Abstract
Introduction
Osteoarthritis (OA) is a prevalent chronic joint disorder. It is characterized by an immune response that maintains a low level of inflammation throughout its progression. During OA, cartilage degradation leads to the release of damage-associated molecular patterns (DAMPs), which intensify the inflammatory response. β-Amyloid is a well-recognized DAMP in OA, can interact with APOE isoforms.Methods
This study identified DAMPs-related genes in OA using bioinformatics techniques. Additionally, we examined the expression levels of β-amyloid and apolipoprotein E (ApoE) isoforms by enzyme-linked immunosorbent assay.Results
We identified 10 key genes by machine learning techniques. Immune infiltration analysis revealed upregulation of various immune cell types in OA cartilage, underscoring the critical role of inflammation in OA pathogenesis. In the validation study, elevated serum levels of β-amyloid in knee osteoarthritis (KOA) patients were confirmed, showing positive correlations with ApoE2 and ApoE4. Notably, ApoE3 was identified as an independent protective factor against KOA.Conclusion
In this bioinformatics analysis, we identified the DAMPs-related genes of KOA and explored their potential functions and regulatory networks. The high expression of β-amyloid in KOA was confirmed by experiments, and the correlation between β-amyloid and ApoE2, ApoE4 in KOA was revealed for the first time, this provides a new way to explore the pathogenesis of KOA and to study the therapeutic targets of KOA.Free full text

Analyzing the Role of Specific Damage-Associated Molecular Patterns-Related Genes in Osteoarthritis and Investigating the Association between β-Amyloid and Apolipoprotein E Isoforms
Abstract
Introduction
Osteoarthritis (OA) is a prevalent chronic joint disorder. It is characterized by an immune response that maintains a low level of inflammation throughout its progression. During OA, cartilage degradation leads to the release of damage-associated molecular patterns (DAMPs), which intensify the inflammatory response. β-Amyloid is a well-recognized DAMP in OA, can interact with APOE isoforms.
Methods
This study identified DAMPs-related genes in OA using bioinformatics techniques. Additionally, we examined the expression levels of β-amyloid and apolipoprotein E (ApoE) isoforms by enzyme-linked immunosorbent assay.
Results
We identified 10 key genes by machine learning techniques. Immune infiltration analysis revealed upregulation of various immune cell types in OA cartilage, underscoring the critical role of inflammation in OA pathogenesis. In the validation study, elevated serum levels of β-amyloid in knee osteoarthritis (KOA) patients were confirmed, showing positive correlations with ApoE2 and ApoE4. Notably, ApoE3 was identified as an independent protective factor against KOA.
Conclusion
In this bioinformatics analysis, we identified the DAMPs-related genes of KOA and explored their potential functions and regulatory networks. The high expression of β-amyloid in KOA was confirmed by experiments, and the correlation between β-amyloid and ApoE2, ApoE4 in KOA was revealed for the first time, this provides a new way to explore the pathogenesis of KOA and to study the therapeutic targets of KOA.
Introduction
Osteoarthritis (OA) is the most common chronic joint disease, characterized by articular cartilage degeneration and secondary bone hyperplasia, predominantly affecting weight-bearing joints such as the knee and hip [1, 2]. Globally, approximately 300 million people suffer from hip and knee OA, with a higher prevalence observed in middle-aged and elderly populations, particularly among women. OA ranks 11th worldwide in terms of disability according to the Years Lived with Disability Index [2]. Diagnosis primarily relies on imaging, with the Kellgren and Lawrence [3] scale assessing disease severity. Despite its prevalence, effective interventions to slow OA progression or prevent cartilage deterioration are lacking, leaving advanced cases reliant on joint replacement surgery [4]. The significant burden OA places on patients underscore the importance of exploring its pathogenesis, early intervention, and therapeutic strategies.
Damage-associated molecular patterns (DAMPs) are endogenous molecules triggering inflammatory responses, associated with various diseases including sepsis and cancer. Released in response to stimuli like physical injury or metabolic disorders, DAMPs interact with cellular pattern recognition receptors, inducing a stress response that exacerbates tissue damage and inflammation [5, 6]. Drugs targeting DAMPs-related inflammation are worth studying. For example, HGMB1 is one of DAMPs, and Toll-like receptors (TLR) antagonist eritoran can attenuate TLR4-dependent HMGB1 release in vivo [7]. In OA, DAMPs contribute to pathogenesis by promoting inflammatory mediator release via signaling pathway activation [8]. Thus, studying DAMPs could lead to novel OA therapies, potentially reducing patient burden.
β-Amyloid, considered a type of DAMP, is a polypeptide formed from amyloid precursor protein (APP) by β- and γ-secretases. It promotes inflammation through binding to advanced glycation end-product receptors (RAGE) and is linked to increased cartilage catabolism in OA [1, 9]. Our prior research identified β-amyloid as an independent risk factor for knee osteoarthritis (KOA), inversely related to hospital stay duration [10]. While β-amyloid’s role in Alzheimer’s disease (AD) is well-documented [11, 12], its mechanisms in OA remain underexplored. Apolipoprotein E (ApoE), differentiated by ε2, ε3, and ε4 alleles, interacts with β-amyloid, affecting its fibrosis and clearance [13, 14]. This interaction, particularly relevant in AD treatment, suggests potential therapeutic targets for OA [15, 16]. This study aims to elucidate the mechanisms of β-amyloid in OA and its interactions with ApoE, providing new insights for OA research and treatment.
Materials and Methods
Date Collection
The technical route of this study is shown in Figure 1. The gene expression date of OA and control samples were obtained from Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/). There are three data sets in this study, including GSE98918, GSE57218, and GSE117999. We used R software to eliminate batch effect and standardize the data. At last, 55 OA samples and 29 control samples were used to verify the differentially expressed genes. To accurately identify DAMPs-related mRNAs, we referred to two reviews (PMID: 33537330 and PMID: 31621566), which provided DAMPs-associated molecules. Subsequently, an in-depth search was conducted through the Genebank database to identify the specific genes corresponding to these DAMPs molecules. By collation, 217 DAMPs-related mRNAs were eventually identified (online suppl. Table 1; for all online suppl. material, see https://doi.org/10.1159/000541542).
Identification of Differentially Expressed DAMPs-Related Genes
The “limma” package in R software was used to screen differentially expressed genes between OA and control samples according to adjusted p < 0.05. Then, we intersect the differentially expressed genes with DAMPs-related genes, and displayed the resulted in Volcano map, Heat map, and box plot.
Functional Enrichment Analysis
Functional enrichment analysis of differentially expressed DAMPs-related genes was conducted using the enricher function in “clusterProfiler” package within R software to analyze Gene Ontology (GO) biological processes and perform Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment. The GO analysis was divided into three categories: molecular function (MF), which includes all gene products’ biochemical activities; biological process (BP), which encompasses broad biological goals, such as mitosis or immune response, that are achieved by ordered assemblies of molecular functions; and cellular component (CC), which involves structural components of a cell or its extracellular environment.
Machine Learning for Key Genes
To identify key genes implicated in OA, we employed three machine learning techniques: Least Absolute Shrinkage and Selection Operator (LASSO) regression, Support Vector Machine – Recursive Feature Elimination, and Random Forest. These methods were chosen for their ability to effectively handle high-dimensional data and select features with the most predictive power. Following the identification of these genes, we performed a correlation analysis to explore the relationships between them. To assess the diagnostic potential of these key genes, receiver operating characteristic (ROC) curves were constructed using the “pROC” package in R. We then calculated the area under the curve (AUC) to quantify the diagnostic accuracy of the selected genes.
Single-Gene Enrichment Analysis
To elucidate the functional roles of the 10 key genes identified in OA, we initially conducted Pearson correlation analyses between these genes and genome-wide expression data. According to p < 0.05, we obtained the correlation gene sets of key genes. Subsequently, we utilized the Reactome database to perform Gene Set Enrichment Analysis (GSEA) for each key gene along with its associated gene sets. This approach allows us to explore the potential BPs and pathways in which these key genes are involved, providing deeper insights into their roles in OA pathogenesis.
Analysis of Immune Cell Infiltration
We employed single-sample gene set enrichment analysis (ssGSEA) to assess the infiltration of immune cells in both OA and healthy joint tissues. This analysis was conducted using the gene set variation analysis (tool implemented in R software). ssGSEA allows for the quantification of immune cell presence and activity levels across individual samples, facilitating a detailed comparison of immune system involvement between diseased and normal conditions.
MicroRNA-Transcription Factor-mRNA Network
To elucidate the regulatory mechanisms influencing key genes in OA, our study concentrated on two crucial regulatory molecule types: microRNAs (miRNAs) and transcription factors (TFs). Using the online platform NetworkAnalyst (https://www.NetworkAnalyst.ca), we predicted upstream miRNAs and TFs that may interact with these key genes. Subsequently, we employed Cytoscape software to visualize these complex regulatory interactions. This visualization helps in understanding the intricate regulatory networks that potentially govern gene expression changes associated with OA.
Collection of Clinical Samples
We recruited a total of 101 patients diagnosed with KOA from the Second Affiliated Hospital, YuYing Children’s Hospital of Wenzhou Medical University. The severity of KOA in these patients was assessed using the Kellgren-Lawrence (K-L) grading system based on X-ray findings. Additionally, a control group comprised of 51 patients without KOA was established. For all participants, we collected demographic and clinical information, including sex, age, and body mass index (BMI). The inclusion criteria for the test group required that all patients meet the KOA diagnostic guidelines as established by the scientific societies of the Chinese Medical Association.
The Expression of β-Amyloid, ApoE2, ApoE3, and ApoE4
Serum samples were collected from all study participants to measure the expression levels of β-amyloid, ApoE2, ApoE3, and ApoE4. These measurements were conducted using the enzyme-linked immunosorbent assay (ELISA) technique (Shanghai Fusheng Industrial Co., Ltd., China, A099548, A57404, A57405, A57406).
Result
Detection of Differentially Expressed DAMPs-Related Genes
To identify differentially expressed genes between OA and control samples, we utilized the “limma” package in R software. Following the removal of batch effects and data normalization, we applied a significance threshold of adjusted p value <0.05. This analysis led to the identification of 1,338 up-regulated and 1,319 down-regulated genes. Upon comparing these genes with known DAMPs-related genes, we identified 20 differentially expressed genes, including 15 up-regulated and 5 down-regulated genes. These genes are posited to play a significant role in the pathogenesis of OA (online suppl. Fig. S1).
Functional Enrichment Analysis
GO and KEGG pathway enrichment analysis were performed to further understand the function of differentially expressed DAMPs-related genes. GO analysis revealed the major roles of these genes in CC, MF, and BP. In the aspect of CC, these genes are mainly related to proton-transporting two-sector ATPase complex and apical part of cell; In the aspect of MF, they are mainly involved in ATPase-coupled ion transmembrane transporter activity and ATPase activity coupled to transmembrane these genes dominate. In the aspect of BP, these genes mainly affect cellular cation homeostasis and proton transmembrane transport (online suppl. Fig. S2a–c). The KEGG pathway was mainly concentrated in human papillomavirus infection and vibrio cholerae infection (online suppl. Fig. S2d). These findings not only increased our understanding of the underlying pathological mechanisms of OA but may also indicated new therapeutic targets.
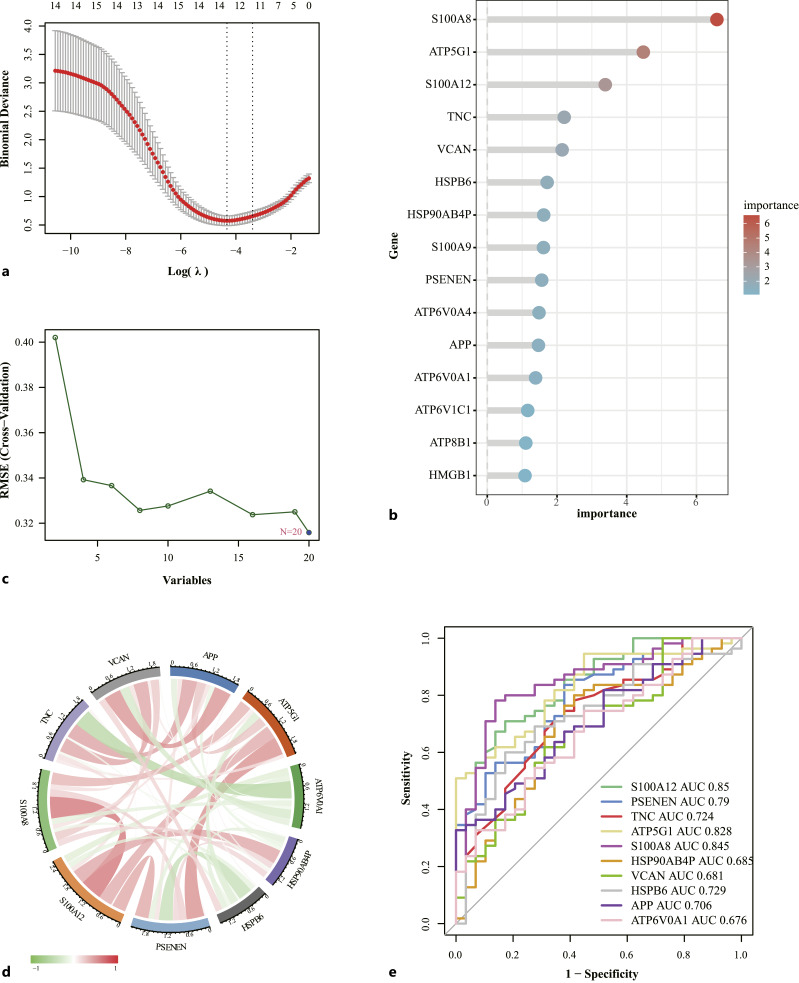
Identification of Key Genes
To identify key genes, three machine-learning methods were used to screen key genes. In LASSO regression, 13 genes were screened (Fig. 2a). In the random forest, the genes were ranked by importance and 12 key genes were selected (Fig. 2b). The SVM identified 20 genes (Fig. 2c). The three machine learning methods were crossed to obtain 10 key genes, including S100A12, PSENEN, TNC, ATP5G1, S100A8, HSP90AB4P, VCAN, HSPB6, APP, ATP6V0A1. To understand the interactions between these genes, correlation analyses were performed and the results of these analyses are shown in Figure 2d. Meanwhile, “pROC” in R software were used to evaluate the diagnostic efficacy of 10 key genes in the diagnosis of OA (Fig. 2e). The AUC of S100a12 and S100A8 were 0.85 and 0.845, indicating that they have high diagnostic efficacy. AUC of ATP5G1 was 0.828, AUC of PSENEN was 0.79, AUC of HSPB6 was 0.729, AUC of TNC was 0.724, AUC of APP was 0.706, AUC of HSP90AB4P was 0.685, AUC of VCAN was 0.681, AUC of ATP6V0A1 was 0.676, which also showed some diagnostic potential.
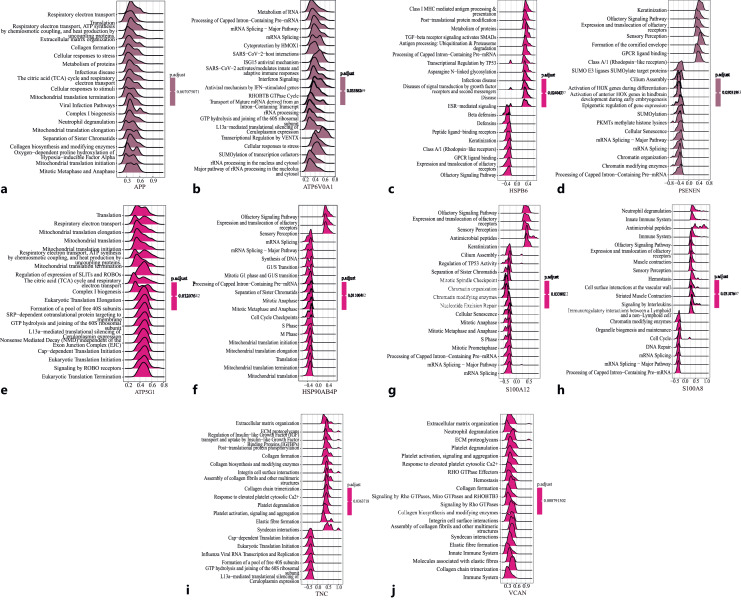
Single-Gene Function Enrichment Analysis of Key Genes
In order to further study the function of the key genes, 10 key genes were correlated with all genes, and then single-gene GSEA analysis was performed based on the reactome database using the R package “Cluster profiler” (Fig. 3). The results showed that APP was positively correlated with respiratory electron transport and translation, ATP5G1 was positively correlated with translation and respiratory electron transport, ATP6V0A1 was positively correlated with metabolism of RNA and processing of capped intron-containing pre-mRNA. HSP90AB4 was positively correlated with olfactory signaling pathway, expression, and translocation of olfactory receptors, and negatively correlated with mitochondrial translation and mitochondrial translation termination. HSPB6 was positively correlated with class I MHC mediated antigen processing and presentation, post-translational protein modification, and negatively correlated with olfactory signaling pathway and expression and translocation of olfactory receptors. S100A8 was positively correlated with neutrophil degranulation and innate immune system, and negatively correlated with processing of capped intron-containing pre-mRNA and mRNA splicing-major pathway. TNC was positively correlated with extracellular matrix and ECM proteoglycans, and negatively correlated with L13a-mediated translation silencing of Ceruloplasmin expression, GTP hydrolysis and joining of the 60S ribosomal subunits. VCAN was positively correlated with extracellular matrix organization and neutrophil degranulation, S100A12 was positively correlated with olfactory signaling pathway, expression and translocation of olfactory receptors, and negatively correlated with mRNA splicing-major pathway and mRNA splicing.
Analysis of Immune Infiltration
In this paper, single-sample Gene Set Enrichment Analysis (ssGSEA) was used to analyze immune infiltration. Correlation analysis of infiltrating immune cells in cartilage tissue was performed first (online suppl. Fig. S3a). Plasmacytoid dendritic cell was negatively correlated with eosinophil and activated B cell. The activated dendritic cell was mainly associated with type 1 helper T cell, and regulatory T cell was positively correlated with myeloid suppressor cell. The difference in immune cell infiltration between the OA group and the normal control group was shown in online supplementary Figure S3b, where we can see activated B cell, activated CD8 T cell, CD56 bright natural killer cell, CD56dim natural killer cell, gamma delta T cell, immature B cell, MDSC, macrophage, natural killer cell, T follicular helper cell, type 1 T helper cell were more highly expressed in OA Group. It was found that many kinds of immune cells were up-regulated in OA cartilage, which revealed the important role of inflammation in OA.
miRNA-TF-mRNA Network Diagram
In this study, we utilized the Regnetwork database to predict possible upstream regulation of key genes, including miRNAs and TFs. This step helps us understand the regulatory mechanisms of these genes in OA. To visualize these complex interactions, we built a network diagram using “Cytoscape” software (online suppl. Fig. S4). The network contains 106 miRNAs, 70 TFs, and 8 mRNAs, revealing a complex regulatory framework that collectively acts on key genes that may influence the development of OA. By deciphering online supplementary Figure S4, we can see how specific miRNAs and TFs interact with specific mRNAs, which is crucial for revealing the molecular mechanisms of disease and finding potential therapeutic targets.
The Expression of β-Amyloid, ApoE2, ApoE3, and ApoE4 in OA and Control Group
A total of 152 patients were included in this study, including 101 patients with KOA and 51 controls. There were 29 male and 72 female patients in the KOA group, 19 male and 32 female patients in the control group. There was no significant difference in sex and age between the two groups. BMI in the KOA group is higher than that of the control group (25.48 [4.4] vs. 23.33 [2.88], p = 0.001). The expression of β-amyloid, ApoE2, ApoE3, and ApoE4 in serum were measured with ELISA. The results showed that β-amyloid in the KOA group were significantly higher than those in the control group (86.96 [41.33] vs. 53.92 [73.49], p = 0.001), ApoE3 and ApoE4 were significantly lower than those in the control group (38.85 [17.63] vs. 49.94 ([39.8], p < 0.001; 44.63 [28.24] vs. 57.81 [30.73], p = 0.022) (Table 1).
Table 1.
Comparison between KOA group and control group
| KOA (n = 101) | control (n = 51) | p value | |
|---|---|---|---|
| Age | 68 (9) | 67 (12) | 0.177 |
| Sex (male/female) | 29/72 | 19/32 | 0.221 |
| BMI, kg/m2 | 25.48 (4.4) | 23.44 (2.88) | 0.001 |
| β-Amyloid, pg/mL | 86.96 (41.33) | 53.92 (73.49) | 0.001 |
| ApoE2, ng/mL | 65.76 (48.44) | 66.65 (52.83) | 0.777 |
| ApoE3, ng/mL | 38.85 (17.63) | 49.94 (39.8) | <0.001 |
| ApoE4, ng/mL | 44.63 (28.24) | 57.81 (30.73) | 0.022 |
Part of the measurement data does not satisfy the normality. All measurement data are presented as median (interquartile range).
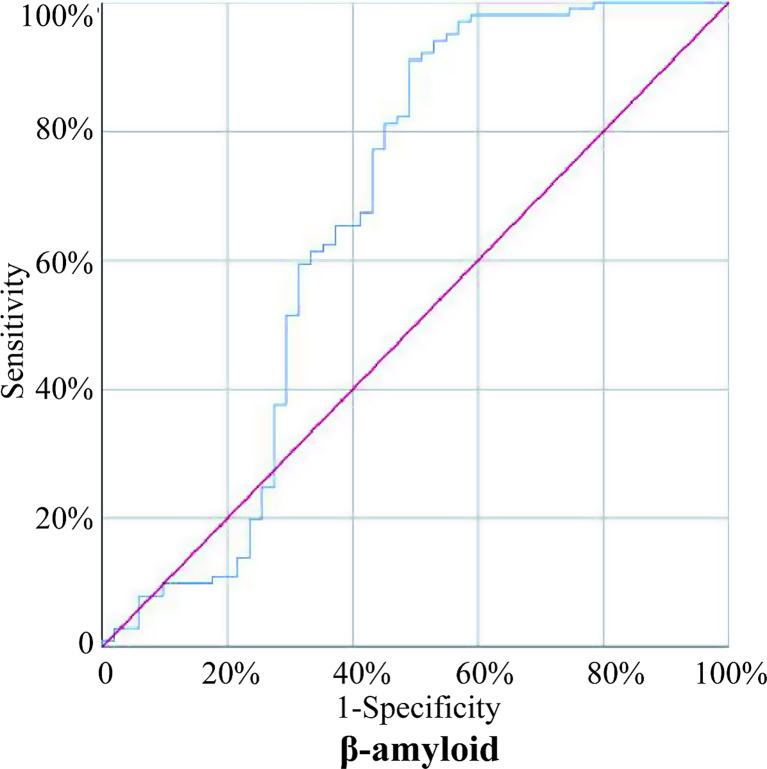
Logistic regression was used to analyze the above indicators with statistical difference between the two groups. The results showed that ApoE3 (OR = 0.966, p = 0.001) was an independent protective factor for KOA, and β-amyloid (OR = 1.011, p = 0.003) and BMI (OR = 1.142, p = 0.027) were independent risk factors for KOA (Table 2). The ROC curve of β-amyloid was drawn (Fig. 4), the AUC was calculated as 0.668 (95% CI: 0.5596–0.7751), the optimal cutoff was 54.9 pg/mL, the specificity was 50.98%, and the sensitivity was 91.09%.
Table 2.
Multifactor logistics regression
| Variable | Multifactor logistics regression | p value |
|---|---|---|
| OR (95% CI) | ||
| ApoE3 | 0.966 (0.944~0.986) | 0.001 |
| ApoE4 | 0.988 (0.972~1.005) | 0.172 |
| β-Amyloid | 1.011 (1.003~1.018) | 0.003 |
| BMI | 1.142 (1.016~1.284) | 0.027 |
The Expression of ApoE2, ApoE3, ApoE4, and β-Amyloid in Different Severity of KOA
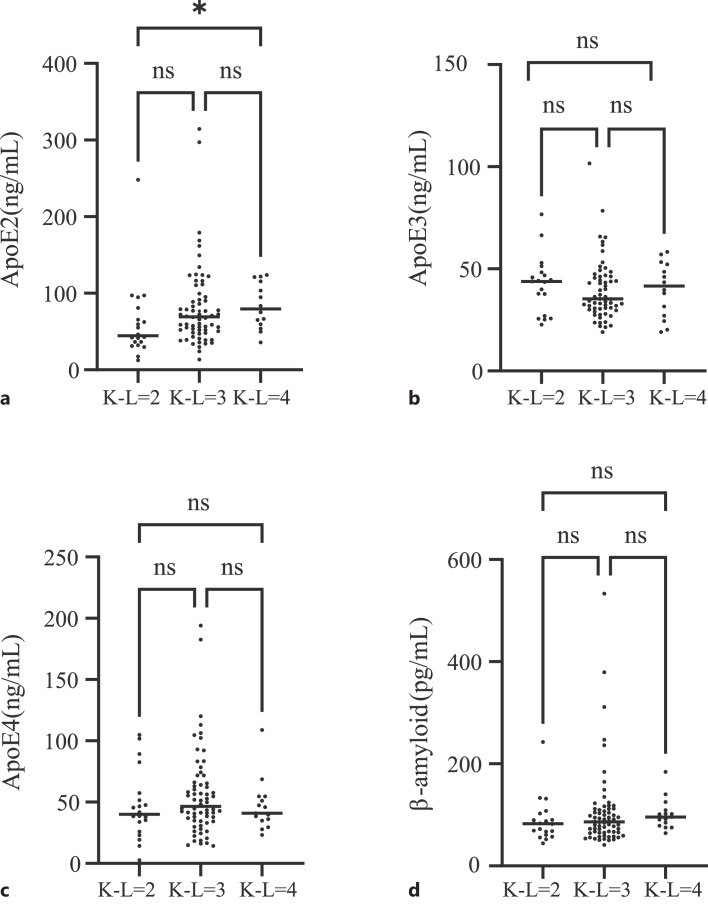
101 patients with KOA were divided into three grades: K-L 2 (n = 20), K-L 3 (n = 66), and K-L 4 (n = 15). K-W test was used to analyze the differences between the three groups (Fig. 5). There was significant difference in ApoE2 between K-L2 and K-L4 grades (p = 0.018). There was no significant difference in the expression levels of β-amyloid, ApoE2, ApoE3, and ApoE4 among the other groups. Postoperative changes in biomarkers were assessed in 45 patients who underwent joint replacement surgery for KOA. Serum samples collected after the surgery were compared to preoperative levels to evaluate the impact of the operation on these markers. The analysis revealed that serum levels of ApoE3 and ApoE4 showed a significant increase post-surgery. In contrast, levels of ApoE2 and β-amyloid remained unchanged. These findings are illustrated in online supplementary Figure S5.
Correlation Analysis of ApoE2, ApoE3, ApoE4, and β-Amyloid
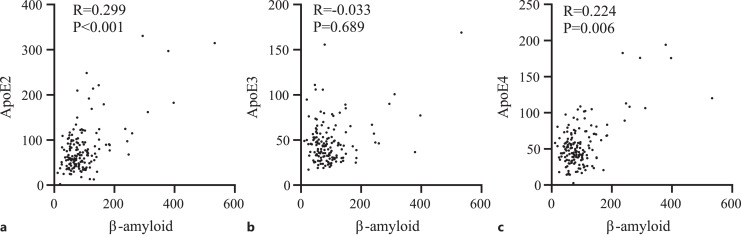
Spearman correlation analysis of β-amyloid with ApoE2, ApoE3, and ApoE4 showed that β-amyloid was positively correlated with ApoE2 (R = 0.299, p < 0.001) and ApoE4 (R = 0.224, p = 0.006). There was no significant association between β-amyloid and ApoE3 (p = 0.689) (Fig. 6).
Discussion
At present, it is generally accepted that long-term and low-grade inflammation is a key factor in the development of OA, which includes the interaction of the innate immune system and inflammatory mediators [17]. Under the influence of other risk factors, such as obesity, advanced age, metabolic disorder, imbalance of bioenergy sensor, and some genetic factors, it can cause local tissue injury of joint, tissue repair failure and inflammation cycle, leads to further cartilage loss and progressive joint degeneration [18]. Damps are a key part of this process. Damps are endogenous molecules released by the body’s own cell death. Articular chondrocytes and cartilage damage release DAMPs, which bind to pattern recognition receptor receptors, including TLRs, NOD-like receptor,s and glycosylation end-products receptors (RAGEs), initiating downstream signaling cascades, leads to transcription of multiple activators, such as NF-κB. And this activation leads to the release of various factors, such as matrix metalloproteinase −1, −3, −9, and −13, cytokines, etc [8].
The rapid development of gene chip technology and bioinformatics has been instrumental in dissecting the molecular underpinnings of diseases like OA. By analyzing OA data sets from the GEO database, we identified 20 differentially expressed DAMPs-related genes. GO analysis linked these genes primarily to ATP-related energy metabolism, mRNA synthesis, and immune inflammation processes. This highlights ATP’s multifaceted role in immune system modulation – from stimulating lymphocytes to cytokine secretion [19]. Additionally, immune cell upregulation in OA cartilage underscores inflammation’s critical role, revealed through our immune infiltration analysis. Interestingly, KEGG analysis suggested an association between DAMPs-related genes and infections like HPV and Vibrio cholerae, areas previously unexplored in OA research. This novel finding suggests potential, yet unexplored, infectious components in OA pathogenesis. In order to further screen the key genes, LASSO regression, SVM, and random forest machine learning methods were used and obtained 10 key genes, including S100A12, PSENEN, TNC, ATP5G1, S100A8, HSP90AB4P, VCAN, HSPB6, APP, ATP6V0A1.
HSPB6 and HSP90AB4P belong to shock protein family, and HSP90AB4P is a pseudogene. Under physiological conditions, HSP exists as a chaperone during protein synthesis or clearance of misfolded proteins. HSPB6, also known as HSP20, belongs to the small-molecular-weight HSP and often exists in the form of dimers in humans, which are up-regulated in different cellular stress or injury responses to protect cells from injury [20]. It has been shown that small HSP, including HSPB6, plays a central role in neuroinflammation in AD and, it is associated with the deposition of β-amyloid [21]. ATP5G1 is a subunit that encodes mitochondrial ATP synthase, which catalyzes ATP synthesis. Some studies have shown that extracellular ATP can induce pro-inflammatory effects by activating the low-affinity receptor P2X7 in the micromolar range, but in the micromolar range, extracellular ATP can exert immunosuppressive effects through activation of high-affinity P2Y11 receptors [22]. ATP6V0A1 encodes a component of vacuolar ATPase (V-ATP) and is associated with innate immune system [23]. TNC is a gene encoding Tenascin-C (TN-C). TN-C is an extracellular matrix glycoprotein. In this study, the results of single-gene functional analysis showed that it was positively correlated with extracellular matrix and ECM proteoglycans. Current studies have shown that TN-C expression is increased in cartilage, synovium, and synovial fluid in patients with OA, but the mechanism is not clear [24]. Exogenous TN-C can slow articular cartilage degeneration in mice with OA. However, paradoxically, specific TN-C fragments can mediate cartilage degradation by inducing aggregase activity [25]. VCAN encodes versican. Currently, ADAMTS-5, a polymerase that can modulate the degradation of versican has been studied as a therapeutic target for OA, and drugs have been developed for clinical trials [26, 27].
Both APP and PSENEN are key genes for β-amyloid production. β-Amyloid protein is derived from APP by hydrolysis of β- and γ-secretase. At present, β-amyloid is widely studied in AD. β-Amyloid usually exists as aggregates in human body. The most common subtypes of β-amyloid are Aβ1-40 and Aβ1-42. Aβ1-42 is more toxic and aggregates more easily [28]. This study shows that APP and PSENEN are up-regulated in OA. APP was mainly associated with respiratory electron transport and transcription, but PSENEN was not enriched to the obvious pathway. Respiratory electron transport is related to ATP production and energy metabolism. On the other hand, current research shows that β-amyloid can promote the production of reactive oxygen species, induce oxidative stress, and promote chondrocyte apoptosis [29, 30]. In a mouse model of AD, knee OA was found to accelerate the deposition of β-amyloid [31].
There is no evidence to support that these genes (except APP and PSENEN) have a direct relationship between ApoE and β-amyloid in OA. However, we notice that APP, S100A8, S100A12 are all ligands of RAGE [32]. Recently, RAGE-specific inhibitors have become available for both research and clinical use in AD [33]. The effects of these drugs on OA deserve further study.
Synovial joint immune cells, such as neutrophils, macrophages, monocytes, dendritic cells, usually interact with DAMPs, and then active the innate immune system [34]. Lindblad’s and Hedfors [35] study showed that areas of the synovial membrane that lay near the cartilage in OA contained foci of T lymphocytes, which were bordered by immunoglobulin-carrying B lymphocytes and plasma cells, as well as strongly HLA-DR positive dendritic-like cells adjoined to alpha Leu-3a+ T helper lymphocytes. In addition, studies showed that neutrophils, monocytes and macrophages play different role in OA [36–38]. However, in our analysis of immune infiltration, there were no significant differences in neutrophils and monocytes between the two groups. Perhaps, more experiments are needed to support the above conclusions in order to study drugs which target the autoimmune system.
Whether the potential role of β-amyloid in the pathogenesis of OA is similar to that of AD deserves further study. In AD patients, ApoE interacts with amyloid deposition. Our study aimed to understand whether there is also an interaction between ApoE and β-amyloid in OA. We collected serum from patients with OA and non-OA to test the expression of β-amyloid and ApoE isoforms. β-Amyloid was found to be an independent risk factor for KOA (OR = 1.011). The AUC value for the diagnosis of OA was 0.668. There was no significant difference in the expression of β-amyloid between different severity groups. β-Amyloid may not be able to differentiate the severity of KOA. Also, there was no significant change in β-amyloid before and after operation. In this study, we found that the expression of ApoE3 and ApoE4 in the KOA group was significantly lower than those in the control group, and ApoE3 was a protective factor in the occurrence of KOA. Edlund’s et al. [39] study suggested that ApoE3 may be involved in insulin-independent regulation of glucose levels. We speculate that the role of ApoE3 in OA may be related to metabolic disorders. In correlation analysis, we found a positive correlation between β-amyloid and ApoE2, ApoE4. In neurons from patients with AD, clearance of β-amyloid plaques depends on uptake of the ApoE/β-amyloid complex by LRP1 receptor [40]. Because of the reduced stability of the complex between ApoE4 and β-amyloid, clearance of β-amyloid is significantly reduced in the presence of ApoE4 [40, 41]. ApoE4 may also contribute to the decrease of β-amyloid clearance in OA. However, this study only measured serum levels of ApoE and β-amyloid protein. Further studies on articular cartilage or chondrocytes are needed to explore the mechanism of β-amyloid and ApoE in OA.
Conclusion
In this study, we identified key DAMPs-related genes in OA using bioinformatics and machine learning methods. Our findings suggest that β-amyloid could be a promising target for diagnosing and treating OA. Additionally, we discovered distinct roles for ApoE isoforms, with ApoE3 acting as a protective factor against knee OA. The interactions between β-amyloid and ApoE isoforms, particularly ApoE2 and ApoE4, merit further research to understand their impact on OA progression.
Statement of Ethics
All experiments were approved by the Ethics Committee of Wenzhou Medical University (No. LCKY2019-34). Written informed consent was obtained from all the participants prior to the enrollment. The study was performed in accordance with the World Medical Association Declaration of Helsinki.
Funding Sources
This work was partially supported by grants from the Natural Science Foundation of China (NSFC81601849), Zhejiang Provincial Medicine and Health Technology Project (2019RC217), the Natural Science Foundation of Zhejiang province (LY22H160005).
Author Contributions
Fangling Yuan and Qipeng Xie designed the study. Fangling Yuan, Yatian Tang, and Feifei Zheng did the bioinformatics analysis. Yatian Tang and Feifei Zheng collected patient information and serum. Fangling Yuan performed the ELISA assay. Fangling Yuan written the article. All authors read and approved the final version of the manuscript.
Funding Statement
This work was partially supported by grants from the Natural Science Foundation of China (NSFC81601849), Zhejiang Provincial Medicine and Health Technology Project (2019RC217), the Natural Science Foundation of Zhejiang province (LY22H160005).
Data Availability Statement
All data created and analyzed during this current work are involved in this published article (and its supplementary information files). Further inquiries can be directed to the corresponding author.
References
 3/
3/ 4 carriers. J Alzheimers Dis. 2021;81(1):339–54.
[Europe PMC free article] [Abstract] [Google Scholar]
4 carriers. J Alzheimers Dis. 2021;81(1):339–54.
[Europe PMC free article] [Abstract] [Google Scholar]Articles from Journal of Innate Immunity are provided here courtesy of Karger Publishers
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Human ApoE Isoforms Differentially Modulate Glucose and Amyloid Metabolic Pathways in Female Brain: Evidence of the Mechanism of Neuroprotection by ApoE2 and Implications for Alzheimer's Disease Prevention and Early Intervention.
J Alzheimers Dis, 48(2):411-424, 01 Jan 2015
Cited by: 49 articles | PMID: 26402005 | PMCID: PMC5485924
Plasma ApoE4 Levels Are Lower than ApoE2 and ApoE3 Levels, and Not Associated with Plasma Aβ40/42 Ratio as a Biomarker of Amyloid-β Amyloidosis in Alzheimer's Disease.
J Alzheimers Dis, 93(1):333-348, 01 Jan 2023
Cited by: 2 articles | PMID: 36970894
iPSC-derived blood-brain barrier modeling reveals APOE isoform-dependent interactions with amyloid beta.
Fluids Barriers CNS, 21(1):79, 11 Oct 2024
Cited by: 0 articles | PMID: 39394110 | PMCID: PMC11468049
The interaction of amyloid-beta with ApoE.
Subcell Biochem, 38:255-272, 01 Jan 2005
Cited by: 43 articles | PMID: 15709483
Review