that the combination regimen of HAIC and PD-1 inhibitors has good tolerability.
In this study, the concomitance of HBV infection, extrahepatic metastasis, and sole administration of HAIC therapy emerged as independent adverse prognostic factors for worse OS according to multivariate analysis, while the presence of HBV infection, high AFP level, extrahepatic metastasis, and sole administration of HAIC therapy were identified as influencing factors for poor PFS. HBV infection stands out as one of the high-risk factors for HCC, as it facilitates the development of HCC through various epigenetic changes. Of particular interest is the role of HBV in inducing immune imbalance and contributing to the deterioration of the tumor immune microenvironment during the progression of HCC, often mediated by sustained immune responses resulting from chronic liver damage.28,29 This underscores the rationale for employing immunotherapy in the management of HCC. In recent years, AFP has been widely utilized for assessing the invasiveness of HCC and making clinical decisions. Positive AFP levels have been unequivocally associated with lower tumor differentiation, tumor progression, and poor survival rates compared to AFP negativity.30 For years, metastasis has been regarded as the primary driving force behind aggressive tumor progression. Similarly, our subgroup analysis reveals that in the context of MVI, late-stage HCC exhibiting metastasis demonstrates significantly worse survival and faster tumor progression. Furthermore, the mechanisms underlying the beneficial effects of HAIC combined with PD-1 inhibitors have been elucidated as described above.
The current study acknowledges several limitations that deserve recognition. Firstly, despite using propensity score matching to address baseline differences, there remains inherent heterogeneity, and the retrospective nature of the study necessitates additional randomized controlled trials to strengthen the evidence. Secondly, although previous studies have shown efficacy profiles of different PD-1 inhibitors in combination therapy for HCC,31 the various types of available immunosuppressants may have different potential effects on HCC based on immunophenotypic characteristics, highlighting the necessity of subsequent research to confirm the effectiveness of specific immunotherapy methods. Moreover, our study focused on HBV-induced HCC, while HCC linked to HCV and alcohol abuse in Western populations may have different survival outcomes, suggesting the need to consider other causal factors in overall and subgroup survival analyses. Therefore, it is imperative to conduct more international randomized controlled trials to establish a higher level of evidence.
Conclusion
Based on the study results, the incorporation of PD1 immune checkpoint inhibitors enhances the antitumor efficacy of mFOLFOX-based HAIC. Despite being employed in the management of very late-stage HCC with both MVI and extrahepatic spread, this combination regimen offers advantages and favorable safety profiles, and is a promising treatment modality.
Funding Statement
This research was supported by the Education (Health) Science and Technology Project Fund, a sub-project fund for technology research and creative design in Nanshan District, Shenzhen (NS2024098).
Disclosure
The authors of this manuscript assert non-affiliation or financial association with companies whose products or services are pertinent to the subject matter discussed in the article. The authors report no conflicts of interest in this work.
References
Articles from Journal of Hepatocellular Carcinoma are provided here courtesy of Dove Press
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Efficiency and safety of hepatic arterial infusion chemotherapy (HAIC) combined with anti-PD1 therapy versus HAIC monotherapy for advanced hepatocellular carcinoma: A multicenter propensity score matching analysis.
Cancer Med, 13(1):e6836, 09 Jan 2024
Cited by: 3 articles | PMID: 38196277 | PMCID: PMC10807563
[Efficacy and safety of transcatheter arterial chemoembolization followed by hepatic arterial infusion chemotherapy combined with TKI and PD-1 inhibitors as first-line treatment for advanced hepatocellular carcinoma].
Nan Fang Yi Ke Da Xue Xue Bao, 44(9):1831-1838, 01 Sep 2024
Cited by: 0 articles | PMID: 39505352
Hepatic artery infusion chemotherapy combined with camrelizumab plus rivoceranib for hepatocellular carcinoma with portal vein tumor thrombosis: a multicenter propensity score-matching analysis.
Hepatol Int, 18(4):1286-1298, 08 May 2024
Cited by: 1 article | PMID: 38717693 | PMCID: PMC11297837
Efficacy and safety of HAIC-FOLFOX plus tyrosine kinase inhibitors and immune checkpoint inhibitors as first-line treatment for unresectable advanced hepatocellular carcinoma: A systematic review and meta-analysis.
Acad Radiol, S1076-6332(24)00710-4, 08 Oct 2024
Cited by: 0 articles | PMID: 39384510
Review