Abstract
Free full text

Biochemical evidence that starch breakdown by Bacteroides thetaiotaomicron involves outer membrane starch-binding sites and periplasmic starch-degrading enzymes.
Abstract
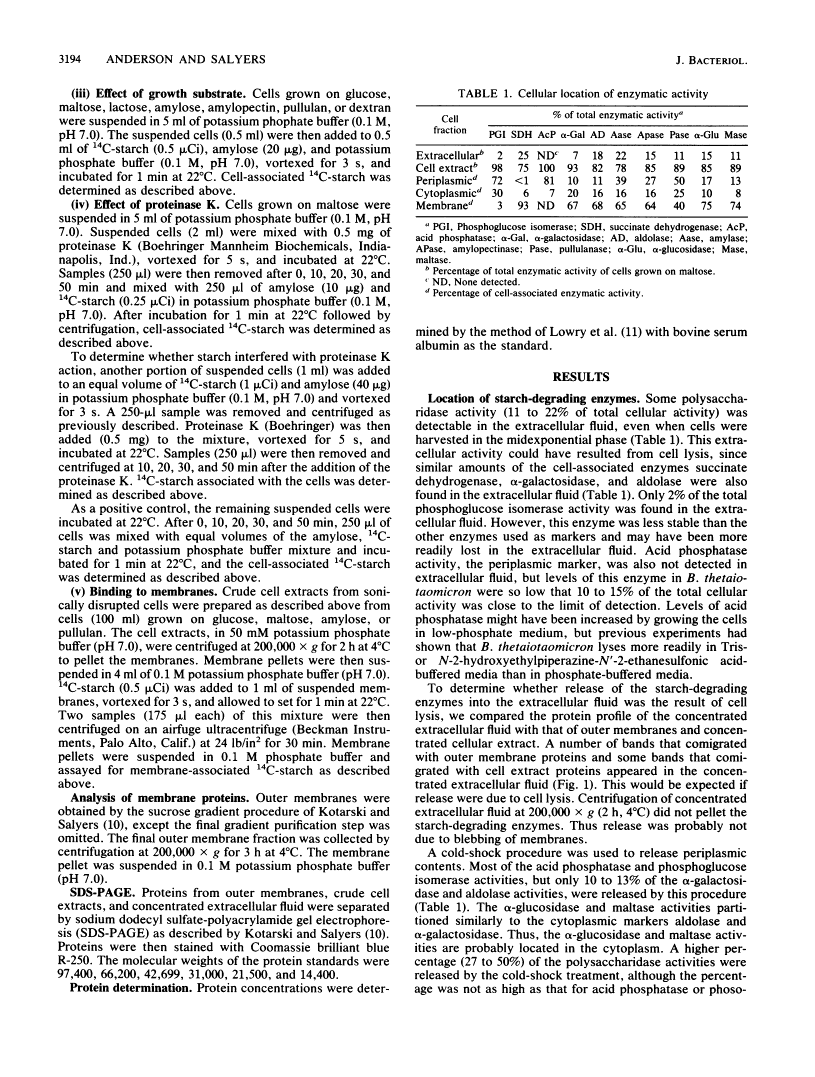
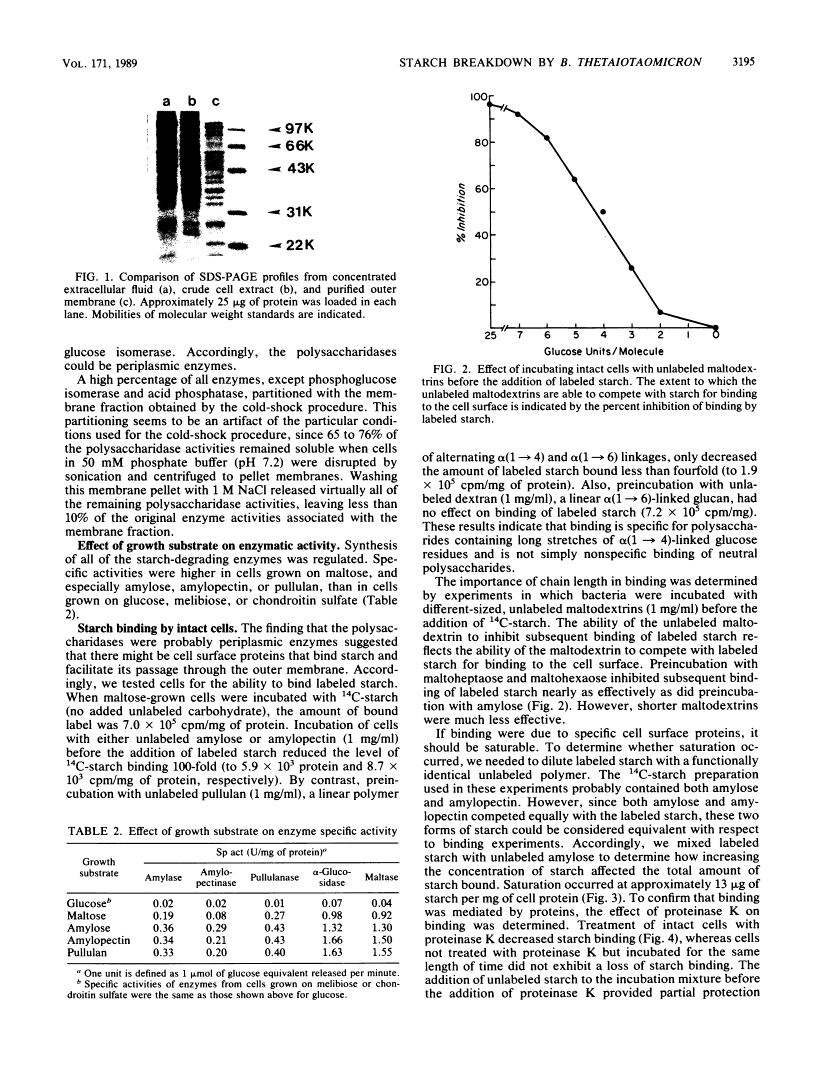
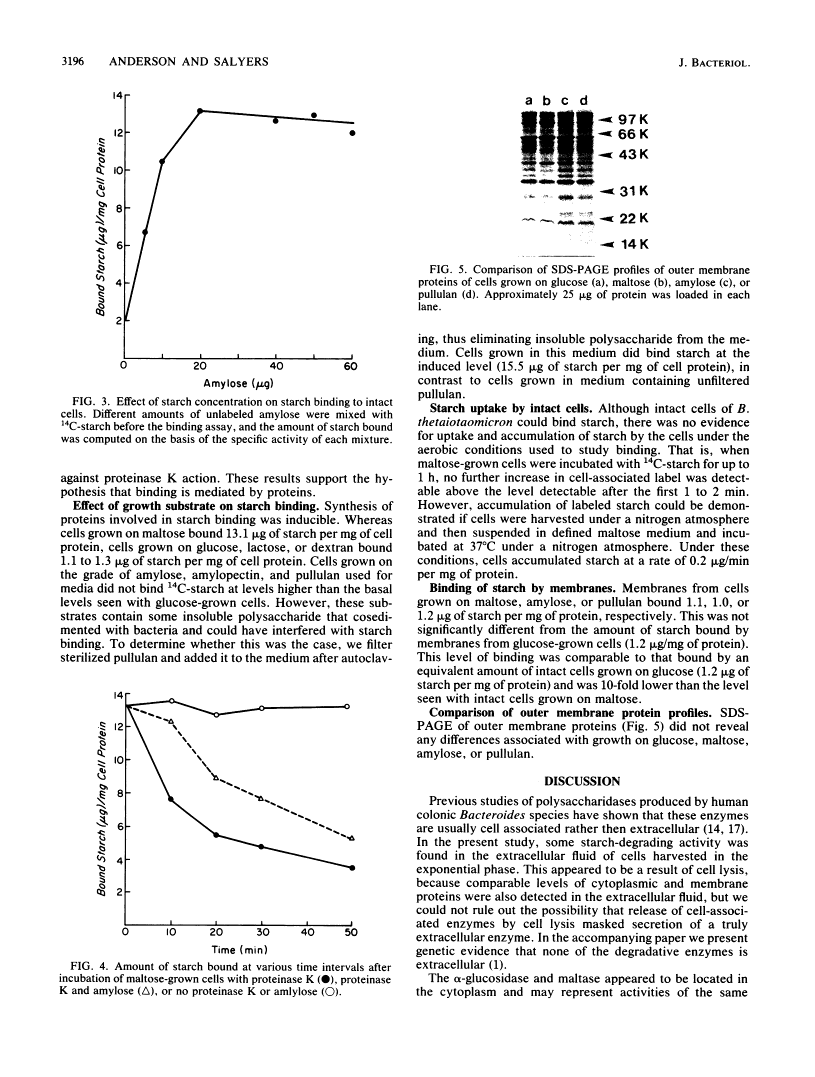
Bacteroides thetaiotaomicron can utilize amylose, amylopectin, and pullulan as sole sources of carbon and energy. The enzymes that degrade these polysaccharides were found to be primarily cell associated rather than extracellular. Although some activity was detected in extracellular fluid, this appeared to be the result of cell lysis. The cell-associated amylase, amylopectinase, and pullulanase activities partitioned similarly to the periplasmic marker, acid phosphatase, when cells were exposed to a cold-shock treatment. Two other enzymes associated with starch breakdown, alpha-glucosidase and maltase, appeared to be located in the cytoplasm. Intact cells of B. thetaiotaomicron were found to bind 14C-starch. Binding was probably mediated by a protein because it was saturable and was decreased by treatment of cells with proteinase K. Results of competition experiments showed that the starch-binding proteins had a preference for maltodextrins larger than maltohexaose and a low affinity for maltose and maltotriose. Both the degradative enzymes and starch binding were induced by maltose. These findings indicate that starch utilization by B. thetaiotaomicron apparently does not involve secretion of extracellular enzymes. Rather, binding of the starch molecule to the cell surface appears to be a first step to passing the molecule through the outer membrane and into the periplasmic space.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.3M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson KL, Salyers AA. Genetic evidence that outer membrane binding of starch is required for starch utilization by Bacteroides thetaiotaomicron. J Bacteriol. 1989 Jun;171(6):3199–3204. [Europe PMC free article] [Abstract] [Google Scholar]
- Beacham IR. Periplasmic enzymes in gram-negative bacteria. Int J Biochem. 1979;10(11):877–883. [Abstract] [Google Scholar]
- Dygert S, Li LH, Florida D, Thoma JA. Determination of reducing sugar with improved precision. Anal Biochem. 1965 Dec;13(3):367–374. [Abstract] [Google Scholar]
- Ford JR, Nunley JA, 2nd, Li YT, Chambers RP, Cohen W. A continuously monitored spectrophotometric assay of glycosidases with nitrophenyl glycosides. Anal Biochem. 1973 Jul;54(1):120–128. [Abstract] [Google Scholar]
- Gherardini F, Babcock M, Salyers AA. Purification and characterization of two alpha-galactosidases associated with catabolism of guar gum and other alpha-galactosides by Bacteroides ovatus. J Bacteriol. 1985 Feb;161(2):500–506. [Europe PMC free article] [Abstract] [Google Scholar]
- Huang L, Forsberg CW. Isolation of a Cellodextrinase from Bacteroides succinogenes. Appl Environ Microbiol. 1987 May;53(5):1034–1041. [Europe PMC free article] [Abstract] [Google Scholar]
- Kasahara M, Anraku Y. Succinate dehydrogenase of Escherichia coli membrane vesicles. Activation and properties of the enzyme. J Biochem. 1974 Nov;76(5):959–966. [Abstract] [Google Scholar]
- Kotarski SF, Linz J, Braun DM, Salyers AA. Analysis of outer membrane proteins which are associated with growth of Bacteroides thetaiotaomicron on chondroitin sulfate. J Bacteriol. 1985 Sep;163(3):1080–1086. [Europe PMC free article] [Abstract] [Google Scholar]
- Kotarski SF, Salyers AA. Isolation and characterization of outer membranes of Bacteroides thetaiotaomicron grown on different carbohydrates. J Bacteriol. 1984 Apr;158(1):102–109. [Europe PMC free article] [Abstract] [Google Scholar]
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [Abstract] [Google Scholar]
- Macy JM, Ljungdahl LG, Gottschalk G. Pathway of succinate and propionate formation in Bacteroides fragilis. J Bacteriol. 1978 Apr;134(1):84–91. [Europe PMC free article] [Abstract] [Google Scholar]
- MALAMY M, HORECKER BL. The localization of alkaline phosphatase in E. coli K12. Biochem Biophys Res Commun. 1961 Jun 2;5:104–108. [Abstract] [Google Scholar]
- McCarthy RE, Kotarski SF, Salyers AA. Location and characteristics of enzymes involved in the breakdown of polygalacturonic acid by Bacteroides thetaiotaomicron. J Bacteriol. 1985 Feb;161(2):493–499. [Europe PMC free article] [Abstract] [Google Scholar]
- Moore WE, Holdeman LV. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. [Europe PMC free article] [Abstract] [Google Scholar]
- Salyers AA, O'Brien M. Cellular location of enzymes involved in chondroitin sulfate breakdown by Bacteroides thetaiotaomicron. J Bacteriol. 1980 Aug;143(2):772–780. [Europe PMC free article] [Abstract] [Google Scholar]
- Smith KA, Salyers AA. Cell-associated pullulanase from Bacteroides thetaiotaomicron: cloning, characterization, and insertional mutagenesis to determine role in pullulan utilization. J Bacteriol. 1989 Apr;171(4):2116–2123. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Bacteriology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jb.171.6.3192-3198.1989
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc210036?pdf=render
Free to read at jb.asm.org
http://jb.asm.org/cgi/content/abstract/171/6/3192
Free after 4 months at jb.asm.org
http://jb.asm.org/cgi/reprint/171/6/3192
Citations & impact
Impact metrics
Citations of article over time
Article citations
In vivo manipulation of human gut Bacteroides fitness by abiotic oligosaccharides.
Nat Chem Biol, 23 Oct 2024
Cited by: 0 articles | PMID: 39443715
Determinants of raffinose family oligosaccharide use in <i>Bacteroides</i> species.
J Bacteriol, 206(10):e0023524, 27 Sep 2024
Cited by: 0 articles | PMID: 39330254 | PMCID: PMC11501099
Metagenome-derived SusD-homologs affiliated with Bacteroidota bind to synthetic polymers.
Appl Environ Microbiol, 90(7):e0093324, 02 Jul 2024
Cited by: 0 articles | PMID: 38953372 | PMCID: PMC11267923
Polysaccharide utilization loci encoded DUF1735 likely functions as membrane-bound spacer for carbohydrate active enzymes.
FEBS Open Bio, 14(7):1133-1146, 12 May 2024
Cited by: 0 articles | PMID: 38735878 | PMCID: PMC11216935
Multiple TonB homologs are important for carbohydrate utilization by Bacteroides thetaiotaomicron.
J Bacteriol, 205(11):e0021823, 24 Oct 2023
Cited by: 2 articles | PMID: 37874167 | PMCID: PMC10662123
Go to all (83) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Genetic evidence that outer membrane binding of starch is required for starch utilization by Bacteroides thetaiotaomicron.
J Bacteriol, 171(6):3199-3204, 01 Jun 1989
Cited by: 68 articles | PMID: 2722748 | PMCID: PMC210037
Contribution of a neopullulanase, a pullulanase, and an alpha-glucosidase to growth of Bacteroides thetaiotaomicron on starch.
J Bacteriol, 178(24):7173-7179, 01 Dec 1996
Cited by: 74 articles | PMID: 8955399 | PMCID: PMC178630
Characterization of four outer membrane proteins that play a role in utilization of starch by Bacteroides thetaiotaomicron.
J Bacteriol, 179(3):643-649, 01 Feb 1997
Cited by: 122 articles | PMID: 9006015 | PMCID: PMC178742
The Sus operon: a model system for starch uptake by the human gut Bacteroidetes.
Cell Mol Life Sci, 73(14):2603-2617, 02 May 2016
Cited by: 114 articles | PMID: 27137179 | PMCID: PMC4924478
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIAID NIH HHS (1)
Grant ID: AI 17876
NIDDK NIH HHS (1)
Grant ID: DK 07497