Abstract
Unlabelled
Upper body obesity (UB Ob) is associated with a reduced net free fatty acid (FFA) response to epinephrine compared with nonobese (Non Ob) and lower-body obese (LB Ob) women. Because catecholamines regulate some of the metabolic responses to exercise, we hypothesized that UB Ob would have a reduced net FFA response to exercise. Plasma FFA rate of appearance (Ra) ([1-14C]palmitate) and fatty acid oxidation (indirect calorimetry) were therefore measured during 2.5 h of stationary bicycle exercise (45% VO2 peak) in 13 UB Ob, 11 LB Ob, and 8 Non Ob premenopausal women. 10 UB Ob and 8 LB Ob women were retested after an approximately 8-kg weight loss.Results
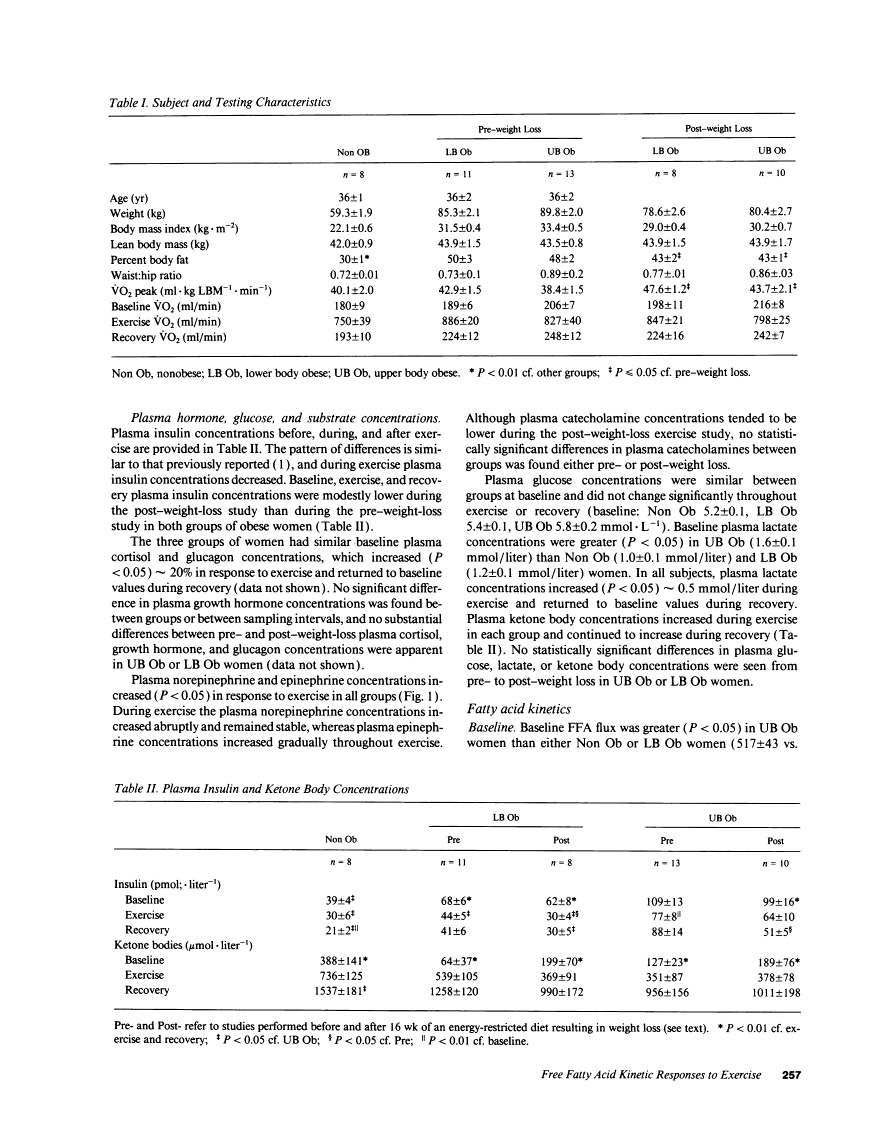
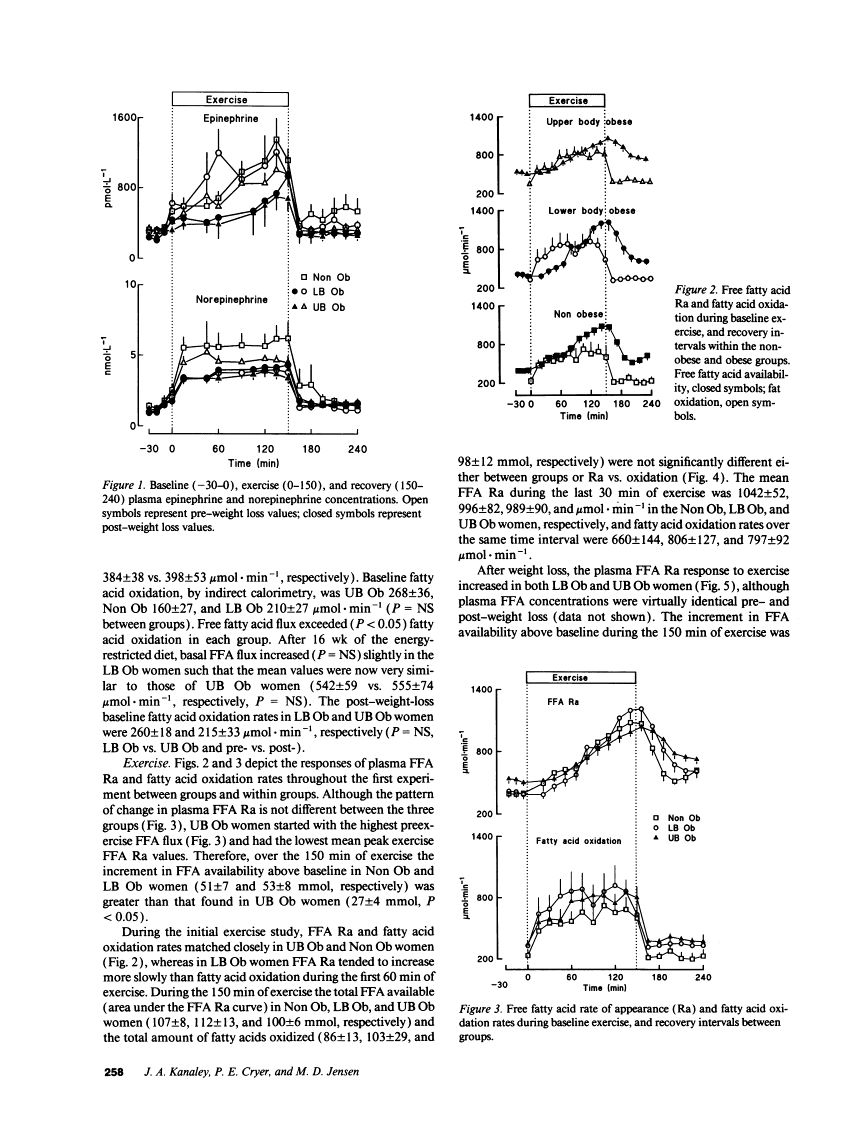
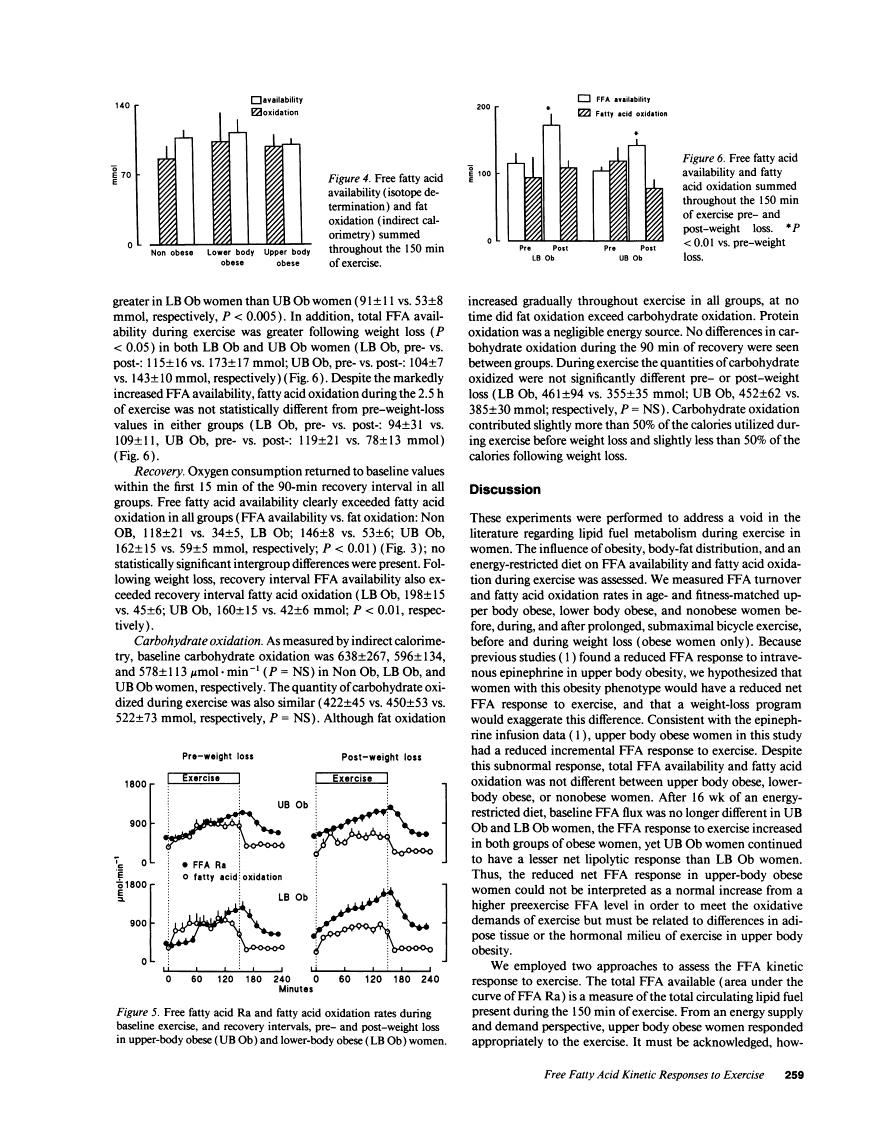
During exercise Non Ob and LB Ob women had greater increments in FFA availability (51 +/- 7 and 53 +/- 8 mmol, respectively) than UB Ob women (27 +/- 4 mmol, P < 0.05). Total exercise FFA availability and fatty acid oxidation were not different between Non Ob, LB Ob, and UB Ob women, however. Following weight loss (approximately 8 kg), the FFA response to exercise increased (P < 0.01) and remained greater (P < 0.05) in LB Ob than in UB Ob women. In conclusion, the FFA response to exercise was reduced in UB Ob women before and after weight loss, but no effects on fatty acid oxidation were apparent.Free full text

Fatty acid kinetic responses to exercise. Effects of obesity, body fat distribution, and energy-restricted diet.
Abstract
Upper body obesity (UB Ob) is associated with a reduced net free fatty acid (FFA) response to epinephrine compared with nonobese (Non Ob) and lower-body obese (LB Ob) women. Because catecholamines regulate some of the metabolic responses to exercise, we hypothesized that UB Ob would have a reduced net FFA response to exercise. Plasma FFA rate of appearance (Ra) ([1-14C]palmitate) and fatty acid oxidation (indirect calorimetry) were therefore measured during 2.5 h of stationary bicycle exercise (45% VO2 peak) in 13 UB Ob, 11 LB Ob, and 8 Non Ob premenopausal women. 10 UB Ob and 8 LB Ob women were retested after an approximately 8-kg weight loss. Results: During exercise Non Ob and LB Ob women had greater increments in FFA availability (51 +/- 7 and 53 +/- 8 mmol, respectively) than UB Ob women (27 +/- 4 mmol, P < 0.05). Total exercise FFA availability and fatty acid oxidation were not different between Non Ob, LB Ob, and UB Ob women, however. Following weight loss (approximately 8 kg), the FFA response to exercise increased (P < 0.01) and remained greater (P < 0.05) in LB Ob than in UB Ob women. In conclusion, the FFA response to exercise was reduced in UB Ob women before and after weight loss, but no effects on fatty acid oxidation were apparent.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Jensen MD, Haymond MW, Rizza RA, Cryer PE, Miles JM. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest. 1989 Apr;83(4):1168–1173. [Europe PMC free article] [Abstract] [Google Scholar]
- Galster AD, Clutter WE, Cryer PE, Collins JA, Bier DM. Epinephrine plasma thresholds for lipolytic effects in man: measurements of fatty acid transport with [l-13C]palmitic acid. J Clin Invest. 1981 Jun;67(6):1729–1738. [Europe PMC free article] [Abstract] [Google Scholar]
- Wahrenberg H, Engfeldt P, Bolinder J, Arner P. Acute adaptation in adrenergic control of lipolysis during physical exercise in humans. Am J Physiol. 1987 Oct;253(4 Pt 1):E383–E390. [Abstract] [Google Scholar]
- Marker JC, Hirsch IB, Smith LJ, Parvin CA, Holloszy JO, Cryer PE. Catecholamines in prevention of hypoglycemia during exercise in humans. Am J Physiol. 1991 May;260(5 Pt 1):E705–E712. [Abstract] [Google Scholar]
- Wood PD, Stefanick ML, Williams PT, Haskell WL. The effects on plasma lipoproteins of a prudent weight-reducing diet, with or without exercise, in overweight men and women. N Engl J Med. 1991 Aug 15;325(7):461–466. [Abstract] [Google Scholar]
- HAVEL RJ, NAIMARK A, BORCHGREVINK CF. Turnover rate and oxidation of free fatty acids of blood plasma in man during exercise: studies during continuous infusion of palmitate-1-C14. J Clin Invest. 1963 Jul;42:1054–1063. [Europe PMC free article] [Abstract] [Google Scholar]
- Ahlborg G, Felig P, Hagenfeldt L, Hendler R, Wahren J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest. 1974 Apr;53(4):1080–1090. [Europe PMC free article] [Abstract] [Google Scholar]
- Gollnick PD. Free fatty acid turnover and the availability of substrates as a limiting factor in prolonged exercise. Ann N Y Acad Sci. 1977;301:64–71. [Abstract] [Google Scholar]
- Wolfe RR, Klein S, Carraro F, Weber JM. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am J Physiol. 1990 Feb;258(2 Pt 1):E382–E389. [Abstract] [Google Scholar]
- Tarnopolsky LJ, MacDougall JD, Atkinson SA, Tarnopolsky MA, Sutton JR. Gender differences in substrate for endurance exercise. J Appl Physiol (1985) 1990 Jan;68(1):302–308. [Abstract] [Google Scholar]
- Issekutz B, Jr, Bortz WM, Miller HI, Wroldsen A. Plasma free fatty acid response to exercise in obese humans. Metabolism. 1967 Jun;16(6):492–502. [Abstract] [Google Scholar]
- Minuk HL, Hanna AK, Marliss EB, Vranic M, Zinman B. Metabolic response to moderate exercise in obese man during prolonged fasting. Am J Physiol. 1980 Apr;238(4):E322–E329. [Abstract] [Google Scholar]
- Scheen A, Cession-Fossion A, Scheen-Lavigne M, Luyckx A. Effect of protein-supplemented fasting on metabolic and hormonal responses to epinephrine infusion in obese subjects. Horm Metab Res. 1982 May;14(5):240–245. [Abstract] [Google Scholar]
- Jensen MD, Haymond MW, Gerich JE, Cryer PE, Miles JM. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. J Clin Invest. 1987 Jan;79(1):207–213. [Europe PMC free article] [Abstract] [Google Scholar]
- Miles JM, Ellman MG, McClean KL, Jensen MD. Validation of a new method for determination of free fatty acid turnover. Am J Physiol. 1987 Mar;252(3 Pt 1):E431–E438. [Abstract] [Google Scholar]
- Jensen MD, Rogers PJ, Ellman MG, Miles JM. Choice of infusion-sampling mode for tracer studies of free fatty acid metabolism. Am J Physiol. 1988 May;254(5 Pt 1):E562–E565. [Abstract] [Google Scholar]
- Herbert V, Lau KS, Gottlieb CW, Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. [Abstract] [Google Scholar]
- Shah SD, Clutter WE, Cryer PE. External and internal standards in the single-isotope derivative (radioenzymatic) measurement of plasma norepinephrine and epinephrine. J Lab Clin Med. 1985 Dec;106(6):624–629. [Abstract] [Google Scholar]
- Cahill GF, Jr, Herrera MG, Morgan AP, Soeldner JS, Steinke J, Levy PL, Reichard GA, Jr, Kipnis DM. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. [Europe PMC free article] [Abstract] [Google Scholar]
- Jensen MD, Braun JS, Vetter RJ, Marsh HM. Measurement of body potassium with a whole-body counter: relationship between lean body mass and resting energy expenditure. Mayo Clin Proc. 1988 Sep;63(9):864–868. [Abstract] [Google Scholar]
- Jensen MD, Heiling V, Miles JM. Measurement of non-steady-state free fatty acid turnover. Am J Physiol. 1990 Jan;258(1 Pt 1):E103–E108. [Abstract] [Google Scholar]
- Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):628–634. [Abstract] [Google Scholar]
- HAVEL RJ, CARLSON LA, EKELUND LG, HOLMGREN A. TURNOVER RATE AND OXIDATION OF DIFFERENT FREE FATTY ACIDS IN MAN DURING EXERCISE. J Appl Physiol. 1964 Jul;19:613–618. [Abstract] [Google Scholar]
- Havel RJ, Ekelund LG, Holmgren A. Kinetic analysis of the oxidation of palmitate-1-14C in man during prolonged heavy muscular exercise. J Lipid Res. 1967 Jul;8(4):366–373. [Abstract] [Google Scholar]
- Björntorp P, Bergman H, Varnauskas E. Plasma free fatty acid turnover rate in obesity. Acta Med Scand. 1969 Apr;185(4):351–356. [Abstract] [Google Scholar]
- Basso LV, Havel RJ. Hepatic metabolism of free fatty acids in normal and diabetic dogs. J Clin Invest. 1970 Mar;49(3):537–547. [Europe PMC free article] [Abstract] [Google Scholar]
- Martin ML, Jensen MD. Effects of body fat distribution on regional lipolysis in obesity. J Clin Invest. 1991 Aug;88(2):609–613. [Europe PMC free article] [Abstract] [Google Scholar]
- Wahren J, Sato Y, Ostman J, Hagenfeldt L, Felig P. Turnover and splanchnic metabolism of free fatty acids and ketones in insulin-dependent diabetics at rest and in response to exercise. J Clin Invest. 1984 May;73(5):1367–1376. [Europe PMC free article] [Abstract] [Google Scholar]
- Jones NL, Heigenhauser GJ, Kuksis A, Matsos CG, Sutton JR, Toews CJ. Fat metabolism in heavy exercise. Clin Sci (Lond) 1980 Dec;59(6):469–478. [Abstract] [Google Scholar]
- Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol. 1984 Apr;56(4):831–838. [Abstract] [Google Scholar]
- Koivisto V, Hendler R, Nadel E, Felig P. Influence of physical training on the fuel-hormone response to prolonged low intensity exercise. Metabolism. 1982 Feb;31(2):192–197. [Abstract] [Google Scholar]
- Hirsch IB, Marker JC, Smith LJ, Spina RJ, Parvin CA, Holloszy JO, Cryer PE. Insulin and glucagon in prevention of hypoglycemia during exercise in humans. Am J Physiol. 1991 May;260(5 Pt 1):E695–E704. [Abstract] [Google Scholar]
- Bukowiecki L, Lupien J, Follea N, Paradis A, Richard D, LeBlanc J. Mechanism of enhanced lipolysis in adipose tissue of exercise-trained rats. Am J Physiol. 1980 Dec;239(6):E422–E429. [Abstract] [Google Scholar]
- Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, Adams PW. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982 Feb;54(2):254–260. [Abstract] [Google Scholar]
Associated Data
Articles from The Journal of Clinical Investigation are provided here courtesy of American Society for Clinical Investigation
Full text links
Read article at publisher's site: https://doi.org/10.1172/jci116559
Read article for free, from open access legal sources, via Unpaywall:
http://www.jci.org/articles/view/116559/files/pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
β-Cell function during a high-fat meal in young versus old adults: role of exercise.
Am J Physiol Regul Integr Comp Physiol, 325(2):R164-R171, 12 Jun 2023
Cited by: 0 articles | PMID: 37306399 | PMCID: PMC10393366
Early chronotype with metabolic syndrome favours resting and exercise fat oxidation in relation to insulin-stimulated non-oxidative glucose disposal.
Exp Physiol, 107(11):1255-1264, 19 Sep 2022
Cited by: 9 articles | PMID: 36123314 | PMCID: PMC9633545
Metabolic profile in women differs between high versus low energy spenders during a low intensity exercise on a cycle-desk.
Sci Rep, 12(1):9928, 15 Jun 2022
Cited by: 2 articles | PMID: 35705612 | PMCID: PMC9200836
Similar rates of fat oxidation during graded submaximal exercise in women of different body composition.
PLoS One, 15(11):e0242551, 18 Nov 2020
Cited by: 2 articles | PMID: 33206727 | PMCID: PMC7673546
Effect of High-Intensity Interval Training on Body Composition, Cardiorespiratory Fitness, Blood Pressure, and Substrate Utilization During Exercise Among Prehypertensive and Hypertensive Patients With Excessive Adiposity.
Front Physiol, 11:558910, 19 Oct 2020
Cited by: 7 articles | PMID: 33192554 | PMCID: PMC7604322
Go to all (44) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Effects of body fat distribution on regional lipolysis in obesity.
J Clin Invest, 88(2):609-613, 01 Aug 1991
Cited by: 151 articles | PMID: 1864970 | PMCID: PMC295396
Regional postprandial fatty acid metabolism in different obesity phenotypes.
Diabetes, 48(8):1586-1592, 01 Aug 1999
Cited by: 139 articles | PMID: 10426377
The effect of low-intensity exercise training on fat metabolism of obese women.
Obes Res, 9(2):86-96, 01 Feb 2001
Cited by: 31 articles | PMID: 11316351
Fate of fatty acids at rest and during exercise: regulatory mechanisms.
Acta Physiol Scand, 178(4):385-390, 01 Aug 2003
Cited by: 44 articles | PMID: 12864743
Review
Funding
Funders who supported this work.
NCRR NIH HHS (1)
Grant ID: RR-00035
NIDDK NIH HHS (2)
Grant ID: DK1-107352
Grant ID: DK1-140484