Abstract
Purpose of review
Pulmonary artery hypertension (PAH) in children contributes significantly to morbidity and mortality in diverse pediatric cardiac, lung, hematologic and other diseases. Advances in pulmonary vascular biology over the past few decades have significantly expanded therapeutic strategies; however, many unique issues persist regarding our understanding of pediatric PAH.Recent findings
Recent studies of pediatric PAH include those that highlight gaps in our understanding of pediatric diseases associated with PAH from those of adult onset, emphasizing the strong need for specific studies regarding unique aspects of the pathogenesis and treatment of children with PAH. Registries have begun to provide new data showing differences in physiology, course, and genetics between adult and pediatric forms of PAH. Unfortunately, therapeutic strategies in pediatric pulmonary hypertension are often limited to small observational studies in children and are dependent on results from larger adult studies. In addition, clinical endpoints for studies and care remain poorly defined in infants and children.Summary
Despite many advances, long-term outcomes for children with PAH remain guarded and substantial challenges persist, especially with regard to understanding mechanisms and approach to severe PAH. Future studies are needed to develop novel biomarkers, clinical endpoints and interventions for young children with diverse causes of PAH.Free full text

Recent progress in understanding pediatric pulmonary hypertension
Abstract
Purpose of review
Pulmonary artery hypertension (PAH) in children contributes significantly to morbidity and mortality in diverse pediatric cardiac, lung, hematologic and other diseases. Advances in pulmonary vascular biology over the past few decades have significantly expanded therapeutic strategies; however, many unique issues persist regarding our understanding of pediatric PAH.
Recent findings
Recent studies of pediatric PAH include those that highlight gaps in our understanding of pediatric diseases associated with PAH from those of adult onset, emphasizing the strong need for specific studies regarding unique aspects of the pathogenesis and treatment of children with PAH. Registries have begun to provide new data showing differences in physiology, course, and genetics between adult and pediatric forms of PAH. Unfortunately, therapeutic strategies in pediatric pulmonary hypertension are often limited to small observational studies in children and are dependent on results from larger adult studies. In addition, clinical endpoints for studies and care remain poorly defined in infants and children.
Summary
Despite many advances, long-term outcomes for children with PAH remain guarded and substantial challenges persist, especially with regard to understanding mechanisms and approach to severe PAH. Future studies are needed to develop novel biomarkers, clinical endpoints and interventions for young children with diverse causes of PAH.
Introduction
Pulmonary arterial hypertension (PAH) in children is a critical determinant of morbidity and mortality in diverse pediatric cardiac, lung, hematologic, and other diseases. PAH is a disease of the entire pulmonary circulation. The small pulmonary arteries are characterized by vascular narrowing due to high tone, structural remodeling of the vessel wall, intraluminal obstruction, and decreased vascular growth and surface area. Recent studies have begun to highlight that vascular stiffness of the larger arteries also contributes to right ventricular afterload. Without therapy, high pulmonary vascular resistance (PVR) causes progressive right ventricular failure, low cardiac output, and high mortality. In the recent past, therapeutic strategies were markedly limited to primarily supportive care, but advances in basic pulmonary vascular biology over the past few decades have led directly to several novel therapies, which have significantly expanded therapeutic choices for patients with PAH. Despite these improvements, long-term outcomes in many settings remain guarded and substantial challenges persist, especially with regard to understanding how best to approach children with PAH [1]. This review will provide a brief and selective overview of recent publications that provide new information regarding our current understanding and approach to PAH in children.
Etiologies, classification, and epidemiology
Historically, idiopathic pulmonary arterial hypertension (IPAH) is associated with extremely poor survival in adults, but even a worse prognosis in children, with an estimated median survival in children of only 10 months [2,3]. Beyond IPAH, pulmonary vascular disease continues to have significant morbidity and mortality in many settings, including pulmonary, cardiac, and hematologic disorders. In the past, pulmonary hypertension was generally categorized as either ‘primary’ (now idiopathic) or ‘secondary’ (associated with other diseases). The World Health Organization (WHO) Classification helped discern different types of pulmonary hypertension [4]. More recently, experts with extensive experience in experimental and clinical aspects of pulmonary hypertension gathered at the 4th World Symposium on Pulmonary Hypertension, Dana Point, California, USA (see below list), to review progress in our understanding of the pathobiology, diagnosis, and treatment of pulmonary hypertension and to update the classification system [5,6].
- (1)
PAH:
Idiopathic.
Heritable:
Bone morphogenetic receptor type 2 (BMPR2).
Activin receptor-like kinase type 1 (ALK1), endoglin (with or without hereditary hemorrhagic telangiectasia).
Unknown.
Drugs and toxins induced.
Associated with [associated pulmonary arterial hypertension (APAH)]:
Connective tissue diseases.
HIV infection.
Portal hypertension.
Congenital heart disease (CHD).
Schistosomiasis.
Chronic hemolytic anemia.
Persistent pulmonary hypertension of the newborn.
- (1)
Pulmonary veno-occlusive disease and/or pulmonary capillary hemangiomatosis.
- (2)
Pulmonary hypertension due to left heart disease:
Systolic dysfunction.
Diastolic dysfunction.
Valvular disease.
- (3)
Pulmonary hypertension due to lung diseases and/or hypoxemia:
Chronic obstructive pulmonary disease.
Interstitial lung disease.
Other pulmonary diseases with mixed restrictive and obstructive pattern.
Sleep-disordered breathing.
Alveolar hypoventilation disorders.
Chronic exposure to high altitude.
Developmental abnormalities.
- (4)
Chronic thromboembolic pulmonary hypertension.
- (5)
Pulmonary hypertension with unclear and/or multi-factorial mechanisms:
Hematological disorders: myeloproliferative disorders, splenectomy.
Systemic disorders: sarcoidosis, pulmonary Langerhans cell histiocytosis, lymphangioleiomyomatosis, neurofibromatosis, vasculitis.
Metabolic disorders: glycogen storage disease, Gaucher disease, thyroid disorders.
Others: tumoral obstruction, fibrosing mediastinitis, chronic renal failure on dialysis.
As with past classifications, the newest version outlined five major categories of disease, based on physiologic and histologic patterns or clinical settings. Recent changes reflect growing recognition of heritable forms of PAH that may lack familial patterns, as related to the BMPR2, ALK1, and unknown genes [6]. The category of ‘systemic to pulmonary shunt’ has been replaced by ‘CHD’, recognizing the many different factors involved. In addition, schistosomiasis, perhaps the most common cause of pulmonary hypertension worldwide, and chronic hemolytic anemias now appear as subgroups within the type 1 category. Although often with different clinical signs and therapeutic approaches, pulmonary veno-occlusive disease and/or capillary hemangiomatosis were maintained within type 1 disease but as a distinct subgroup, as these patients respond poorly to vasodilator therapy.
Although many aspects of disease are similar between adult and pediatric pulmonary hypertension [7••], distinct differences exist, including etiologic mechanisms, disease course, frequent associations with genetic syndromes, and treatment responses [8,9••,10•]. Whether the Dana Point Classification is sufficient for use in children with pulmonary hypertension is uncertain for several reasons. First, pediatric pulmonary hypertension is intrinsically linked to issues of lung growth and development, including many prenatal and early postnatal influences [11–16]. The development of pulmonary hypertension in the neonate and young infant is often related to impaired functional and structural adaptation of the pulmonary circulation during transition from fetal to postnatal life. In particular, the timing of pulmonary vascular injury is a critical determinant of the subsequent response of the developing lung to such adverse stimuli as hyperoxia, hypoxia, hemodynamic stress, inflammation, and others. In fact, adult pulmonary hypertension may have its origins in disruptions or alterations that take place during development, perhaps related to genetic, epigenetic, or environmental (e.g., hemodynamic, inflammatory, or others) triggers [17••,18••].
Abnormalities of the lung circulation are significant beyond the adverse hemodynamic effects of pulmonary hypertension alone. The developing lung circulation plays critical roles in lung organogenesis and development of the distal airspace, maintenance of lung structure, metabolism, gas exchange, the ability to tolerate increased workloads imposed by exercise, and others. Experimentally, disruption of lung vascular growth can impair distal airspace structure during development and contributes to the pathobiology of diverse lung diseases, especially as related to premature infants [11–14]. Finally, there are apparent differences in primary diseases and vascular function, structure, genetics, and perhaps responsiveness to therapies between adults and children with pulmonary hypertension [19,20]. Therapeutic strategies for adult pulmonary hypertension have not been sufficiently studied in children, especially regarding potential toxicities or optimal dosing, and age-appropriate endpoints for clinical and research use are lacking.
Although the recent Dana Point Classification has excluded PVR from the definition [6], most physicians taking care of children continue to embrace the inclusion of PVR index at least 3 Woods units × m2 as an important criterion. As infants and young children can often have mean systemic blood pressure 50–70 mmHg or less, it may be more appropriate to define pulmonary hypertension according to the value of the ratio of the pulmonary arterial systolic pressure to the systemic arterial systolic pressure.
Data on pediatric epidemiology remain scarce and the exact incidence and prevalence of pulmonary hypertension in children is not known. Although several registries of adult patients exist, such registries for children with pulmonary hypertension are less well established and underpowered for sufficient analysis [9••,10•,21]. PAH may be idiopathic or heritable or associated with specified diseases (‘associated PAH’). On the basis of the available data in children, the predominant diagnoses are pulmonary hypertension associated with CHD and IPAH. However, this likely reflects local patterns of clinical practice, as many pulmonary hypertension centers, especially those with multidisciplinary teams, are managing care for a growing population of patients with bronchopulmonary dysplasia (BPD), congenital diaphragmatic hernia (CDH), sickle cell anemia, and other diseases other than IPAH or CHD [22,23,24••]. Pulmonary vascular disease is often a silent contributor to morbidity and mortality of many disorders in pediatrics, including BPD, cystic fibrosis, sickle cell disease (SCD), and various interstitial lung diseases. Pulmonary hypertension appears to predict early death in adults with SCD and is already present by echocardiogram in about 20% of the children with SCD [25,26]. Clinical strategies that anticipate the development of PAH in these diverse clinical settings may allow earlier recognition and more aggressive therapy, thereby slowing the development of PAH in many chronic lung parenchymal and cardiovascular diseases.
Pathology and pathophysiology
Recent laboratory and patient-oriented studies have implicated novel mechanisms underlying the pathogenesis and pathophysiology of PAH (see [17••,27]). The pathogenesis of PAH is complex and multifactorial, often resulting from interactions between genetic susceptibility and environmental or acquired factors, including hemodynamic stress, inflammation, hypoxia, and others. For example, the use of appetite-suppressant drugs, methamphetamines, antidepressants, and other agents is associated with an increased risk for PAH, yet this may be partly dependent on genetic susceptibility factors [17••].
General mechanisms that cause PAH include increased vascular tone and reactivity, vessel wall structural remodeling and thrombosis, and impaired vascular growth. Pulmonary vasoconstriction and altered reactivity is often considered as one of the earliest components of PAH, followed over time with alterations of vascular structure. Increased vasoconstriction is likely related to an imbalance between the impaired production of endogenous vasodilators [including nitric oxide, prostacyclin (PgI2), and others] and excessive production of vasoconstrictors [such as endothelin-1 (ET-1) and serotonin (5HT)]. These changes reflect endothelial cell dysfunction, which results from injury due to several mechanisms, including hypoxia, hemodynamic stress, inflammation, oxidative stress, and altered growth factor production. Abnormal smooth muscle cell growth and function due to altered potassium and calcium channel expression and activity, increased calcium sensitization via enhanced rho kinase activity, changes in mitochondrial or metabolic functions, and other mechanisms are critical in PAH. With advanced disease, plexiform lesions (due to disorganized proliferation of endothelial cells within the vessel lumen), thrombosis and increased intimal growth of smooth muscle cells and fibroblasts contribute to striking obstructive lesions, which are often recalcitrant to therapy [17••,27,28].
Finally, impaired or aberrant angiogenesis or vasculogenesis has been increasingly recognized as playing a significant role in the progression and severity of PAH, especially in the setting of developmental lung disorders in children [11–15]. In addition, impaired angiogenesis during development can cause marked PAH later in life, with persistent signs of pulmonary arterial remodeling, reduced vessel density, and right ventricular hypertrophy [29]. Paradoxically, excessive angiogenic signaling may enhance abnormalities of increased endothelial proliferation in plexiform lesions, contributing to disease severity in rodent models. The summary statement from a recent National Institutes of Health (NIH) workshop on the pulmonary circulation highlighted many exciting new findings and research opportunities; however, very little discussion focused on the developing lung circulation and issues regarding pediatric pulmonary vascular disease [17••].
Recent studies have explored the potential role of circulating endothelial cells (CECs) or endothelial progenitor cells (EPCs) during lung development, in the pathobiology of pulmonary vascular disease in neonates and CHD, or perhaps as biomarkers for disease risk and severity [30,31,32•]. Smajda et al. [33,34] have published a series of papers that suggest that CEC and EPC levels are associated with disease severity in childhood PAH, and that prolonged treprostinil therapy may increase EPC levels in patients. Several reports have examined the application and potential impact of noninvasive studies, such as echocardiograms and 6-min walk tests, in pediatric pulmonary hypertension [35•,36–38,39••,40].
Genetics
Genetic studies have shown that mutations in receptors of the transforming growth factor beta (TGF-β) receptor family have been identified in most cases of inherited (familial) and some cases of sporadic IPAH [41]. The underlying pathobiology of altered BMPR2 function is complex and incompletely understood, but an imbalance in TGF-β–BMP signaling favors the pro-proliferative phenotype of IPAH. Clinically, adults with BMPR2 mutations may have worse disease, as characterized by younger age at diagnosis (34 vs. 46 years), worse hemodynamics at diagnosis, and shorter time to death or transplant [42].
In children with IPAH, the genetics are less clear, probably due to the fewer and smaller numbers of patients included in these early studies. One study described only rare findings of BMPR2 mutations in pediatric IPAH [43]. In another report, four of 18 (22%) children with IPAH had mutations of BMPR2, ALK1, or endoglin [44]. Rosenzweig et al. reported that eight of 78 (10%) children with IPAH had BMPR2 mutations [19]. As observed in adults with IPAH, children with BMPR2 mutation were less likely than those without BMPR2 mutation to respond during acute vasoreactivity testing during cardiac catheterization (13 vs. 44%), suggesting important differences in disease severity.
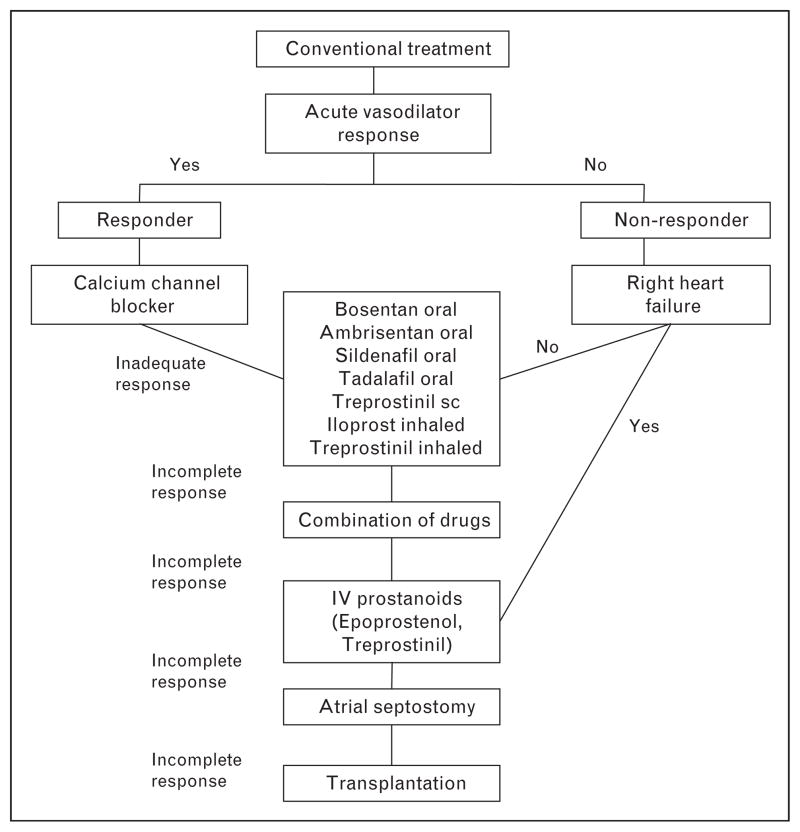
Therapeutic options
A recent review has summarized medical therapies for pediatric patients with PAH [45•]. Current approaches are primarily based on three key signaling cascades, involving endothelial-derived agents: PgI2, ET-1, and nitric oxide pathways (Fig. 1).
Prostacyclin analogues
As in adults, chronic, continuous intravenous infusion of epoprostenol, a prostacyclin analogue, can improve hemodynamics, quality of life, exercise capacity, and survival in children with IPAH and APAH [46,47]. Line sepsis, local infection, and catheter dislodgement, however, are not unusual [48] and can lead to life-threatening rebound pulmonary hypertension due to abrupt discontinuation of therapy. The use of closed hub may reduce septic complications in children [49].
Iloprost, a relatively stable prostacyclin analogue, has been administered as an inhalational agent in children and adults, but its short-term and long-term efficacies remain unproven [45•]. In a small series in children, inhaled iloprost improved WHO functional class in only 35% of cases, and efficacy was often not sustained [50]. Acute bronchoconstriction has been recognized as an adverse event and compliance can be poor due to the need for frequent aerosol administrations (six to eight times daily). Treprostinil, another PgI2 analogue, is approved by the US Food and Drug Association (FDA) for use by continuous subcutaneous or intravenous infusion and by inhalation in adults. Subcutaneous treprostinil causes short-term improvement in exercise tolerance and hemodynamics in some adults with PAH, but local site discomfort can limit its tolerance. Subcutaneous treprostinil has been used to transition some children who were chronically stable on intravenous epoprostenol and may improve clinical course in children as an add-on therapy [51,52].
ET-1 receptor antagonists
ET-1, a potent vasoconstrictor peptide and smooth muscle cell mitogen, is produced primarily by endothelial cells and acts through stimulation of two receptor subtypes, ETA and ETB. ETA and ETB receptors on vascular smooth muscle mediate vasoconstriction, whereas ETB receptors on endothelial cells cause release of nitric oxide and PgI2, and act as clearance receptors for circulating ET-1. Bosentan, an oral dual ET-1 receptor antagonist (ETRA), can lower pulmonary arterial pressure (PAP) and PVR and may improve functional status and survival estimates at 1 and 2 years (98 and 91%, respectively) in children with PAH [53]. Nevertheless, in children and adults with PAH and systemic-to-pulmonary shunt, bosentan produces only short-term improvement in functional class and 6-min walk distance [20]. A progressive decline in function was observed within 1 year of bosentan therapy in both groups, with a more pronounced decline noted in children, who tended to have more severe disease at baseline. Ambrisentan, an oral daily ETRA that selectively inhibits the ETA receptor, can have beneficial effects on exercise capacity and functional class in adult patients, but few data are available for children. Sitaxsentan was recently removed from the market due to concerns of liver toxicity.
Phosphodiesterase type 5 inhibitors
On the basis of extensive preclinical and clinical studies, the phosphodiesterase type 5 inhibitor (PDE5i) sildenafil has been approved by the FDA for the treatment of moderate and severe PAH in adult patients and is widely used in children. Recent studies suggest additional benefit in the setting of chronic lung disease and CHD [54–57]. Interestingly, PDE5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition with sildenafil may improve right ventricular contractility [57]. More recent studies have provided new information on the potential role for intravenous sildenafil in children with persistent pulmonary hypertension of the newborn (PPHN) and postoperative cardiac disease [58•,59]. Tadalafil, a long-acting PDE5i, was recently approved by the FDA for adults with chronic PAH and may improve the clinical course in adults with severe PAH on intravenous prostacyclin therapy. However, experience with tadalafil use in children is limited.
New approaches
In theory, combined therapy that targets each of these major signaling pathways may provide greater efficacy than monotherapy approaches. Several studies have suggested that the use of various combinations, such as epoprostenol and sildenafil, may improve exercise capacity, hemodynamics, time to clinical worsening, and quality of life. Whether combination therapy should be used as a first step by simultaneous initiation of two or more drugs or by addition of a second treatment to a previous therapy once insufficient remains unknown.
Recent insights from basic studies on the pathobiology of PAH have suggested novel targets for the development of new therapies that include vascular tone but more specifically target cell growth, proliferation, and apoptosis [17••]. Such approaches include the potential roles for platelet-derived growth factor receptor blockade, soluble guanylate cyclase activators and stimulators, rho kinase, statins, serotonin signaling, and others. Whether these observations will successfully translate to successful human therapy is unknown, but these important studies will likely provide critical leads to enhance long-term outcomes.
Conclusion
Overall, pediatric pulmonary hypertension has been understudied and little is understood regarding the natural history, mechanisms of disease, and treatment of childhood pulmonary hypertension. Limitations regarding current translational approaches to children with pulmonary hypertension are partly due to the relatively small numbers of patients with pulmonary hypertension associated with specific pediatric disorders at each center; the small number of well established, multi-disciplinary programs in pediatric pulmonary hypertension; little communication between translational and clinician-scientists; and limited interactions between existing pulmonary hypertension programs [1]. There is clearly a need to develop clinical infrastructure to better define the natural history and course of pediatric pulmonary hypertension, to develop new strategies to identify at-risk patients early in their course, and to establish novel approaches to diagnose, monitor disease progression, and treat children with pulmonary hypertension.
Acknowledgments
This work was supported in part by grants from the NHLBI HL68702, HL085703, and P50 HL084923 (S.H.A.) and the Jayden DeLuca Fund. S.H.A. has no significant conflicts of interest. D.D.I. has served as a consultant for Actelion, Gilead, Pfizer, and United Therapeutics.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 360–361).
Full text links
Read article at publisher's site: https://doi.org/10.1097/mop.0b013e3283464a52
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3128451?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1097/mop.0b013e3283464a52
Article citations
Surgical versus Non-Surgical Management of Obstructive Sleep-disordered Breathing in Children: A Meta-analysis.
Open Respir Med J, 14:47-52, 26 Nov 2020
Cited by: 0 articles | PMID: 33299493 | PMCID: PMC7705953
Risk Factors for Obstructive Sleep Apnea Syndrome in Children: State of the Art.
Int J Environ Res Public Health, 16(18):E3235, 04 Sep 2019
Cited by: 79 articles | PMID: 31487798 | PMCID: PMC6765844
Review Free full text in Europe PMC
Prevalence of Pulmonary Hypertension in Pediatric Patients With Obstructive Sleep Apnea and a Cardiology Evaluation: A Retrospective Analysis.
J Clin Sleep Med, 15(8):1081-1087, 01 Aug 2019
Cited by: 13 articles | PMID: 31482829 | PMCID: PMC6707049
Surgery for partial atrioventricular septal defect with pulmonary hypertension in an adult dog.
J Vet Med Sci, 80(7):1183-1189, 06 Jun 2018
Cited by: 2 articles | PMID: 29877312 | PMCID: PMC6068305
A Retrospective Review of Infants Receiving Sildenafil.
J Pediatr Pharmacol Ther, 23(2):100-105, 01 Mar 2018
Cited by: 5 articles | PMID: 29720910 | PMCID: PMC5916436
Go to all (26) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Advances in pediatric pulmonary arterial hypertension.
Curr Opin Cardiol, 27(2):70-81, 01 Mar 2012
Cited by: 15 articles | PMID: 22274573 | PMCID: PMC3319159
Review Free full text in Europe PMC
Pediatric pulmonary arterial hypertension.
Curr Hypertens Rep, 15(6):606-613, 01 Dec 2013
Cited by: 4 articles | PMID: 24163011
Review
Advances in targeted therapy for pulmonary arterial hypertension in children.
Eur J Pediatr, 182(5):2067-2076, 17 Dec 2022
Cited by: 3 articles | PMID: 36527480
Review
Phosphodiesterase 5 inhibitors for pulmonary hypertension.
Cochrane Database Syst Rev, 1:CD012621, 31 Jan 2019
Cited by: 63 articles | PMID: 30701543 | PMCID: PMC6354064
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NHLBI NIH HHS (7)
Grant ID: HL68702
Grant ID: P50 HL084923-05
Grant ID: R01 HL085703
Grant ID: R01 HL068702
Grant ID: P50 HL084923
Grant ID: HL085703
Grant ID: R56 HL068702