Abstract
Free full text

Transient expression of genes introduced into cultured cells of Drosophila.
Abstract
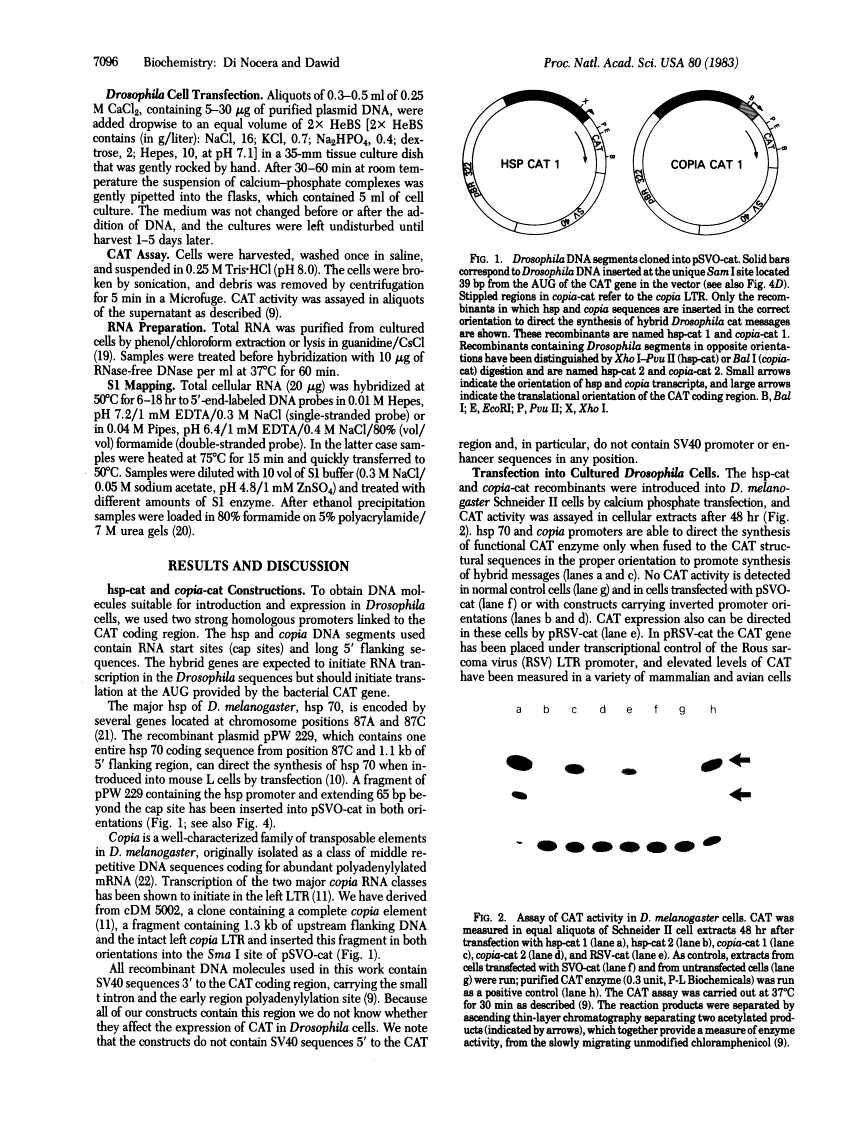
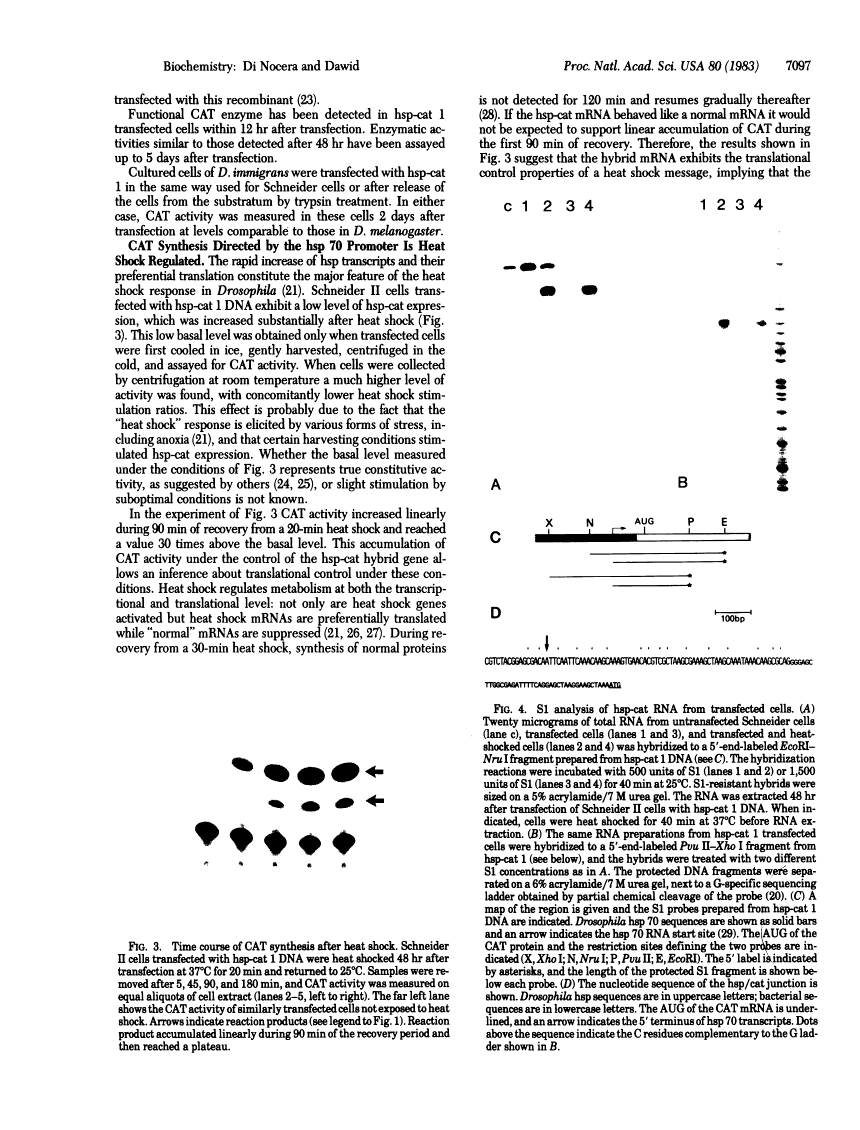
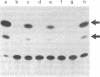
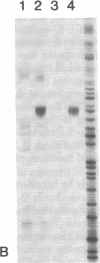
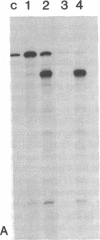
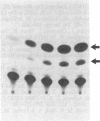
Recombinant plasmids in which the sequence encoding the bacterial chloramphenicol acetyltransferase (CAT; acetyl-CoA:chloramphenicol 3-O-acetyltransferase, EC 2.3.1.28) has been placed under the control of Drosophila heat shock protein 70 (hsp 70) or copia promoters have been introduced into cultured cells of two Drosophila species (Schneider II line of Drosophila melanogaster and D. immigrans) as calcium-phosphate complexes. Within 1-2 days after transfection functional CAT enzyme was detected in cells exposed to either CAT recombinant. The expression of the bacterial information depends on the activity of the Drosophila promoters because plasmids in which the Drosophila DNA fragments were fused to the CAT coding sequence in inverted orientation did not support the synthesis of CAT enzyme activity. Low levels of CAT activity and of hybrid mRNA were detected in cells transformed with hsp-cat recombinants when the cells were maintained at room temperature, and both mRNA levels and CAT activity increased substantially after a brief exposure to 37 degrees C. hsp-cat mRNA has the same 5' terminus as authentic Drosophila hsp 70 messenger. These experiments document a practical system for the introduction and expression of isolated genes in cultured cells of Drosophila.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Mandel M, Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. [Abstract] [Google Scholar]
- Hinnen A, Hicks JB, Fink GR. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. [Europe PMC free article] [Abstract] [Google Scholar]
- Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. [Abstract] [Google Scholar]
- Schaffner W. Direct transfer of cloned genes from bacteria to mammalian cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2163–2167. [Europe PMC free article] [Abstract] [Google Scholar]
- Gurdon JB, Melton DA. Gene transfer in amphibian eggs and oocytes. Annu Rev Genet. 1981;15:189–218. [Abstract] [Google Scholar]
- Capecchi MR. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980 Nov;22(2 Pt 2):479–488. [Abstract] [Google Scholar]
- Gallagher TM, Friesen PD, Rueckert RR. Autonomous replication and expression of RNA 1 from black beetle virus. J Virol. 1983 May;46(2):481–489. [Europe PMC free article] [Abstract] [Google Scholar]
- Gorman CM, Moffat LF, Howard BH. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. [Europe PMC free article] [Abstract] [Google Scholar]
- Corces V, Holmgren R, Freund R, Morimoto R, Meselson M. Four heat shock proteins of Drosophila melanogaster coded within a 12-kilobase region in chromosome subdivision 67B. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5390–5393. [Europe PMC free article] [Abstract] [Google Scholar]
- Flavell AJ, Levis R, Simon MA, Rubin GM. The 5' termini of RNAs encoded by the transposable element copia. Nucleic Acids Res. 1981 Dec 11;9(23):6279–6291. [Europe PMC free article] [Abstract] [Google Scholar]
- Grunstein M, Hogness DS. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. [Europe PMC free article] [Abstract] [Google Scholar]
- Birnboim HC, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. [Europe PMC free article] [Abstract] [Google Scholar]
- Katz L, Kingsbury DT, Helinski DR. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. [Europe PMC free article] [Abstract] [Google Scholar]
- Hoopes BC, McClure WR. Studies on the selectivity of DNA precipitation by spermine. Nucleic Acids Res. 1981 Oct 24;9(20):5493–5504. [Europe PMC free article] [Abstract] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972 Apr;27(2):353–365. [Abstract] [Google Scholar]
- Ullrich A, Shine J, Chirgwin J, Pictet R, Tischer E, Rutter WJ, Goodman HM. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. [Abstract] [Google Scholar]
- Maxam AM, Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. [Abstract] [Google Scholar]
- Finnegan DJ, Rubin GM, Young MW, Hogness DS. Repeated gene families in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1053–1063. [Abstract] [Google Scholar]
- Gorman CM, Merlino GT, Willingham MC, Pastan I, Howard BH. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. [Europe PMC free article] [Abstract] [Google Scholar]
- Findly RC, Pederson T. Regulated transcription of the genes for actin and heat-shock proteins in cultured Drosophila cells. J Cell Biol. 1981 Feb;88(2):323–328. [Europe PMC free article] [Abstract] [Google Scholar]
- Kloetzel PM, Bautz EK. Heat-shock proteins are associated with hnRNA in Drosophila melanogaster tissue culture cells. EMBO J. 1983;2(5):705–710. [Europe PMC free article] [Abstract] [Google Scholar]
- Lindquist S. Translational efficiency of heat-induced messages in Drosophila melanogaster cells. J Mol Biol. 1980 Feb 25;137(2):151–158. [Abstract] [Google Scholar]
- Storti RV, Scott MP, Rich A, Pardue ML. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. [Abstract] [Google Scholar]
- DiDomenico BJ, Bugaisky GE, Lindquist S. Heat shock and recovery are mediated by different translational mechanisms. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6181–6185. [Europe PMC free article] [Abstract] [Google Scholar]
- Török I, Karch F. Nucleotide sequences of heat shock activated genes in Drosophila melanogaster. I. Sequences in the regions of the 5' and 3' ends of the hsp 70 gene in the hybrid plasmid 56H8. Nucleic Acids Res. 1980 Jul 25;8(14):3105–3123. [Europe PMC free article] [Abstract] [Google Scholar]
- Pelham HR. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. [Abstract] [Google Scholar]
- Bourouis M, Jarry B. Vectors containing a prokaryotic dihydrofolate reductase gene transform Drosophila cells to methotrexate-resistance. EMBO J. 1983;2(7):1099–1104. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.80.23.7095
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc389999?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Transfection of Sponge Cells and Intracellular Localization of Cancer-Related MYC, RRAS2, and DRG1 Proteins.
Mar Drugs, 21(2):119, 10 Feb 2023
Cited by: 2 articles | PMID: 36827160 | PMCID: PMC9964533
1,25-dihydroxyvitamin D3 and its nuclear receptor repress human α1 (I) collagen expression and type I collagen formation.
Liver Int, 33(5):677-686, 17 Feb 2013
Cited by: 41 articles | PMID: 23413886 | PMCID: PMC3707129
The Birt-Hogg-Dubé tumor suppressor Folliculin negatively regulates ribosomal RNA synthesis.
Hum Mol Genet, 22(2):284-299, 16 Oct 2012
Cited by: 9 articles | PMID: 23077212 | PMCID: PMC3526160
Reconstitution of a frizzled8.Wnt3a.LRP6 signaling complex reveals multiple Wnt and Dkk1 binding sites on LRP6.
J Biol Chem, 285(12):9172-9179, 21 Jan 2010
Cited by: 133 articles | PMID: 20093360 | PMCID: PMC2838336
New aminoacyl-tRNA synthetase-like protein in insecta with an essential mitochondrial function.
J Biol Chem, 285(49):38157-38166, 24 Sep 2010
Cited by: 14 articles | PMID: 20870726 | PMCID: PMC2992249
Go to all (168) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Transient expression of Drosophila melanogaster rDNA promoter into cultured Drosophila cells.
Nucleic Acids Res, 14(16):6417-6432, 01 Aug 1986
Cited by: 13 articles | PMID: 3092185 | PMCID: PMC311655
Expression of Drosophila hsp 70-CAT hybrid gene in Aedes cells induced by heat shock.
Somat Cell Mol Genet, 11(4):371-377, 01 Jul 1985
Cited by: 12 articles | PMID: 3927494
Transient expression of the chloramphenicol acetyltransferase gene in cultured mosquito cells.
Gene, 36(1-2):173-178, 01 Jan 1985
Cited by: 20 articles | PMID: 3864716
Expression of the prokaryotic gene for chloramphenicol acetyl transferase in Drosophila under the control of larval serum protein 1 gene promoters.
J Mol Biol, 189(1):13-24, 01 May 1986
Cited by: 16 articles | PMID: 3097322