Abstract
Background
Combining antegrade stenting (AGS) and hepaticogastrostomy (HGS) is an increasingly used endoscopic ultrasound-guided intervention when stenting by endoscopic retrograde cholangiopancreatography is impossible.Objectives
We comprehensively assessed the benefits and downsides of combined AGS and HGS (HGS procedure with AGS, HGAS).Data sources and methods
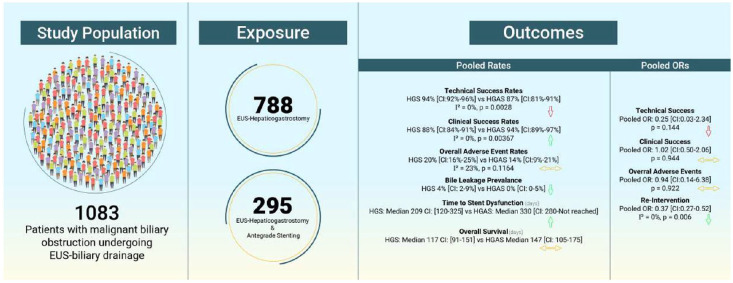
From 788 HGS and 295 HGAS cases, a random-effects meta-analysis was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol. Five electronic databases were searched for studies on HGS with or without AGS from inception until May 2024. The odds ratio (OR) and pooled rates were used for single and two-arm comparisons with 95% confidence intervals (CI).Results
From 26 eligible studies. The pooled technical and clinical success was 94% (CI: 92%-96%) and 88% (CI: 84%-91%) for HGS and 89% (CI: 83%-93%) and 94% (CI: 89%-97%) for HGAS, respectively. Pooled OR of HGAS and HGS showed an OR = 0.38 (CI: 0.07-2.00) for technical success and an OR = 1.02 (CI: 0.50-2.06) for clinical success. The pooled adverse event rates were 20% (CI: 16%-25%) for HGS and 14% (CI: 9%-20%) for HGAS, whereas pooled OR showed an OR = 1.09 (CI: 0.30-3.94). For re-intervention, an OR = 0.37 (CI: 0.27-0.52) was found. Time to stent dysfunction increased, HGAS 333 (CI: 280-Not reached) and HGS 209 (CI: 120-325) with no change in overall survival HGS 117 (CI: 94-147) and 140 (CI: 105-170).Conclusion
The use of HGAS appears to increase clinical success and reduce the need for re-intervention. Overall adverse event rates were similar but bile leakage prevalence was decreased. Time to stent dysfunction seems to increase with no change in overall survival.Trial registration
Our protocol was prospectively registered with PROSPERO (CRD42024509412).Free full text

Hepaticogastrostomy versus hepaticogastrostomy with antegrade stenting for malignant biliary obstruction: a systematic review and meta-analysis
Abstract
Background:
Combining antegrade stenting (AGS) and hepaticogastrostomy (HGS) is an increasingly used endoscopic ultrasound-guided intervention when stenting by endoscopic retrograde cholangiopancreatography is impossible.
Objectives:
We comprehensively assessed the benefits and downsides of combined AGS and HGS (HGS procedure with AGS, HGAS).
Data sources and methods:
From 788 HGS and 295 HGAS cases, a random-effects meta-analysis was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol. Five electronic databases were searched for studies on HGS with or without AGS from inception until May 2024. The odds ratio (OR) and pooled rates were used for single and two-arm comparisons with 95% confidence intervals (CI).
Results:
From 26 eligible studies. The pooled technical and clinical success was 94% (CI: 92%–96%) and 88% (CI: 84%–91%) for HGS and 89% (CI: 83%–93%) and 94% (CI: 89%–97%) for HGAS, respectively. Pooled OR of HGAS and HGS showed an OR =
= 0.38 (CI: 0.07–2.00) for technical success and an OR
0.38 (CI: 0.07–2.00) for technical success and an OR =
= 1.02 (CI: 0.50–2.06) for clinical success. The pooled adverse event rates were 20% (CI: 16%–25%) for HGS and 14% (CI: 9%–20%) for HGAS, whereas pooled OR showed an OR
1.02 (CI: 0.50–2.06) for clinical success. The pooled adverse event rates were 20% (CI: 16%–25%) for HGS and 14% (CI: 9%–20%) for HGAS, whereas pooled OR showed an OR =
= 1.09 (CI: 0.30–3.94). For re-intervention, an OR
1.09 (CI: 0.30–3.94). For re-intervention, an OR =
= 0.37 (CI: 0.27–0.52) was found. Time to stent dysfunction increased, HGAS 333 (CI: 280–Not reached) and HGS 209 (CI: 120–325) with no change in overall survival HGS 117 (CI: 94–147) and 140 (CI: 105–170).
0.37 (CI: 0.27–0.52) was found. Time to stent dysfunction increased, HGAS 333 (CI: 280–Not reached) and HGS 209 (CI: 120–325) with no change in overall survival HGS 117 (CI: 94–147) and 140 (CI: 105–170).
Conclusion:
The use of HGAS appears to increase clinical success and reduce the need for re-intervention. Overall adverse event rates were similar but bile leakage prevalence was decreased. Time to stent dysfunction seems to increase with no change in overall survival.
Trial registration:
Our protocol was prospectively registered with PROSPERO (CRD42024509412).
Introduction
Endoscopic retrograde cholangiopancreatography has long been considered the gold standard for palliation in patients with malignant biliary obstruction (MBO), with over 500,000 procedures performed annually in the U.S. alone. 1 The technical failure of the Endoscopic Retrograde Cholangiopancreatography (ERCP) procedure varies between 5% and 15%. 2 Failure is exceptionally high in a subset of patients, such as those with surgically altered anatomy and duodenal obstruction. 3 In case of ERCP failure, percutaneous biliary drainage (PTBD) can be an alternative procedure of choice.
Since first described by Giovannini et al.4,5 in 2001 and 2003, endoscopic ultrasound-guided (EUS) biliary drainage has been gaining traction over the last decade. A recent meta-analysis suggests that compared to PTBD, EUS-biliary drainage offers better clinical success and fewer adverse events. 6 Combined with its higher cost-effectiveness, it is predictable that soon, EUS-biliary drainage can replace PTBD in case of primary ERCP failure or even become an alternative to primary ERCP. 7 The umbrella term EUS-biliary drainage consists of different procedures with different bilio-enteric anastomoses such as choledochoduodenostomy, hepaticogastrostomy (HGS), gallbladder drainage, or lack of anastomoses such as the rendezvous and antegrade stenting (AGS) techniques. 8 A consensus on when each procedure should be performed has emerged, individual patient anatomy and malignancy play an important role. 9
In 2011, Imai et al. 10 were the first to combine the HGS procedure with AGS (HGAS). The rationale is that the combination can minimize bile leakage that could be caused by each procedure separately while increasing stent patency. The existence of two drainage routes allows the bile to keep draining in the event the HGS is occluded by sludge or debris, as well as in stent dysfunction due to migration. Therefore, the aim of our study was to compare the combined procedure of HGAS versus HGS alone in terms of early outcomes such as clinical and technical success, overall adverse events, re-intervention rates, and explore outcomes such as time to stent dysfunction and overall survival.
Methods
We report our systematic review and meta-analysis based on the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines (see Supplemental Table S1), while we followed the Cochrane Handbook. The protocol of the study was registered on PROSPERO (CRD42022371848).11–13
Eligibility criteria
We used the PICO framework (population, intervention, comparison, outcomes) for our eligibility criteria. 14 We included studies of patients with MBO who underwent EUS-biliary drainage with HGS with or without the AGS procedure. Randomized controlled trials (RCTs), cohort studies, or case series with more than five patients were analyzed that measured technical success, clinical success, overall adverse events rates, re-intervention rates, time to stent dysfunction, and overall survival. Studies of patients undergoing EUS-biliary drainage for benign biliary obstruction and abstracts were excluded.
Outcome definitions
The present study’s primary outcomes are the technical success rate, clinical success, overall adverse event rates, and re-intervention rates. Secondary outcomes were time to stent dysfunction and overall survival.
Technical success was defined as a successful stent placement among all studies. Clinical success was defined as a reduction in bilirubin concentration to at least 40% of pretreatment value within 4 weeks post-procedure. Re-intervention was defined as any endoscopic or percutaneous procedure that was required to improve symptoms after stent placement. The time to stent dysfunction was defined as days from stent deployment to a biliary re-intervention due to stent dysfunction from stent migration or sludge accumulation. Overall survival was measured as the number of days from successful procedure to patient death.
weeks post-procedure. Re-intervention was defined as any endoscopic or percutaneous procedure that was required to improve symptoms after stent placement. The time to stent dysfunction was defined as days from stent deployment to a biliary re-intervention due to stent dysfunction from stent migration or sludge accumulation. Overall survival was measured as the number of days from successful procedure to patient death.
Information sources
A systematic search was conducted from inception to the 5 November 2022 in the following medical databases: MEDLINE (via PubMed), Embase, Scopus, Web of Science, and Cochrane Central Register of Controlled Trials (CENTRAL). In addition, backward and forward citation searching was conducted on the 15 January 2023, and we updated our search on the 28 May 2024 to identify all eligible articles.
Search strategy
During the systematic search, the following search key was used: (Hepaticogastrostomy or hepatogastrostomy or (transmural and hepat*)) (Supplemental Table S2).
Selection process
The selection was performed by two independent review authors (P.P. and D.B.); after duplicates were removed, published articles were selected first by title, then by abstract and full text. In case of disagreements, a third independent reviewer (M.O.) was also involved.
Data collection process
Data were manually collected from the eligible articles into a predefined data table in preparation for analysis. The data were collected independently by two authors (P.P. and D.B.) and compared to resolve disagreements.
Data items
The following data were extracted: first author, year of publication, intervention, number of patients, gender ratio, age, clinical success, technical success, re-intervention, adverse events, time to stent dysfunction, overall survival, types of stents, and dilatation device.
Study risk of bias assessment and quality of evidence
Two authors independently performed the risk of bias assessment, with disagreements resolved by a third independent reviewer (M.O.). The “Risk of Bias In Non-randomized Studies—of Interventions” tool was used for studies included in the direct comparative meta-analysis, and the “Methodological index for non-randomized studies” tool for the studies included in the proportional meta-analysis. We followed the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) handbook. The GRADEpro tool was used to assess the quality of evidence.15–17
Synthesis methods
We assumed considerable between-study heterogeneity in all cases, a random-effects model was used to pool effect sizes. Pooled odds ratio (OR) based on raw data was calculated using the Mantel–Haenszel method. 18 Small-study publication bias was assessed by visual inspection of funnel plots and calculation of modified Egger’s test p-values. We assumed possible small-study bias if the p-value was less than 10%.
We performed two main kinds of analyses for pooling proportions. “Classical 2-level” meta-analyses were performed for pooling when different studies were included. If more results were available in separate categories (subgroups) in the same study, a “3-level” model was used. To meta-analyze the time to stent dysfunction curves and overall survival curves, we digitized the available Kaplan–Meier (KM) curves and calculated summary KM curves with 95% confidence interval (CI) using the method proposed by Combescure et al. 19 Using this model, we estimated the median time to stent dysfunction and the median overall survival time based. 19
All statistical analyses were performed using R with meta Schwarzer 2023, v6.2.1 package for basic meta-analysis calculations and plots, metafor Viechtbauer 2023, v4.2.0 for 3-level models, IPDfromKM v0.1.10 and metaSurvival v0.1.0 packages for the summary survival curves, and dmetar package for additional influential analysis calculations and plots.20–24
For additional details, please see the Synthesis Methods section in the Supplemental Material on pages 3–4.
Results
Search and selection
Of a total of 4466, we identified 2601 records after duplicate removal. Of these, 1974 were excluded in the title and abstract selection phase. Of the 88 articles sought for retrieval, 32 could not be retrieved, which left us with 56 articles. Of these, 23 were excluded because they did not contain relevant information (Supplemental Table S3). Finally, of these 23 articles, further 811 were assessed from the reference and citation chasing. Three of these articles were assessed, and two were included, leaving us with 25 articles. A detailed description of the screening and selection is provided in the PRISMA flowchart (Supplemental Figure S1).
Basic characteristics of included studies
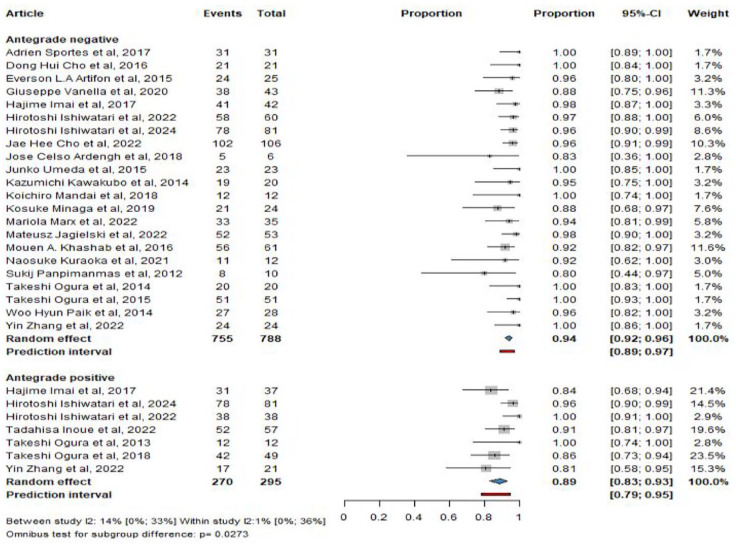
In total, 3 case series, 20 cohort studies, 5 prospective, 15 retrospective, and 3 RCTs were included; 3 studies were exclusively with HGAS, 18 with HGS, and 5 with HGS and HGAS. From these studies, 788 patients underwent HGS and 295 HGAS. The main characteristics of the included studies are summarized in Supplemental Table S4.
Pooled ORs
Four studies were included for technical success, clinical success, and overall adverse events, and three for re-intervention. Results are summarized in Figure 1.
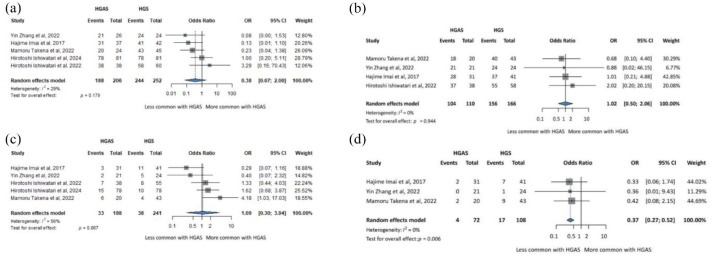
Summary figure for direct comparisons of HGS and HGAS. (a) Technical success. (b) Clinical success. (c) Overall adverse events rates. (d) Re-intervention rates.
CI, confidence interval; HGAS, hepaticogastrostomy with antegrade stent; HGS, hepaticogastrostomy; OR, odds ratio.
Overall, technical success between HGS and HGAS showed an OR =
= 0.38 (CI: 0.07–2.00), p
0.38 (CI: 0.07–2.00), p =
= 0.179 (Figure 1(a)). Clinical success showed an OR
0.179 (Figure 1(a)). Clinical success showed an OR =
= 1.02 (CI: 0.50–2.06), p
1.02 (CI: 0.50–2.06), p =
= 0.944 (Figure 1(b)). The overall adverse events showed an OR
0.944 (Figure 1(b)). The overall adverse events showed an OR =
= 1.09 (CI: 0.30–3.94), p
1.09 (CI: 0.30–3.94), p =
= 0.867 (Figure 1(c)). For the re-intervention, comparison of HGS and HGAS showed an OR
0.867 (Figure 1(c)). For the re-intervention, comparison of HGS and HGAS showed an OR =
= 0.37 (CI: 0.27–0.52), p
0.37 (CI: 0.27–0.52), p =
= 0.006 (Figure 1(d)).
0.006 (Figure 1(d)).
Pooled rates
Pooled technical and clinical success
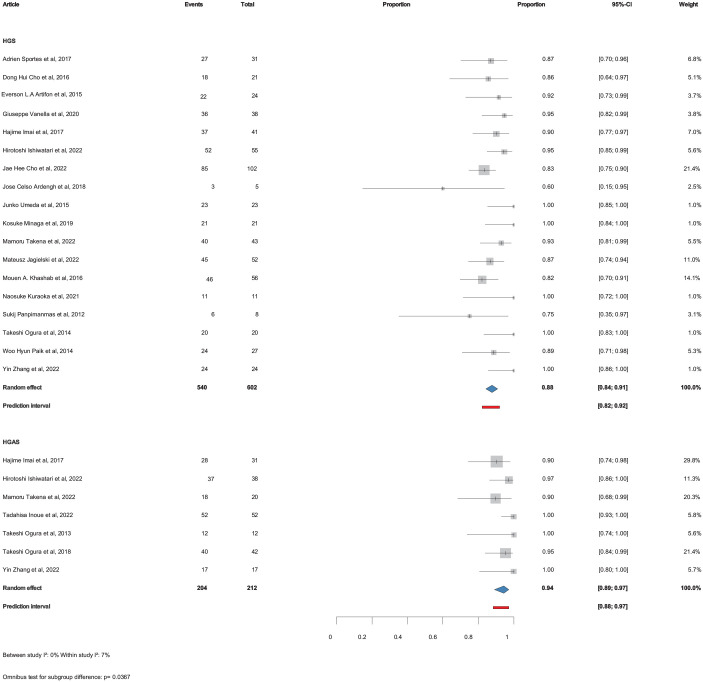
All 26 studies included information on technical success and a total of 22 studies included information on clinical success. The pooled technical success was 94% (CI: 92%–96%) for HGS and 89% (CI: 83%–93%) for HGAS, p =
= 0.00273 (Figure 2). The pooled clinical success was 88% (CI: 84%–91%) for HGS and 94% (CI: 89%–97%) for HGAS, p
0.00273 (Figure 2). The pooled clinical success was 88% (CI: 84%–91%) for HGS and 94% (CI: 89%–97%) for HGAS, p =
= 0.0367 (Figure 3).
0.0367 (Figure 3).
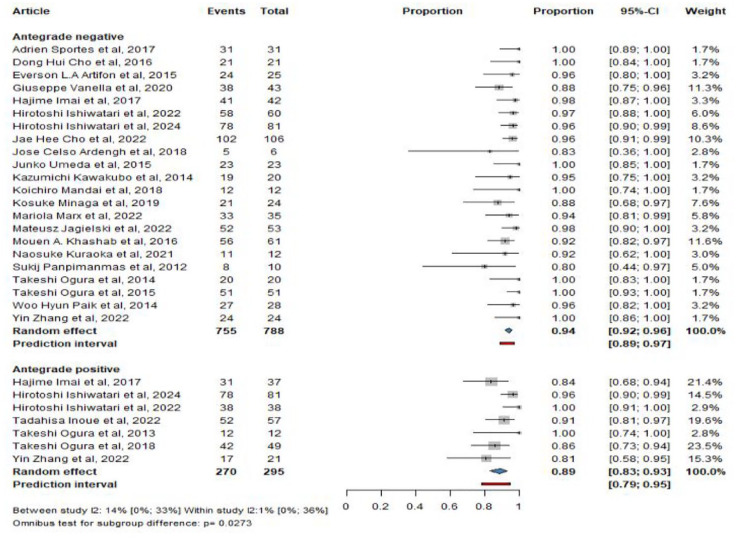
Pooled technical success with (HGAS) and without (HGS) antegrade stenting.
CI, confidence interval; HGS, hepaticogastrostomy; HGAS, hepaticogastrostomy with antegrade stent.
Pooled overall adverse events
Seventeen studies included information on pooled overall adverse events. The pooled adverse events were 20% (CI: 16%–25%) for HGS and 14% (CI: 9%–20%) for HGAS, p =
= 0.0728 (Figure 4).
0.0728 (Figure 4).
Bile leakage and overall adverse events without pancreatitis prevalence
In the case of HGS, 28 events were observed among 477 patients, while the addition of AGS resulted in the observation of a single event among 212 patients. Thus, bile leakage in the HGS group had a 4% prevalence (CI: 2%–9%), whereas the prevalence in the HGAS group was calculated at 0% (CI: 0%–5%) (Figure 5(a)).
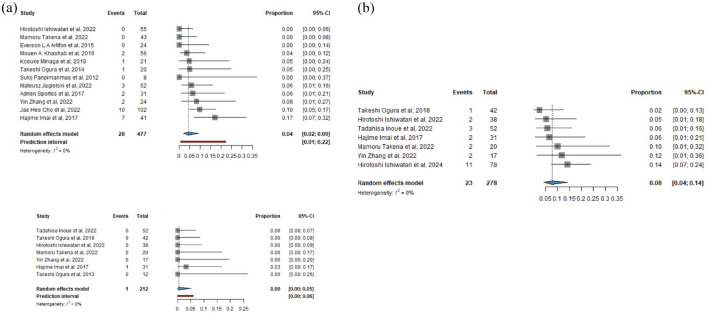
(a) Bile leakage prevalence with (HGAS) and without (HGS) antegrade stenting. (b) Overall adverse events prevalence without pancreatitis for HGAS.
CI, confidence interval; HGAS, hepaticogastrostomy with antegrade stent; HGS, hepaticogastrostomy.
For the prevalence of overall adverse events for HGAS, excluding pancreatitis, the rate was 8% (CI: 4%–14%) (Figure 5(b)).
Time to stent dysfunction and overall survival
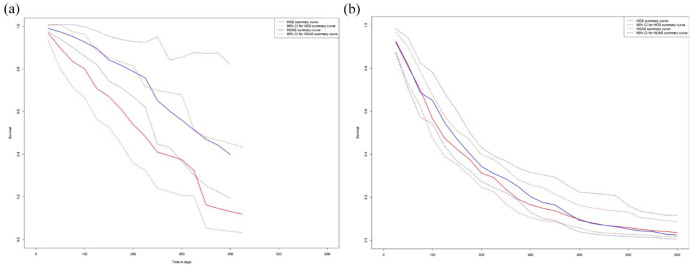
Four articles, 2 two-arm and 2 one-arm with a total of 208 patients for HGAS and 5 articles, 2 two-arm and 2 one-arm with a total of 193 for HGS were used to generate survival curves for time to stent dysfunction. For HGS, 5 articles with 248 patients for HGAS and 9 articles with 361 patients for HGS were used to compare survival plots for overall survival outcomes.
The time to stent dysfunction was 217 (CI: 120–308) days for HGS and 333 (CI: 280–not reached)
days for HGS and 333 (CI: 280–not reached) days for HGAS (Figure 6(a)). The overall survival for HGS and HGAS was 117 (CI: 94–147) and 140 (CI: 105–170)
days for HGAS (Figure 6(a)). The overall survival for HGS and HGAS was 117 (CI: 94–147) and 140 (CI: 105–170) days, respectively (Figure 6(b)).
days, respectively (Figure 6(b)).
Risk of bias and assessment and quality of evidence
According to the GRADE system, the certainty of evidence regarding technical success was high. The certainty of evidence was considered low for clinical success and overall adverse events (Supplemental Table S5). Overall, no significant bias was observed, results of the risk of bias assessment are summarized in Supplemental Table S6 and Supplemental Figure S2.
Publication bias and heterogeneity
No significant small-study publication bias was observed in the funnel plots for all outcomes (Supplemental Figures S3–S9). For direct comparisons, heterogeneity was I2 =
= 18% for technical success. For clinical success and overall adverse event rates, heterogeneity was calculated as I2
18% for technical success. For clinical success and overall adverse event rates, heterogeneity was calculated as I2 =
= 0% and I2
0% and I2 =
= 64%, respectively. Re-intervention showed I2
64%, respectively. Re-intervention showed I2 =
= 0%. The between- and within-study heterogeneity was I2
0%. The between- and within-study heterogeneity was I2 =
= 0% for pooled technical success. For clinical success, between-study heterogeneity was I2
0% for pooled technical success. For clinical success, between-study heterogeneity was I2 =
= 0% and the within-study was calculated at I2
0% and the within-study was calculated at I2 =
= 7%. The between-study heterogeneity for the overall adverse event rates was I2
7%. The between-study heterogeneity for the overall adverse event rates was I2 =
= 0% and within-study heterogeneity was calculated at I2
0% and within-study heterogeneity was calculated at I2 =
= 23%. Bile leakage showed a heterogeneity of I2
23%. Bile leakage showed a heterogeneity of I2 =
= 0%.
0%.
Discussion
This meta-analysis suggests that although more technically challenging, the addition of AGS is more successful in relieving biliary obstruction as well as increasing stent patency than HGS alone, together with its lower re-intervention rates and reduced bile leakage prevalence, the technical difficulties associated with HGAS could be justified for the patients’ benefits. The lower re-intervention rates may be attributed to a decrease in sludge and debris formation. In our exploratory analysis, median time to stent dysfunction appears to increase with no change in overall survival. Further research must be conducted to conclusively prove the increased time to stent dysfunction.
The technical success between HGS and HGAS were 94% (CI: 92%–96%) and 89% (CI: 83%–93%), respectively. This is not surprising as combined procedures are always more challenging than single ones. The results of the clinical success, which is measured as a reduction in bilirubin levels of at least 40% within a month of the procedure, suggests that the addition of AGS was better than HGS alone, 94% (CI: 89%–97%) to 88% (CI: 84%–91%), respectively. In terms of overall adverse events, HGAS performed similarly to HGS, 14% (CI: 9%–20%) and 20% (CI: 16%–25%), respectively. The deployment of AGS can lead to pancreatitis which does not occur during HGS, this might eventually balance the overall adverse event rates as shown in our analysis where the prevalence of adverse events without pancreatitis for HGAS was 8% (CI: 4%–14%). Moreover, the hypothesis of reduced bile leakage which can lead to serious complications such as bile peritonitis appears to be true with 1 event in 212 patients for HGAS compared to 28 events in 477 patients for HGS.
The need for re-intervention after HGAS compared to HGS was significantly decreased OR =
= 0.37 (CI: 0.27–0.52), this can be attributed to a decrease in sludge and debris formation in the HGS stent thus decreasing the need for endoscopic removal. Thus, although the addition of AGS comes with increased hospital costs and fluoroscopic radiation dosage, at the same time it can end up decreasing future costs and radiation that are associated with re-interventions.
25
Although an exploratory analysis, median time to stent dysfunction appears to increase, with HGAS median 333 (CI: 280–not reached) and HGS median 217 (CI: 120–308), since the existence of two drainage routes alleviates the intraductal pressure by said malignancies thus relieving the stress in the HGS stent. Since the stomach is a movable organ, with the addition of the AGS the bile can continue flowing in the physiological direction, thus the burden on the HGS stent is reduced which allows it to remain in place longer and not migrate into the abdominal cavity. No difference in overall survival was observed HGS median 117 CI: (94–147) and HGAS median 140 CI: (105–170). For this reason, to re-affirm the justification for the use of HGAS, we suggest that more long-term follow-up studies should be conducted examining the time to stent dysfunction. Lastly, during data collection, it was noted that many studies would report patient death as the time to stent dysfunction outcome. Our results only used those studies that didn’t confound the two outcomes. Future studies should always treat them separately.
0.37 (CI: 0.27–0.52), this can be attributed to a decrease in sludge and debris formation in the HGS stent thus decreasing the need for endoscopic removal. Thus, although the addition of AGS comes with increased hospital costs and fluoroscopic radiation dosage, at the same time it can end up decreasing future costs and radiation that are associated with re-interventions.
25
Although an exploratory analysis, median time to stent dysfunction appears to increase, with HGAS median 333 (CI: 280–not reached) and HGS median 217 (CI: 120–308), since the existence of two drainage routes alleviates the intraductal pressure by said malignancies thus relieving the stress in the HGS stent. Since the stomach is a movable organ, with the addition of the AGS the bile can continue flowing in the physiological direction, thus the burden on the HGS stent is reduced which allows it to remain in place longer and not migrate into the abdominal cavity. No difference in overall survival was observed HGS median 117 CI: (94–147) and HGAS median 140 CI: (105–170). For this reason, to re-affirm the justification for the use of HGAS, we suggest that more long-term follow-up studies should be conducted examining the time to stent dysfunction. Lastly, during data collection, it was noted that many studies would report patient death as the time to stent dysfunction outcome. Our results only used those studies that didn’t confound the two outcomes. Future studies should always treat them separately.
Strengths and limitations
This meta-analysis is the first examining the effects the addition of AGS has on the HGS procedure, although it has limitations, mainly because it includes retrospective cohort studies with no RCTs found investigating the clinical question. Moreover, factors that affect stent patency, such as type of stent (plastic, metallic) as well as the length and diameter of the stent itself were not analyzed individually. 26 Nonetheless, homogeneity was found among study results and along the definition of measured outcomes apart from the case of clinical success. For that reason, an inclusive definition of clinical success that would fit all the different definitions was decided upon. Finally, in many of the included studies, the number of participants for each outcome can be misleading, as outcomes such as clinical success and overall adverse events need only be measured from the total successful procedures. We identified this discrepancy between the results of the different studies and applied them to our results. Lastly, our analysis contains information in the form of pooled results from the studies and direct comparisons; in this way, all the different analyses that could be performed were conducted.
Implication for practice and research
MBO is usually found in terminally ill patients; the survival rates are low, and palliation is usually the only available approach. Hence, the use of a technique with the best results and the fewest adverse events is crucial, improving quality of life.27,28 Our meta-analysis shows that HGAS is justified over HGS due to the increased clinical success and lower re-intervention rates, as the overall adverse events remain practically unchanged and bile leakage which can lead to serious events such as bile peritonitis is reduced. In our exploratory analysis, time to stent dysfunction was increased, if more studies demonstrate the increase in time to recurrent biliary obstruction of HGAS over HGS, then this combinatory procedure should be preferred. Future research should focus on studies such as propensity-matched analysis or RCTs and late outcomes such as time to stent dysfunction. It is essential to note that time to patient death should not be measured as time to stent dysfunction outcome and should be treated separately.
Conclusion
Our findings guide endoscopists in the decision to use HGAS EUS-biliary drainage. Although technically more challenging, the increased time to stent dysfunction, higher rate of clinical success, and reduction in re-interventions together with the decrease in bile leakage prevalence with no change in the rate of overall adverse events argue in favor of the benefits associated with HGAS.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848241273085 for Hepaticogastrostomy versus hepaticogastrostomy with antegrade stenting for malignant biliary obstruction: a systematic review and meta-analysis by Panagiotis Paraskevopoulos, Mahmoud Obeidat, Dániel Bednárik, Petrana Martinekova, Dániel Sándor Veres, Nándor Faluhelyi, Alexandra Mikó, Péter Mátrai, Péter Hegyi and Bálint Erőss in Therapeutic Advances in Gastroenterology
Footnotes
ORCID iD: Bálint Erőss  https://orcid.org/0000-0003-3658-8427
https://orcid.org/0000-0003-3658-8427
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Panagiotis Paraskevopoulos, Centre for Translational Medicine, Semmelweis University, Budapest, Hungary.
Mahmoud Obeidat, Centre for Translational Medicine, Semmelweis University, Budapest, Hungary.
Dániel Bednárik, Centre for Translational Medicine, Semmelweis University, Budapest, Hungary. Heim Pál National Pediatric Institute, Budapest, Hungary.
Petrana Martinekova, Centre for Translational Medicine, Semmelweis University, Budapest, Hungary.
Dániel Sándor Veres, Centre for Translational Medicine, Semmelweis University, Budapest, Hungary. Department of Biophysics and Radiation Biology, Semmelweis University, Budapest, Hungary.
Nándor Faluhelyi, Centre for Translational Medicine, Semmelweis University, Budapest, Hungary. Department of Medical Imaging, Medical School, University of Pécs, Pécs, Hungary.
Alexandra Mikó, Centre for Translational Medicine, Semmelweis University, Budapest, Hungary. Institute for Translational Medicine, Medical School, University of Pécs, Pécs, Hungary. Institute of Medical Genetics, Medical School, University of Pécs, Pécs, Hungary.
Péter Mátrai, Institute for Translational Medicine, Medical School, University of Pécs, Pécs, Hungary.
Péter Hegyi, Centre for Translational Medicine, Semmelweis University, Budapest, Hungary. Institute for Translational Medicine, Medical School, University of Pécs, Pécs, Hungary. Translational Pancreatology Research Group, Interdisciplinary Centre of Excellence for Research Development and Innovation, University of Szeged, Szeged, Hungary. Institute of Pancreatic Diseases, Semmelweis University, Budapest, Hungary.
Bálint Erőss, Centre for Translational Medicine, Semmelweis University, Üllői út 26, Budapest H-1085, Hungary. Institute for Translational Medicine, Medical School, University of Pécs, Pécs, Hungary. Institute of Pancreatic Diseases, Semmelweis University, Budapest, Hungary.
Declarations
Ethics approval and consent to participate: No ethical approval was required for this systematic review with meta-analysis, as all data were already published in peer-reviewed journals. No patients were involved in the design, conduct, or interpretation of our study.
Consent for publication: All authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript.
Author contributions: Panagiotis Paraskevopoulos: Conceptualization; Formal analysis; Methodology; Project administration; Writing – original draft.
Mahmoud Obeidat: Conceptualization; Methodology; Project administration; Writing – original draft.Dániel Bednárik: Conceptualization; Writing – review & editing.
Petrana Martinekova: Conceptualization; Writing – review & editing.
Dániel Sándor Veres: Conceptualization; Data curation; Formal analysis; Writing – review & editing.
Nándor Faluhelyi: Conceptualization; Writing – review & editing.
Alexandra Mikó: Conceptualization; Writing – review & editing.
Péter Mátrai: Conceptualization; Data curation; Formal analysis; Writing – review & editing.
Péter Hegyi: Conceptualization; Writing – review & editing.
Bálint Erőss: Conceptualization; Supervision; Validation; Writing – original draft.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: The datasets used in this study can be found in the full-text articles included in the systematic review and meta-analysis.
References
Articles from Therapeutic Advances in Gastroenterology are provided here courtesy of SAGE Publications
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/169797019
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Application of endoscopic ultrasound-guided hepaticogastrostomy combined with antegrade stenting in patients with malignant biliary obstruction after failed ERCP.
Surg Endosc, 36(8):5930-5937, 17 Feb 2022
Cited by: 4 articles | PMID: 35178592
Endoscopic ultrasound-guided hepaticogastrostomy versus hepaticogastrostomy with antegrade stenting for malignant distal biliary obstruction.
J Hepatobiliary Pancreat Sci, 29(6):703-712, 07 Feb 2022
Cited by: 9 articles | PMID: 35094496
Utility of Endoscopic Ultrasound-Guided Hepaticogastrostomy with Antegrade Stenting for Malignant Biliary Obstruction after Failed Endoscopic Retrograde Cholangiopancreatography.
Oncology, 93 Suppl 1:69-75, 20 Dec 2017
Cited by: 25 articles | PMID: 29258066
Is Endoscopic Ultrasound-Guided Hepaticogastrostomy Safe and Effective after Failed Endoscopic Retrograde Cholangiopancreatography?-A Systematic Review and Meta-Analysis.
J Clin Med, 13(13):3883, 01 Jul 2024
Cited by: 0 articles | PMID: 38999449
Review