Abstract
Free full text

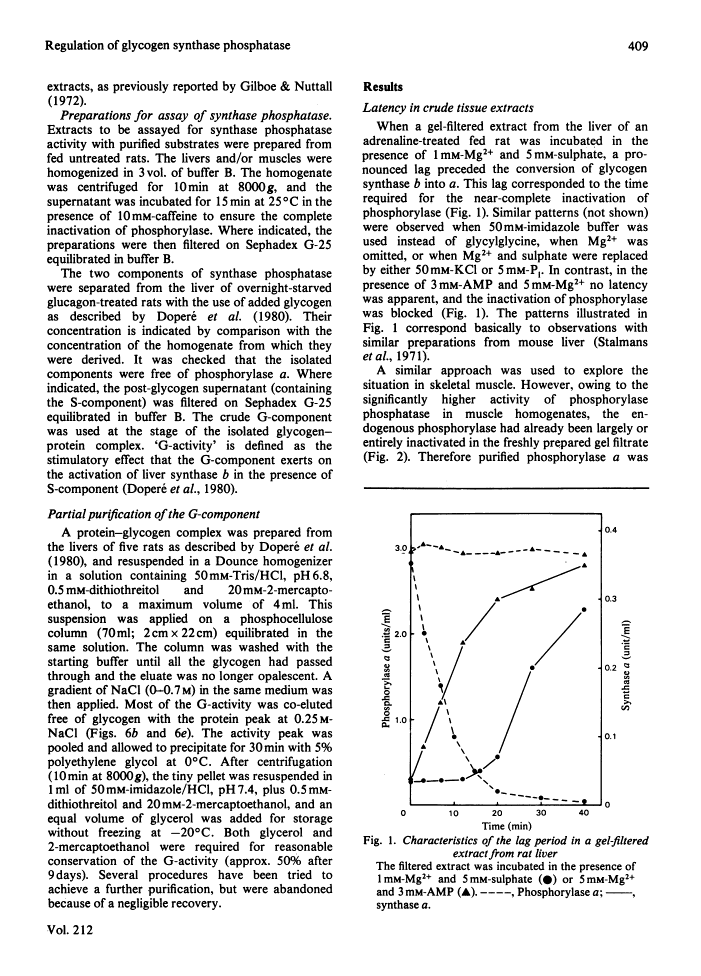
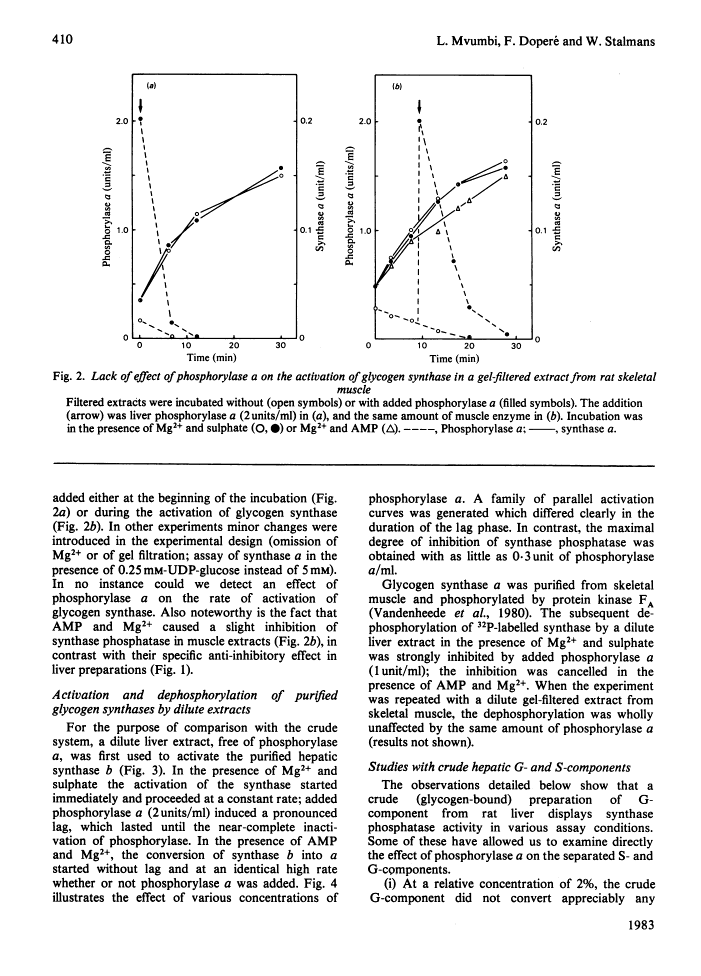
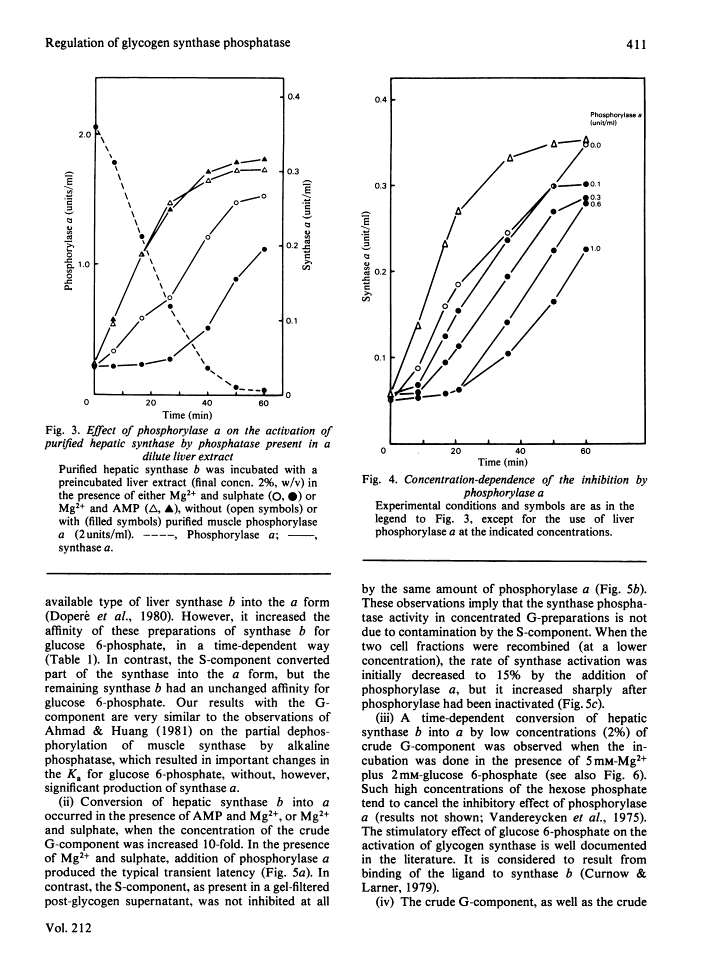
The inhibitory effect of phosphorylase a on the activation of glycogen synthase depends on the type of synthase phosphatase.
Abstract
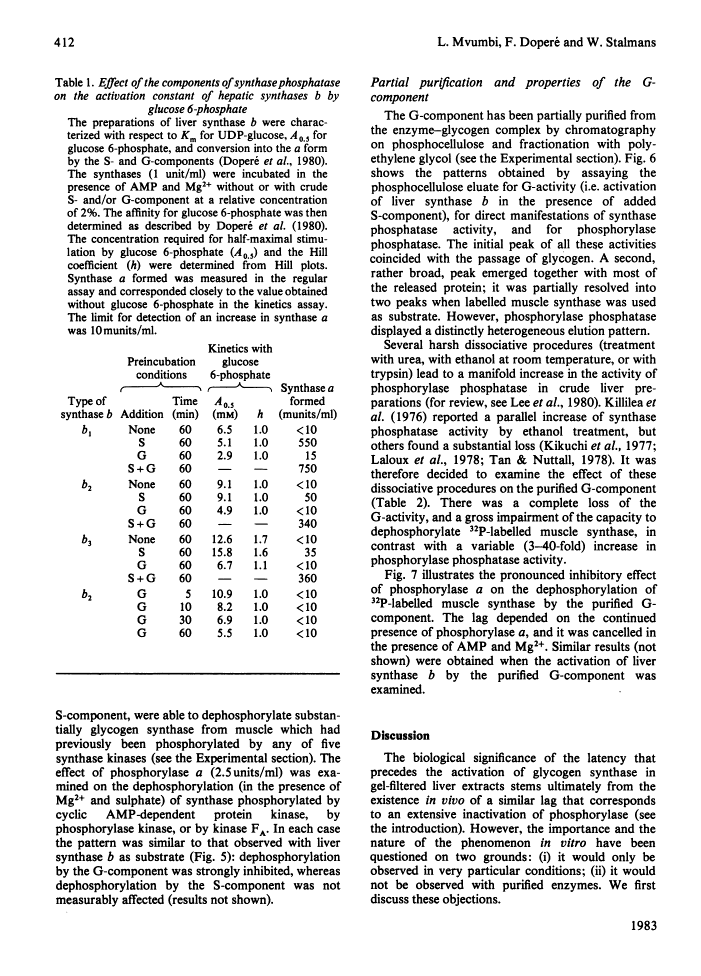
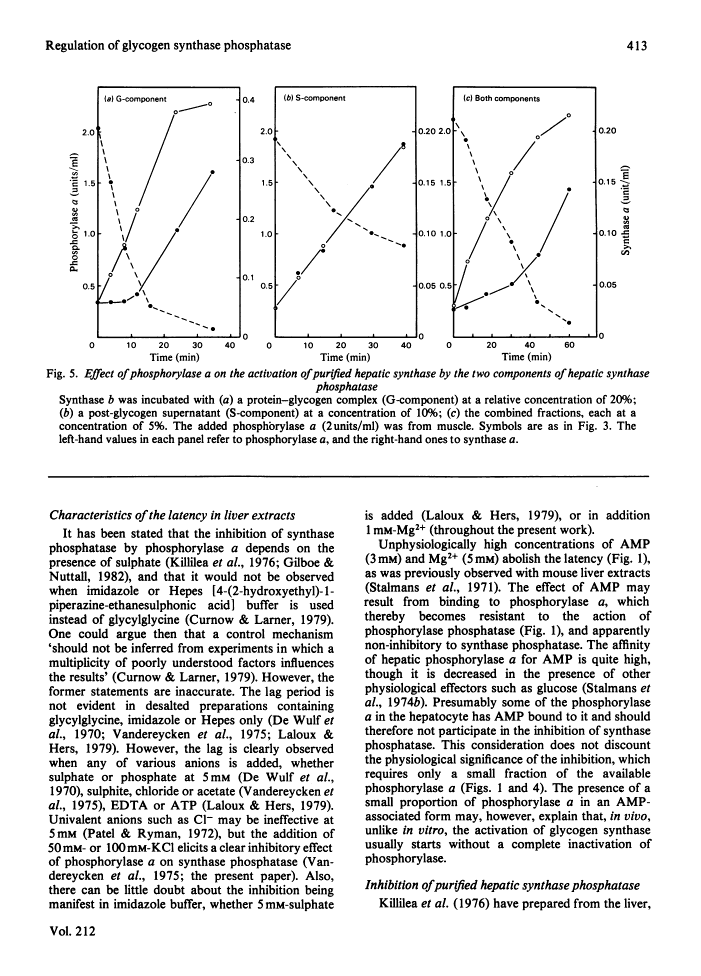
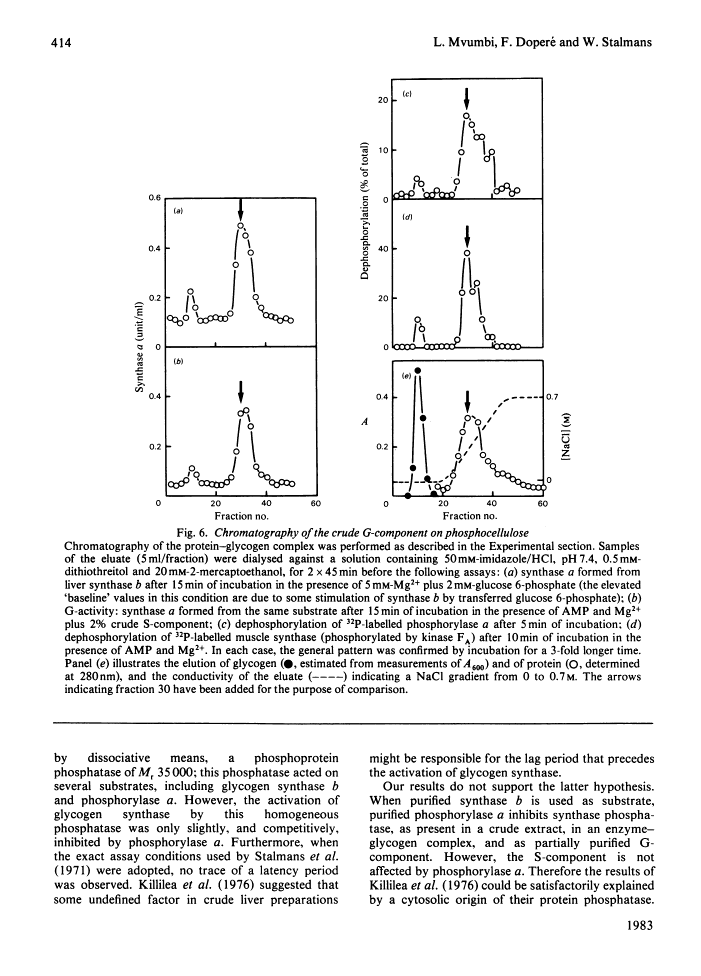
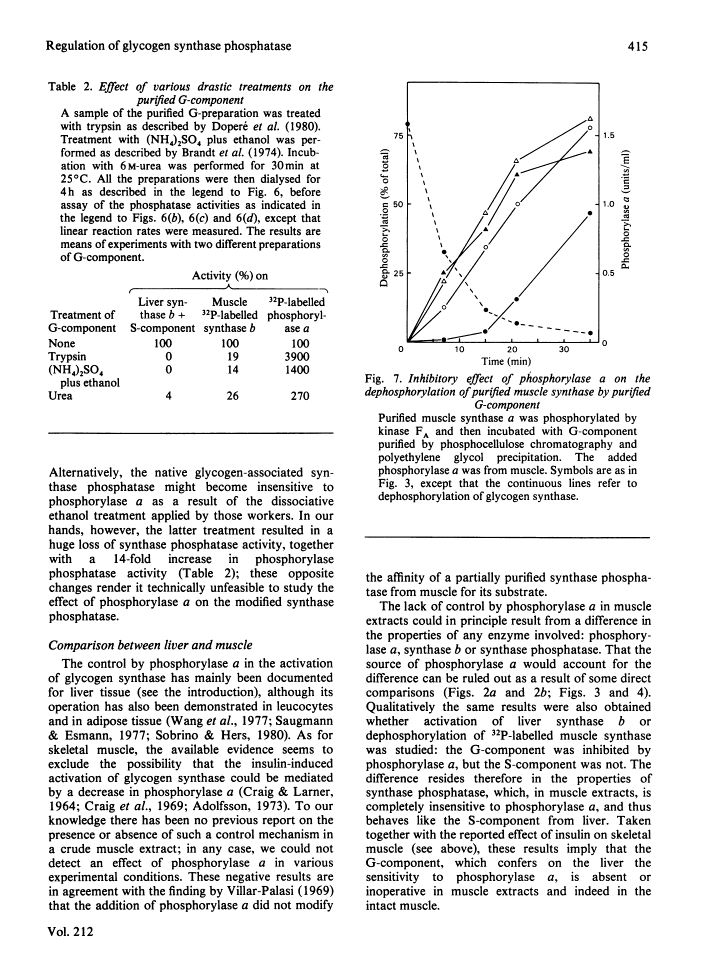
The activity of glycogen synthase phosphatase in rat liver stems from the co-operation of two proteins, a cytosolic S-component and a glycogen-bound G-component. It is shown that both components possess synthase phosphatase activity. The G-component was partially purified from the enzyme-glycogen complex. Dissociative treatments, which increase the activity of phosphorylase phosphatase manyfold, substantially decrease the synthase phosphatase activity of the purified G-component. The specific inhibition of glycogen synthase phosphatase by phosphorylase a, originally observed in crude liver extracts, was investigated with purified liver synthase b and purified phosphorylase a. Synthase phosphatase is strongly inhibited, whether present in a dilute liver extract, in an isolated enzyme-glycogen complex, or as G-component purified therefrom. In contrast, the cytosolic S-component is insensitive to phosphorylase a. The activation of glycogen synthase in crude extracts of skeletal muscle is not affected by phosphorylase a from muscle or liver. Consequently we have studied the dephosphorylation of purified muscle glycogen synthase, previously phosphorylated with any of three protein kinases. Phosphorylase a strongly inhibits the dephosphorylation by the hepatic G-component, but not by the hepatic S-component or by a muscle extract. These observations show that the inhibitory effect of phosphorylase a on the activation of glycogen synthase depends on the type of synthase phosphatase.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolfesson S. Glycogen synthesis in rat diaphragm in vivo: a biphasic effect of insulin on glycogen synthetase enzyme. Acta Physiol Scand. 1973 Apr;87(4):465–473. [Abstract] [Google Scholar]
- Ahmad Z, Huang KP. Dephosphorylation of rabbit skeletal muscle glycogen synthase (phosphorylated by cyclic AMP-independent synthase kinase 1) by phosphatases. J Biol Chem. 1981 Jan 25;256(2):757–760. [Abstract] [Google Scholar]
- Brandt H, Killilea SD, Lee EY. Activation of phosphorylase phosphatase by a novel procedure: evidence for a regulatory mechanism involving the release of a catalytic subunit from enxyme-inhibitor complex(es) of higher molecular weight. Biochem Biophys Res Commun. 1974 Nov 27;61(2):598–604. [Abstract] [Google Scholar]
- CRAIG JW, LARNER J. INFLUENCE OF EPINEPHRINE AND INSULIN ON URIDINE DIPHOSPHATE GLUCOSE-ALPHA-GLUCAN TRANSFERASE AND PHOSPHORYLASE IN MUSCLE. Nature. 1964 Jun 6;202:971–973. [Abstract] [Google Scholar]
- Craig JW, Rall TW, Larner J. The influence of insulin and epinephrine on adenosine 3',5'-phosphate and glycogen transferase in muscle. Biochim Biophys Acta. 1969 Apr 1;177(2):213–219. [Abstract] [Google Scholar]
- Devos P, Hers HG. Glycogen metabolism in the liver of the foetal rat. Biochem J. 1974 May;140(2):331–340. [Europe PMC free article] [Abstract] [Google Scholar]
- De Wulf H, Stalmans W, Hers HG. The effect of glucose and of a treatment by glucocorticoids on the activation in vitro of liver glycogen synthetase. Eur J Biochem. 1970 Jul;15(1):1–8. [Abstract] [Google Scholar]
- Doperé F, Stalmans W. Release and activation of phosphorylase phosphatase upon rupture of organelles from rat liver. Biochem Biophys Res Commun. 1982 Jan 29;104(2):443–450. [Abstract] [Google Scholar]
- Doperé F, Vanstapel F, Stalmans W. Glycogen-synthase phosphatase activity in rat liver. Two protein components and their requirement for the activation of different types of substrate. Eur J Biochem. 1980 Feb;104(1):137–146. [Abstract] [Google Scholar]
- Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980 Jun;107(2):519–527. [Abstract] [Google Scholar]
- Gilbert M, Bourbon J. Effects of acute variation of fetal glycemia on glycogen storage and on glycogen synthase and phosphorylase activities in the liver of the rat fetus. Diabetes. 1980 Apr;29(4):266–271. [Abstract] [Google Scholar]
- Gilboe DP, Nuttall FQ. The role of ATP and glucose 6-phosphate in the regulation of glycogen synthetase D phosphatase. Biochem Biophys Res Commun. 1972 Aug 21;48(4):898–906. [Abstract] [Google Scholar]
- Huang KP, Itarte E, Singh TJ, Akatsuka A. Phosphorylation of glycogen synthase by cyclic AMP-independent casein kinase-2 from rabbit skeletal muscle. J Biol Chem. 1982 Mar 25;257(6):3236–3242. [Abstract] [Google Scholar]
- Hue L, Bontemps F, Hers H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem J. 1975 Oct;152(1):105–114. [Europe PMC free article] [Abstract] [Google Scholar]
- Itarte E, Mor A, Pena JM, Salavert A, Cussó R, Guinovart JJ. Cyclic AMP-independent glycogen synthase kinase from rat liver. FEBS Lett. 1979 May 15;101(2):347–350. [Abstract] [Google Scholar]
- Katz J, Golden S, Wals PA. Glycogen synthesis by rat hepatocytes. Biochem J. 1979 May 15;180(2):389–402. [Europe PMC free article] [Abstract] [Google Scholar]
- Kikuchi K, Tamura S, Hiraga A, Tsuiki S. Glycogen synthase phosphatase of rat liver. Its separation from phosphorylase phosphatase on DE-52 columns. Biochem Biophys Res Commun. 1977 Mar 7;75(1):29–37. [Abstract] [Google Scholar]
- Killilea SD, Brandt H, Lee EY, Whelan WJ. Evidence for the coordinate control of activity of liver glycogen synthase and phosphorylase by a single protein phosphatase. J Biol Chem. 1976 Apr 25;251(8):2363–2368. [Abstract] [Google Scholar]
- Laloux M, Hers HG. The role of phosphorylase in the inhibitory effect of EDTA and ATP on liver glycogen synthase phosphatase. Biochem Biophys Res Commun. 1979 Feb 14;86(3):762–768. [Abstract] [Google Scholar]
- Laloux M, Stalmans W, Hers HG. Native and latent forms of liver phosphorylase phosphatase. The non-identity of native phosphorylase phosphatase and synthase phosphatase. Eur J Biochem. 1978 Dec 1;92(1):15–24. [Abstract] [Google Scholar]
- Lee EY, Silberman SR, Ganapathi MK, Petrović S, Paris H. The phosphoprotein phosphatases: properties of the enzymes involved in the regulation of glycogen metabolism. Adv Cyclic Nucleotide Res. 1980;13:95–131. [Abstract] [Google Scholar]
- Nimmo HG, Proud CG, Cohen P. The purification and properties of rabbit skeletal muscle glycogen synthase. Eur J Biochem. 1976 Sep;68(1):21–30. [Abstract] [Google Scholar]
- Postle AD, Bloxham DP. The use of tritiated water to measure absolute rates of hepatic glycogen synthesis. Biochem J. 1980 Oct 15;192(1):65–73. [Europe PMC free article] [Abstract] [Google Scholar]
- Saugmann P, Esmann V. Glycogen metabolism: the integrated cellular response to a bi-directional metabolic stimulus. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1520–1527. [Abstract] [Google Scholar]
- Sobrino F, Hers HG. The inactivation of phosphorylase and activation of glycogen synthase in the adipose tissue. Eur J Biochem. 1980 Aug;109(1):239–246. [Abstract] [Google Scholar]
- Stalmans W. The role of the liver in the homeostasis of blood glucose. Curr Top Cell Regul. 1976;11:51–97. [Abstract] [Google Scholar]
- Stalmans W, Gevers G. The catalytic activity of phosphorylase b in the liver. With a note on the assay in the glycogenolytic direction. Biochem J. 1981 Nov 15;200(2):327–336. [Europe PMC free article] [Abstract] [Google Scholar]
- Stalmans W, Hers HG. The stimulation of liver phosphorylase b by AMP, fluoride and sulfate. A technical note on the specific determination of the a and b forms of liver glycogen phosphorylase. Eur J Biochem. 1975 Jun;54(2):341–350. [Abstract] [Google Scholar]
- Stalmans W, de Wulf H, Hers HG. The control of liver glycogen synthetase phosphatase by phosphorylase. Eur J Biochem. 1971 Feb;18(4):582–587. [Abstract] [Google Scholar]
- Stalmans W, De Wulf H, Hue L, Hers HG. The sequential inactivation of glycogen phosphorylase and activation of glycogen synthetase in liver after the administration of glucose to mice and rats. The mechanism of the hepatic threshold to glucose. Eur J Biochem. 1974 Jan 3;41(1):127–134. [Abstract] [Google Scholar]
- Stalmans W, Laloux M, Hers HG. The interaction of liver phosphorylase a with glucose and AMP. Eur J Biochem. 1974 Nov 15;49(2):415–427. [Abstract] [Google Scholar]
- Tan AW, Nuttall FQ. Evidence for the non-identity of proteins having synthase phosphatase, phosphorylase phosphatase and histone phosphatase activity in rat liver. Biochim Biophys Acta. 1978 Jan 12;522(1):139–150. [Abstract] [Google Scholar]
- Vandenheede JR, Yang SD, Goris J, Merlevede W. ATP x Mg-dependent protein phosphatase from rabbit skeletal muscle. II. Purification of the activating factor and its characterization as a bifunctional protein also displaying synthase kinase activity. J Biol Chem. 1980 Dec 25;255(24):11768–11774. [Abstract] [Google Scholar]
- Vandereycken G, Keppens S, De Wulf H. Ionic requirements for the inhibition of liver glycogen synthetase phosphatase by phosphorylase a. Arch Int Physiol Biochim. 1975 Dec;83(5):1013–1014. [Abstract] [Google Scholar]
- Vanstapel F, Doperé F, Stalmans W. The role of glycogen synthase phosphatase in the glucocorticoid-induced deposition of glycogen in foetal rat liver. Biochem J. 1980 Nov 15;192(2):607–612. [Europe PMC free article] [Abstract] [Google Scholar]
- Villar-Palasi C. Oligo- and polysaccharide inhibition of muscle transferase D phosphatase. Ann N Y Acad Sci. 1969 Oct 14;166(2):719–730. [Abstract] [Google Scholar]
- Wang P, Bantle G, Sorensen NB. Effect of metabolites and phosphorylase on the D to I conversion of glycogen synthase from human polymorphonuclear leukocytes. Biochim Biophys Acta. 1977 Feb 28;496(2):436–447. [Abstract] [Google Scholar]
- Yang SD, Vandenheede JR, Goris J, Merlevede W. ATP x Mg-dependent protein phosphatase from rabbit skeletal muscle. I. Purification of the enzyme and its regulation by the interaction with an activating protein factor. J Biol Chem. 1980 Dec 25;255(24):11759–11767. [Abstract] [Google Scholar]
Associated Data
Articles from Biochemical Journal are provided here courtesy of The Biochemical Society
Full text links
Read article at publisher's site: https://doi.org/10.1042/bj2120407
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1152061?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Immunochemical Study of the Effect of F2Glc on Glycogen Synthase Translocation and Glycogen Synthesis in Isolated Rat Hepatocytes.
Appl Biochem Biotechnol, 184(3):909-918, 16 Sep 2017
Cited by: 0 articles | PMID: 28918449
Fasting-induced protein phosphatase 1 regulatory subunit contributes to postprandial blood glucose homeostasis via regulation of hepatic glycogenesis.
Diabetes, 60(5):1435-1445, 06 Apr 2011
Cited by: 41 articles | PMID: 21471512 | PMCID: PMC3292316
Disruption of the allosteric phosphorylase a regulation of the hepatic glycogen-targeted protein phosphatase 1 improves glucose tolerance in vivo.
Cell Signal, 21(7):1123-1134, 09 Mar 2009
Cited by: 24 articles | PMID: 19275933
A novel glycogen-targeting subunit of protein phosphatase 1 that is regulated by insulin and shows differential tissue distribution in humans and rodents.
FEBS J, 272(6):1478-1489, 01 Mar 2005
Cited by: 49 articles | PMID: 15752363
Go to all (37) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Phosphorylase a is an allosteric inhibitor of the glycogen and microsomal forms of rat hepatic protein phosphatase-1.
FEBS Lett, 198(2):194-202, 01 Mar 1986
Cited by: 52 articles | PMID: 3007211
The hepatic defect in glycogen synthesis in chronic diabetes involves the G-component of synthase phosphatase.
Biochem J, 217(2):427-434, 01 Jan 1984
Cited by: 29 articles | PMID: 6320806 | PMCID: PMC1153233
Calcium ions and glycogen act synergistically as inhibitors of hepatic glycogen-synthase phosphatase.
Biochem J, 232(3):697-704, 01 Dec 1985
Cited by: 21 articles | PMID: 3004415 | PMCID: PMC1152941
Short-term hormonal control of protein phosphatases involved in hepatic glycogen metabolism.
Adv Enzyme Regul, 30:305-327, 01 Jan 1990
Cited by: 4 articles | PMID: 2169698
Review