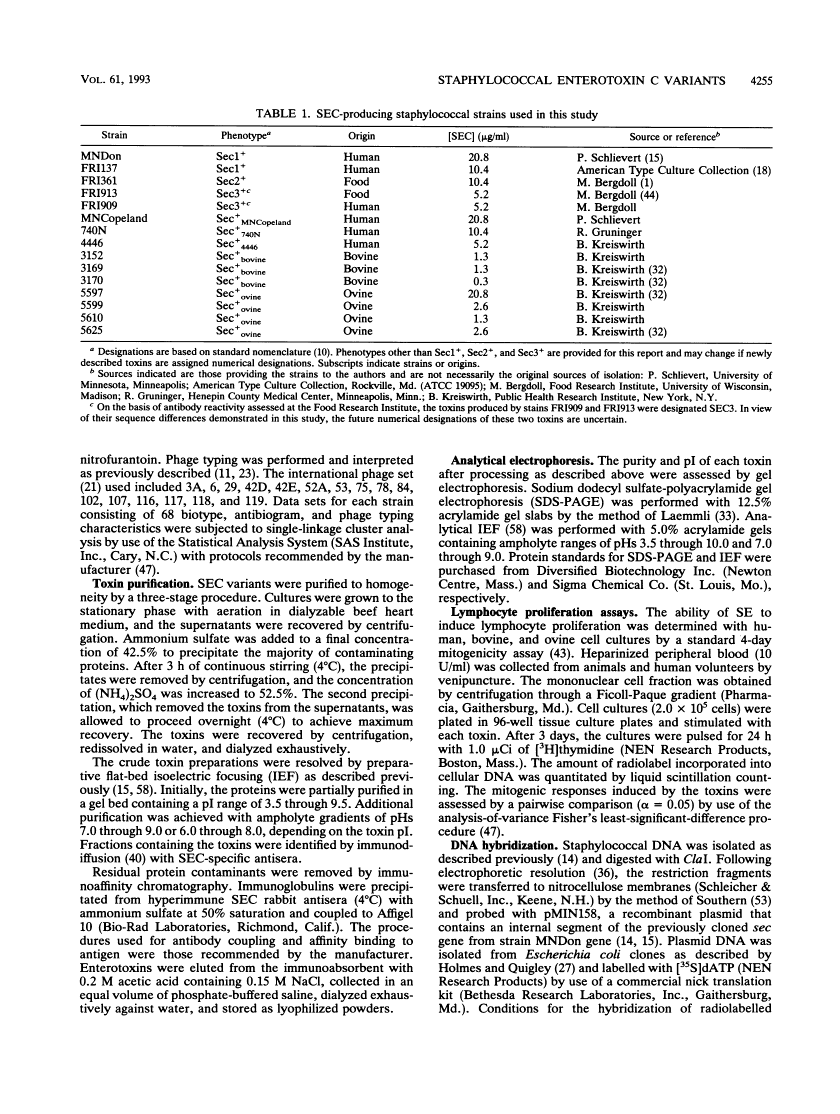
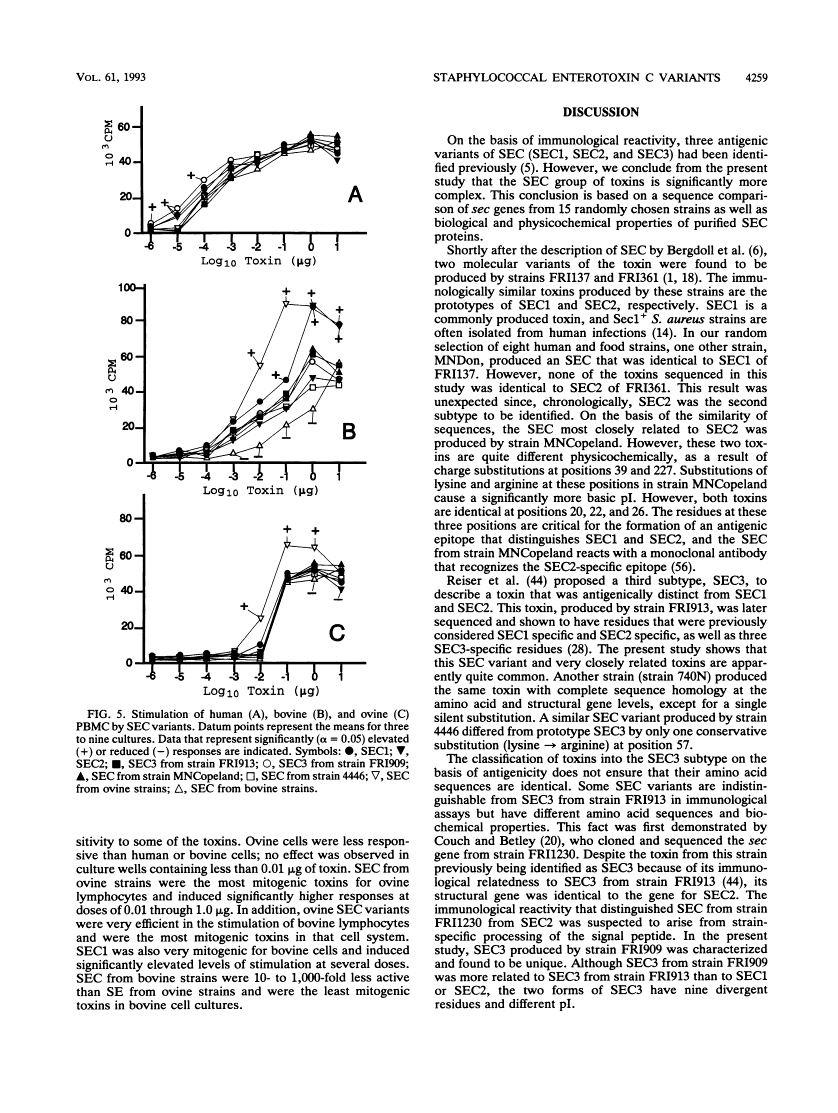
Abstract
Free full text

Characterization of novel type C staphylococcal enterotoxins: biological and evolutionary implications.
Abstract
The type C staphylococcal enterotoxins (SEC) are a group of highly conserved proteins with significant immunological cross-reactivity. Although three antigenically distinct SEC subtypes (SEC1, SEC2, and SEC3) have been reported in the literature, we observed that the isoelectric points of SEC from several Staphylococcus aureus isolates are different from those of any of these three subtypes. This observation led us to propose that additional SEC molecular variants exist. For assessment of this possibility, the sec genes from representative human, animal, and food isolates were cloned and sequenced. The toxins encoded by the 18 isolates used in this study included five unique SEC proteins in addition to SEC1, SEC2, and SEC3. Six of the SEC proteins (including SEC1, SEC2, and SEC3) were produced by human and food isolates. Analysis of seven bovine and ovine isolates showed that isolates from each animal species produced a unique host-specific SEC. All of the SEC caused lymphocyte proliferation, although some of the toxins differed in their ability to stimulate cells from several animal species. An explanation for these results, which is supported by our phenotypic analysis of Sec+ staphylococcal isolates, is that toxin heterogeneity is due to selection for modified SEC sequences that facilitate the survival of S. aureus isolates in their respective hosts.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.9M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avena RM, Bergdoll MS. Purification and some physicochemical properties of enterotoxin C, Staphylococcus aureus strain 361. Biochemistry. 1967 May;6(5):1474–1480. [Abstract] [Google Scholar]
- Barsumian EL, Cunningham CM, Schlievert PM, Watson DW. Heterogeneity of group A streptococcal pyrogenic exotoxin type B. Infect Immun. 1978 May;20(2):512–518. [Europe PMC free article] [Abstract] [Google Scholar]
- Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [Abstract] [Google Scholar]
- Bayles KW, Iandolo JJ. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J Bacteriol. 1989 Sep;171(9):4799–4806. [Europe PMC free article] [Abstract] [Google Scholar]
- Bergdoll MS, Borja CR, Avena RM. Identification of a new enterotoxin as enterotoxin C. J Bacteriol. 1965 Nov;90(5):1481–1485. [Europe PMC free article] [Abstract] [Google Scholar]
- Bergdoll MS, Robbins RN, Weiss K, Borja CR, Huang Y, Chu FS. The staphylococcal enterotoxins: similarities. Contrib Microbiol Immunol. 1973;1:390–396. [Abstract] [Google Scholar]
- Betley MJ, Mekalanos JJ. Staphylococcal enterotoxin A is encoded by phage. Science. 1985 Jul 12;229(4709):185–187. [Abstract] [Google Scholar]
- Bohach GA, Fast DJ, Nelson RD, Schlievert PM. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17(4):251–272. [Abstract] [Google Scholar]
- Bohach GA, Hovde CJ, Handley JP, Schlievert PM. Cross-neutralization of staphylococcal and streptococcal pyrogenic toxins by monoclonal and polyclonal antibodies. Infect Immun. 1988 Feb;56(2):400–404. [Europe PMC free article] [Abstract] [Google Scholar]
- Bohach GA, Kreiswirth BN, Novick RP, Schlievert PM. Analysis of toxic shock syndrome isolates producing staphylococcal enterotoxins B and C1 with use of southern hybridization and immunologic assays. Rev Infect Dis. 1989 Jan-Feb;11 (Suppl 1):S75–S82. [Abstract] [Google Scholar]
- Bohach GA, Schlievert PM. Expression of staphylococcal enterotoxin C1 in Escherichia coli. Infect Immun. 1987 Feb;55(2):428–432. [Europe PMC free article] [Abstract] [Google Scholar]
- Bohach GA, Schlievert PM. Nucleotide sequence of the staphylococcal enterotoxin C1 gene and relatedness to other pyrogenic toxins. Mol Gen Genet. 1987 Aug;209(1):15–20. [Abstract] [Google Scholar]
- Bohach GA, Schlievert PM. Conservation of the biologically active portions of staphylococcal enterotoxins C1 and C2. Infect Immun. 1989 Jul;57(7):2249–2252. [Europe PMC free article] [Abstract] [Google Scholar]
- Borja CR, Bergdoll MS. Purification and partial characterization of enterotoxin C produced by Staphylococcus aureus strain 137. Biochemistry. 1967 May;6(5):1467–1473. [Abstract] [Google Scholar]
- Choi YW, Kotzin B, Herron L, Callahan J, Marrack P, Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. [Europe PMC free article] [Abstract] [Google Scholar]
- Couch JL, Betley MJ. Nucleotide sequence of the type C3 staphylococcal enterotoxin gene suggests that intergenic recombination causes antigenic variation. J Bacteriol. 1989 Aug;171(8):4507–4510. [Europe PMC free article] [Abstract] [Google Scholar]
- Hewick RM, Hunkapiller MW, Hood LE, Dreyer WJ. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [Abstract] [Google Scholar]
- Ho G, Campbell WH, Carlson E. Ovine-associated Staphylococcus aureus protein with immunochemical similarity to toxic shock syndrome toxin 1. J Clin Microbiol. 1989 Jan;27(1):210–212. [Europe PMC free article] [Abstract] [Google Scholar]
- Holmes DS, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. [Abstract] [Google Scholar]
- Hovde CJ, Hackett SP, Bohach GA. Nucleotide sequence of the staphylococcal enterotoxin C3 gene: sequence comparison of all three type C staphylococcal enterotoxins. Mol Gen Genet. 1990 Jan;220(2):329–333. [Abstract] [Google Scholar]
- Hynes WL, Weeks CR, Iandolo JJ, Ferretti JJ. Immunologic cross-reactivity of type A streptococcal exotoxin (erythrogenic toxin) and staphylococcal enterotoxins B and C1. Infect Immun. 1987 Mar;55(3):837–838. [Europe PMC free article] [Abstract] [Google Scholar]
- Johnson LP, Tomai MA, Schlievert PM. Bacteriophage involvement in group A streptococcal pyrogenic exotoxin A production. J Bacteriol. 1986 May;166(2):623–627. [Europe PMC free article] [Abstract] [Google Scholar]
- Kim YB, Watson DW. A purified group A streptococcal pyrogenic exotoxin. Physiochemical and biological properties including the enhancement of susceptibility to endotoxin lethal shock. J Exp Med. 1970 Mar 1;131(3):611–622. [Europe PMC free article] [Abstract] [Google Scholar]
- Kreiswirth BN, Projan SJ, Schlievert PM, Novick RP. Toxic shock syndrome toxin 1 is encoded by a variable genetic element. Rev Infect Dis. 1989 Jan-Feb;11 (Suppl 1):S83–S89. [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Lee PK, Kreiswirth BN, Deringer JR, Projan SJ, Eisner W, Smith BL, Carlson E, Novick RP, Schlievert PM. Nucleotide sequences and biologic properties of toxic shock syndrome toxin 1 from ovine- and bovine-associated Staphylococcus aureus. J Infect Dis. 1992 Jun;165(6):1056–1063. [Abstract] [Google Scholar]
- Metzger JF, Johnson AD, Spero L. Intrinsic and chemically produced microheterogeneity of Staphylococcus aureus enterotoxin type C. Infect Immun. 1975 Jul;12(1):93–97. [Europe PMC free article] [Abstract] [Google Scholar]
- Mollick JA, Cook RG, Rich RR. Class II MHC molecules are specific receptors for staphylococcus enterotoxin A. Science. 1989 May 19;244(4906):817–820. [Abstract] [Google Scholar]
- Musser JM, Schlievert PM, Chow AW, Ewan P, Kreiswirth BN, Rosdahl VT, Naidu AS, Witte W, Selander RK. A single clone of Staphylococcus aureus causes the majority of cases of toxic shock syndrome. Proc Natl Acad Sci U S A. 1990 Jan;87(1):225–229. [Europe PMC free article] [Abstract] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. [Abstract] [Google Scholar]
- Park YH, Fox LK, Hamilton MJ, Davis WC. Bovine mononuclear leukocyte subpopulations in peripheral blood and mammary gland secretions during lactation. J Dairy Sci. 1992 Apr;75(4):998–1006. [Abstract] [Google Scholar]
- Park YH, Fox LK, Hamilton MJ, Davis WC. Suppression of proliferative response of BoCD4+ T lymphocytes by activated BoCD8+ T lymphocytes in the mammary gland of cows with Staphylococcus aureus mastitis. Vet Immunol Immunopathol. 1993 Mar;36(2):137–151. [Abstract] [Google Scholar]
- Poindexter NJ, Schlievert PM. Toxic-shock-syndrome toxin 1-induced proliferation of lymphocytes: comparison of the mitogenic response of human, murine, and rabbit lymphocytes. J Infect Dis. 1985 Jan;151(1):65–72. [Abstract] [Google Scholar]
- Reiser RF, Robbins RN, Noleto AL, Khoe GP, Bergdoll MS. Identification, purification, and some physicochemical properties of staphylococcal enterotoxin C3. Infect Immun. 1984 Sep;45(3):625–630. [Europe PMC free article] [Abstract] [Google Scholar]
- Roberson JR, Fox LK, Hancock DD, Besser TE. Evaluation of methods for differentiation of coagulase-positive staphylococci. J Clin Microbiol. 1992 Dec;30(12):3217–3219. [Europe PMC free article] [Abstract] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. [Europe PMC free article] [Abstract] [Google Scholar]
- Schlievert PM. Purification and characterization of staphylococcal pyrogenic exotoxin type B. Biochemistry. 1980 Dec 23;19(26):6204–6208. [Abstract] [Google Scholar]
- Schlievert PM, Blomster DA. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J Infect Dis. 1983 Feb;147(2):236–242. [Abstract] [Google Scholar]
- Schlievert PM, Schoettle DJ, Watson DW. Purification and physicochemical and biological characterization of a staphylococcal pyrogenic exotoxin. Infect Immun. 1979 Mar;23(3):609–617. [Europe PMC free article] [Abstract] [Google Scholar]
- Shafer WM, Iandolo JJ. Genetics of staphylococcal enterotoxin B in methicillin-resistant isolates of Staphylococcus aureus. Infect Immun. 1979 Sep;25(3):902–911. [Europe PMC free article] [Abstract] [Google Scholar]
- Smith HO. Recovery of DNA from gels. Methods Enzymol. 1980;65(1):371–380. [Abstract] [Google Scholar]
- Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. [Abstract] [Google Scholar]
- Spero L, Morlock BA. Cross-reactions between tryptic polypeptides of staphylococcal enterotoxins B and C. J Immunol. 1979 Apr;122(4):1285–1289. [Abstract] [Google Scholar]
- Thompson NE, Ketterhagen MJ, Bergdoll MS. Monoclonal antibodies to staphylococcal enterotoxins B and C: cross-reactivity and localization of epitopes on tryptic fragments. Infect Immun. 1984 Jul;45(1):281–285. [Europe PMC free article] [Abstract] [Google Scholar]
- Turner TN, Smith CL, Bohach GA. Residues 20, 22, and 26 determine the subtype specificities of staphylococcal enterotoxins C1 and C2. Infect Immun. 1992 Feb;60(2):694–697. [Europe PMC free article] [Abstract] [Google Scholar]
- WATSON DW. Host-parasite factors in group A streptococcal infections. Pyrogenic and other effects of immunologic distinct exotoxins related to scarlet fever toxins. J Exp Med. 1960 Feb 1;111:255–284. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.61.10.4254-4262.1993
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/iai/61/10/4254.full.pdf
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/reprint/61/10/4254
Free to read at iai.asm.org
http://iai.asm.org/cgi/content/abstract/61/10/4254
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/iai.61.10.4254-4262.1993
Article citations
Staphylococcal enterotoxin C as a novel strategy for treating lumbar spondylolysis in adolescents: Description of technique.
Medicine (Baltimore), 102(39):e35224, 01 Sep 2023
Cited by: 0 articles | PMID: 37773848 | PMCID: PMC10545280
Bidirectional Functional Effects of Staphylococcus on Carcinogenesis.
Microorganisms, 10(12):2353, 28 Nov 2022
Cited by: 6 articles | PMID: 36557606 | PMCID: PMC9783839
Review Free full text in Europe PMC
Effect of Temperature on the Expression of Classical Enterotoxin Genes among Staphylococci Associated with Bovine Mastitis.
Pathogens, 10(8):975, 02 Aug 2021
Cited by: 4 articles | PMID: 34451439 | PMCID: PMC8398761
Mild Lactic Acid Stress Causes Strain-Dependent Reduction in SEC Protein Levels.
Microorganisms, 9(5):1014, 08 May 2021
Cited by: 3 articles | PMID: 34066749 | PMCID: PMC8151770
Staphylococcal Enterotoxin C Subtypes Are Differentially Associated with Human Infections and Immunobiological Activities.
mSphere, 6(1):e01153-20, 27 Jan 2021
Cited by: 4 articles | PMID: 33504664 | PMCID: PMC7885323
Go to all (52) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Vbeta-dependent stimulation of bovine and human T cells by host-specific staphylococcal enterotoxins.
Infect Immun, 65(10):4048-4054, 01 Oct 1997
Cited by: 28 articles | PMID: 9317006 | PMCID: PMC175582
Characterization of the canine type C enterotoxin produced by Staphylococcus intermedius pyoderma isolates.
Infect Immun, 65(6):2346-2352, 01 Jun 1997
Cited by: 35 articles | PMID: 9169773 | PMCID: PMC175325
Subtype-specific interactions of type C staphylococcal enterotoxins with the T-cell receptor.
Mol Microbiol, 22(3):523-534, 01 Nov 1996
Cited by: 30 articles | PMID: 8939435
Immunoregulatory effects of superantigens: interactions of staphylococcal enterotoxins with host MHC and non-MHC products.
Immunol Rev, 131:27-42, 01 Feb 1993
Cited by: 18 articles | PMID: 8486393
Review