Abstract
Free full text

The −KTS isoform of Wt1 induces the transformation of Leydig cells into granulosa-like cells
Dear Editor,
In mammals, the testis and ovary both originate from a common bipotential gonad primordium, the genital ridge, composed of multipotential somatic progenitor cells. Testicular development is initiated by testis-determining factors Sry and Sox91,2, which induce the Sertoli cell lineage differentiation and formation of testicular cords. Conversely, in the absence of Sry in female gonads, the ovary-specific pathways including RSPO1/WNT4/β-catenin signaling3 and FOXL24 commit the differentiation of pre-granulosa cells and direct ovarian fate. Leydig cells are steroidogenic cells crucial for spermatogenesis and maintaining secondary sex characteristics by producing steroid hormones in males.
The Wilms tumor gene (Wt1) is present in the entire urogenital ridges at embryonic day (E) 9.5, where it plays a regulatory role in the formation of the genital ridges and kidneys5. Wt1 has two major isoforms, +KTS and −KTS, distinguished by the presence or absence of three amino acids (lysine-threonine-serine (KTS)) between the third and fourth zinc fingers. The +KTS variant has been identified as an essential regulator for Sry expression during sex determination. The –KTS isoform, while not necessary for sex determination, is crucial for the survival of the gonadal primordium6,7. A recent study published in Science reported that the Wt1–KTS isoform is also an ovary-determining factor, and its overexpression in male gonads, using a transgenic mouse model, leads to the transformation of Sertoli into granulosa cells8.
Our previous studies demonstrated that Wt1 is required for lineage specification and maintenance of supporting cells in gonad development, and inactivation of Wt1 results in Sertoli to Leydig-like cells transformation9–11. To investigate whether the overexpression of Wt1 can induce the transformation of Leydig into Sertoli cells, a Wt1–KTS conditional transgenic mouse model (Wt1+/Ctg) was generated. The expression of the Wt1–KTS was controlled by the CAG promoter, with flox sites flanking a poly A stop codon inserted between the CAG promoter and coding sequence, and modulated by Cre activation (Supplementary Fig. S1a). The effectiveness of Wt1–KTS induction by Cre recombinase was evaluated using mouse embryonic fibroblast cells isolated from Wt1+/Ctg;Cre-ERTM mice treated with tamoxifen. These results indicated a significant increase in both mRNA (Supplementary Fig. S1b) and protein (Supplementary Fig. S1c) levels of WT1–KTS after tamoxifen treatment, confirming the effective activation of Wt1–KTS by Cre recombinase.
To induce the overexpression of Wt1–KTS in undifferentiated gonadal somatic cells, Wt1+/Ctg mice were bred with Sf1-Cre mice. It has been reported that Sf1 (also known as Nr5a1) is expressed in the adrenogonadal primordium starting from E9.512. Real-time PCR analysis demonstrated a significant increase in the mRNA level of Wt1–KTS in Wt1+/Ctg;Sf1-Cre testes at E12.5 (Supplementary Fig. S2a). Western blot results showed a substantial increase in the protein level of WT1 (Supplementary Fig. S2b, c). These findings confirmed the effective activation of Wt1–KTS in undifferentiated gonadal somatic cells mediated by Sf1-Cre during sex determination.
Adult Wt1+/Ctg;Sf1-Cre males were infertile, and the size of testes was significantly reduced (Fig. (Fig.1a).1a). Histological analysis revealed well-organized seminiferous tubules in Wt1+/Ctg;Sf1-Cre mice, with SOX9-positive Sertoli cells located at the periphery region of seminiferous tubules, similar to control testes (Fig. (Fig.1a,1a, black arrowheads). Interestingly, no 3β-HSD-positive Leydig cells were observed in Wt1+/Ctg;Sf1-Cre testes compared to the control (Fig. (Fig.1a,1a, black arrows).
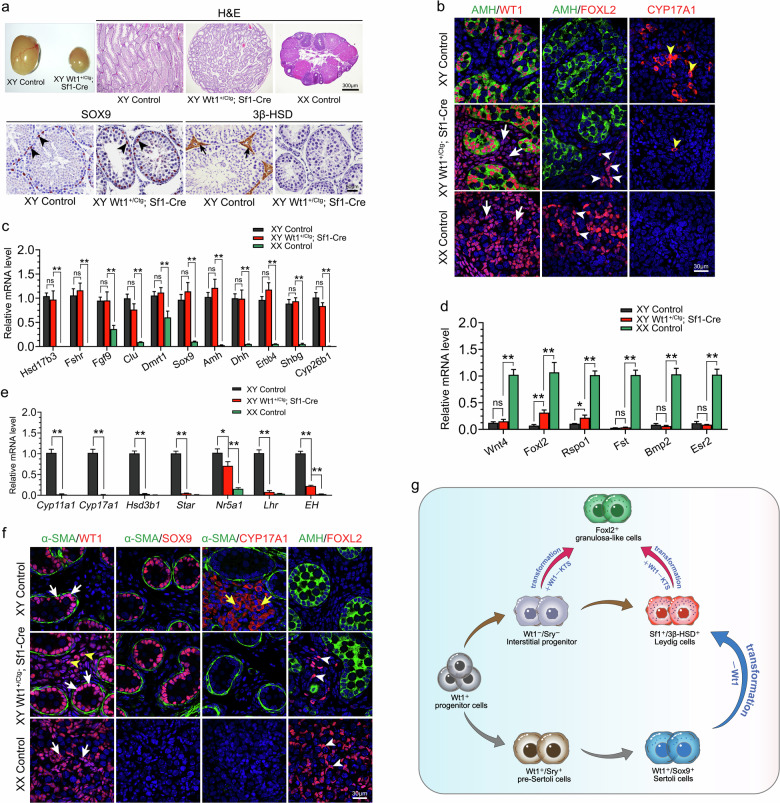
a Histological analysis of gonads from control males, Wt1+/Ctg;Sf1-Cre males, and control females at 2 months of age. b Immunostaining of WT1, AMH, FOXL2, and CYP17A1 on control males, Wt1+/Ctg;Sf1-Cre males, and control females at E12.5. c–e The mRNA levels of Sertoli cell-specific genes (c), granulosa cell-specific genes (d), and Leydig cell-specific genes (e) in control testes, Wt1+/Ctg;Sf1-Cre testes and control ovaries at E12.5 (n =
= 6). f The expression of WT1, SOX9, CYP17A1, α-SMA, and FOXL2 in gonads of control males, Wt1+/Ctg;Sf1-Cre males, and control females at E16.5 was examined by immunofluorescence. g Schematic diagram illustrating the proposed role of Wt1 in regulating gonadal somatic cell differentiation. Data are presented as mean
6). f The expression of WT1, SOX9, CYP17A1, α-SMA, and FOXL2 in gonads of control males, Wt1+/Ctg;Sf1-Cre males, and control females at E16.5 was examined by immunofluorescence. g Schematic diagram illustrating the proposed role of Wt1 in regulating gonadal somatic cell differentiation. Data are presented as mean ±
± SEM. Two-tailed Student’s t-test. *P
SEM. Two-tailed Student’s t-test. *P <
< 0.05; **P
0.05; **P <
< 0.01.
0.01.
To further investigate the functions of Wt1–KTS in gonadal somatic cell differentiation during sex determination, the expression of Sertoli- and pre-granulosa cell-specific genes was examined at E12.5 by immunostaining and real-time PCR analyses. AMH, a specific marker for Sertoli cells, was expressed in the Sertoli cells of both control and Wt1+/Ctg;Sf1-Cre testes, with the structure of testicular cords being intact (Fig. (Fig.1b).1b). FOXL2, abundantly expressed in control ovaries, was absent in control testes (Fig. (Fig.1b).1b). However, FOXL2-positive cells were also observed in testes of Wt1+/Ctg;Sf1-Cre mice (Fig. (Fig.1b,1b, white arrowheads), albeit in fewer number compared to a recent study8. Additionally, the WT1 signal in Wt1+/Ctg;Sf1-Cre testes was not only noted in AMH-positive Sertoli cells but also obviously detected in the interstitial cells (Fig. (Fig.1b,1b, white arrowheads) situated in the testis interstitium, indicating the Wt1–KTS expression was significantly increased during sex determination. Furthermore, FOXL2-positive granulosa-like cells were located in the testis interstitium, distinct from AMH-positive Sertoli cells (Fig. (Fig.1b,1b, white arrowheads). Meanwhile, the number of CYP17A1-positive (Fig. (Fig.1b,1b, yellow arrowhead) Leydig cells was dramatically reduced in Wt1+/Ctg;Sf1-Cre testes. We further examined the expression of other Sertoli, granulosa, and Leydig cell-specific genes using real-time PCR in Wt1+/Ctg;Sf1-Cre testes. The mRNA levels of several Sertoli cell-specific genes, including Hsd17b3, Fshr, Fgf9, Clu, Dmrt1, Sox9, Amh, Dhh, Erbb4, Shbg, and Cyp26b1, remained unchanged in Wt1+/Ctg;Sf1-Cre testes compared to control (Fig. (Fig.1c).1c). This suggests that the Sertoli cell differentiation was not affected by the overexpression of Wt1–KTS in gonadal somatic cells before sex determination. The mRNA levels of Foxl2 and Rspo1 were upregulated in Wt1+/Ctg;Sf1-Cre testes compared to control (Fig. (Fig.1d).1d). However, the expression of other granulosa cell-specific genes, such as Wnt4, Fst, Bmp2, and Esr2, was not increased in the Wt1+/Ctg;Sf1-Cre testes (Fig. (Fig.1d).1d). In line with the immunostaining results, the expression of steroidogenic cell-specific genes (e.g., Cyp11a1, Cyp17a1, Hsd3b1, Star, Nr5a1, Lhr, and EH) was obviously decreased (Fig. (Fig.1e1e).
To examine whether the Sertoli cells were transformed into granulosa-like cells at later developmental stages, the expression of Sox9 and Foxl2 was examined at E16.5 by immunostaining. Notably, seminiferous tubules outlined by peritubular myoid cell marker α-SMA and SOX9-positive Sertoli cells were observed at the periphery region of testicular cords in both control and Wt1+/Ctg;Sf1-Cre testes but not in control ovaries (Fig. (Fig.1f).1f). Similar to the findings at E12.5, CYP17A1-positive Leydig cells were absent in Wt1+/Ctg;Sf1-Cre testes and control ovaries compared to control testes (Fig. (Fig.1f,1f, yellow arrows). In addition, AMH was detected in the testicular cords of both control and Wt1+/Ctg;Sf1-Cre testes. FOXL2-positive granulosa cells (white arrowheads) were noted in the control ovary but absent in the control testis (Fig. (Fig.1f).1f). By contrast, several FOXL2-positive granulosa-like cells (Fig. (Fig.1f,1f, white arrowheads) were detected in the interstitium of Wt1+/Ctg;Sf1-Cre testes, distinct from the AMH signal. Importantly, FOXL2-positive granulosa-like cells were also observed in adult Wt1+/Ctg;Sf1-Cre testes, located outside of α-SMA-marked seminiferous tubules (Supplementary Fig. S3d, white arrows).
Our previous studies demonstrated that inactivation of Wt1 causes supporting to steroidogenic cell transformation9–11. We hypothesized that the emergence of FOXL2-positive granulosa-like cells in Wt1–KTS-overexpressing testes might originate from Leydig cell progenitors. To test this hypothesis, we induced the overexpression of Wt1–KTS, specifically in Leydig cells, using Cyp17a1-Cre mice. To ascertain the specificity of Cyp17a1-Cre, we crossed these mice with mT-mG reporting mice. The results revealed that the GFP signal was exclusively detected in 3β-HSD-positive Leydig cells in mT-mG;Cyp17a1-Cre testes at E16.5 (Supplementary Fig. S4c, yellow arrows), indicating the specific activation of Cyp17a1-Cre in Leydig cells. Subsequently, we examined the expression of WT1 in control (mT-mG;Cyp17a1-Cre) and Wt1+/Ctg;mT-mG; Cyp17a1-Cre testes through immunostaining. In control testes, WT1 was observed in Sertoli cells, while no GFP and WT1 double-positive cells were detected (Supplementary Fig. S4f, white arrows). Conversely, in Wt1+/Ctg;mT-mG;Cyp17a1-Cre testes, WT1 was expressed not only in Sertoli cells but also in GFP-positive Leydig cells (Supplementary Fig. S4i, white arrowheads), suggesting the exclusive activation of Cyp17a1-Cre in Leydig cells. To analyze the fate of Leydig cells after Wt1–KTS overexpression, we examined the expression of 3β-HSD, SOX9, and FOXL2 by immunofluorescence at E16.5. In both control and Wt1+/Ctg;mT-mG;Cyp17a1-Cre testes, SOX9-positive Sertoli cells (yellow arrows) were observed within the seminiferous tubules, while no SOX9-positive cells were detected in control ovaries (Supplementary Fig. S5). The strong 3β-HSD signal was observed in the control testis interstitium and colocalized with the GFP signal (Supplementary Fig. S5b, white arrows), indicative of Leydig cells. Interestingly, a weaker 3β-HSD signal was detected in the interstitium of Wt1+/Ctg;mT-mG;Cyp17a1-Cre testes and colocalized with GFP signal (Supplementary Fig. S5e, white arrowheads). Notably, a small number of FOXL2-positive cells were observed in the interstitium of Wt1+/Ctg;mT-mG;Cyp17a1-Cre testes and colocalized with GFP signal (Supplementary Fig. S5f, yellow arrowheads). Real-time PCR analysis showed that the expression of Sertoli cell-specific genes (e.g., Sox9, Amh, Dhh, and Fgf9) was not significantly altered in Wt1+/Ctg;Cyp17a1-Cre testes, while the expression of steroidogenic cell-specific genes (e.g., Cyp11a1, Star, Hsd3b1, and Cyp17a1) was significantly downregulated. The mRNA levels of Foxl2 and Rspo1 were upregulated in Wt1+/Ctg;Cyp17a1-Cre testes, but other granulosa cell-specific genes (e.g., Fst and Wnt4) were not increased (Supplementary Fig. S5j). Furthermore, examination of Wt1+/Ctg;mT-mG;Cyp17a1-Cre testes at the adult stage revealed the absence of 3β-HSD-positive Leydig cells and the presence of FOXL2 and GFP double-positive cells (yellow arrows) in the interstitium (Supplementary Fig. S6), further confirming the transformation of Leydig into granulosa-like cells induced by Wt1–KTS overexpression.
To further test whether overexpression of Wt1–KTS could induce Sertoli to Foxl2-expressing granulosa-like cell transformation, Wt1+/Ctg mice were crossed with AMH-Cre transgenic mice. Co-expression of GFP and AMH was observed in mT-mG;AMH-Cre testis at E14.5 (Supplementary Fig. S7c, white arrows), indicating that AMH-Cre was activated explicitly in Sertoli cells. The mRNA level of Wt1–KTS in Wt1+/Ctg;AMH-Cre was significantly increased compared with control testes (Supplementary Fig. S7d), confirming the successful overexpression of Wt1–KTS in Sertoli cells.
At E16.5, seminiferous tubules were well-organized, and SOX9-positive Sertoli cells (yellow arrows) were located within the seminiferous tubules in both control and Wt1+/Ctg;AMH-Cre testes (Supplementary Fig. S8). 3β-HSD-positive Leydig cells (white arrows) were also observed in the interstitium of both control (Supplementary Fig. S8b) and Wt1+/Ctg;AMH-Cre (Supplementary Fig. S8e) testes, while no SOX9 (Supplementary Fig. S8g) and 3β-HSD (Supplementary Fig. S8h) signals were noted in control ovaries. FOXL2-positive cells were detected in control ovaries (white arrowheads) but not in control and Wt1+/Ctg;AMH-Cre testes (Supplementary Fig. S8). Moreover, the mRNA levels of Sertoli cells-specific genes (e.g., Sox9, Amh, Dhh, and Fgf9), steroidogenic cells-specific genes (e.g., Cyp11a1, Star, Hsd3b1, and Cyp17a1), and pre-granulosa cells-specific genes (e.g., Foxl2, Rspo1, Fst, and Wnt4) were not changed in Wt1+/Ctg;AMH-Cre testes (Supplementary Fig. S8j). Furthermore, the cell fate of Wt1–KTS-overexpressing Sertoli cells was examined by co-staining of α-SMA and SOX9, α-SMA and FOXL2, and α-SMA and 3β-HSD in Wt1+/Ctg;AMH-Cre testes at 2 weeks of age. Seminiferous tubules in Wt1+/Ctg;AMH-Cre testes were well-organized, and SOX9-positive Sertoli cells were appropriately located at the periphery region (Supplementary Fig. S9d). Similar to the results observed at the embryonic stage, FOXL2-positive granulosa cells were not detected (Supplementary Fig. S9e), and abundant 3β-HSD-positive steroidogenic cells were present in the interstitium of Wt1+/Ctg;AMH-Cre testes (Supplementary Fig. S9f). These results collectively demonstrate that the overexpression of Wt1–KTS does not induce transdifferentiation of Sertoli into granulosa-like cells.
In the study, we also observed FOXL2-positive granulosa-like cells in the testes of Wt1+/Ctg;Sf1-Cre mouse model. However, we did not observe male-to-female sex reversal, and the differentiation of testes was not affected by Wt1–KTS overexpression. The integrity of testicular cords remained intact, and the expression of the SOX9 in Seroli cells was unchanged. Importantly, we did not detect FOXL2-positive cells inside the seminiferous tubules, indicating that the fate of Sertoli cells remained unaltered with Wt1–KTS overexpression. Surprisingly, the development of gonads after sex determination, particularly regarding follicle development, was not examined in the study by Gregoire et al. despite the reported male-to-female sex reversal8.
The androgens produced by Leydig cells are crucial for the development of the male reproductive system and spermatogenesis. Deficiency in testosterone production has been associated with defects in spermatogenesis in mouse models and human patients13. Therefore, the absence of Leydig cells could explain the spermatogenic defects and male infertility observed in our mouse model. In the present study, we observed a blockade in the differentiation of Leydig cells upon overexpression of Wt1–KTS in undifferentiated gonadal somatic cells. This finding aligns with our previous research9–11, reinforcing the notion that Wt1 represses the differentiation of Leydig cells. Furthermore, our mouse model also exhibited FOXL2-positive granulosa-like cells, consistent with the recent study by Gregoire et al.8. However, the specific origin of these FOXL2-positive cells remained a critical question left unanswered by their research. They concluded that these cells are derived from Wt1–KTS-overexpressing Sertoli cells, but they did not conduct lineage tracing experiments to validate this assertion8. Additionally, they did not explore the fate of Leydig cells following Wt1–KTS overexpression. Given that a subset of Leydig cells shares a common origin with Sertoli cells and that Wt1 is a critical factor in their differentiation, it becomes imperative to ascertain whether Leydig cells undergo normal differentiation upon Wt1–KTS overexpression.
As proposed in the schematic diagram illustrated in Fig. Fig.1g,1g, it is widely acknowledged that both Sertoli and Leydig cells originate from Wt1-positive progenitor cells within undifferentiated gonads14. During sex determination, a subset of gonadal somatic cells retains Wt1 expression and initiates the expression of Sry, leading to their differentiation into Sertoli cells. Conversely, for reasons yet to be fully understood, a small fraction of somatic cells lose Wt1 and do not express Sry, consequently undergoing differentiation into Leydig cells. In our hypothesis, we postulate that if Wt1–KTS is overexpressed in all progenitor cells, the differentiation of Leydig cells would be impeded, while the differentiation of Sertoli cells would remain unaffected. Furthermore, the progenitors of Leydig cells would be unable to differentiate into Sertoli cells under conditions of Wt1–KTS overexpression due to the absence of pro-testis factors such as Sry and Sox9. Instead, they would likely differentiate into granulosa cells because Sertoli and granulosa cells share a common progenitor pool, and the absence of Sry or Sox9 prompts the differentiation of gonadal somatic cells into granulosa cells in male gonads15. However, our study did not provide direct evidence to support this conclusion due to the inability to overexpress Wt1–KTS, specifically in Leydig cell progenitors. Nonetheless, crucial findings emerged from our research and we observed the transformation of Leydig cells into FOXL2-positive granulosa cells following the overexpression of Wt1–KTS specific in Leydig cells after sex determination. By contrast, the specific overexpression of Wt1–KTS in Sertoli cells after sex determination did not disrupt testis development, and no FOXL2-positive cells were detected. Based on these data, we inferred that the FOXL2-positive granulosa cells originated from Leydig cell progenitors rather than Sertoli cells. These findings provide valuable insights into the cellular dynamics underlying gonadal development and differentiation.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82421003, 32270902, and 32170855); The Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0840000); The Faculty Resources Project of the College of Life Sciences, Inner Mongolia University (2022-104). We thank Dr Humphrey Hung-Chang Yao from the National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA, for the gift of the Sf1-Cre mice.
Author contributions
F.G., M.C., and K.M. designed the experiments. C.C., B.L., L.L., N.W., and Z.S. performed the experiments. C.C. and L.Z. wrote the manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Changhuo Cen, Bowen Liu.
Contributor Information
Kai Meng, Email: moc.621@888125iakgnem.
Min Chen, Email: nc.ca.zoi@nimnehc.
Fei Gao, Email: nc.ca.zoi@foag.
Supplementary information
The online version contains supplementary material available at 10.1038/s41421-024-00732-6.
References
Articles from Cell Discovery are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1038/s41421-024-00732-6
Read article for free, from open access legal sources, via Unpaywall:
https://www.nature.com/articles/s41421-024-00732-6.pdf

 5
5 


