Abstract
Background
Respiratory syncytial virus (RSV) pneumonia after hematopoietic cell transplant (HCT) is associated with severe morbidity. Although RSV RNA has been detected in serum from patients with RSV lower respiratory disease (LRD) after HCT, the association with clinical outcomes has not been well established in multivariable models. Additionally, the role of antiviral treatment in HCT recipients has not been previously analyzed in multivariable models.Methods
We retrospectively identified HCT recipients with virologically confirmed RSV LRD and tested stored plasma/serum samples by quantitative reverse transcription polymerase chain reaction for RSV RNA. Risk factors for RSV RNA detection and the impact of RSV RNA in serum and antiviral therapy on outcomes were analyzed using multivariable Cox models.Results
RSV RNA was detected in plasma or serum from 28 of 92 (30%) patients at a median of 24.5 days following HCT and 2 days following LRD. In multivariable models, neutropenia, monocytopenia, thrombocytopenia, and mechanical ventilation increased the risk of plasma/serum RSV RNA detection; lymphopenia and steroid use did not. RSV RNA detection increased the risk of overall mortality in multivariable models (adjusted hazard ratio [aHR], 2.09 [P = .02]), whereas treatment with aerosolized ribavirin decreased the risk of overall mortality and pulmonary death (aHR, 0.33 [P = .001] and aHR 0.31 [P = .003], respectively).Conclusions
RSV RNA detection in plasma or serum may be a marker for lung injury and poor outcomes in HCT recipients with RSV LRD. Treatment with aerosolized ribavirin appeared to be protective against overall and pulmonary mortality.Free full text

Respiratory Syncytial Virus Lower Respiratory Disease in Hematopoietic Cell Transplant Recipients: Viral RNA Detection in Blood, Antiviral Treatment, and Clinical Outcomes
Abstract
Background. Respiratory syncytial virus (RSV) pneumonia after hematopoietic cell transplant (HCT) is associated with severe morbidity. Although RSV RNA has been detected in serum from patients with RSV lower respiratory disease (LRD) after HCT, the association with clinical outcomes has not been well established in multivariable models. Additionally, the role of antiviral treatment in HCT recipients has not been previously analyzed in multivariable models.
Respiratory syncytial virus (RSV) pneumonia after hematopoietic cell transplant (HCT) is associated with severe morbidity. Although RSV RNA has been detected in serum from patients with RSV lower respiratory disease (LRD) after HCT, the association with clinical outcomes has not been well established in multivariable models. Additionally, the role of antiviral treatment in HCT recipients has not been previously analyzed in multivariable models.
Methods. We retrospectively identified HCT recipients with virologically confirmed RSV LRD and tested stored plasma/serum samples by quantitative reverse transcription polymerase chain reaction for RSV RNA. Risk factors for RSV RNA detection and the impact of RSV RNA in serum and antiviral therapy on outcomes were analyzed using multivariable Cox models.
We retrospectively identified HCT recipients with virologically confirmed RSV LRD and tested stored plasma/serum samples by quantitative reverse transcription polymerase chain reaction for RSV RNA. Risk factors for RSV RNA detection and the impact of RSV RNA in serum and antiviral therapy on outcomes were analyzed using multivariable Cox models.
Results. RSV RNA was detected in plasma or serum from 28 of 92 (30%) patients at a median of 24.5 days following HCT and 2 days following LRD. In multivariable models, neutropenia, monocytopenia, thrombocytopenia, and mechanical ventilation increased the risk of plasma/serum RSV RNA detection; lymphopenia and steroid use did not. RSV RNA detection increased the risk of overall mortality in multivariable models (adjusted hazard ratio [aHR], 2.09 [P = .02]), whereas treatment with aerosolized ribavirin decreased the risk of overall mortality and pulmonary death (aHR, 0.33 [P = .001] and aHR 0.31 [P = .003], respectively).
RSV RNA was detected in plasma or serum from 28 of 92 (30%) patients at a median of 24.5 days following HCT and 2 days following LRD. In multivariable models, neutropenia, monocytopenia, thrombocytopenia, and mechanical ventilation increased the risk of plasma/serum RSV RNA detection; lymphopenia and steroid use did not. RSV RNA detection increased the risk of overall mortality in multivariable models (adjusted hazard ratio [aHR], 2.09 [P = .02]), whereas treatment with aerosolized ribavirin decreased the risk of overall mortality and pulmonary death (aHR, 0.33 [P = .001] and aHR 0.31 [P = .003], respectively).
Conclusions. RSV RNA detection in plasma or serum may be a marker for lung injury and poor outcomes in HCT recipients with RSV LRD. Treatment with aerosolized ribavirin appeared to be protective against overall and pulmonary mortality.
RSV RNA detection in plasma or serum may be a marker for lung injury and poor outcomes in HCT recipients with RSV LRD. Treatment with aerosolized ribavirin appeared to be protective against overall and pulmonary mortality.
Respiratory syncytial virus (RSV) causes significant morbidity and mortality, with a hospitalization rate of 55.3 per 100 000 person-years and death rate of 4.3 per 100 000 persons annually [1, 2]. Immunocompromised hosts are particularly affected, and recipients of hematopoietic cell transplant (HCT) have high rates of progression from upper respiratory disease (URD) to potentially severe lower respiratory disease (LRD) [3–7]. Overall mortality following development of RSV LRD remains high without well-defined, specific risk factors for death.
Centers caring for HCT patients have employed a variety of treatment strategies for RSV including ribavirin (aerosolized, intravenous, or oral) with or without pooled intravenous immunoglobulin (IVIG), RSV-specific immunoglobulin, or palivizumab [8–12]. Early treatment appears to be more effective, although studies have been limited by small numbers of patients and have not accounted for multiple risk factors such as disease severity [11–14]. No randomized trials examining ribavirin for treatment of RSV LRD have been reported, although 2 small randomized trials examined its use as preemptive therapy [9, 15]. Cost and difficulty in drug administration have also contributed to controversy in the use of ribavirin.
Identifying biological markers of RSV disease severity and poor outcomes is an important goal that may allow for improved therapeutic intervention. Although it is clear that presence of viral nucleic acid in blood is relevant for viruses such as cytomegalovirus and adenovirus, its role is less clear for respiratory viruses. Several studies have described the presence of viral nucleic acid in serum, whole blood, and peripheral blood monocytic cells of patients infected with respiratory viruses including severe acute respiratory syndrome coronavirus, influenza virus, avian H5N1 influenza virus, rhinovirus, and RSV, and have suggested a correlation with disease severity and poor outcomes [16–25]. Recently, evaluation of serum influenza RNA in HCT recipients found that influenza RNA detection was associated with hypoxemia, respiratory failure, and increased overall and influenza-related death [26]. Initial studies at our center reported that RSV RNA in serum is detectable and may be associated with poor outcomes, but formal risk factor and outcome analyses are lacking [27].
The purpose of this retrospective study was to examine the association of plasma or serum RSV RNA detection with disease outcomes in HCT recipients with RSV LRD. Additionally, we evaluated the clinical and virologic factors associated with outcomes in a large cohort that allowed for analysis using multivariable models.
METHODS
Patients and Samples
HCT patients with virologically confirmed RSV LRD between January 1990 and April 2011 were included in this study. Clinical data were collected from databases and supplemental review of the medical record. This study was approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center. Subjects signed informed consent permitting the use of data and stored samples for research.
RSV LRD was defined as RSV detection from bronchoalveolar lavage (BAL), lung biopsy, or autopsy specimens in association with lower respiratory tract clinical symptoms and/or radiographic changes. The day of RSV LRD diagnosis was defined as the date that RSV was detected in the lower tract specimen. Virologic confirmation was obtained by direct fluorescent antibody, shell vial centrifugation, or conventional viral culture in lower respiratory tract samples; after 2007 patients also had RSV detected by reverse transcription polymerase chain reaction (RT-PCR). Stored serum or plasma samples were available in a subset of patients. Weekly plasma or serum samples collected within 4 weeks before and after diagnosis of LRD were tested for RSV RNA by real-time RT-PCR.
RSV RNA Detection Methods
Serum or plasma frozen at ≤−20°C was thawed and extracted in duplicate. Total nucleic acids were isolated from 280 µL of specimen (QIAamp Viral RNA Mini Kit, Qiagen) and analyzed for the presence of RSV A and RSV B viral RNA by quantitative, real-time RT-PCR assays targeting the RSV matrix genes [28]. The RT-PCR assays were performed in duplicate to assess low levels of viral RNA and optimize detection. Thus, a total of 4 RT-PCR results were obtained for each specimen. To ensure that negative results were not due to poor nucleic acid extraction or inhibition of the PCR assay, 1000 copies per reaction of a specimen processing control was added to the lysis buffer during extraction. All negative samples had evidence of amplification of the control. One low-positive control containing 200–1000 copies per reaction of RSV and 1 negative control consisting of cultured, uninfected human epithelial cells were processed with each batch of patient specimens. Each assay reliably detected 10 viral copies per reaction, which provided a sensitivity of 200 copies/mL (10 µL of nucleic acid specimen added per reaction). All PCR assays were performed according to the standards of the College of American Pathologists, and the laboratories passed proficiency testing in viral diagnostics.
Statistical Analysis
Patients were analyzed based on their first episode of RSV LRD. The probability of overall survival was estimated using the Kaplan-Meier method; the probability of mortality due to respiratory failure (pulmonary death) was estimated with cumulative incidence curves, with death from other reasons as a competing risk. The log-rank test was used to compare mortality curves between subgroups. Cox proportional hazards models were used to evaluate unadjusted and adjusted hazard ratios (HRs) for RSV RNA detection, and for overall mortality and pulmonary death at day 30 and day 90 following RSV LRD. Poisson regression was used to evaluate unadjusted and adjusted relative risk (RR) for respiratory failure, defined as the requirement for mechanical ventilation. Covariates evaluated as candidate risk factors for inclusion in multivariable models are listed in Table Table1.1. Variables with P ≤ .2 in univariate analysis were candidates for multivariable models. For model formation, risk factors of interest were selected a priori; factors significant in univariate models at P ≤ .2 were added one by one to those factors for evaluation. Models presented are those with significant confounding variables. Given the extended study period and the associated changes in transplant practices, year of transplant was evaluated over 2 time periods as well as continuously. Time-dependent steroid use and mechanical ventilation were included in all models for RSV RNA detection. Treatment with ribavirin, oxygen requirement at diagnosis, and cell source were included in all models for overall mortality; treatment with ribavirin and oxygen requirement at diagnosis were included in all models for pulmonary death. Oxygen requirement at diagnosis and presence of RSV RNA were included in all models of respiratory failure. Two-sided P values <.05 were considered to be statistically significant. Separate multivariable models were used to minimize the number of covariates per model due to the limited number of outcome events. All statistical analyses were performed using SAS 9.3 for Windows (SAS Institute, Inc, Cary, North Carolina).
Table 1.
Characteristics of Patients With Respiratory Syncytial Virus (RSV) Lower Respiratory Disease, Including Subgroups Tested for Presence of RSV RNA in Blood
| Variable | All Patients | Patients With RSV RNA Detection Performed | Patients Without RSV RNA Detection Performed |
|---|---|---|---|
| (N = 118) | (n = 92) | (n = 26) | |
| Age, y, median (IQR) | 42 (32–52) | 42 (32.5–52) | 39 (24–51) |
| Age, y | |||
 <21 <21 | 11 (9%) | 6 (7%) | 5 (19%) |
 21–60 21–60 | 94 (80%) | 75 (81%) | 19 (73%) |
 >60 >60 | 13 (11%) | 11 (12%) | 2 (8%) |
| Sexa | |||
 Female Female | 43 (36%) | 29 (32%) | 14 (54%) |
 Male Male | 75 (64%) | 63 (68%) | 12 (46%) |
| Year of transplant | |||
 1989–1997 1989–1997 | 69 (59%) | 56 (61%) | 13 (50%) |
 1998–2010 1998–2010 | 49 (41%) | 36 (39%) | 13 (50%) |
| Race | |||
 Unknown Unknown | 4 (3%) | 3 (3%) | 1 (4%) |
 White White | 99 (85%) | 77 (84%) | 22 (88%) |
 Nonwhite Nonwhite | 14 (12%) | 12 (13%) | 2 (8%) |
| Disease riskb | |||
 High High | 58 (49%) | 47 (51%) | 11 (42%) |
 Standard Standard | 60 (51%) | 45 (49%) | 15 (58%) |
| Cell source groups | |||
 PBSC PBSC | 51 (43%) | 36 (39%) | 15 (58%) |
 BM/CBc BM/CBc | 67 (57%) | 56 (61%) | 11 (42%) |
| Donor type | |||
 Allogeneic Allogeneic | 96 (81%) | 76 (83%) | 20 (77%) |
 Autologous Autologous | 22 (19%) | 16 (17%) | 6 (23%) |
| Conditioning regimen | |||
 MA ± low TBI MA ± low TBI | 44 (37%) | 34 (37%) | 10 (39%) |
 MA + TBI (≥12 Gy) MA + TBI (≥12 Gy) | 56 (48%) | 44 (48%) | 12 (46%) |
 Nonmyeloablative Nonmyeloablative | 18 (15%) | 14 (15%) | 4 (15%) |
| Pulmonary infiltrates | |||
 None None | 23 (19%) | 19 (21%) | 4 (15%) |
 CXR or CT infiltrate CXR or CT infiltrate | 95 (81%) | 73 (79%) | 22 (85%) |
| WBC count, 106 cells/L | |||
 >1000 >1000 | 75 (64%) | 58 (63%) | 17 (65%) |
 ≤1000 ≤1000 | 43 (36%) | 34 (37%) | 9 (35%) |
| Lymphocyte count, 106 cells/L | |||
 ≥100 ≥100 | 83 (70%) | 63 (69%) | 20 (77%) |
 <100 <100 | 35 (30%) | 29 (31%) | 6 (23%) |
| Neutrophil count, 106 cells/L | |||
 ≥100 ≥100 | 90 (76%) | 72 (78%) | 18 (69%) |
 <100 <100 | 28 (24%) | 20 (22%) | 8 (31%) |
| Monocyte count, 106 cells/L | |||
 ≥100 ≥100 | 53 (49%) | 38 (46%) | 15 (58%) |
 <100 <100 | 55 (51%) | 44 (54%) | 11 (42%) |
| Platelet count, 106 cells/L | |||
 ≥10 000 ≥10 000 | 85 (72%) | 90 (76%) | 21 (81%) |
 <10 000 <10 000 | 33 (28%) | 28 (24%) | 5 (19%) |
| Copathogend | |||
 None None | 69 (59%) | 55 (60%) | 14 (54%) |
 Any copathogen Any copathogen | 49 (41%) | 37 (40%) | 12 (46%) |
| Oxygen at diagnosis of LRD | |||
 None to ≤2 L None to ≤2 L | 70 (59%) | 53 (58%) | 17 (65%) |
 >2 L/ventilatore >2 L/ventilatore | 48 (41%) | 39 (42%) | 9 (35%) |
| Steroid use at diagnosis of LRD | |||
 ≤2 mg/kg ≤2 mg/kg | 106 (90%) | 82 (89%) | 24 (92%) |
 >2 mg/kg >2 mg/kg | 12 (10%) | 10 (11%) | 2 (8%) |
| Palivizumab | |||
 No No | 76 (64%) | 60 (65%) | 16 (62%) |
 Yes Yes | 42 (36%) | 32 (35%) | 10 (38%) |
| IVIGf | |||
 No No | 69 (58%) | 57 (62%) | 12 (46%) |
 Yes Yes | 49 (42%) | 35 (38%) | 14 (54%) |
| Preemptive ribavirin prior to LRD | |||
 None None | 92 (78%) | 69 (75%) | 23 (88%) |
 <5 d <5 d | 10 (8%) | 9 (10%) | 1 (4%) |
 ≥5 d ≥5 d | 16 (14%) | 14 (15%) | 2 (8%) |
| Ribavirin treatment for LRD | |||
 None None | 17 (14%) | 16 (17%) | 1 (4%) |
 Systemicg Systemicg | 13 (11%) | 11 (12%) | 2 (8%) |
 Aerosolizedh Aerosolizedh | 88 (75%) | 65 (71%) | 23 (88%)i |
| FEV/FVC % prior to RSV LRD | |||
 ≥70 ≥70 | 90 (81%) | 71 (81%) | 19 (83%) |
 <70 <70 | 21 (19%) | 17 (19%) | 4 (17%) |
| TLC % prior to RSV LRD | |||
 ≥80 ≥80 | 96 (95%) | 78 (95%) | 18 (95%) |
 <80 <80 | 5 (5%) | 4 (5%) | 1 (5%) |
Data are presented No. (%) unless otherwise specified. Patients' demographic characteristics were compared using χ2 test or Fisher exact test for categorical variables, as appropriate.
Abbreviations: BM/CB, bone marrow/cord blood; CT, computed tomography; CXR, chest radiography; FEV, forced expiratory volume; FVC, forced vital capacity; IQR, interquartile range; IVIG, intravenous immunoglobulin; LRD, lower respiratory disease; MA, myeloablative; PBSC, peripheral blood stem cell; RSV, respiratory syncytial virus; TBI, total body irradiation; TLC, total lung capacity; WBC, white blood cell.
a P = .037.
b Underlying disease risk defined as previously described [29].
c Four cord blood, 63 bone marrow.
d Copathogens defined as pathogenic bacteria, fungi, or opportunistic viruses from bronchoalveolar lavage and/or blood obtained within 2 days of RSV LRD diagnosis.
e Including those requiring continuous positive airway pressure and mechanical ventilation.
f IVIG was administered at the discretion of the attending physician.
g Intravenously dosed as a loading dose (35 mg/kg in 3 divided doses every 8 hours) followed by a maintenance dose (25 mg/kg in 3 divided doses every 8 hours for 6 days) [30] or orally dosed as a loading dose (10 mg/kg) followed by 400 mg every 8 hours on day 2 and 600 mg every 8 hours on day 3 [31]. Oral, n = 4; intravenous, n = 9.
h Current practice guidelines at our center involve treatment with aerosolized ribavirin in all patients with RSV LRD, dosed as 6 g as a single dose over 18 hours or 2 g three times a day over 2 hours [15].
i Ten patients also received palivizumab; 9 patients also received IVIG.
RESULTS
Characteristics of Patients and Samples
We identified 118 patients with RSV LRD from January 1990 through April 2011. Characteristics of the cohort are outlined in Table Table1.1. The median time to LRD after HCT was 52.5 days (range, −1 to 3957 days). Among patients with URD prior to LRD (n = 86), the median duration between URD and LRD diagnoses was 5 days (range, 0–74 days). Twenty-six (22%) patients received antiviral therapy prior to the development of RSV LRD. Antiviral treatment for LRD was initiated at a median of 1 day (range, 0–9 days) following LRD diagnosis.
Of the 118 patients with RSV LRD, 92 (78%) had serum samples available for testing (Supplementary Figure 1). The patients with samples available for serum testing were representative of the entire cohort; sex was the only variable with significant variation between the groups (P = .037; Table Table1).1). A total of 380 samples were tested for RSV RNA; an average of 4 samples were tested per patient (range, 1–10).
Frequency and Timing of Viral RNA Detection in Serum/Plasma
Overall, RSV RNA was detected in serum samples from 28 of 92 (30%) patients in 41 of 380 (11%) samples tested. The detection rate varied by year when analyzed over equal time periods: 1990–1994 (13/31 [42%] samples; 1995–1999 (4/28 [14%] samples); 2000–2004 (1/8 [13%] samples); and 2005–2011 (10/24 [42%] samples). RSV RNA detection occurred a median of 4 days following LRD diagnosis (range, −20 to 20 days; Figure Figure1).1). The median number of positive samples per patient was 1 (range, 1–5). The median maximum RNA load among positive results was 2.74 log10 copies/mL (range, 2.03–4.17 log10 copies/mL). Eleven of the 41 (27%) positive samples were positive in both extraction aliquots. The median viral load for samples positive in both duplicates was statistically higher than the median viral load for single positive samples (3.11 vs 2.55 log10 copies/mL; P = .007).
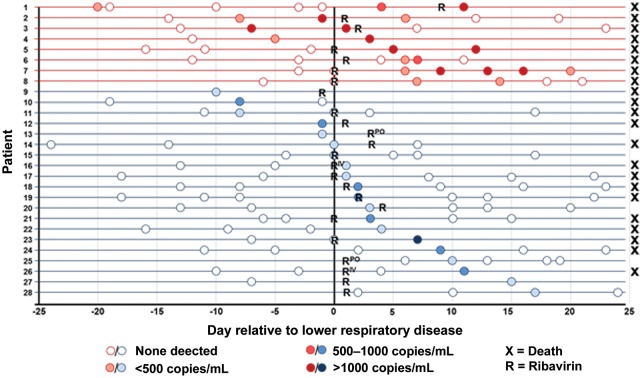
Detection of respiratory syncytial virus (RSV) RNA in blood relative to lower respiratory disease. Horizontal lines represent individual patients; circles represent serum sample time points. Patients with multiple days of RSV RNA detection are shown in red; patients with single positive serum samples are shown in blue. Abbreviations: R = initiation of aerosolized ribavirin; RPO = initiation of oral ribavirin; RIV = initiation of intravenous ribavirin.
Twenty-four of the 28 patients with RSV RNA detected received antiviral therapy (4 systemic ribavirin; 20 aerosolized ribavirin). In 14 patients, RSV RNA was detected only once (n = 11) or twice (n = 3) during the course of antiviral therapy. One patient had 5 positive samples while on therapy (6, 9, 13, 16, and 20 days after starting ribavirin; Figure Figure11).
Risk Factors for Viral RNA Detection
In univariate analysis, treatment with ribavirin, steroid use of >2 mg/kg, mechanical ventilation at time of diagnosis, leukopenia, neutropenia, monocytopenia, and thrombocytopenia were associated with increased risk of RSV RNA detection in 92 patients with serum samples available; lymphopenia was not significantly associated with increased risk (Table (Table2).2). RSV RNA was detected in 15 of 34 patients (44%) with profound leukopenia (white blood cell [WBC] counts ≤1000 × 106/L), compared with 13 of 58 (22%) patients with WBC counts >1000 × 106 cells/L (HR, 3.34 [95% confidence interval {CI}, 1.55–7.17], P = .002). In multivariable models adjusting for steroid use and either lymphopenia, neutropenia, monocytopenia, or thrombocytopenia, mechanical ventilation consistently showed an association with RSV RNA detection; neutropenia, monocytopenia and severe thrombocytopenia (platelet count ≤10 000 × 106 cells/L) also each showed increased risk for RSV RNA detection (Table (Table33).
Table 2.
Univariate Analysis of Risk Factors for Respiratory Syncytial Virus RNA Detection in Blood (n = 92)
| Covariate | HR (95% CI) | P Value |
|---|---|---|
| Transplant year | ||
 1989–1997 1989–1997 | 1.00 | |
 1998–2010 1998–2010 | 0.65 (.30–1.44) | .289 |
| Transplant year as continuous covariate | 0.96 (.89–1.02) | .186 |
| Age at transplant as continuous covariate | 1.00 (.98–1.03) | .78 |
| Cell source | ||
 PBSC PBSC | 1.00 | |
 BM/CB BM/CB | 2.06 (.90–4.75) | .088 |
| Donor type | ||
 Autologous Autologous | 1.00 | |
 Allogeneic Allogeneic | 1.01 (.37–2.79) | .98 |
| Conditioning regimen | ||
 MA + TBI (≥12 Gy) MA + TBI (≥12 Gy) | 1.00 | |
 MA ± low TBI MA ± low TBI | 1.58 (.70–3.59) | .271 |
 Nonmyeloablative Nonmyeloablative | 0.81 (.26–2.57) | .727 |
| WBC count, 106 cells/L | ||
 >1000 >1000 | 1.00 | |
 ≤1000 ≤1000 | 3.34 (1.55–7.17) | .002 |
| Lymphocyte count, 106 cells/L | ||
 ≥100 ≥100 | 1.00 | |
 <100 <100 | 1.54 (.70–3.38) | .282 |
| Neutrophil count, 106 cells/L | ||
 ≥100 ≥100 | 1.00 | |
 <100 <100 | 3.82 (1.76–8.28) | <.001 |
| Monocyte count, 106 cells/L | ||
 ≥100 ≥100 | 1.00 | |
 <100 <100 | 4.01 (1.59–10.1) | .003 |
| Platelet count, 106 cells/L | ||
 ≥10 000 ≥10 000 | 1.00 | |
 <10 000 <10 000 | 2.52 (1.18–5.38) | .017 |
| Day of RSV LRD following HCT | ||
 ≥30 ≥30 | 1.00 | |
 <30 <30 | 2.88 (1.33–6.21) | .007 |
| Copathogena | ||
 None None | 1.00 | |
 Any copathogen Any copathogen | 0.87 (.40–1.91) | .735 |
| Oxygen at diagnosis of LRD | ||
 None to ≤2 L None to ≤2 L | 1.00 | |
 >2 L/ventilatorb >2 L/ventilatorb | 2.85 (1.30–6.24) | .009 |
| Mechanical ventilation as time-dependent | 18.9 (7.80–45.7) | <.001 |
| Steroid use >2 mg/kg as time-dependent | 5.20 (1.51–18.0) | .009 |
| Palivizumab treatment | ||
 No No | 1.00 | |
 Yes Yes | 0.86 (.39–1.88) | .698 |
| Preemptive ribavirin prior to LRD | ||
 None None | 1.00 | |
 <5 d <5 d | 0.50 (.12–2.15) | .354 |
 ≥5 d ≥5 d | 0.46 (.14–1.53) | .204 |
| Ribavirin treatment as time-dependentc,d | 2.89 (1.26–6.62) | .012 |
| Acute GVHD as time-dependente (grade 0–2 vs 3–4) | 1.55 (.63–3.77) | .339 |
| FEV/FVC % prior to RSV LRD | ||
 ≥70 ≥70 | 1.00 | |
 <70 <70 | 0.49 (.15–1.62) | .241 |
| TLC % prior to RSV LRD | ||
 ≥80 ≥80 | 1.00 | |
 <80 <80 | 2.25 (.53–9.57) | .273 |
| IVIGf | ||
 None None | 1.00 | |
 Weekly Weekly | 0.80 (.30–2.18) | .668 |
 High High | 1.08 (.40–2.94) | .877 |
Abbreviations: BM/CB, bone marrow/cord blood; CI, confidence interval; FEV, forced expiratory volume; FVC, forced vital capacity; GVHD, graft-vs-host disease; HCT, hematopoietic cell transplant; HR, hazard ratio; IVIG, intravenous immunoglobulin; LRD, lower respiratory disease; MA, myeloablative; PBSC, peripheral blood stem cell; RSV, respiratory syncytial virus; TBI, total body irradiation; TLC, total lung capacity; WBC, white blood cell.
a Copathogens defined as pathogenic bacteria, fungi, or opportunistic viruses from bronchoalveolar lavage and/or blood obtained within 2 days of RSV LRD diagnosis. Copathogens in 28 patients with RSV RNA detected: 2 rhinovirus, 1 adenovirus, 2 fungal, 3 bacterial, 4 multiple pathogens.
b Including those requiring continuous positive airway pressure and mechanical ventilation.
c Intravenously dosed as a loading dose (35 mg/kg in 3 divided doses every 8 hours) followed by a maintenance dose (25 mg/kg in 3 divided doses every 8 hours for 6 days) [30] or orally dosed as a loading dose (10 mg/kg) followed by 400 mg every 8 hours on day 2 and 600 mg every 8 hours on day 3 [31].
d Current practice guidelines at our center involve treatment with aerosolized ribavirin in all patients with RSV LRD, dosed as 6 g as a single dose over 18 hours or 2 g three times a day over 2 hours [15].
e Acute GVHD defined as previously described [32].
f IVIG was administered at the discretion of the attending physician.
Table 3.
Multivariable Analyses of Risk Factors for Respiratory Syncytial Virus RNA Detection (n = 92)
| Covariates | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Steroid use >2 mg/kg as time-dependent | 4.02 (.95–17.1) | .059 | 2.92 (.71–12.0) | .136 | 2.65 (.63–11.2) | .184 | 2.61 (.68–10.0) | .163 |
| Mechanical ventilation as time-dependent | 12.6 (4.81–32.8) | <.001 | 13.6 (5.09–36.4) | <.001 | 16.1 (6.22–41.5) | <.001 | 16.5 (6.73–40.5) | <.001 |
| Neutrophil count, 106 cells/L (<100 vs ≥100) | 3.46 (1.47–8.18) | .005 | … | … | … | … | … | … |
| Monocyte count, 106 cells/L (<100 vs ≥100) | … | … | 2.86 (1.07–7.65) | .036 | … | … | … | … |
| Lymphocyte count, 106 cells/L (<100 vs ≥100) | … | … | … | … | 1.05 (.44–2.47) | .915 | … | … |
| Platelet count, 106 cells/L (<10000 vs ≥10000) | … | … | … | … | … | … | 2.49 (1.13–5.47) | .023 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
BAL viral loads were available in 30 of the 92 patients with serum tested for RSV RNA. Among these patients, BAL viral load above the median (>1.8 × 106 copies/mL) was associated with an increased risk of serum RSV RNA detection (HR, 1.74 [95% CI, .74–4.13], P = .21) in univariate analysis. Multivariable analysis could not be performed due to the limited number of subjects and events.
RSV RNA Detection as a Risk Factor for Outcomes
Among 92 patients with serum available for testing, RSV RNA detection was evaluated in a time-dependent manner in univariate and multivariable models. Detection of RSV RNA was a statistically significant risk factor for death at day 90 following RSV LRD in multiple models adjusting for ribavirin treatment plus 1 other covariate (eg, adjusted HR [aHR] of 2.09 [95% CI, 1.15–3.81], P = .02, after adjusting for transplant year), with the exception of the model including oxygen use (Figure (Figure22A). In models for pulmonary death, statistical significance was not reached. Of the 28 patients with RSV RNA detected, 22 died. All 8 patients with detection of RSV RNA in >1 sample died.
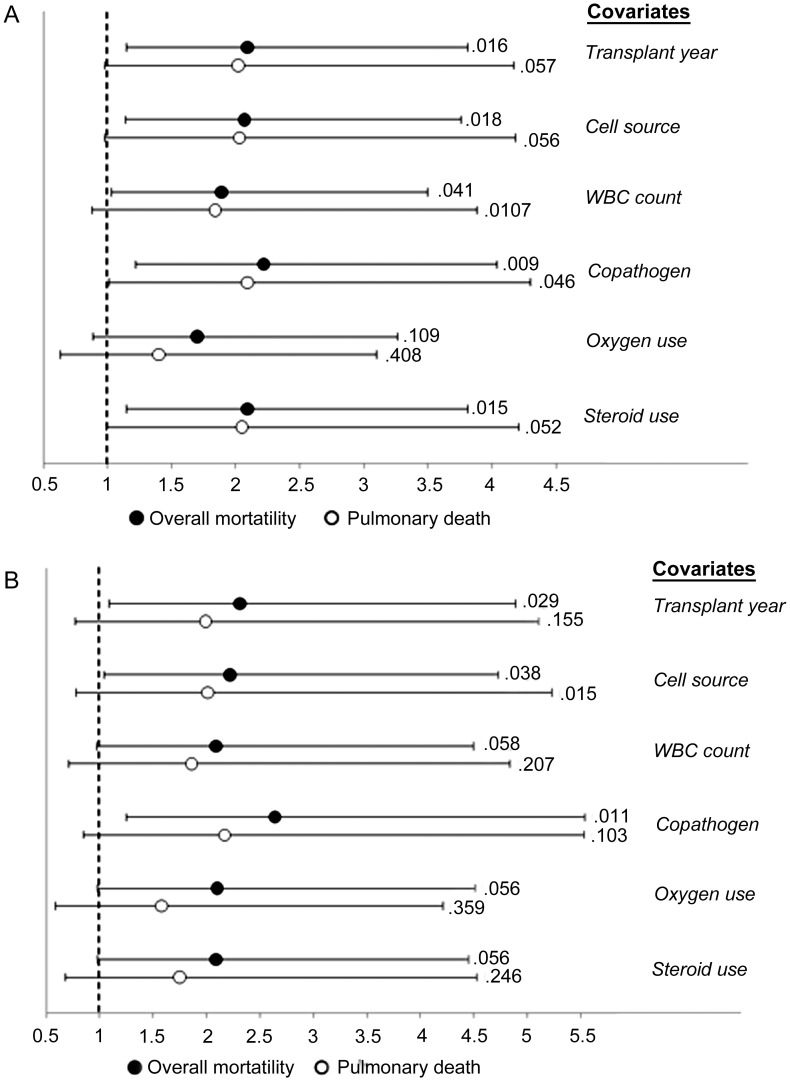
Hazard ratios and 95% confidence intervals from multivariable models evaluating respiratory syncytial virus (RSV) RNA detection in blood as a risk factor for overall mortality and pulmonary death by day 90 following RSV lower respiratory disease (LRD; n = 92), including P values. Hazard ratios and P values shown are for RSV RNA detection. Models for outcomes at day 30 following RSV LRD similarly showed RSV RNA detection as a risk factor for overall mortality and pulmonary death (data not shown). A, All models included presence of any RSV RNA and ribavirin treatment plus 1 other covariate. B, All models include presence of RSV RNA above median (2.74 log10 copies/mL) and ribavirin treatment plus 1 other covariate. Abbreviation: WBC, white blood cell.
When comparing patients with peak serum viral loads above vs below the median and adjusting for the variables above, higher RSV viral load was associated with increased risk of overall death (Figure (Figure22B). Additionally, we compared the effect of 1 vs 2 positive RSV RNA detection results on mortality and did not see significant changes in HRs (data not shown).
In univariate analysis, RSV RNA detection was associated with an increased risk of respiratory failure (RR, 1.83 [95% CI, .99–3.38], P = .055). When multivariable models were performed adjusting for oxygen use and 1 other covariate at a time (copathogens, WBC count, platelet count, day of LTD after transplant, age at transplant, and conditioning regimen), the effect of RSV RNA detection was no longer significant (data not shown).
The effect of RSV RNA detection on length of hospitalization was evaluated by comparing days alive while not hospitalized within the first 28 days following diagnosis of LTD. For patients with RSV RNA detected on or before LTD diagnosis (n = 11), the median number of days was 0 (range 0–16), whereas the median number of days was 4 (range, 0–24) for patients without RSV RNA detected (P = .038). After adjusting for oxygen use, RSV RNA detection remained a significant factor affecting days alive and not hospitalized (mean difference, −5.49 days [95% CI, 10.5 to −.47], P = .032).
Host and Transplant Factors Associated With Mortality
Among all 118 patients, overall mortality at day 30 and day 90 following RSV LRD was 39% (n = 46) and 47% (n = 56), respectively. Death from respiratory failure was observed in 29% (n = 34) and 34% (n = 40) of patients at day 30 and day 90 following RSV LRD, respectively. In unadjusted models, patients who received aerosolized ribavirin treatment had improved survival compared with those who did not receive any ribavirin treatment after LRD (60% vs 24% for overall death and 73% vs 35% for pulmonary death; P < .0001 for both; Supplementary Figure 2A and 2B). Survival curves stratified by patients who did and did not receive oxygen therapy at LRD diagnosis for those patients who received none or systemic ribavirin only are shown in Supplementary Figures 2C–F.
Univariate analysis at day 90 following RSV LRD revealed several factors that were statistically significantly associated with overall mortality and pulmonary death including transplant year, cell source, conditioning regimen, WBC count, monocyte count, platelet count, oxygen requirement at diagnosis, steroid use at diagnosis, palivizumab treatment, and ribavirin treatment following LRD (Supplementary Table 1). Treatment with aerosolized ribavirin showed a protective effect against mortality at day 90 in multiple models when adjusting for cell source, oxygen requirement at diagnosis, WBC count, and presence of copathogens (Table (Table4).4). The protective effect of aerosolized ribavirin was also demonstrated in multivariable models determining risk for pulmonary death (Table (Table55).
Table 4.
Multivariable Analyses of Risk Factors and Treatment Efficacy for Overall Mortality by Day 90 Following Respiratory Syncytial Virus Lower Respiratory Disease (n = 118)
| Covariates | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Ribavirin treatment for LRD | ||||||
 Systemica vs none Systemica vs none | 0.71 (.28–1.76) | .454 | 0.61 (.24–1.56) | .303 | 0.86 (.34–2.17) | .751 |
 Aerosolizedb vs none Aerosolizedb vs none | 0.33 (.17–.64) | .001 | 0.28 (.14–.55) | <.001 | 0.39 (.20–.77) | .006 |
| Oxygen at diagnosis of LRD | ||||||
 >2 L/ventilatorc vs none/≤2 L >2 L/ventilatorc vs none/≤2 L | 2.73 (1.58–4.71) | <.001 | 2.50 (1.46–4.29) | <.001 | 2.63 (1.53–4.51) | <.001 |
| Cell source | ||||||
 BM/CB vs PBSC BM/CB vs PBSC | 2.44 (1.28–4.64) | .006 | 2.35 (1.23–4.49) | .01 | 2.81 (1.49–5.30) | .001 |
| Steroid use at diagnosis of LRD | ||||||
 >2 mg/kg vs ≤2 mg/kg >2 mg/kg vs ≤2 mg/kg | 2.46 (1.24–4.92) | .01 | … | … | … | … |
| WBC count, 106 cells/L | ||||||
 ≤1000 vs >1000 ≤1000 vs >1000 | … | … | 1.97 (1.14–3.41) | .016 | … | … |
| Copathogend | ||||||
 Any vs none Any vs none | … | … | … | … | 1.86 (1.07–3.22) | .027 |
Analysis of shorter-term mortality (day 30 following respiratory syncytial virus [RSV] LRD) showed similar results (data not shown).
Abbreviations: BM/CB, bone marrow/cord blood; CI, confidence interval; HR, hazard ratio; LRD, lower respiratory disease; PBSC, peripheral blood stem cell; WBC, white blood cell.
a Intravenously dosed as a loading dose (35 mg/kg in 3 divided doses every 8 hours) followed by a maintenance dose (25 mg/kg in 3 divided doses every 8 hours for 6 days) [30] or orally dosed as a loading dose (10 mg/kg) followed by 400 mg every 8 hours on day 2 and 600 mg every 8 hours on day 3 [31].
b Current practice guidelines at our center involve treatment with aerosolized ribavirin in all patients with RSV LRD, dosed as 6 g as a single dose over 18 hours or 2 g three times a day over 2 hours [15].
c Including those requiring continuous positive airway pressure and mechanical ventilation.
d Copathogens defined as pathogenic bacteria, fungi or opportunistic viruses from bronchoalveolar lavage and/or blood obtained within 2 days of RSV LRD diagnosis.
Table 5.
Multivariable Analyses of Risk Factors and Treatment Efficacy for Pulmonary Death by Day 90 Following Respiratory Syncytial Virus Lower Respiratory Disease (n = 118)
| Covariates | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| HR 95% CI | P Value | HR 95% CI | P Value | HR 95% CI | P Value | |
| Ribavirin treatment for LRD | ||||||
 Systemica vs none Systemica vs none | 0.55 (.18–1.68) | .296 | 0.47 (.16–1.37) | .165 | 0.31 (.10–.89) | .031 |
 Aerosolizedb vs none Aerosolizedb vs none | 0.26 (.12–.55) | <.001 | 0.31 (.15–.67) | .003 | 0.19 (.09–.39) | <.001 |
| Oxygen at diagnosis of LRD | ||||||
 >2 L/ventilatorc vs none/≤2 L >2 L/ventilatorc vs none/≤2 L | 3.42 (1.78–6.57) | <.001 | 2.05 (.96–4.35) | .063 | 3.13 (1.63–6.00) | <.001 |
| Cell source | ||||||
 BM/CB vs PBSC BM/CB vs PBSC | 2.48 (1.15–5.31) | .02 | … | … | … | … |
| Steroid use at diagnosis of LRD | ||||||
 >2 mg/kg vs ≤2 mg/kg >2 mg/kg vs ≤2 mg/kg | … | … | 2.62 (1.20–5.72) | .016 | … | … |
| WBC count, 106 cells/L | ||||||
 ≤1000 vs >1000 ≤1000 vs >1000 | … | … | … | … | 2.15 (1.13–4.06) | .019 |
Analysis of shorter-term mortality (day 30 following RSV LRD) showed similar results (data not shown).
Abbreviations: BM/CB, bone marrow/cord blood; CI, confidence interval; HR, hazard ratio; LRD, lower respiratory disease; PBSC, peripheral blood stem cell; WBC, white blood cell.
a Intravenously dosed as a loading dose (35 mg/kg in 3 divided doses every 8 hours) followed by a maintenance dose (25 mg/kg in 3 divided doses every 8 hours for 6 days) [30] or orally dosed as a loading dose (10 mg/kg) followed by 400 mg every 8 hours on day 2 and 600 mg every 8 hours on day 3 [31].
b Current practice guidelines at our center involve treatment with aerosolized ribavirin in all patients with RSV LRD, dosed as 6 g as a single dose over 18 hours or 2 g three times a day over 2 hours [15].
c Including those requiring continuous positive airway pressure and mechanical ventilation.
DISCUSSION
We have demonstrated several host, transplant, virologic, and treatment factors associated with increased overall and pulmonary death in HCT recipients with RSV LRD. In particular, we have shown the presence of RSV RNA in blood in a significant proportion (30%) of patients with RSV LRD and correlated RSV RNA detection with mortality in multivariable models. Additionally, we have shown a protective effect of aerosolized ribavirin for overall and pulmonary mortality in multivariable models.
Risk factors for RSV RNA detection in blood included mechanical ventilation, suggesting that acute lung injury plays a role in pathogenesis of RSV infection and possibly in dissemination of viral nucleic acid into the bloodstream. This hypothesis is also supported by the association between thrombocytopenia and RSV RNA detection, as well as the association between higher BAL viral load and serum RSV RNA detection. Other risk factors for RSV RNA detection in plasma/serum include neutropenia and monocytopenia, implying that the inflammatory response plays a part in viral containment within the respiratory system. Pathological examinations in autopsy specimens have shown alveolar mononuclear cell infiltration in children with RSV [33], and BAL specimens in children with RSV bronchiolitis have shown that neutrophils comprise >80% of the inflammatory cell infiltrate at the height of clinical symptoms [34]. Furthermore, RSV nucleic acid has been demonstrated in monocytes and neutrophils in BAL and blood specimens in adults and children with RSV infection [24, 25, 35, 36]. Interestingly, our study did not show a substantial effect of lymphopenia or steroid use up to 2 mg/kg on RSV RNA detection, indicating that moderate immune suppression does not impact blood RSV RNA detection.
The higher rate of serum RSV RNA detection in this study compared to previous reports by our group may be explained by duplicate extraction and RT-PCR testing [26, 27]. Although duplicate testing is not a clinically practical method, it was performed to maximize detection of viral loads near the limit of detection (approximately 2.3 log10 copies/mL) and to account for stochastic distribution. Patients with duplicate positive samples had higher viral loads, and patients with viral loads above the median have an increased risk for death compared to patients with viral loads below the median.
Detection of RSV RNA in serum or plasma was associated with an increased risk of overall death in multivariable models, as well as decreased time alive and not hospitalized. Serum RSV RNA detection and its association with mortality and hospitalization suggest a role of viral dissemination in disease severity; however, this study did not directly investigate viral replication outside of the respiratory tract. Future studies will examine messenger RNA in peripheral blood samples to determine if a true viremic phase occurs in severe RSV infection.
This study allowed us to evaluate several clinical factors in univariate and multivariable analysis. Factors associated with increased mortality included use of oxygen at diagnosis, receipt of a bone marrow or cord blood transplant, steroid use >2 mg/kg prior to diagnosis, leukopenia, and presence of copathogens (Supplementary Table 1). Additionally, we were interested in the effect of ribavirin on clinical outcomes. We demonstrated that treatment of RSV LRD with aerosolized ribavirin in HCT recipients was consistently associated with decreased probability of overall death and death due to respiratory failure in multivariable models. Although our results suggest a stronger protective effect for aerosolized ribavirin over systemic ribavirin (Supplementary Figures 2A and 2B), the number of patients who received systemic ribavirin in this cohort is small (n = 13) and therefore these results should be interpreted cautiously.
Ribavirin has been available for the treatment of RSV infection for >30 years; its use in HCT recipients has been controversial due to the low quality of evidence to support its use. Treatment of RSV LRD with ribavirin has never been evaluated in randomized trials; however, many centers administer ribavirin with or without pooled IVIG, RSV-specific immunoglobulin, or palivizumab [8, 10]. A pooled analysis by Shah and Chemaly suggested that HCT recipients treated with ribavirin (with or without immunomodulators) had lower rates of mortality in a review of 21 separate studies [8]. Our study is the first analysis of a single cohort to show the protective effect of ribavirin on overall and pulmonary mortality in HCT recipients using multivariable models. Our data also show that all patients who required oxygen therapy and were not treated with ribavirin died.
HCT recipients with RSV who were transplanted earlier in our study had worse outcomes than more recent patients. This may be explained by improvement in supportive care and changes in transplantation practices in recent years, and suggests that earlier patients experienced more acute lung injury related to RSV LRD. Similarly, patients requiring increased oxygen support at time of LRD diagnosis were more likely to die. Oxygen use may serve as a marker for extent of lung injury, and its association with poor outcomes confirms previous findings [26, 29, 37]. Patients who received peripheral blood stem cell transplants had improved survival compared to patients who received bone marrow or cord blood transplants, suggesting an important role of immune reconstitution.
Several factors were not significantly associated with mortality. Most notably, steroid therapy up to 2 mg/kg/day at the time of diagnosis (given for treatment of GVHD) did not correlate with increased risk of overall or pulmonary death. Although palivizumab treatment was protective in univariate models, this is likely due to inclusion of earlier patients who received neither ribavirin nor palivizumab therapy. Palivizumab was not protective in multivariable models, a finding that has recently been analyzed separately in a homogenous cohort of patients who received aerosolized ribavirin [29]. Interestingly, although lymphopenia has been suggested as a risk factor for progression from URD to LRD [4, 12], we did not find lymphopenia to be associated with increased risk for death in patients with established RSV pneumonia. Low total WBC count did correlate with increased risk of death in multivariable analyses; monocytopenia correlated with increased death in univariate analysis. These findings suggest a role for other immune responses in disease severity.
Limitations of this study include the retrospective design and the lack of randomization in evaluating the ribavirin effect. The study period is long, and there have been changes in supportive care practices. We accounted for this by including time and transplant-related changes in our models. The relatively small number of patients meeting clinical endpoints limited our ability to establish a single model to account for all covariates. However, separate multivariable models showed a protective effect of ribavirin and the impact of serum/plasma RSV RNA detection on overall and pulmonary death when adjusting for important covariates. RNA degradation may have played a role in observed differences in detection levels over time. Our study tested only serum and plasma, whereas other studies have recovered RSV RNA from whole blood, peripheral blood monocytes, and neutrophils [23, 25, 38, 39]. Currently, prospective sample collection is under way to specifically evaluate these additional cellular compartments for the presence of RSV genomic RNA and RSV messenger RNA.
In conclusion, our data demonstrate the protective effect of ribavirin on overall mortality and pulmonary death at day 30 and day 90 following RSV LRD in HCT recipients in multivariable models. Detection of RSV RNA in blood was associated with an increased risk of death among HCT recipients with RSV LRD, suggesting that RSV RNA detection in blood may serve as a biomarker for poor overall outcomes and may indicate the need for more aggressive therapy in this population.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Chris Davis and Zach Stednick for database services, Terry Stevens-Ayers and Anne Cent for specimen processing, and Rohit Shankar and Reggie Sampoleo for laboratory testing.
We thank Chris Davis and Zach Stednick for database services, Terry Stevens-Ayers and Anne Cent for specimen processing, and Rohit Shankar and Reggie Sampoleo for laboratory testing.
Author contributions. A. W. performed the research, collected data, analyzed data, and wrote the manuscript; S. S. collected data and critically reviewed the manuscript; H. X. and W. L. performed statistical analyses, generated tables and figures, and critically reviewed the manuscript; A. P. C. and J. A. E. contributed to the analysis plan and critically reviewed the manuscript; J. K. contributed to assay development, provided resources, and critically reviewed the manuscript; K. R. J. provided resources and critically reviewed the manuscript; M. B. designed and performed the research, analyzed data, provided resources, and wrote the manuscript.
A. W. performed the research, collected data, analyzed data, and wrote the manuscript; S. S. collected data and critically reviewed the manuscript; H. X. and W. L. performed statistical analyses, generated tables and figures, and critically reviewed the manuscript; A. P. C. and J. A. E. contributed to the analysis plan and critically reviewed the manuscript; J. K. contributed to assay development, provided resources, and critically reviewed the manuscript; K. R. J. provided resources and critically reviewed the manuscript; M. B. designed and performed the research, analyzed data, provided resources, and wrote the manuscript.
Financial support. This work was supported by the National Institutes of Health (grant numbers CA18029, CA15704, HL081595, HL93294, K23HL091059, and L40AI071572). A. P. C. also received support from the Seattle Children's Center for Clinical and Translational Research and the Clinical and Translational Science Award (grant number ULI RR025014).
This work was supported by the National Institutes of Health (grant numbers CA18029, CA15704, HL081595, HL93294, K23HL091059, and L40AI071572). A. P. C. also received support from the Seattle Children's Center for Clinical and Translational Research and the Clinical and Translational Science Award (grant number ULI RR025014).
Potential conflicts of interest. J. A. E. has received research funding from Novartis and Gilead and served as a consultant for GSK. M. B. received research support from GSK and served as a consultant for Novartis, GSK, and Gilead Sciences. All other authors report no potential conflicts.
J. A. E. has received research funding from Novartis and Gilead and served as a consultant for GSK. M. B. received research support from GSK and served as a consultant for Novartis, GSK, and Gilead Sciences. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed
References
Articles from Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/cid/cit639
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/cid/article-pdf/57/12/1731/17350990/cit639.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/cid/cit639
Article citations
The Impact of Pretransplant Respiratory Virus Detection on Posttransplant Outcomes in Children Undergoing Hematopoietic Cell Transplantation.
Clin Infect Dis, 79(3):761-771, 01 Sep 2024
Cited by: 0 articles | PMID: 38666501
SARS-CoV-2 RNA and Nucleocapsid Antigen Are Blood Biomarkers Associated With Severe Disease Outcomes That Improve in Response to Remdesivir.
J Infect Dis, 230(3):624-634, 01 Sep 2024
Cited by: 2 articles | PMID: 38657001 | PMCID: PMC11420797
Evolving Epidemiology of Pediatric Respiratory Syncytial Virus (RSV) Cases Around COVID-19 Pandemic: Impact and Clinical Insights, Retrospective Cohort Study.
J Epidemiol Glob Health, 14(2):319-326, 04 Apr 2024
Cited by: 0 articles | PMID: 38573464
From Forgotten Pathogen to Target for New Vaccines: What Clinicians Need to Know about Respiratory Syncytial Virus Infection in Older Adults.
Viruses, 16(4):531, 29 Mar 2024
Cited by: 2 articles | PMID: 38675874 | PMCID: PMC11053843
Review Free full text in Europe PMC
Respiratory Syncytial Virus Infections in Recipients of Bone Marrow Transplants: A Systematic Review and Meta-Analysis.
Infect Dis Rep, 16(2):317-355, 29 Mar 2024
Cited by: 3 articles | PMID: 38667752 | PMCID: PMC11050314
Review Free full text in Europe PMC
Go to all (77) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Outcome of respiratory syncytial virus lower respiratory tract disease in hematopoietic cell transplant recipients receiving aerosolized ribavirin: significance of stem cell source and oxygen requirement.
Biol Blood Marrow Transplant, 19(4):589-596, 05 Jan 2013
Cited by: 46 articles | PMID: 23298855 | PMCID: PMC3667608
Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease.
J Infect Dis, 209(8):1195-1204, 23 Dec 2013
Cited by: 82 articles | PMID: 24368837 | PMCID: PMC3969549
Respiratory virus pneumonia after hematopoietic cell transplantation (HCT): associations between viral load in bronchoalveolar lavage samples, viral RNA detection in serum samples, and clinical outcomes of HCT.
J Infect Dis, 201(9):1404-1413, 01 May 2010
Cited by: 50 articles | PMID: 20350162 | PMCID: PMC2853730
Treatment of respiratory syncytial virus pneumonia in a lung transplant recipient: case report and review of the literature.
Pharmacotherapy, 24(7):932-938, 01 Jul 2004
Cited by: 13 articles | PMID: 15303457
Review
Funding
Funders who supported this work.
NCI NIH HHS (4)
Grant ID: P01 CA018029
Grant ID: P30 CA015704
Grant ID: CA15704
Grant ID: CA18029
NCRR NIH HHS (2)
Grant ID: UL1 RR025014
Grant ID: ULI RR025014
NHLBI NIH HHS (6)
Grant ID: R01 HL081595
Grant ID: HL081595
Grant ID: K24 HL093294
Grant ID: K23 HL091059
Grant ID: HL93294
Grant ID: K23HL091059
NIAID NIH HHS (2)
Grant ID: L40 AI071572
Grant ID: L40AI071572





