Abstract
Free full text

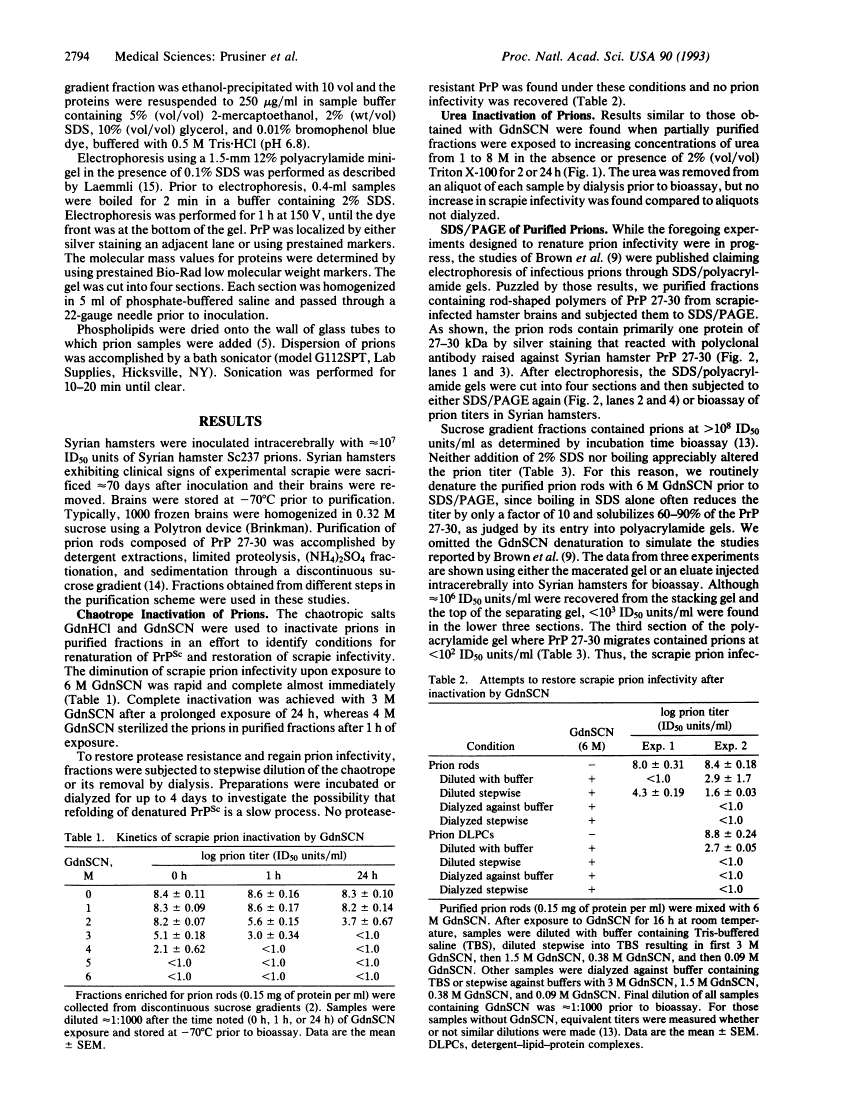
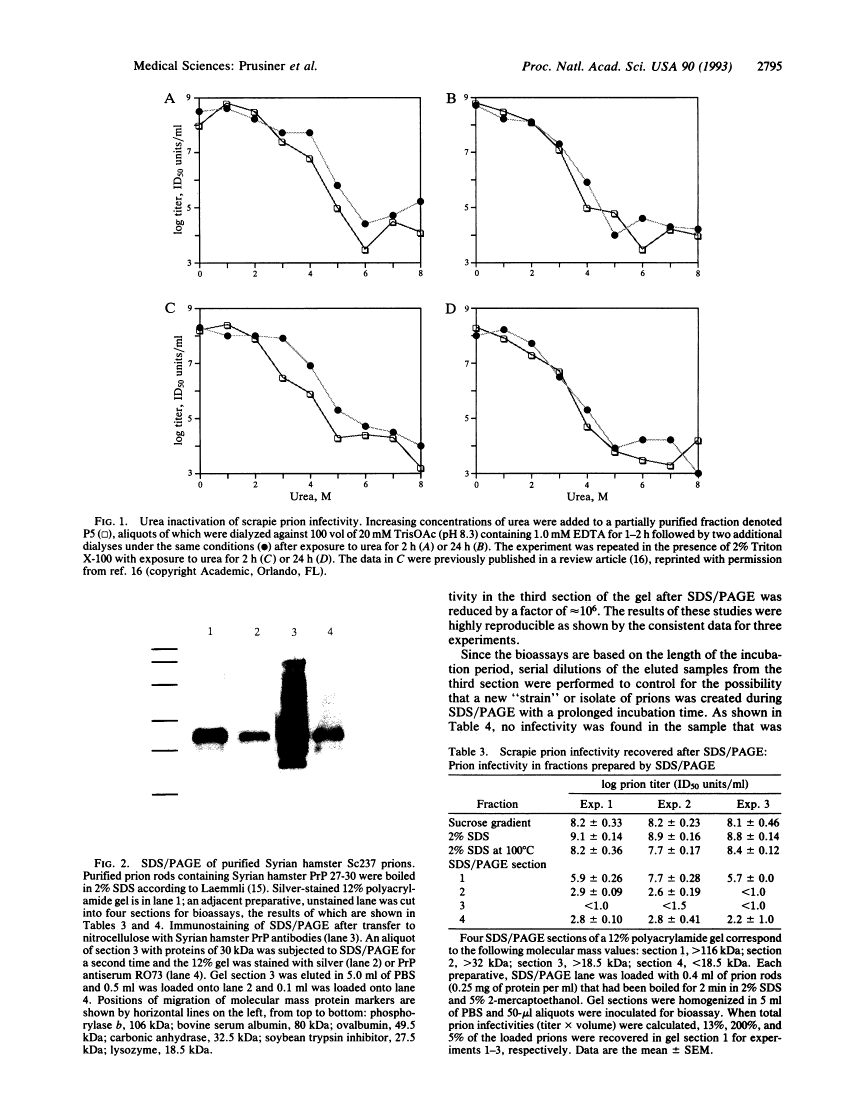
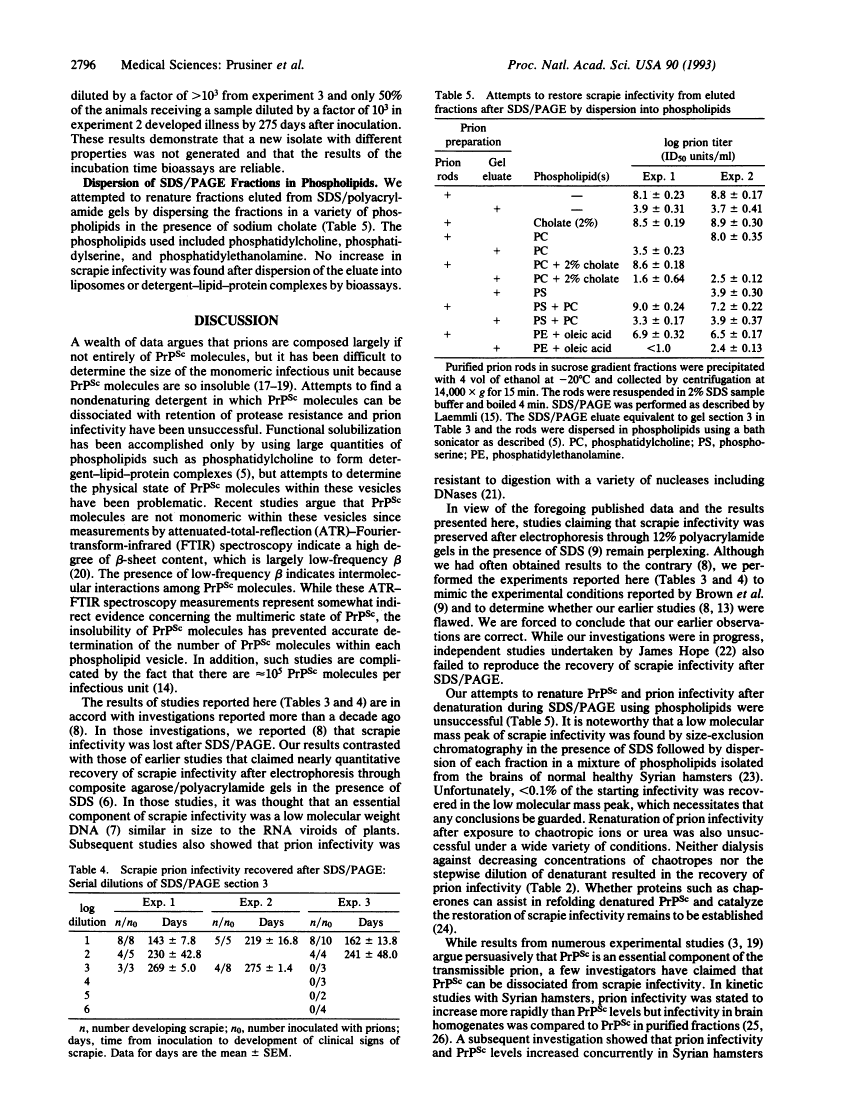
Attempts to restore scrapie prion infectivity after exposure to protein denaturants.
Abstract
A wealth of experimental evidence argues that infectious prions are composed largely, if not entirely, of the scrapie isoform of the prion protein. We attempted to restore scrapie infectivity after exposure to protein denaturants including urea, chaotropic salts, and SDS. None of the procedures restored infectivity. Dialysis to remove slowly chaotropic ions and urea failed to restore scrapie infectivity. Attempts to create monomers of the scrapie isoform of the prion protein under nondenaturing conditions using a wide variety of detergents have been unsuccessful, to date, except for one report claiming that scrapie infectivity could be recovered from 12% polyacrylamide gels after SDS/PAGE [Brown, P., Liberski, P. P., Wolff, A. & Gajdusek, D. C. (1990) Proc. Natl. Acad. Sci. USA 87, 7240-7244]. We found that < 0.001% of the infectious prion titer could be recovered from the region of a polyacrylamide gel where the denatured proteinase K-resistant core of the scrapie isoform of the prion protein and other 30-kDa proteins migrate. We conclude that under the denaturing conditions used for SDS/PAGE, the scrapie isoform of the prion protein is denatured and little or no renaturation occurs upon injection of fractions eluted from gels into animals for bioassays.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982 Dec 24;218(4579):1309–1311. [Abstract] [Google Scholar]
- Prusiner SB, Bolton DC, Groth DF, Bowman KA, Cochran SP, McKinley MP. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. [Abstract] [Google Scholar]
- Prusiner SB. Chemistry and biology of prions. Biochemistry. 1992 Dec 15;31(49):12277–12288. [Abstract] [Google Scholar]
- Borchelt DR, Scott M, Taraboulos A, Stahl N, Prusiner SB. Scrapie and cellular prion proteins differ in their kinetics of synthesis and topology in cultured cells. J Cell Biol. 1990 Mar;110(3):743–752. [Europe PMC free article] [Abstract] [Google Scholar]
- Gabizon R, McKinley MP, Prusiner SB. Purified prion proteins and scrapie infectivity copartition into liposomes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4017–4021. [Europe PMC free article] [Abstract] [Google Scholar]
- Malone TG, Marsh RF, Hanson RP, Semancik JS. Membrane-free scrapie activity. J Virol. 1978 Mar;25(3):933–935. [Europe PMC free article] [Abstract] [Google Scholar]
- Marsh RF, Malone TG, Semancik JS, Lancaster WD, Hanson RP. Evidence for an essential DNA component in the Scrapie agent. Nature. 1978 Sep 14;275(5676):146–147. [Abstract] [Google Scholar]
- Prusiner SB, Groth DF, Bildstein C, Masiarz FR, McKinley MP, Cochran SP. Electrophoretic properties of the scrapie agent in agarose gels. Proc Natl Acad Sci U S A. 1980 May;77(5):2984–2988. [Europe PMC free article] [Abstract] [Google Scholar]
- Brown P, Liberski PP, Wolff A, Gajdusek DC. Conservation of infectivity in purified fibrillary extracts of scrapie-infected hamster brain after sequential enzymatic digestion or polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7240–7244. [Europe PMC free article] [Abstract] [Google Scholar]
- Scott M, Foster D, Mirenda C, Serban D, Coufal F, Wälchli M, Torchia M, Groth D, Carlson G, DeArmond SJ, et al. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell. 1989 Dec 1;59(5):847–857. [Abstract] [Google Scholar]
- Marsh RF, Kimberlin RH. Comparison of scrapie and transmissible mink encephalopathy in hamsters. II. Clinical signs, pathology, and pathogenesis. J Infect Dis. 1975 Feb;131(2):104–110. [Abstract] [Google Scholar]
- Kimberlin RH, Walker C. Characteristics of a short incubation model of scrapie in the golden hamster. J Gen Virol. 1977 Feb;34(2):295–304. [Abstract] [Google Scholar]
- Prusiner SB, Cochran SP, Groth DF, Downey DE, Bowman KA, Martinez HM. Measurement of the scrapie agent using an incubation time interval assay. Ann Neurol. 1982 Apr;11(4):353–358. [Abstract] [Google Scholar]
- Prusiner SB, McKinley MP, Bowman KA, Bolton DC, Bendheim PE, Groth DF, Glenner GG. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Prusiner SB. Prions: novel infectious pathogens. Adv Virus Res. 1984;29:1–56. [Abstract] [Google Scholar]
- Bellinger-Kawahara CG, Kempner E, Groth D, Gabizon R, Prusiner SB. Scrapie prion liposomes and rods exhibit target sizes of 55,000 Da. Virology. 1988 Jun;164(2):537–541. [Abstract] [Google Scholar]
- Gabizon R, Prusiner SB. Prion liposomes. Biochem J. 1990 Feb 15;266(1):1–14. [Europe PMC free article] [Abstract] [Google Scholar]
- Prusiner SB. Molecular biology of prion diseases. Science. 1991 Jun 14;252(5012):1515–1522. [Abstract] [Google Scholar]
- Gasset M, Baldwin MA, Fletterick RJ, Prusiner SB. Perturbation of the secondary structure of the scrapie prion protein under conditions that alter infectivity. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):1–5. [Europe PMC free article] [Abstract] [Google Scholar]
- Diener TO, McKinley MP, Prusiner SB. Viroids and prions. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5220–5224. [Europe PMC free article] [Abstract] [Google Scholar]
- Safar J, Wang W, Padgett MP, Ceroni M, Piccardo P, Zopf D, Gajdusek DC, Gibbs CJ., Jr Molecular mass, biochemical composition, and physicochemical behavior of the infectious form of the scrapie precursor protein monomer. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6373–6377. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhi W, Landry SJ, Gierasch LM, Srere PA. Renaturation of citrate synthase: influence of denaturant and folding assistants. Protein Sci. 1992 Apr;1(4):522–529. [Europe PMC free article] [Abstract] [Google Scholar]
- Czub M, Braig HR, Diringer H. Pathogenesis of scrapie: study of the temporal development of clinical symptoms, of infectivity titres and scrapie-associated fibrils in brains of hamsters infected intraperitoneally. J Gen Virol. 1986 Sep;67(Pt 9):2005–2009. [Abstract] [Google Scholar]
- Czub M, Braig HR, Diringer H. Replication of the scrapie agent in hamsters infected intracerebrally confirms the pathogenesis of an amyloid-inducing virosis. J Gen Virol. 1988 Jul;69(Pt 7):1753–1756. [Abstract] [Google Scholar]
- Jendroska K, Heinzel FP, Torchia M, Stowring L, Kretzschmar HA, Kon A, Stern A, Prusiner SB, DeArmond SJ. Proteinase-resistant prion protein accumulation in Syrian hamster brain correlates with regional pathology and scrapie infectivity. Neurology. 1991 Sep;41(9):1482–1490. [Abstract] [Google Scholar]
- Xi YG, Ingrosso L, Ladogana A, Masullo C, Pocchiari M. Amphotericin B treatment dissociates in vivo replication of the scrapie agent from PrP accumulation. Nature. 1992 Apr 16;356(6370):598–601. [Abstract] [Google Scholar]
- Taraboulos A, Jendroska K, Serban D, Yang SL, DeArmond SJ, Prusiner SB. Regional mapping of prion proteins in brain. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7620–7624. [Europe PMC free article] [Abstract] [Google Scholar]
- Hecker R, Taraboulos A, Scott M, Pan KM, Yang SL, Torchia M, Jendroska K, DeArmond SJ, Prusiner SB. Replication of distinct scrapie prion isolates is region specific in brains of transgenic mice and hamsters. Genes Dev. 1992 Jul;6(7):1213–1228. [Abstract] [Google Scholar]
- Fradkin JE, Schonberger LB, Mills JL, Gunn WJ, Piper JM, Wysowski DK, Thomson R, Durako S, Brown P. Creutzfeldt-Jakob disease in pituitary growth hormone recipients in the United States. JAMA. 1991 Feb 20;265(7):880–884. [Abstract] [Google Scholar]
- Buchanan CR, Preece MA, Milner RD. Mortality, neoplasia, and Creutzfeldt-Jakob disease in patients treated with human pituitary growth hormone in the United Kingdom. BMJ. 1991 Apr 6;302(6780):824–828. [Europe PMC free article] [Abstract] [Google Scholar]
- Pocchiari M, Peano S, Conz A, Eshkol A, Maillard F, Brown P, Gibbs CJ, Jr, Xi YG, Tenham-Fisher E, Macchi G. Combination ultrafiltration and 6 M urea treatment of human growth hormone effectively minimizes risk from potential Creutzfeldt-Jakob disease virus contamination. Horm Res. 1991;35(3-4):161–166. [Abstract] [Google Scholar]
- Taylor DM, Dickinson AG, Fraser H, Robertson PA, Salacinski PR, Lowry PJ. Preparation of growth hormone free from contamination with unconventional slow viruses. Lancet. 1985 Aug 3;2(8449):260–262. [Abstract] [Google Scholar]
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. [Abstract] [Google Scholar]
- Chesebro B. Spongiform encephalopathies. PrP and the scrapie agent. Nature. 1992 Apr 16;356(6370):560–560. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.90.7.2793
Read article for free, from open access legal sources, via Unpaywall:
http://www.pnas.org/content/90/7/2793.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.90.7.2793
Article citations
Evidence for preexisting prion substrain diversity in a biologically cloned prion strain.
PLoS Pathog, 19(9):e1011632, 05 Sep 2023
Cited by: 5 articles | PMID: 37669293 | PMCID: PMC10503715
Heat inactivation of stable proteinaceous particles for future sample return mission architecture.
Front Microbiol, 13:911091, 09 Aug 2022
Cited by: 0 articles | PMID: 36016789 | PMCID: PMC9396123
Chronic Wasting Disease (CWD) in Cervids and the Consequences of a Mutable Protein Conformation.
ACS Omega, 7(15):12474-12492, 04 Apr 2022
Cited by: 5 articles | PMID: 35465121 | PMCID: PMC9022204
Review Free full text in Europe PMC
Detecting Differences in Prion Protein Conformation by Quantifying Methionine Oxidation.
ACS Omega, 7(3):2649-2660, 07 Jan 2022
Cited by: 2 articles | PMID: 35097263 | PMCID: PMC8793083
Quantifying the Role of Lysine in Prion Replication by Nano-LC Mass Spectrometry and Bioassay.
Front Bioeng Biotechnol, 8:562953, 23 Sep 2020
Cited by: 1 article | PMID: 33072723 | PMCID: PMC7542330
Go to all (60) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Disruption of prion rods generates 10-nm spherical particles having high alpha-helical content and lacking scrapie infectivity.
J Virol, 70(3):1714-1722, 01 Mar 1996
Cited by: 61 articles | PMID: 8627692 | PMCID: PMC189995
Immunoaffinity purification and neutralization of scrapie prions.
Prog Clin Biol Res, 317:583-600, 01 Jan 1989
Cited by: 2 articles | PMID: 2574871
Review
Immunoaffinity purification and neutralization of scrapie prion infectivity.
Proc Natl Acad Sci U S A, 85(18):6617-6621, 01 Sep 1988
Cited by: 91 articles | PMID: 3137571 | PMCID: PMC282028
Conservation of infectivity in purified fibrillary extracts of scrapie-infected hamster brain after sequential enzymatic digestion or polyacrylamide gel electrophoresis.
Proc Natl Acad Sci U S A, 87(18):7240-7244, 01 Sep 1990
Cited by: 26 articles | PMID: 2119503 | PMCID: PMC54719
Funding
Funders who supported this work.
NIA NIH HHS (2)
Grant ID: AG02132
Grant ID: AG08967
NINDS NIH HHS (1)
Grant ID: NS14069