Abstract
Free full text

Mammographic density does not differ between unaffected BRCA1/2 mutation carriers and women at low-to-average risk of breast cancer
Abstract
Elevated mammographic density (MD) is one of the strongest risk factors for sporadic breast cancer. Epidemiologic evidence suggests that MD is, in part, genetically determined; however, the relationship between MD and BRCA1/2 mutation status is equivocal. We compared MD in unaffected BRCA1/2 mutation carriers enrolled in the U.S. National Cancer Institute’s Clinical Genetics Branch’s Breast Imaging Study (n = 143) with women at low-to-average breast cancer risk enrolled in the same study (n = 29) or the NCI/National Naval Medical Center’s Susceptibility to Breast Cancer Study (n = 90). The latter were BRCA mutation-negative members of mutation-positive families or women with no prior breast cancer, a Pedigree Assessment Tool score <8 (i.e., low risk of a hereditary breast cancer syndrome) and a Gail score <1.67. A single experienced mammographer measured MD using a computer-assisted thresholding method. We collected standard breast cancer risk factor information in both studies. Unadjusted mean percent MD was higher in women with BRCA1/2 mutations compared with women at low-to-average breast cancer risk (37.3% vs. 33.4%; P = 0.04), but these differences disappeared after adjusting for age and body mass index (34.9% vs. 36.3%; P = 0.43). We explored age at menarche, nulliparity, age at first birth, menopausal status, number of breast biopsies, and exposure to exogenous hormonal agents as potential confounders of the MD and BRCA1/2 association. Taking these factors into account did not significantly alter the results of the age/body mass index-adjusted analysis. Our results do not provide support for an independent effect of BRCA1/2 mutation status on mammographic density.
Introduction
The tissue composition of the breast is reflected mammographically by the pattern of distribution of fibroglandular and fatty tissue. The higher the component of fat, the lower the mammographic density (MD). Conversely, the higher the proportion of fibroglandular tissue, the greater the density (reviewed in [1]). Since MD is a noninvasive, reliable and quantitative measure that is strongly associated with breast cancer risk [2, 3], MD is viewed as a compelling candidate for use as an intermediate marker in studies aimed at understanding breast cancer etiology and prevention [4].
Epidemiologic risk factors strongly associated with MD, such as age and body mass index (BMI), explain only 20–30% of the variation in density (reviewed in [5]). The remaining variation in MD is thought to be, in part, genetically regulated (or genetically determined) (reviewed in [6]). Increased MD has been positively associated with family history of breast cancer in most [3, 7–16], but not all [17–21], studies, which have included women with and without a personal history of breast cancer and have used a variety of methods to measure MD. Family studies [22, 23], including studies of sisters [24–27] and twins [23, 27–29], provide further support for a genetic influence on MD. In fact, a large twin study conducted in Australia and North America estimated that up to 67% of the variation in MD may be attributed to common genetic factors [29].
In contrast to the evidence from family studies, findings from gene association studies have been largely inconsistent, and the relationship between MD and BRCA1/2 mutation status is also equivocal [6]. Helvie et al. [30] were the first to suggest that MD might be greater in carriers of germline mutations in the major breast cancer susceptibility gene, BRCA1, after finding dense mammographic patterns in four of eight women with BRCA1 mutations. However, these findings provided little evidence for differences in density patterns between mutation carriers and the general population. Subsequently, four small studies have compared MD between affected (i.e., diagnosed with breast cancer) BRCA1/2 mutation carriers and women with sporadic breast cancer, offering conflicting results [31–34]. When compared with women at low risk of developing breast cancer, 30 age-matched BRCA1/2 carriers had higher percent MD in a Chicago study [35, 36]. In contrast, a European study of BRCA1/2 mutation families compared MD in 206 BRCA1/2 carriers (96 affected; 110 unaffected) with 136 noncarriers (3 affected; 133 unaffected) and found no difference in MD by mutation status [37].
We aimed to further evaluate potential differences in MD between 143 BRCA1/2 mutation carriers without breast cancer enrolled in the U.S. National Cancer Institute (NCI) Clinical Genetics Branch’s Breast Imaging Study and 119 women at low-to-average risk of developing breast cancer participating in the same study or in the NCI/National Naval Medical Center’s Susceptibility to Breast Cancer Study.
Materials and methods
Study populations
The NCI Clinical Genetic Branch’s Breast Imaging Study is a study of breast cancer screening modalities in women who are at high genetic risk of breast cancer. The study design and methodology have been described previously [38, 39]. Briefly, eligible women were ages 25–56 years and carried a known deleterious BRCA1/2 mutation, or were first- or second-degree relatives of BRCA1/2 mutation carriers, or were first- or second-degree relatives of individuals with BRCA-associated cancers in BRCA1/2 mutation-positive families. Participants were seen at the NIH Clinical Center (NCI Protocol #01-C-009; NCT00012415); and at baseline (2001–2007) underwent a physical examination, breast MRI, and a standard four-view mammogram, which was reviewed by the study radiologist (CKC). Two hundred women enrolled in this study, including 170 women with deleterious BRCA1/2 mutations and 30 mutation-negative women. The NCI Institutional Review Board (IRB) approved this study.
The NCI/National Naval Medical Center’s (NNMC) Susceptibility to Breast Cancer Study is a cross-sectional study of the association between MD and genes involved in estrogen metabolism. Participants were enrolled from the patient population at the NNMC and other referring institutions (NNMC Protocol #NNMC.2000.0010) and the NIH Clinical Center (NCI Protocol #00-C-0079). Eligible participants included women with a documented personal history of breast cancer and a comparison group with no personal history of any cancer with the exception of non-melanoma skin cancer and cervical cancer in situ. Participants were enrolled from 2000 to 2006 and received four-view mammograms, as part of standard health care, which were reviewed by two study radiologists (CKC and CEG). The craniocaudal views of the film-screen mammograms obtained within the year prior to enrollment were obtained for analysis. Enrolled were 737 women, of whom 707 were deemed to be evaluable (i.e., provided a bio-specimen for the purposes of the primary study endpoint), including 219 breast cancer cases and 488 controls. The IRBs of the NNMC and NCI approved this study.
Mammogram digitization
Analog mammographic films from both studies were digitized as follows: the craniocaudal views were photographed in the NIH Medical Arts and Photography Branch on a Nikon 4 × 5 inch format optical stand with a light box and additional top light illumination using a Better Light digital scanning back camera (Better Light, Inc., San Carlos, CA), Model Super6K-HS. The images were acquired with BetterLight ViewFinder 7.4.1 software set at 267 dots per inch and 25% scanning resolution, yielding a red-green-blue TIFF file. The files were then converted to gray scale and the “Levels” function was employed to adjust brightness and contrast in Adobe Photoshop 3.0.4 (San Jose, CA). If the pectoralis major muscle was visible in the image, the technician used Photoshop’s Dodge and Burn Tool to “burn,” or darken, any radio-opaque muscle tissue to exclude the muscle tissue from the MD calculation. Any image alterations were made under the supervision of the study mammographer.
Assessment of mammographic density
Participants from both studies had standardized, quantitative calculations of MD measured from digitized mammograms by the same experienced study mammographer (CKC) using an interactive computerized thresholding method developed at the NIH Clinical Center (Version 3.44, MEDx, Medical Numerics, Germantown, MD). After segmenting the breast from background, a threshold gray-level value was selected such that all pixel values above the threshold were tinted to optimally cover the breast parenchyma (Fig. 1). The summed area occupied by dense pixels divided by the total breast area constituted the percent MD. The radiologist was masked to BRCA1/2 mutation status, and the MD results from both breasts were averaged for analysis, unless the patient had only one side available for imaging. In that case, the density reading from one breast was used for analysis (n = 1 BRCA2 mutation carrier and n = 9 NCI/NNMC study participants with only one side available for MD assessment). We assessed the internal reliability of the radiologist’s readings by randomly submitting a masked set of 100 mammograms (30 from the Susceptibility to Breast Cancer Study and 70 from the Breast Imaging Study) for re-review.
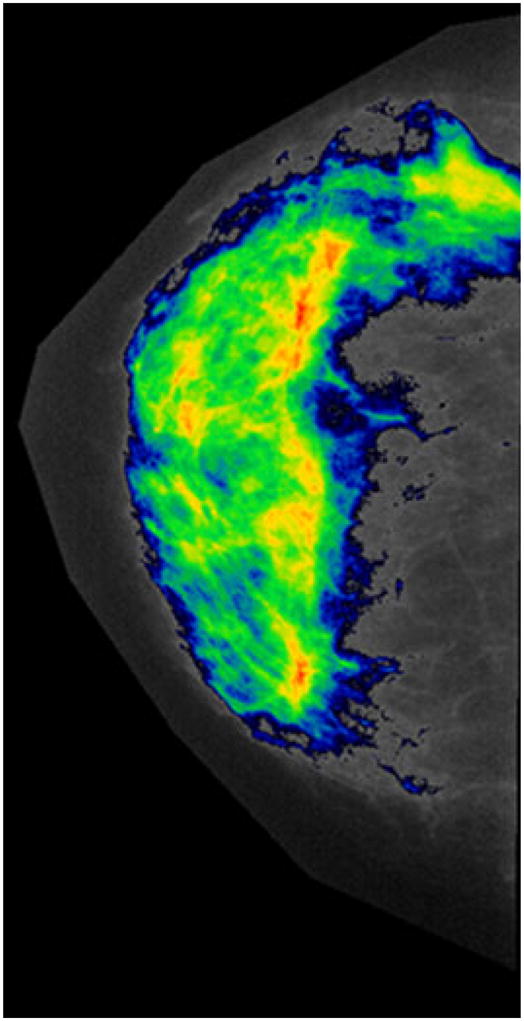
Representative digitized mammogram showing tinted pixels for MD calculation. The colors (ranging from the coolest blue to the hottest red) indicate increasing density, where red is representative of the densest tissue
Among the Breast Imaging Study participants only, the study radiologist also visually scored all mammograms using the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) as follows: The breast (1) is almost entirely fat; (2) contains scattered fibroglandular densities; (3) is heterogeneously dense; and (4) is extremely dense [40]. The BI-RADS classification of MD has been associated with breast cancer risk in a dose–response fashion [41–43]. Among Breast Imaging Study participants with available BI-RADS and percent MD data (n = 170), percent MD increased with increasing BI-RADS density categories as expected (Supplementary Table). For the purposes of this report, however, we used percent MD as the measure of primary interest because it was available for participants from both studies.
Association of MEDx-derived mammographic density with Cumulus density
Because the quantitative measure of MD used in this study has not been validated with respect to its association with breast cancer risk, we selected a subset of images to be assessed using Cumulus™, a computer-assisted thresholding program [44] that is routinely and widely used to measure MD; previous studies have demonstrated that Cumulus-measured MD is strongly associated with breast cancer risk in a variety of populations [2, 14, 45, 46]. We randomly selected 18 low-to-average risk women and 22 mutation carriers in order to mirror the proportions represented in the main analysis. Since a random sample may not include women in the tails of the MD distribution, we selected an additional 5 women (two low-to-average risk and three mutation carriers) from both the lowest and highest quartiles of MD. Masked images from these 50 women were sent to the Mayo Clinic for Cumulus review by a trained programmer using previously described methods [45]. One case was deleted as left and right mammographic images could not be read due to an inability within the digitized images to set the Cumulus threshold that separates the breast from the background. Additionally, one left and four right images were identified by the Cumulus programmer as being problematic; images from the opposite breast were read and included in the MEDx-MD comparison. In addition, to assess the internal reliability of the programmer, four masked duplicate images were reviewed. The NCI IRB approved this study.
Assessment of breast cancer risk factors and covariates
Participants completed self-administered questionnaires which captured demographic characteristics, current weight and height, smoking status, medical and reproductive history, menopausal status, use of exogenous hormonal medications, and personal and familial history of cancer. “Postmenopausal” status was defined as having had no menstrual cycles in the 12 months prior to enrollment or a history of bilateral oophorectomy. Questionnaire items were compared between studies, and common response categories were combined in order to create a harmonized analytic database. Five-year Gail [47] and Pedigree Assessment Tool (PAT) [48] scores were calculated for all controls participating in the Susceptibility to Breast Cancer Study. The PAT is a point-scoring system that uses family cancer history to identify women who are at increased risk of hereditary breast cancer, including potential BRCA mutation carriers; a PAT score of 8.0 has been associated with 100% sensitivity and 93% specificity [48].
Analytic sample
The NCI Clinical Genetic Branch’s Breast Imaging Study: After excluding five women with missing MD readings (3 BRCA1 carriers, 1 BRCA2 carrier, and 1 noncarrier), 22 women with prevalent breast cancer (11 BRCA1 carriers and 11 BRCA2 carriers), and one BRCA1 carrier with prevalent ovarian cancer, the study population included 143 mutation carriers and 29 mutation-negative women eligible for analysis.
The NCI/NNMC Susceptibility to Breast Cancer Study: For the purposes of this report, the analytic sample was restricted to controls with available MD readings who were determined to be at low-to-average breast cancer risk. After excluding women with prior breast cancer (n = 219) and women missing MD readings (n = 226), 262 potentially eligible women remained. Of these, 153 women had a 5-year Gail score ≥1.67, three women were missing Gail scores, 15 women had PAT scores ≥8, and one women was determined to have a personal history of skin cancer, type unspecified; these women were excluded, resulting in 90 low-to-average risk women for analysis. Given the rarity of BRCA mutations in the general population, and the low PAT scores, these 90 women were assumed to be mutation-negative.
Statistical analysis
Intra-class correlation coefficients (ICC) were calculated to assess the intra-reader reliability of the MD assessments. Spearman’s rank correlation coefficients were used to describe the correlation between MD measured by MEDx with that measured by Cumulus. Baseline characteristics were compared between BRCA1/2 mutation carriers and women at low-to-average risk using the two-sample t-test for independent samples, with assumed equal variances for continuous measures, and the chi-square test for discrete measures. Characteristics were compared across quartiles of percent MD, using analysis of variance (ANOVA) for continuous measures and the chi-square test for discrete measures. The two-sample t-test for independent samples with assumed equal variances was used to compare mean percent MD between BRCA1/2 mutation carriers and women at low-to-average breast cancer risk. ANCOVA was used to compare means of percent MD between the two groups, while controlling for potential confounding factors. Since age and BMI have been previously shown to have strong inverse associations with percent MD [5], the multivariate models assessing the relation between mutation status and MD were first adjusted for age, and then for age and BMI. Finally, ANCOVA was used to compare means of MD between the two groups, additionally controlling for covariates determined to be significantly associated (P < 0.05) with mutation status and MD in univariate analyses. MD values were approximately normally distributed. Probability values of <0.05 were considered statistically significant. All tests of statistical significance were two-tailed. Analyses were performed using SAS software release 9.1.3 (SAS Institute Inc., Cary, NC).
Results
The participant demographics by study are shown in Table 1. BRCA1/2 mutation carriers included 143 women with complete MD measures and no personal history of breast or ovarian cancer. Women determined to be at low-to-average breast cancer risk (n = 119) included (a) 29 mutation-negative women with complete MD measures and no prior breast or ovarian cancer, and (b) 90 women with complete MD measures and without cancer, a PAT score <8 and a 5-year Gail score <1.67.
Table 1
Participant risk status by study
| NCI Clinical Genetic Branch’s Breast Imaging Study, 2001–2007 (n = 172) | No. women |
|---|---|
| Risk status | |
 BRCA1 BRCA1 | 91 |
 BRCA2 BRCA2 | 52 |
 Mutation-negative, from mutation-positive family Mutation-negative, from mutation-positive family | 29 |
 Personal history of breast cancer Personal history of breast cancer | 0 |
|
| |
| NCI/NNMC Susceptibility to Breast Cancer Study, 2000–2006 (n = 90) | Median (range; IQR) |
|
| |
| Risk score | |
 Maternal PAT Maternal PAT | 0 (0, 7; 3) |
 Paternal PAT Paternal PAT | 0 (0, 5; 0) |
 5-year Gail score [46] 5-year Gail score [46] | 1.2 (0.3, 1.6; 0.5) |
| Number of first-degree relatives with breast cancer | 0 (0, 1; 0) |
| Personal history of breast cancer | 0 |
IQR inter-quartile range, NCI National Cancer Institute, NNMC National Naval Medical Center, PAT Pedigree Assessment Tool [47]
The BRCA1/2 mutation carriers were statistically significantly younger than the women at low-to-average breast cancer risk (Table 2). Compared with low-to-average risk women, the mutation carriers were more likely to be white, nulliparous, and/or to have a later age at first birth. In addition, the mutation carriers were more likely to have ever used oral contraceptives and to have undergone surgical menopause.
Table 2
Baseline characteristics of the study populations
| Variable | Low-to-average risk of breast cancer (n = 119)
| Unaffected BRCA1/2 carriers (n = 143)
| P-value | ||
|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | ||
| Age (years), mean (SD) | 48.4 (9.3) | 25–79 | 38.0 (8.6) | 22–55 | <.0001 |
| Body mass index (BMI)a, mean (SD) | 26.3 (5.8) | 18.0–49.5 | 25.5 (5.4) | 17.9–48.2 | 0.23 |
|
| |||||
| n | %b | n | % | ||
|
| |||||
| Race | |||||
 Other Other | 19 | 16.0 | 0 | 0.0 | <.0001 |
 White, non-Hispanic White, non-Hispanic | 100 | 84.0 | 143 | 100.0 | |
| Education level | |||||
 High school High school | 28 | 23.5 | 32 | 22.4 | 0.28 |
 College degree College degree | 39 | 32.8 | 60 | 42.0 | |
 Graduate work Graduate work | 52 | 43.7 | 51 | 35.7 | |
| Cigarette smoking | |||||
 Never Never | 81 | 68.1 | 95 | 66.4 | 0.74 |
 Former Former | 31 | 26.1 | 36 | 25.2 | |
 Current Current | 7 | 5.9 | 12 | 8.4 | |
| Age at menarche (years) | |||||
 <12 <12 | 18 | 15.3 | 19 | 13.4 | 0.91 |
 12–13 12–13 | 73 | 61.9 | 90 | 63.4 | |
 ≥14 ≥14 | 27 | 22.9 | 33 | 23.2 | |
 Missing Missing | 1 | 1 | |||
| Parous | |||||
 No No | 31 | 26.1 | 66 | 46.2 | 0.001 |
 Yes Yes | 88 | 73.9 | 77 | 53.8 | |
| Age at first birth (years) | |||||
 <30 <30 | 65 | 54.6 | 54 | 37.8 | 0.006 |
 ≥30 or nulliparous ≥30 or nulliparous | 54 | 45.4 | 89 | 62.2 | |
| Breastfed | |||||
 Never Never | 52 | 45.2 | 75 | 52.4 | 0.25 |
 Ever Ever | 63 | 54.8 | 68 | 47.6 | |
 Missing Missing | 4 | 0 | |||
| Oral contraceptives | |||||
 Never Never | 30 | 25.4 | 16 | 11.2 | 0.002 |
 Former Former | 78 | 66.1 | 99 | 69.2 | |
 Current Current | 10 | 8.5 | 28 | 19.6 | |
 Missing Missing | 1 | 0 | |||
| Menopausal status | |||||
 Premenopausal Premenopausal | 68 | 61.3 | 81 | 56.6 | <.0001 |
 Postmenopausal, natural Postmenopausal, natural | 9 | 8.1 | 10 | 7.0 | |
 Postmenopausal, surgical Postmenopausal, surgical | 16 | 14.4 | 51 | 35.7 | |
 Postmenopausal, unknown Postmenopausal, unknown | 18 | 16.2 | 1 | 0.7 | |
 Missing Missing | 8 | 0 | |||
| Hormone therapy | |||||
 Never Never | 81 | 68.6 | 101 | 70.6 | 0.93 |
 Former Former | 17 | 14.4 | 20 | 14.0 | |
 Current Current | 20 | 16.9 | 22 | 15.4 | |
 Missing Missing | 1 | 0 | |||
| SERMs | |||||
 Never Never | 115 | 97.5 | 133 | 93.0 | 0.24 |
 Former Former | 1 | 0.8 | 5 | 3.5 | |
 Current Current | 2 | 1.7 | 5 | 3.5 | |
 Missing Missing | 1 | 0 | |||
| Prior breast biopsy | |||||
 0 0 | 83 | 69.7 | 108 | 75.5 | 0.57 |
 1 1 | 25 | 21.0 | 25 | 17.5 | |
 2+ 2+ | 11 | 9.2 | 10 | 7.0 | |
SERM selective estrogen receptor modulator
P-values < 0.05 are in bold type
The ICC for intra-observer agreement for MD assessed in the 100 paired sets using MEDx was 0.889 and in the four paired sets using Cumulus was 0.997, documenting high reliability of each method. For the 49 women with Cumulus measures, percent MD was strongly and positively correlated with that measured by MEDx (r = 0.84, P < 0.0001). Excluding the five women with problematic images from one breast did not significantly alter the results (r = 0.86, P < 0.0001).
Table 3 shows the baseline characteristics of the study populations by quartiles of percent MD. Women with higher MD had a lower BMI and were more likely to be college graduates, nulliparous, and younger, and thus were more likely to be premenopausal and to have never used menopausal hormone therapy.
Table 3
Baseline characteristics of the study populations by quartiles of mammographic density
| Variable | Mammographic density (%) quartiles
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 (0–25.9)
| Q2 (26.0–36.3)
| Q3 (36.4–46.4)
| Q4 (46.8–84.6)
| P-value | |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||||
| Age (years) | 48.5 (11.5) | 42.9 (9.5) | 39.2 (8.8) | 40.4 (8.8) | <.0001 | ||||
| Body mass index (BMI)a | 30.0 (7.1) | 26.1 (4.7) | 24.3 (3.9) | 23.0 (3.4) | <.0001 | ||||
|
| |||||||||
| n | %b | n | % | n | % | n | % | ||
|
| |||||||||
| White | 60 | 92.3 | 62 | 93.9 | 62 | 95.4 | 59 | 89.4 | 0.59 |
| College graduate | 43 | 66.2 | 54 | 81.8 | 48 | 73.8 | 57 | 86.4 | 0.03 |
| Ever smoker | 21 | 32.3 | 20 | 30.3 | 24 | 36.9 | 21 | 31.8 | 0.87 |
| Age at menarche (years) | |||||||||
 <12 <12 | 11 | 17.5 | 12 | 18.2 | 6 | 9.2 | 8 | 12.1 | 0.66 |
 12–13 12–13 | 41 | 65.1 | 39 | 59.1 | 42 | 64.6 | 41 | 62.1 | |
 ≥14 ≥14 | 11 | 17.5 | 15 | 22.7 | 17 | 26.2 | 17 | 25.8 | |
 Missing Missing | 2 | 0 | 0 | 0 | |||||
| Age at first birth ≥30 years or nulliparous | 29 | 44.6 | 32 | 48.5 | 38 | 58.5 | 44 | 66.7 | 0.049 |
| Ever breastfed | 36 | 56.3 | 34 | 53.1 | 29 | 45.3 | 32 | 49.2 | 0.61 |
 Missing Missing | 1 | 2 | 1 | 0 | |||||
| Oral contraceptives | |||||||||
 Never Never | 12 | 18.8 | 14 | 21.2 | 11 | 16.9 | 9 | 13.6 | 0.90 |
 Former Former | 45 | 70.3 | 42 | 63.6 | 44 | 67.7 | 46 | 69.7 | |
 Current Current | 7 | 10.9 | 10 | 15.2 | 10 | 15.4 | 11 | 16.7 | |
 Missing Missing | 1 | 0 | 0 | 0 | |||||
| Menopausal status | |||||||||
 Premenopausal Premenopausal | 25 | 38.5 | 34 | 54.8 | 48 | 75.0 | 42 | 66.7 | 0.0002 |
 Postmenopausal, surgical Postmenopausal, surgical | 21 | 32.3 | 17 | 27.4 | 13 | 20.3 | 16 | 25.4 | |
 Postmenopausal, natural/type unknown Postmenopausal, natural/type unknown | 19 | 29.2 | 11 | 17.7 | 3 | 4.7 | 5 | 7.9 | |
 Missing Missing | 0 | 4 | 1 | 3 | |||||
| Hormone therapy | |||||||||
 Never Never | 31 | 48.4 | 47 | 71.2 | 55 | 84.6 | 49 | 74.2 | 0.001 |
 Former Former | 17 | 26.6 | 8 | 12.1 | 6 | 9.2 | 6 | 9.1 | |
 Current Current | 16 | 25.0 | 11 | 16.7 | 4 | 6.2 | 11 | 16.7 | |
 Missing Missing | 1 | 0 | 0 | 0 | |||||
| Ever use of SERMs | 3 | 4.7 | 4 | 6.1 | 2 | 3.1 | 4 | 6.1 | 0.84 |
 Missing Missing | 1 | 0 | 0 | 0 | |||||
| Prior breast biopsy | |||||||||
 0 0 | 43 | 66.2 | 44 | 66.7 | 52 | 80.0 | 52 | 78.8 | 0.22 |
 1 1 | 18 | 27.7 | 15 | 22.7 | 9 | 13.8 | 8 | 12.1 | |
 2+ 2+ | 4 | 6.2 | 7 | 10.6 | 4 | 6.2 | 6 | 9.1 | |
SERM selective estrogen receptor modulator
P-values < 0.05 are in bold type
While unadjusted mean MD was higher in women with BRCA1/2 mutations versus women at low-to-average risk (Table 4), there was no statistically significant difference in MD after adjusting for age and BMI. In fact, after adjustment for age and BMI, mean MD was marginally, albeit not significantly, lower among carriers than among low-to-average risk women (Fig. 2). We explored age at menarche, nulliparity, age at first birth, menopausal status, number of breast biopsies, and exposure to exogenous hormonal agents as covariates of potential interest with regard to modulating MD. Taking these factors into account did not significantly alter the results of the age-/BMI-adjusted analysis (data not shown). Results were also similar when BRCA1 and BRCA2 mutation carriers were analyzed separately. Compared with low-to-average risk women, mean MD was 2.1% lower among BRCA1 carriers and 0.94% lower among BRCA2 carriers; after adjustment for age and BMI, these differences were not statistically significant.
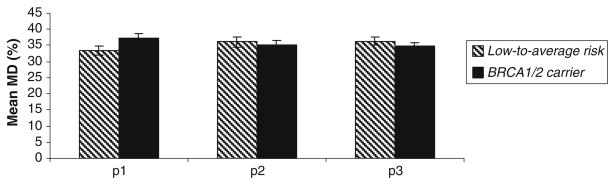
Unadjusted and adjusted mean (±SE) baseline MD (%) in unaffected BRCA1/2 mutation carriers (n = 143) versus women at low-to-average risk of breast cancer (n = 119)
Table 4
Mean baseline mammographic density in BRCA1/2 mutation carriers versus women at low-to-average risk of breast cancer
| Variable | Low-to-average risk of breast cancer (n=119)
| Unaffected BRCA1/2 carriers (n=143)
| P-value
| ||||
|---|---|---|---|---|---|---|---|
| Mean (SE) | Range | Mean (SE) | Range | p1 | p2 | p3 | |
| Mammographic density (%) | 33.4 (1.4) | (2.0–84.6) | 37.3 (1.3) | (2.8–70.8) | 0.04 | 0.65 | 0.43 |
P-values < 0.05 are in bold type
p1. P-value for two-sample t-test
p2. P-value for ANCOVA, adjusting for age (continuous)
p3. P-value for ANCOVA, adjusting for age (continuous) and BMI (continuous)
Because age was such a strong confounder in our analysis, we conducted post hoc subgroup analyses using a restricted age range. MD is strongly and inversely associated with age, and, by virtue of the Breast Imaging Study inclusion criteria, the BRCA1/2 mutation carriers were on average approximately 10 years younger than the low-to-average risk group. Thus, if MD were truly lower among BRCA1/2 mutation carriers, such a relationship could be obscured in age-adjusted analyses. We therefore truncated age at the upper age limit of the Breast Imaging Study participants (55 years) and reanalyzed the data. Among women ≤55 years of age, mean MD did not differ between mutation carriers (n = 143) and women at low-to-average risk (n = 101) either before (P = 0.44) or after (P = 0.68) age adjustment.
Discussion
We investigated the association between MD and BRCA1/2 mutation status among women without breast cancer. Compared with women at low-to-average breast cancer risk, we observed no difference in percent MD among BRCA1 and BRCA2 mutation carriers, after accounting for age, BMI, and other potential confounders. Our results do not provide support for an independent effect of BRCA1/2 mutation status on MD.
Our null findings for a difference in MD by mutation status are consistent with those reported by three smaller studies of women with breast cancer [32–34] and the larger European study of BRCA1/2 mutation families [37]. However, our results differ from those from a small study of Asian breast cancer patients diagnosed before age 40 that found a higher BI-RADS MD score among 9 BRCA1 mutation carriers relative to 19 age-matched sporadic cases [31]. Our results also differ from a Chicago study which compared MD in 30 BRCA1/2 carriers (including 17 with breast cancer) with that in 142 women at low breast cancer risk (i.e., no family history of breast cancer and lifetime Gail risk<10%) [35, 36]. Percent MD was visually estimated, and a computerized texture analysis was performed. Breast cancer cases were not analyzed separately. Results demonstrated higher percent MD among the BRCA1/2 carriers than in the age-matched low-risk group, although a test of statistical significance was not performed and, with the exception of age, potential confounding factors were not considered in the analysis. Using a single summary score for their computerized texture features, the investigators achieved a high level of performance in distinguishing between mutation carriers and low-risk women [35]. Their results suggested that there may be additional information contained within mammographic images—not necessarily captured by current measurements of MD—which may be used to more accurately assess breast cancer risk. We are currently investigating whether these novel quantitative mammographic characteristics (e.g., texture and contrast) are associated with mutation status in the Breast Imaging Study and the Susceptibility to Breast Cancer Study participants.
Some limitations of this study deserve consideration. The technology used to measure percent MD has not been prospectively validated for its association with breast cancer risk. However, percent MD was positively associated with BI-RADS density among the Breast Imaging Study participants, inversely associated with age and BMI among all participants as expected [5], and strongly, positively correlated with MD measured by Cumulus among a subset of participants, suggesting internal and external validity for the MD measurements. Thus, our use of a quantitative, highly reliable measure of MD has proven to be a strength of the study: our ICC estimate for percent MD (ρ = 0.889) is consistent with that reported by Boyd et al. [46], who observed an ICC of ρ = 0.897 for 150 film sets in the Canadian National Breast Screening Study. The original design of our study had been to use mutation-negative women from the mutation-positive families as the comparison group, which would have provided participants known not to carry BRCA mutations, and controlled for theoretical within-family correlations in MD. However, we were unable to recruit a sufficiently large number of such women, and thus turned to the NCI/NNMC Susceptibility to Breast Cancer Study as an alternative. These participants offered the advantage of having been imaged and having MD estimated by the same mammographer as the Breast Imaging Study participants. Thus, although these women are legitimately classified as “low-to-average-risk” by all available indicators (no personal history of breast or ovarian cancer, and low scores on both the Gail and PAT models), their mutation status was not directly determined. Given that the prevalence of BRCA1/2 mutations in the general white population is estimated as from 1 in 400 to 1 in 800, the expected number of mutation carriers among the 90 NCI/NNMC Susceptibility Breast Cancer Study participants is 0.1–0.2. It is therefore unlikely that this possibility influenced our results.
Despite these limitations, this study is one of the largest to date to have evaluated the association between MD and BRCA1/2 mutation status among women without breast cancer. The study sample size achieved 80% power to detect a mean difference in MD of 5.2% between mutation carriers and low-to-average risk women. Observed mean differences in MD between the two groups were <4% in all univariate and multivariate analyses, including those restricted to women ≤55 years of age; this consistency provides compelling support for our conclusion that no association exists between mutation status and MD.
Although MD does not appear to be associated with BRCA1/2 mutation status, results from the Epidemiological Study of BRCA1 and BRCA2 mutation carriers (EMBRACE) suggest that MD is a breast cancer risk factor among mutation carriers [37], just as it is in the setting of sporadic cancer. MD ≥50% was associated with an increased odds of breast cancer (odds ratio = 2.29, 95% CI 1.23–4.26) [37]; this odds ratio is similar to that observed for the association between MD and breast cancer risk in the general population.
In conclusion, our data, plus those from prior reports, suggest that the increased risk of breast cancer in BRCA1/2 mutation carriers is not mediated through a heritable factor which modulates MD. Given the strong epidemiologic evidence for a large genetic influence on MD in the general population, ongoing genome-wide association studies may reveal novel genes which could improve our understanding of the mechanism by which MD influences susceptibility among women at risk of both hereditary and sporadic breast cancer. Additionally, prospective studies of BRCA1/2 carriers are needed to clarify the association between MD and breast cancer in this high-risk population. Such a study is being planned as an add-on to the National Ovarian Cancer Prevention and Early Detection Study (GOG-199) [49].
Acknowledgments
The Breast Imaging Study (NCI Protocol #01-C-009); The Susceptibility to Breast Cancer Study (NCI Protocol #00-C-0079/NNMC Protocol #NNMC.2000.0010). We wish to thank Ruthann Giusti, Christine Mueller, and Paul Han for clinical support; Nicole Dupree, Jason Hu, Beth Mittl, Usha Singh, and Andrea Wilson for their help in data preparation; Pamela Klein for the original design of the NCI/NNMC Susceptibility to Breast Cancer Study; and Fang Fang Wu for the Cumulus density assessments. Special thanks to all our study participants, without whose cooperation this study could not have been done. Financial Support: This project was supported by the Intramural Research Program of the National Cancer Institute, and by contracts NO2-CP-11019-50 and NO2-CP-65504-50 with Westat, Inc. Dr. Gierach was supported by the NCI Cancer Prevention Fellowship Program. The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
Abbreviations
| ANOVA | Analysis of variance |
| BI-RADS | Breast Imaging Reporting and Data System |
| BMI | Body mass index |
| ICC | Intra-class correlation coefficient |
| IRB | Institutional review board |
| MD | Mammographic density |
| NCI | National Cancer Institute |
| NIH | National Institutes of Health |
| NNMC | National Naval Medical Center |
| PAT | Pedigree Assessment Tool |
Contributor Information
Gretchen L. Gierach, Hormonal and Reproductive Epidemiology Branch, Division of Cancer Epidemiology and Genetics and Cancer Prevention Fellowship Program, Office of Preventive Oncology, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA. 6120 Executive Blvd., Suite 550, Room 5016, Rockville, MD 20892, USA.
Jennifer T. Loud, Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA, 6120 Executive Blvd., Room 7028, Rockville, MD 20892, USA.
Catherine K. Chow, Diagnostic Radiology Department, National Institutes of Health Clinical Center, Bethesda, MD, USA.
Sheila A. Prindiville, Coordinating Center for Clinical Trials, Office of the Director, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Jennifer Eng-Wong, Lombardi Comprehensive Cancer Center, Georgetown University, Washington, DC, USA.
Peter W. Soballe, Uniformed Services University of the Health Sciences, Bethesda, MD, USA.
Claudia Giambartolomei, Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Phuong L. Mai, Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Claudia E. Galbo, Department of Radiological Sciences, Uniformed Services University of the Health Sciences, Bethesda, MD, USA.
Kathryn Nichols, Westat Corporation, Rockville, MD, USA.
Kathleen A. Calzone, Genetics Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Celine Vachon, Department of Health Sciences Research, College of Medicine, Mayo Clinic, Rochester, MN, USA.
Mitchell H. Gail, Biostatistics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Mark H. Greene, Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s10549-010-0749-7
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3125980?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Association between Family History of Breast Cancer and Breast Density in Saudi Premenopausal Women Participating in Mammography Screening.
Clin Pract, 14(1):164-172, 19 Jan 2024
Cited by: 0 articles | PMID: 38391399 | PMCID: PMC10887693
Biological Mechanisms and Therapeutic Opportunities in Mammographic Density and Breast Cancer Risk.
Cancers (Basel), 13(21):5391, 27 Oct 2021
Cited by: 8 articles | PMID: 34771552 | PMCID: PMC8582527
Review Free full text in Europe PMC
RASSF1A Suppression as a Potential Regulator of Mechano-Pathobiology Associated with Mammographic Density in BRCA Mutation Carriers.
Cancers (Basel), 13(13):3251, 29 Jun 2021
Cited by: 1 article | PMID: 34209669 | PMCID: PMC8269117
The TP53 mutation rate differs in breast cancers that arise in women with high or low mammographic density.
NPJ Breast Cancer, 6:34, 07 Aug 2020
Cited by: 4 articles | PMID: 32802943 | PMCID: PMC7414106
Mammographic density: intersection of advocacy, science, and clinical practice.
Curr Breast Cancer Rep, 11(3):100-110, 24 Jul 2019
Cited by: 1 article | PMID: 33312342 | PMCID: PMC7728377
Go to all (29) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Mammographic density and breast cancer in women from high risk families.
Breast Cancer Res, 17:93, 11 Jul 2015
Cited by: 15 articles | PMID: 26163143 | PMCID: PMC4499171
Mammographic density and breast cancer risk in BRCA1 and BRCA2 mutation carriers.
Cancer Res, 66(3):1866-1872, 01 Feb 2006
Cited by: 82 articles | PMID: 16452249
Relationships between computer-extracted mammographic texture pattern features and BRCA1/2 mutation status: a cross-sectional study.
Breast Cancer Res, 16(4):424, 23 Aug 2014
Cited by: 36 articles | PMID: 25159706 | PMCID: PMC4268674
[Medical management of women with inherited predisposition to breast cancer: indications and procedures for mammographic screening].
Bull Cancer, 88(7):677-686, 01 Jul 2001
Cited by: 0 articles | PMID: 11495821
Review
Funding
Funders who supported this work.
Intramural NIH HHS (1)
Grant ID: ZIA CP010145-11
NCI NIH HHS (4)
Grant ID: N02CP65504
Grant ID: N02-CP-11019-50
Grant ID: N02CP11019
Grant ID: N02-CP-65504-50





