Abstract
Free full text

89Zr-Immuno-Positron Emission Tomography in Oncology: State-of-the-Art 89Zr Radiochemistry
Abstract
Immuno-positron emission tomography (immunoPET) with 89Zr-labeled antibodies has shown great potential in cancer imaging. It can provide important information about the pharmacokinetics and tumor-targeting properties of monoclonal antibodies and may help in anticipating on toxicity. Furthermore, it allows accurate dose planning for individualized radioimmunotherapy and may aid in patient selection and early-response monitoring for targeted therapies. The most commonly used chelator for 89Zr is desferrioxamine (DFO). Preclinical studies have shown that DFO is not an ideal chelator because the 89Zr–DFO complex is partly unstable in vivo, which results in the release of 89Zr from the chelator and the subsequent accumulation of 89Zr in bone. This bone accumulation interferes with accurate interpretation and quantification of bone uptake on PET images. Therefore, there is a need for novel chelators that allow more stable complexation of 89Zr. In this Review, we will describe the most recent developments in 89Zr radiochemistry, including novel chelators and site-specific conjugation methods.
Role of 89Zr-ImmunoPET in Drug Development and Personalized Cancer Treatment
In past years, immuno-positron emission tomography (immunoPET) with 89Zr-labeled antibodies (89Zr-immunoPET) has shown great potential in cancer imaging. It can play an important role in early drug development and molecular characterization of tumors for individualized anticancer treatment. 89Zr-immunoPET provides important information about the pharmacokinetics and tumor (and normal tissue) targeting properties of monoclonal antibodies.1−3 A safety issue in drug development is the maldistribution of large-molecule drugs, resulting in an adverse balance between safety (from effects in nontarget tissues) and efficacy (on-target effects in target tissues). 89Zr-immunoPET imaging could help in two ways: (1) in animal models to avoid selection of molecules with a propensity for maldistribution and (2) in clinical development to reduce incidence of maldistribution in trial subjects exposed to the investigational new macromolecular drug. 89Zr-immunoPET is also an important tool to noninvasively assess the expression and accessibility of target antigens in tumors and normal tissues for patient selection and early-response monitoring of targeted therapies.4−6 In this regard, 89Zr-immunoPET has several advantages over conventional approaches such as tumor biopsies and immunohistochemistry. First of all, it allows the measurement of target expression of whole tumor lesions and their metastases, thereby avoiding misinterpretation due to tumor heterogeneity or sampling error. Furthermore, it allows the longitudinal monitoring of target expression, which could be of clinical relevance because target expression can change in time during disease progression or treatment. Finally, in vivo imaging also takes into account target accessibility after systemic administration. Next to target expression, factors such as vascular permeability, interstitial fluid pressure, blood flow, and vessel density affect the uptake of a monoclonal antibodies in a tumor. If target accessibility is low, the therapeutic agent might not reach the tumor cells despite adequate expression of the target.7−9 A final application of 89Zr-immunoPET is accurate dose planning for individualized radioimmunotherapy with 177Lu- or 90Y-labeled antibodies, such as, for example, for 90Y-ibritumomab tiuxetan therapy.10,11
Advantages and Limitations of 89Zr-ImmunoPET
For imaging purposes, immunoPET is preferred over immunoSPECT because of the higher resolution, sensitivity, and more-accurate image quantification. 89Zr is an ideal radionuclide for immunoPET. It decays via positron emission and electron capture to 89mY, which in turn decays via γ ray emission (909 keV) to stable 89Y. The 78.4 h half-life of 89Zr perfectly matches with the pharmacokinetics of monoclonal antibodies that typically show optimal tumor-to-blood ratios several days after injection. The relatively low-energy positrons (Emean 395 keV) provide high-resolution PET images. Importantly, 89Zr is a residualizing radionuclide: upon internalization, it is trapped inside the tumor cell, which results in improved tumor retention and enhanced tumor-to-normal tissue ratios, as compared with nonresidualizing radionuclides such iodine-124 (124I).12
Although 89Zr-immunoPET is a highly attractive technique to measure tumor-associated antigens and to measure the in vivo distribution of monoclonal antibodies, but it also has a few limitations. First of all, the enhanced permeability and retention effect may result in non-receptor-mediated uptake of radiolabeled antibodies leading to false-positive results. Second, low target expression may lead to false-negative results due to low imaging contrast because of the long circulatory half-life of monoclonal antibodies. Third, 89Zr has a low positron abundance of only 23%, compared with those of 18F (97%) and 68Ga (89%) but similar to that of 124I (23%). Finally, for each 89Zr-immunoPET scan, patients are exposed to relatively high doses of radioactivity, which limits the possibility for longitudinal studies, especially for patients who are treated with curative intent.
89Zr-ImmunoPET in Preclinical and Clinical Studies
Meijs et al. were the first to show the potential of 89Zr-labeled antibodies for PET imaging. The 89Zr-labeled anti-EpCam antibody 323/A3 was successfully used to visualize human OVCAR-3 xenografts in immunodeficient mice.13 Since then, numerous 89Zr-labeled antibodies have been developed targeting several tumor-associated antigens, i.e. EGFR, HER2, CD44v6, PSMA, CD20, and VEGF-A.14,15 During the past years, 89Zr-immunoPET has been successfully translated to cancer patient populations in various studies.14
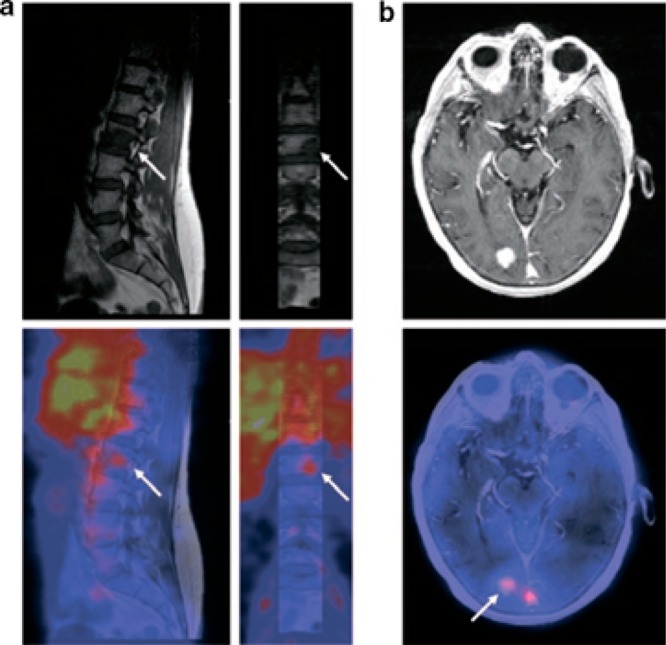
The first clinical 89Zr-immunoPET study was reported by Börjesson et al. They showed that primary squamous cell carcinomas (HNSCC) could be detected by PET imaging using 89Zr-labeled chimeric anti-CD44v6 antibody U36.1 Since then, several studies have successfully used 89Zr-immunoPET for tumor imaging. One prime example is immunoPET with 89Zr-trastuzumab. Dijkers et al. have shown that 89Zr-trastuzumab immunoPET can detect metastatic liver, lung, bone, and even brain lesions in patients with HER2-positive breast cancer (Figure Figure11).2 Furthermore, 89Zr-trastuzumab immunoPET was able to solve a clinical dilemma in a patient with suspected liver and mediastinal metastases, which could not be confirmed by biopsies.5 Finally, 89Zr-trastuzumab immunoPET has been used to predict and monitor therapy response.6,16 It must be noted that for many lesions, HER2 expression was never demonstrated, so future studies should confirm the correlation between 89Zr-trastuzumab uptake and HER2 expression levels. An overview of (pre)clinical 89Zr-immunoPET studies has been described in detail in several excellent reviews.14,15,17,18
Current Challenges in 89Zr Radiochemistry
Although preclinical and clinical studies clearly indicate the potential of 89Zr-immunoPET for cancer imaging, from a chemical point of view, the current radiotracers are suboptimal. An ideal 89Zr-labeled antibody for immunoPET should fulfill the following criteria:
- 1)
safe for clinical use;
- 2)
unchanged pharmacokinetics compared to the unconjugated antibody;
- 3)
unchanged affinity for its target; and
- 4)
no release of 89Zr.
Currently, the most commonly used chelator for 89Zr is desferrioxamine (DFO). Preclinical studies have shown that DFO is a not an ideal chelator because the 89Zr–DFO complex is partly unstable. This results in release of 89Zr from the chelator and the subsequent accumulation of 89Zr in bone tissue that can reach values >10% ID/g.10,19−22 It has been suggested that 89Zr bone uptake can be attributed to a metabolic process, as it seems to be more-pronounced for internalizing antibodies compared with noninternalizing antibodies. After the internalization and catabolization of the 89Zr–DFO–antibody conjugate in the lysosomes, the 89Zr may be released from the chelator and subsequently leave the cell.10,19,20,22
Several studies have shown a mismatch between the 89Zr-labeled antibody and 111In- or 177Lu-labeled antibody with respect to bone uptake. The bone uptake of released 89Zr is undesired because it may hamper the detection of bone metastases. Furthermore, it will overestimate the tracer uptake in bone tissues and could lead to overestimation of the radiation dose to the bone marrow in case of dose planning for radioimmunotherapy. Therefore, there is a need for novel chelators that allow more stable complexation of 89Zr. The importance of the choice of the bifunctional chelating agent in the design of a metal based radiopharmaceutical has been demonstrated in a large number of studies and highlighted in several reviews in the past few years.23−26
Another challenge in 89Zr-immunoPET is the antibody-chelator conjugation without affecting immunoreactivity and binding affinity. Conjugation of antibodies with bifunctional chelators is mostly based on random reactions with the ε-amino group of lysine residues. However, if the antigen binding domain of the antibody contains critical lysines residues, the chelator may alter the antigen binding affinity and reduce the immunoreactivity of the antibody. These challenges may be overcome by site-specific conjugation techniques. These techniques result in chemically defined antibody-chelator conjugates, while random conjugation results in mixtures of antibody-chelator conjugates with different substitution ratios. Several site-specific conjugation strategies have been developed.27,28 In this Review, we will describe the most recent developments in 89Zr-radiochemistry, including site-specific conjugation methods and novel chelators (for previous reviews on 89Zr radiochemistry, see refs (15), (29), and (30)). We will focus on antibody-based radiotracers; however, the described developments are also hihgly relevant for other types of radiotracers, including antibody fragments, nanobodies, affibody molecules, and peptides.
89Zr Bifunctional Chelating Agents: The Current State of the Art
DFO and DFO-Based Bifunctional Chelators
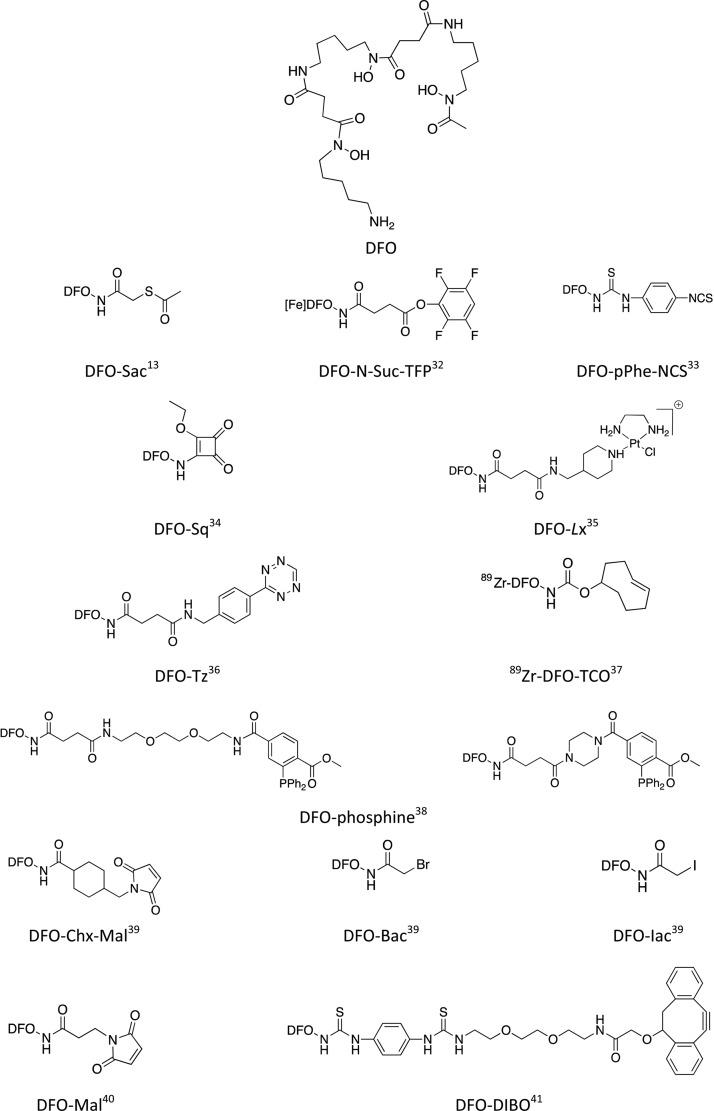
DFO, also called deferoxamine, desferrioxamine B, desferoxamine B, MPO-B, DFOA, DFB, or Desferal (Figure Figure22), is by far the most commonly used chelator for 89Zr-immunoPET. It was found 25 years ago that this natural bacterial siderophore binds 89Zr much more efficiently than diethylenetriaminepentaacetic acid (DTPA).31 Different approaches have been proposed for the conjugation of DFO to antibodies, involving various bifunctional versions of DFO (Figure Figure22).15,30
Random Bioconjugation of DFO-Based Bifunctional Chelators
The most-common approach is the random conjugation to lysine residues of antibodies. Meijs et al. used a two-step procedure to introduce DFO into two different mAbs (E48 and 323/A3).13 In the first step, maleimide groups were incorporated into the mAbs through the reaction of lysines with 4-(N-maleimidomethyl) cyclohexane carboxylic acid N-hydroxysuccinimide ester (SMCC). DFO was converted to its S-acetyl protected thiol derivative (DFO-Sac) by reaction with N-succinimidyl-S-acetylthioacetate (SATA). The two products were reacted with each other in the presence of hydroxylamine, and the resulting bioconjugates were purified by gel filtration to remove the excess of DFO–Sac. The bioconjugates were labeled with 89Zr with a specific activity of 5 mCi/mg. Biodistribution studies in tumor bearing mice showed a high tumor uptake of the conjugates but also a high background accumulation of 89Zr in other tissues. This might be due to the cleavage of the succinimide–thioether bond, which results in the release of the chelator from the antibody.
Most of the in vivo studies reported so far involved antibodies modified with DFO bearing either an activated ester (DFO–N-Suc–TFP) or an isothiocyanate function (DFO–pPhe–NCS).32,42} The activated ester strategy, developed by Verel et al., involves six successive steps: (i) DFO was succinylated; (ii) the modified DFO was metalated with Fe3+; (iii) it was reacted with tetrafluorophenol to give the activated ester DFO–N-Suc–TFP, which was (iv) conjugated to the antibody (an anti-CD44v6 chimeric mAb U36 directed against head and neck cancer in the pioneer work of Verel et al.) with a chelator-to-mAb ratio of about 1:1; and (v) the product was demetalated by transchelation with excess EDTA and (vi) radiolabeled with 89Zr. The protection of DFO by Fe3+ complexation is mandatory to prevent side-reactions during the formation of the activated tetrafluorophenolic ester. The method is synthetically demanding, but the [Fe]DFO–N-Suc–TFP can be stored for at least one year and is commercially available. Also, it has to be mentioned that the removal of iron after conjugation requires low pH (between 4.2 and 4.5), which can have deleterious effects on some proteins. However, this strategy yields 89Zr-labeled antibodies with favorable in vitro and in vivo behaviors, and it has been used for labeling a wide range of mAbs. Another efficient route for incorporating DFO into biologicals, developed more recently by the same research group from Amsterdam, is the use of a p-isothiocyanatophenyl derivative DFO–pPhe–NCS.33,42 They reported on the conjugation of this bifunctional chelating agent to three mAbs (U36, cetuximab, and rituximab) with a 1.5:1 chelator-to-mAb ratio, regardless of the nature of the antibody. Upon storage in NaCl containing buffers, the immunoconjugates appeared to be less stable than the analogs obtained through the DFO–N-Suc–TFP route, probably due to the reaction of thiourea bond with hypochlorite ions formed upon radiation. However, in vivo studies revealed comparable behavior of the conjugates prepared by the two methods and this more straightforward route has become a standard protocol for 89Zr-DFO labeling of antibodies. It has to be noted that the poor water solubility of DFO–pPhe–NCS requires the use of a small amount of DMSO, and precautions have to be taken during the conjugation step to prevent protein degradation. Donnelly and co-workers proposed an alternative to DFO–pPhe–NCS by attaching a squaramide ester to DFO.34 Because this new bifunctional DFO derivative, DFO–Sq can be considered an extended DFO; this work will be discussed in the Hydroxamate-Based Chelators (DFO Analogs) section.
Very recently, Sijbrandi et al. developed a new 89Zr–DFO trastuzumab conjugate using a novel linker technology called Lx.35Lx stands for a metal–organic linker based on ethylenediamineplatinum(II). The platinum complex was first coordinated to a DFO derivative to give a stable and storable compound, with increased water solubility, which was then conjugated to trastuzumab to yield the immunoPET conjugate. The DFO-to-mAb ratio in this conjugate was determined to be 2.6. Interestingly, the characterization of fragments obtained by papain or pepsin digestion shown that 85% of the payload was bound to the Fc region of the mAb, which reduces the chance of affecting the immunoreactivity. It was also shown that the platinum derivative was more likely coordinated to histidine residues, thus offering a valuable alternative to the conventional coupling to lysine or cysteine residues. The conjugates were stable in vivo, a comparative biodistribution study of 89Zr–DFO–Lx–trastuzumab and 89Zr–DFO–trastuzumab prepared by the DFO–N–Suc-TFP route showed similar 89Zr uptake in the tumor and all organs with the exception of the liver (5.4 ± 0.8 and 4.0 ± 0.3%ID/g for 89Zr–DFO–Lx–trastuzumab and 89Zr–DFO–trastuzumab, respectively).
The use of click chemistry has emerged as an alternative method for the design of radiolabeled compounds for both imaging and therapeutic purposes.43 The click chemistry tool box has been incredibly expanded in the last years, providing numerous ligation methods for bioconjugation with many advantages: fast, quantitative, clean, selective, and biorthogonal, allowing site-specific approaches (see the Site-Specific Bioconjugation of DFO-Based Bifunctional Chelators section) and in vivo pretargeting.
Zeglis et al. used this approach for radiolabeling trastuzumab with either 89Zr or 64Cu.36 They used the well-known inverse electron demand Diels–Alder reaction (IEDDA) between norbornene and tetrazine to introduce a bifunctional chelator. In the first step, the mAb was reacted with norbornene succinimidyl ester. The resulting modified antibody, which can be stored at 4 °C for a long period, was then coupled to a tetrazine-containing chelator (DFO–Tz or DOTA–Tz) to yield the final immunoconjugates ready to be metalated with 89Zr or 64Cu. The coupling reaction was performed in mild conditions but required a relatively long incubation time (5 h). The chelator-to-mAb ratio ranged from 1.1 to 3.8, depending on the amount of norbornene–NHS–ester used in the first step. Although the 89Zr-labeled conjugate was stable in human serum at 37 °C for 48 h, in vivo studies in mice showed relatively high bone uptake (up to 15.2% ID/g after 72 h). Above all, this work is a nice proof of concept of the modularity of the click-chemistry approach.
More recently, Meimetis et al. developed a similar strategy for the synthesis of a dual labeled trastuzumab for bimodal imaging (PET and fluorescence imaging).44 In this work, transcyclooctene (TCO)–PEG4–NHS was conjugated to trastuzumab, and then the resulting trastuzumab–TCO was coupled via IEDDA reaction to a bimodal probe containing both DFO, a BODIPY moiety, and a tetrazine unit. The click reaction of TCO with tetrazine was much more rapid than the one with norbornene and was completed within 3 min to yield two conjugates with different degrees of labeling (ca. 1 and 2.5 probes per antibody, these values being determined by mass spectrometry and UV–vis measurements). Surprisingly, the bimodal immunoconjugate exhibited a 4-fold higher tumor uptake in HER2+ tumors bearing mice in comparison to the conjugate prepared by the conventional DFO–N-Suc–TFP method. However, a 2.5-fold higher liver uptake was also observed, probably due to the enhanced lipophilicity introduced by the BODIPY dye. Interestingly, no significant difference between the conjugates prepared by the two different routes was observed for bone uptake, which remained relatively low (3.52% ID/g). The TCO–tetrazine click reaction has been also used in a reverse way (the TCO was appended to DFO) for labeling a peptide with 89Zr.37
Another click-chemistry reaction, the Staudinger ligation, was investigated for labeling chimeric mAb U36 with 89Zr–DFO in a pretargeting approach.38 Up to 8 triazide moieties (24 azide functions) could be incorporated into the mAb without affecting significantly the immunoreactivity. A pair of 89Zr–DFO–phosphine derivatives were synthesized and their Staudinger ligation with the U36-triazide was studied. The reaction was quite slow in PBS (20–25% after 2h at 37 °C) and even slower in human serum and, unfortunately, was not observed in vivo. This study showed that Staudinger ligation is not the method of choice, at least with the aim of in vivo pretargeting.
Site-Specific Bioconjugation of DFO-Based Bifunctional Chelators
One major drawback of the methods described above is that the random conjugation of the probe to amino-acid residues within the antibody (e.g., lysines) leads to poorly chemically defined biologics. This lack of specificity and homogeneity, both with respect to the BFC-to-mAb ratio and the site of conjugation, may result in in vivo behavior of the conjugate that is less efficient. In particular, the attachment of the bifunctional chelator to the antigen-binding domains of the antiboby may lower its immunoreactivity. Inspired by the exponentially growing field of bioconjugation techniques for the synthesis of antibody–drug conjugates (ADC),45,46 increasing attention has been paid in the last years to the use of site-specific labeling of antibodies also for molecular imaging.27,28 A precise control of the number and the location of the chelators on the antibody should result in better-defined and more-efficient immunoconjugates and more easily reproducible procedures and may facilitate the approval process.
Tinianow et al. from Genentech were the first to report a site-specific conjugation route for the synthesis of 89Zr immunoconjugates using maleimide- and halide-modified DFO.39 The authors used a bioengineered mAb, so-called thio-trastuzumab, containing two cysteine residues in the heavy chain. These unpaired cysteines incorporated into nonbioactive sites of the antibody were reacted with either bromoacetyl– or iodoacetyl–DFO (DFO–Bac and DFO–Iac) through nucleophilic substitution or with a maleimide derivative (DFO–Chx–Mal) via Michael addition. Among the three thiol-reactive DFO derivatives investigated, DFO–Chx–Mal proved to be the best candidate in terms of bioconjugation and radiolabeling efficiency. Indeed, the resulting bioconjugate containing exactly two chelators per antibody was obtained after 1 h of incubation of the BFC with thio-trastuzumab at room temperature at pH 7.5. Radiometalation with 89Zr-oxalate gave the 89Zr-labeled conjugate with the highest radiochemical yield (87.5%) and purity (98%), in comparison with the two others site-specifically prepared immunoconjugates and the two analogs prepared by conventional routes (DFO–N-Suc–TFP and DFO–pPhe–NCS). However, in the model employed in this study, the site-specific approach did not show significant advantage over the random conjugation route.
Ma et al. also used a maleimide-modified DFO, the maleimidopropionate–DFO (DFO–Mal), which was conjugated to trastuzumab after reduction of disulfide bonds with Tris(2-carboxyethyl) phosphine (TCEP). The biodistribution of the resulting highly loaded bioconjugate (containing between four and eight chelators per antibody) in normal mice was studied with the aim to compare DFO with a new tripodal chelator (see the Hydroxypyridinone-, Terephthalamide-, and Catecholate-Based Chelators section).40
Schibli and co-workers used a method based on enzymatic modification of antibodies for the site-specific incorporation of DFO into two antibodies, chimeric CE7 and rituximab, targeting, respectively, L1CAM and CD20.47 In this work, the mAbs were first enzymatically deglycosylated to differentiate selectively two glutamine residues in the Fc region, which can undergo further reaction with DFO catalyzed by bacterial transglutaminase (BTG). In a variant of this approach, they also developed a chimeric CE7 mutant in which two asparagines were replaced by glutamines, thus allowing the introduction of four chelators per antibody. In vivo distribution in tumor bearing mice revealed that engineered bioconjugates labeled with 67Ga showed higher tumor-to-liver ratio compared to those prepared by conventional routes. However, the biodistribution study with 89Zr analogs was not reported, and PET images did not evidence a superior behavior of the engineered bioconjugate.
Enzyme-mediated methodologies represent an efficient tool for site-specific introduction of cytotoxic payloads on the heavy chain glycans of mAbs48 and also for the development of site-specifically labeled immunoconjugates.41 In their work, Zeglis et al. used β-1,4-galactosidase to remove terminal galactose residues on the Fc domain of an antibody targeting PSMA (J591), which were then replaced by N-azido-acetylgalactosamine (GalNAz) using a mutant β-1,4-galactosyltransferase Gal-1T1(Y289L) to afford the modified J591 bearing biorthogonal azido groups. The DFO conjugate was finally obtained by clicking on N3-J591, a DFO bearing a dibenzocyclooctyne unit (DFO–DIBO), following the well-known strain promoted alkyne–azide cycloaddition (SPAAC) method. The N3-to-J591 and DFO-to-J591 ratios were roughly identical (2.8 chelators per antibody), showing that the click reaction was quantitative. This study was the first one combining click chemistry and site-specific approaches for the design of DFO immunoconjugates. The 89Zr–DFO–DIBO–GalNAz–J591 conjugate was shown to be as stable as the conventionally prepared 89Zr–DFO–NCS–J591 (>96% after 7 days incubation in serum); however, in vitro and in vivo studies demonstrated that both radioimmunoconjugates behaved in a nearly identical way. The same research group moved a step forward and prepared a site-specifically dual-labeled (PET–optical imaging [OI]) huA33, a mAb-targeting colorectal cancer.49 In this case, the click-chemistry reaction was conducted with a mixture of DFO–DIBO and Alexa Fluor 680-DIBO, providing the hybrid imaging agents with different chelator-to-dye-to-mAb ratios (from 1:1.3:1 to 2.9:0.5:1). They used the same approach to design a PET–OI bimodal immunoconjugate based on a pancreatic ductal adenocarcinoma (PDAC) targeting antibody 5B1.50
These different conjugation strategies may result in different synthesis and radiolabeling efficiencies, but the linker chemistry may also change the stability of the radiolabeled bioconjugate, its immunoreactivity, and biodistribution and, therefore, can impact the quality of the PET images. From the studies conducted so far with different DFO bifunctional chelating agents, it is not obvious to define the better methodology for the design of a DFO-based immunoconjugate, partly because it is also “antibody-dependent”. Although it is the most commonly used nowadays, especially due to the fact that the bifunctional chelating agent is commercially available, the DFO–pPhe–NCS-based strategy is far from perfect. DFO–pPhe–NCS, as is most of the hydroxamate-based chelators, is poorly soluble in water, thus requiring careful handling during the conjugation step. Also, the formed thiourea bond is susceptible to radiation-induced cleavage, although this limitation may be overcome by avoiding the presence of chloride anions. There is also no clear evidence of the added value of site-specific conjugation in comparison to random labeling. In most of the studies comparing both approaches, the site-specifically modified antibody did not outperform the conjugates prepared by conventional routes. However, as stated by Zeglis, one can believe that with some antibodies, it will be highly preferable to use well-defined, chemically controlled constructs. The development of new site-specific bioconjugation methods, together with the increasing number of click-chemistry tools available, should lead to the construction of finely designed immunoconjugates exhibiting higher selectivity and efficiency. Such approaches will also facilitate the design of more-sophisticated conjugates in the future, e.g., PET–OI bimodal imaging agents or imageable ADC.
Despite its extensive use, which makes it the gold standard for 89Zr-immunoPET, DFO and its bifunctional versions suffer from some drawbacks. Beside the issues discussed above regarding the way the DFO is attached to the antibody, the chelating agent by itself is probably not the best ligand for 89Zr complexation, thus being responsible of metal release in vivo. These issues prompted several groups to seek for improved 89Zr chelators in the last 3 years.
Alternatives to DFO
Although no crystal structure of the 89Zr–DFO complex has been reported so far, density functional theory (DFT) calculations predicted that the radiometal is complexed by the three hydroxamate groups of the linear hexadentate ligand and that the coordination sphere of the metal ion was saturated by two water molecules.21 Thus, the highly oxophilic hard cation Zr4+ is stabilized by the octacoordination of eight oxygen atoms. However, it has been hypothesized that DFO is not an ideal chelator for 89Zr because of the presence of only three hydroxamate groups and the need of additional water molecules to allow the saturation of the coordination sphere of Zr4+. The search for more-efficient chelators has been logically oriented toward molecular constructs containing intrinsically eight oxygen donor atoms.
Hydroxamate-Based Chelators (DFO Analogs)
Brechbiel and co-workers investigated the coordination chemistry of Zr4+ with four bidentate hydroxamates ligands and reported the single crystal X-ray structure of the octa-coordinated complex.51 Following this pioneering work, Patra et al. designed an extended DFO, so-called DFO*, by appending an extra hydroxamate group to commercial DFO (Table 1, entry 1).52 DFO* can thus wrap around the metal to give an octadentate complex as predicted by DFT calculations. Vugts et al. prepared a bifunctional version of DFO* (DFO*–pPhe–NCS), which was conjugated to trastuzumab, and the resulting conjugate was compared to the DFO analog.53 DFO* conjugate showed superior characteristics, both in vitro and in vivo. The biodistribution of the two 89Zr-radiolabeled mAbs into N87 tumor-bearing nude mice showed similar blood kinetics and tumor uptake at 24, 72, and 144 h post injection (p.i.). Most importantly, DFO* showed significantly lower uptake in bone and also in liver and spleen tissue at 144 h p.i. Moreover, the ratio of 89Zr–DFO–trastuzumab over 89Zr–DFO*–trastuzumab in femur was 1.5 at 24 h p.i. and increased to 5 at 144 h p.i.
Table 1
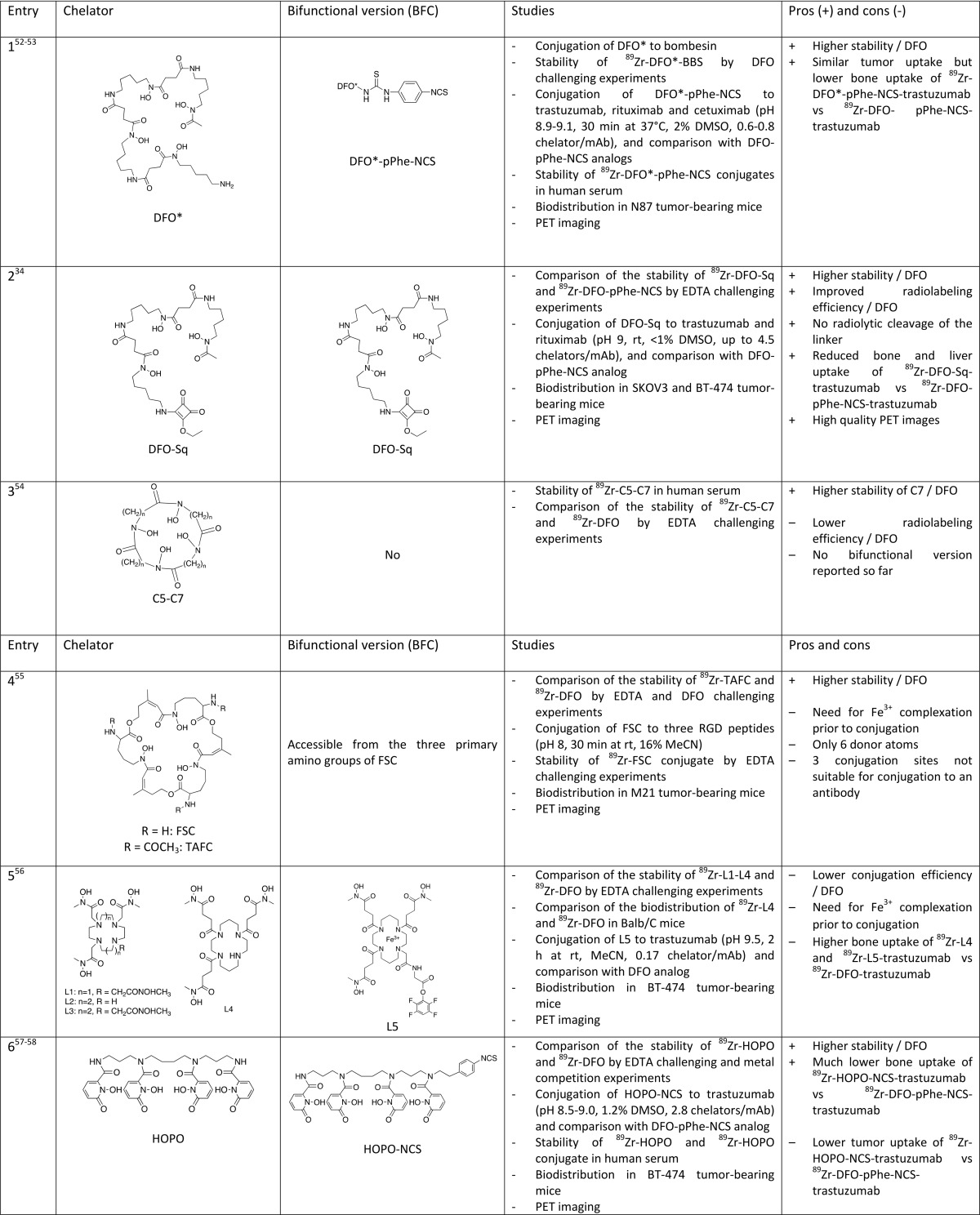
Similar to the DFO* approach, Donnelly and co-workers proposed to use a squaramide ester derivative of DFO (DFO–Sq, Figure Figure22) for conjugation to trastuzumab (Table 1, entry 2).34 The presence of two oxygen atoms in the squaramide group is supposed to complete the coordination of the Zr4+ ion. In vivo comparison of 89Zr–DFO–trastuzumab and 89Zr–DFOSq–trastuzumab also turned to the advantage of the new conjugate, which, after injection in SK-OV-3 xenograft-bearing mice, improved PET imaging and resulted in higher tumor-to-bone ratio. Although there is no evidence of octacoordination of the metal and the difference of the tumor-to-bone ratio is not as pronounced as it is in the case of DFO*, especially 96 h p.i., this new bifunctional version of DFO shows advantages compared to DFO–pPhe–NCS in terms of radiolabeling efficiency. The authors also claimed that the squarate bond is more stable than the thiourea bond. Further comparative studies will be necessary to prove the superiority of this promising DFO analog.
It is well-established that metal complexes with macrocyclic compounds show higher kinetic inertness in comparison to their linear analogs. Brechbiel and co-workers decided to combine this favorable feature of the macrocyclic structure with the presence of eight coordinating oxygen atoms and synthesized a series of macrocyclic chelators of various sizes containing four hydroxamate units through ring closure metathesis of the corresponding linear compounds (Table 1, entry 3).54 The macrocycle with the larger cavity size (C7) gave a much-more-stable Zr complex than DFO. However, no bioconjugatable version of this promising chelator has been prepared yet. Decristoforo et al. also chose to take advantage of the expected higher stability of macrocyclic derivatives and investigated the properties of fusarinine C (FSC), a cyclic natural siderophore containing three hydroxamate groups and its triacetylated derivative (TAFC) (Table 1, entry 4).55 Despite the presence of only six coordinating oxygen atoms, 89Zr–TAFC showed much-higher stability in comparison to 89Zr–DFO, evidenced by challenge experiments with a large excess of EDTA, thus demonstrating the added value of the cyclic structure versus the linear one. The three secondary amines on FSC allowed the conjugation of the chelator to three cyclic RGD peptides. However, a limitation of the use of this candidate for 89Zr-immunoPET is the presence of the three identical secondary amine functions, which make it difficult to conjugate it to only one antibody.
Boros and co-workers have chosen to use macrocyclic tetraamines, i.e. cyclen or cyclam, as a scaffold to bring hydroxamate arms in favorable conformation for optimal 89Zr coordination (Table 1, entry 5).56 A series of cyclen and cyclam derivatives bearing three or four hydroxamate groups of various lengths, including a bifunctional version, have been prepared. Unfortunately, none of them showed significantly improved properties in comparison to DFO.
Hydroxypyridinone-, Terephthalamide-, and Catecholate-Based Chelators
An alternative to hydroxamate groups as bidentate oxygenated ligands are catecholate or hydroxypyridinone groups. In particular, 1-hydroxypyridin-2-one groups exhibit lower pKa values than catechol or hydroxamic acid and are deprotonated at physiological pH, thus offering hard oxygen atoms suitable for Zr coordination. In 2014, Lewis and co-workers, inspired by the work of Raymond in the field of actinide separation,65 proposed a new 89Zr chelator based on four 1-hydroxypyridin-2-one groups appended to a linear tetraamine, i.e. 3,4,3-(LI-1,2-HOPO), also called HOPO (Table 1, entry 6).57 DFT calculations showed that the Zr4+ is octacoordinated by HOPO. This coordination scheme has been confirmed by a crystal structure.58 In their first paper, a comprehensive stability study has demonstrated that 89Zr–HOPO complex exhibited much higher stability than 89Zr–DFO. Indeed, ligand challenge experiments with 100-fold excess EDTA within a 5–8 pH range left the 89Zr–HOPO as >99% intact even after 7 days at 37 °C, while the significant release of 89Zr from DFO was observed. 89Zr–HOPO also showed a good stability upon transmetalation with a large range of metal cations (10-fold excess), although 17% of 89Zr was replaced by Fe3+ after 7 days. However, only 39% of the 89Zr–DFO complex remained intact under the same conditions. This promising behavior prompted the authors to prepare a bifunctional version of HOPO bearing a benzyl NCS group, which enabled its bioconjugation to trastuzumab.58 Surprisingly, 89Zr–HOPO–trastuzumab appeared to be slightly less stable than 89Zr–DFO–trastuzumab in serum, but in vivo studies in BT474 xenograft-bearing mice confirmed the superiority of the new conjugate, especially regarding bone uptake. Indeed, after 336 h p.i., the tumor-to-bone ratio was more than 3-fold higher in the case of 89Zr–HOPO–trastuzumab.
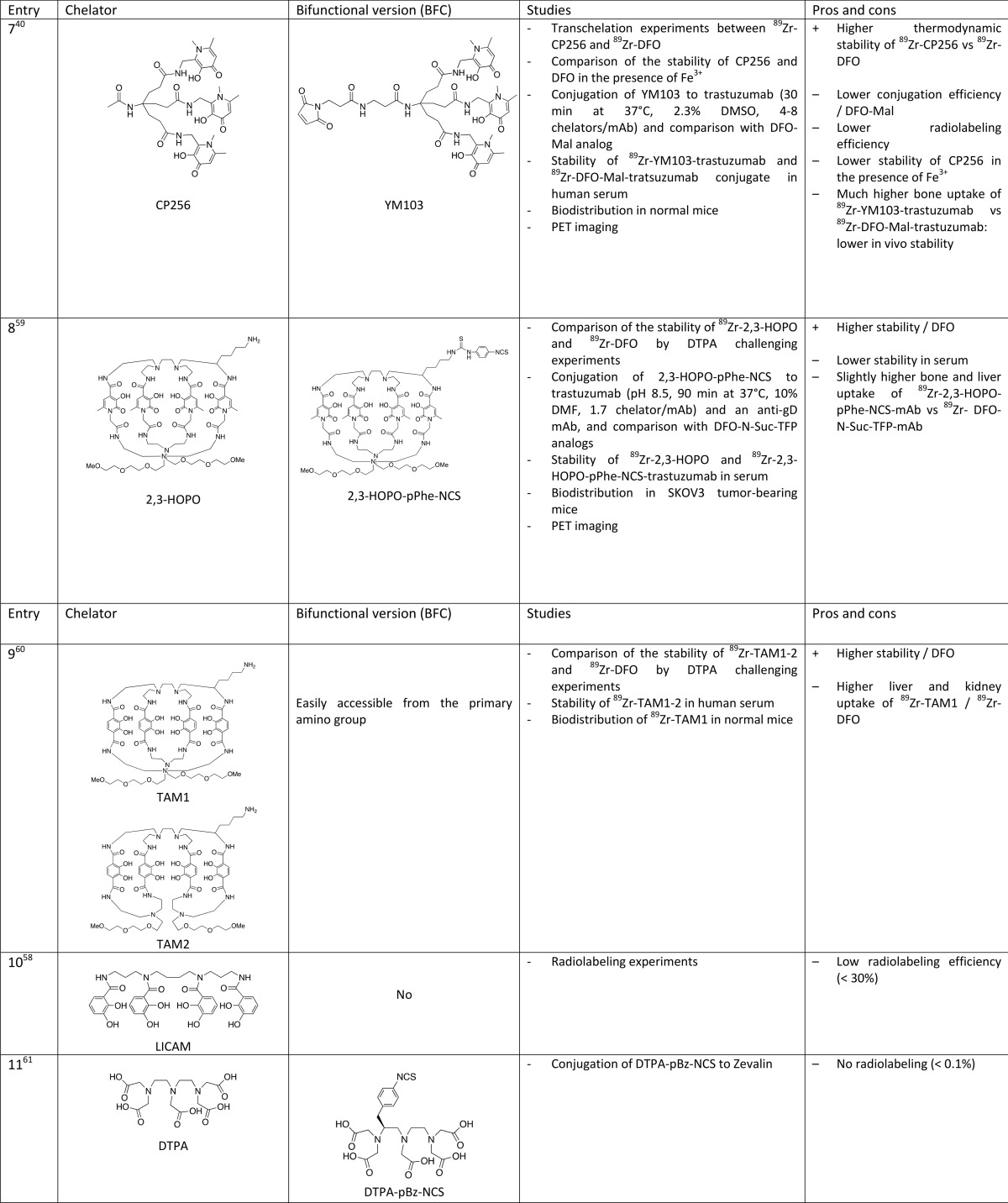
Ma et al. used a tripodal ligand incorporating three 1,6-dimethyl-3-hydroxypyridin-4-one as 89Zr chelator (Table 1, entry 7).40 This chelator, designated as CP256 or THP, has proven to be very effective for 68Ga labeling. A bifunctional derivative of CP256 containing a maleimide group (YM103) has been conjugated to trastuzumab after reducing mAb disulfide bonds and compared to a DFO analog. Both competition studies between DFO and CP256 and in vivo experiments in healthy mice (29% ID/g in bones after 3 days) led to the conclusion that this tripodal hexadentate chelator forms less stable complexes with 89Zr than DFO.
Another hydroxypyridinone isomer, i.e. 3-hydroxypyridin-2-one, has been investigated by Tinianow et al. (Table 1, entry 8).59 A sophisticated macrobicyclic octadentate compound incorporating four 3-hydroxypyridin-2-one groups and a pPhe–NCS function was conjugated to trastuzumab and compared to the DFO analog in vivo in SK-OV-3 tumor bearing mice. The new bioconjugate showed slightly higher uptake in bone and liver compared to the DFO analog. The authors expect that changing the topology of the chelator and, thus, the positioning of the 3-hydroxypyridin-2-one groups could lead to more stable complexes. The same research group has also prepared a pair of structural analogs incorporating four terephthalamide groups instead of hydroxypyridinones TAM1 and TAM2 (Table 1, entry 9).60 The corresponding 89Zr complexes exhibited higher stability in vitro than DFO as evidenced by challenge experiments with excess DTPA and comparable bone uptake after injection in normal mice. However, the liver and kidney uptake of the new complexes were higher than those of 89Zr–DFO. These chelators have not been conjugated to a biomolecule.
A catechol containing analog of HOPO (LICAM) has been investigated by Lewis and co-workers (Table 1, entry 10) but was found to be inefficient for 89Zr complexation (maximum radiochemical yield of <30%), probably due to the too-high pKa value of the catechol group.58
Carboxylate- and Phosphonate-Based Chelators
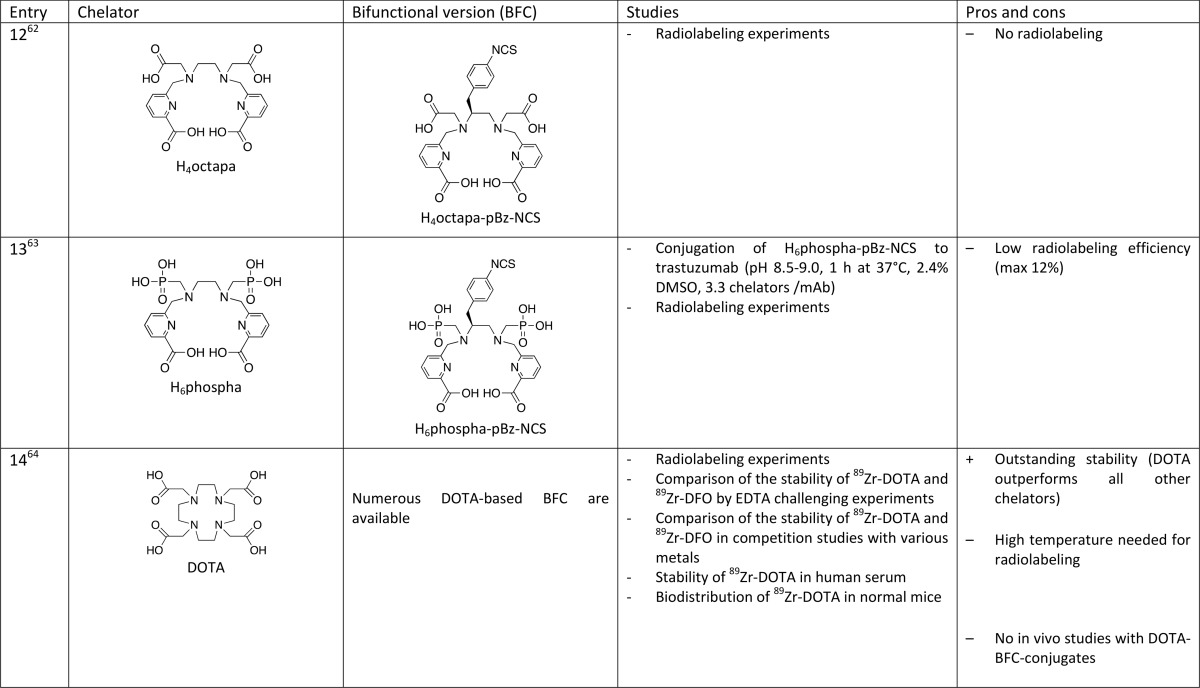
Diethylenetriaminepentaacetic acid (DTPA) was proven to be a poor candidate for 89Zr labeling after bioconjugation of a bifunctional derivative, DTPA–pBz–NCS, to Zevalin (anti-CD20), with a labeling yield of <0.1% (Table 1, entry 11).61 Orvig and co-workers investigated another acyclic chelator containing carboxylate functions designed for 111In labeling, H4octapa (Table 1, entry 12), but their attempts to radiolabel H4octapa–-trastuzumab conjugate with 89Zr failed.62 With the aim of improving radiolabeling kinetics, they chose to replace two carboxylate arms with two phosphonate groups. A bifunctional version of this chelator, H6phospha–pBz–NCS (Table 1, entry 13), was conjugated to trastuzumab (3.3 chelators per antibody) and 89Zr radiolabeling experiments of the conjugate have confirmed their hypothesis. However, the maximum labeling yield (12%) compared poorly to the standard DFO.63
Finally, a very recent study from the Wadas research group could revolutionize the field (Table 1, entry 14–16).64 Contrary to what was stated by many authors, it appeared that 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) could be a powerful alternative to DFO for complexing 89Zr. DOTA is a workhorse chelator that shows extraordinary coordination properties for a wide range of metals including lanthanides (Gd-based contrast agents for MRI) and a large number of radioisotopes used for SPECT (111In), PET (64Cu, and 68Ga), or RIT (177Lu, 90Y, and 225Ac). A tremendous number of studies has been devoted to tetraazacycloalkanes chemistry to finely tune the coordination properties toward a given metal, and different approaches have been devised to provide bifunctional versions of such derivatives for bioconjugation to various biovectors.66−69 Surprisingly, this highly versatile family of chelators remained largely unexplored for Zr complexation, based on the well-reasoned idea that their structure is not well-adapted to the highly oxophilic Zr4+ hard cation. Indeed, the presence of only four hard donor oxygen atoms and four nitrogen atoms in DOTA was believed to give less-stable Zr complexes than the ones in which the metal is surrounded by eight oxygen atoms. In their paper, Wadas and co-workers reported for the first time the single crystal structure of Zr–DOTA, which is typical of a DOTA metal complex, i.e. the metal cation is entrapped in the cage and octacoordinated by the four nitrogen atoms of the cyclic scaffold and the four oxygen atoms of the acetate pendant arms. A comprehensive stability study of 89Zr–DOTA has been performed, demonstrating an excellent stability of the complex. DOTA clearly outperformed all its competitors, including DFO. It also showed exceptional in vivo behavior, with much less liver, kidney, and bone uptake than 89Zr–DFO. Will DOTA replace DFO and end the quest for improved Zr chelators? Probably not, because DOTA suffers from one major drawback. As a counterpart of its exceptional and unexpected kinetic inertness, the formation of the 89Zr–DOTA complex requires conditions, which are not compatible with biovectors such as antibodies. Indeed, 100% incorporation of the radiometal could be reached only after heating at 95 °C during 1 h with 89ZrCl4. 89ZrCl4 was preferred to 89Zr(ox)2, which gave only 65% radiochemical yield after 2h at 99 °C, while the radiometalation of DFO was quantitative at room temperature within 15 min. This is also an issue because 89ZrCl4 has to be prepared starting from commercially available 89Zr(ox)2 prior to radiometalation; however, it is known that 89ZrCl4 is preferred to 89Zr(ox)2 for toxicity reasons. If the conditions needed for the incorporation of 89Zr into DOTA obviously prohibit the use of this chelator for 89Zr-immunoPET in a conventional way, i.e., the first bioconjugation and then radiometalation in the last step, this work opens the door to a hot-conjugation approach, preparing the 89Zr complex of a bifunctional DOTA prior to the bioconjugation step. Although not optimal, this approach may be facilitated by the easy access to a wide range of bifunctional DOTA derivatives and the development of numerous efficient click-chemistry methods for bioconjugation. No doubt that such approach will be investigated in the future and if the gain in stability is demonstrated by in vivo studies and overcomes the less-straightforward labeling procedures, then DOTA could become a serious candidate for 89Zr-immunoPET. It is also a valuable alternative for biomolecules that can resist the high temperatures required for the incorporation of 89Zr into the DOTA macrocycle.
Conclusions and Future Developments
From the studies reported so far, it is evident that numerous parameters may affect the labeling efficiency, stability, and biodistribution of 89Zr-labeled immunoconjugates: the coordination properties of the chelator, the nature of the linker, the way the probe is conjugated to the antibody. It is not obvious to claim which combination of these different factors will be the best, particularly because it may also depend on the nature of the antibody. However, some trends can be drawn from the work done so far.
Regarding the bioconjugation method, routinely used approaches, e.g., involving isothiocyanate or maleimide functions to conjugate, respectively, to lysines or cysteines residues, may not be ideal. Indeed, the resulting bonds linking the 89Zr complex to the biomolecule may be partially cleaved in some cases. Some recent alternatives are appealing. For instance, among the bifunctional DFO derivatives, DFO–Sq showed interesting behavior; however, the promising properties of this BFC need to be validated in subsequent studies. More importantly, one may predict that further improvements will arise from rapid advances in the field of site-specific bioconjugation and click-chemistry. The right combination of a site-specific bioconjugation method and a bioorthogonal reaction should lead to better-defined and stable constructs with higher immunoreactivity. Moreover, these powerful tools, allowing the fine chemical control of the biomolecule modification, will facilitate the design of bimodal imaging (PET–OI) and theranostic agents (imageable ADC), which undoubtedly represent the next generation of immunoconjugates.
It is now generally accepted that DFO is not the best chelator for 89Zr, although (to different levels) bone uptake was observed in all in vivo experiments whatever the DFO-based BFC used. This concern prompted many groups to develop new chelators with improved 89Zr coordination properties. Some of them failed because they were proven to be less efficient than DFO; none of them are perfect, but all of this recent work (in the last three years) has helped to better understand the important features that a good candidate must meet. First, it is clear that eight donor atoms, preferentially oxygen atoms, are necessary to complete the coordination sphere around the Zr4+ metal ion. Thus, extended DFO, such as DFO* or DFO–Sq, proved to be superior to DFO in many aspects, although they still suffer from some drawbacks such as poor water solubility or lower radiolabeling efficiency. Among the alternatives to hydroxamate group that have been evaluated, hydroxypyridinone is probably the most promising one, with HOPO and, to a lesser extent, 2,3-HOPO showing higher in vitro and in vivo properties in comparison to DFO. The well-known macrocyclic effect could also play an important role in improving the 89Zr complex stability, but the synthesis of chelators incorporating in the macrocyclic ring four hydroxamate or hydroxypyridinone moieties is not straightforward, and the preparation of their bifunctional versions is even more difficult. Finally, DOTA is definitively an option that need to be investigated further despite the high temperature required for complexation. The long half-life of 89Zr allows the metalation to be carried out prior to the bioconjugation step, providing that a good kit that is easy to conjugate is developed.
It appears from the recent advances in the field that there is still room for improved chelators and novel bioconjugation approaches to facilitate the development of ever-more-efficient 89Zr immunoconjugates. However, these radioimmunoconjugates will have to demonstrate clear superiority in subsequent preclinical and clinical studies in comparison to the first generation of 89Zr immunoconjugates that are now widely used for many clinical applications. The cost of development of these new 89Zr immunoconjugates will have to be taken into account. The use of new bifunctional chelating agents, if proven to be significantly better than commercially available DFO derivatives, should not impair too much the global cost of the radioimmunoconjugate. The strategies involving engineering of mAb prior to the bioconjugation step could have a stronger financial impact, and the added value of such immunoconjugates compared to the ones prepared by conventional routes will have to be high, which was not clearly demonstrated so far. However, the recent development for clinical applications of site-specifically constructed ADC indicates that this approach is worth being further investigated.
Acknowledgments
The research leading to these results received funding from the Innovative Medicines Initiatives 2 Joint Undertaking under grant agreement no. 116106. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. In addition, it received funding from The Netherlands Organisation for Scientific Research (NWO, project no. 91617039).
Glossary
Abbreviations
| ADC | antibody-drug conjugate |
| BFC | bifunctional chelator |
| BODIPY | boron–dipyrromethene |
| BTG | bacterial transglutaminase |
| CT | computed tomography |
| DFO | desferrioxamine |
| DFT | density functional theory |
| DIBO | dibenzocyclooctyne |
| DOTA | 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| DTPA | diethylenetriaminepentaacetic acid |
| EDTA | ethylenediaminetetraacetic acid |
| EGFR | epidermal growth factor receptor |
| EpCam | epithelial cell adhesion molecule |
| FDG | fluorodeoxyglucose |
| GalNaz | N-azidoacetylgalactosamine |
| HNSCC | head and neck squamous cell carcinoma |
| HSP | heat-shock protein |
| ID | injected dose |
| IEDDA | inverse electron demand Diels–Alder reaction |
| mAb | monoclonal antibody |
| MRI | magnetic resonance imaging |
| NHS | N-hydroxysuccinimide |
| OI | optical imaging |
| PBS | phosphate-buffered saline |
| PDAC | pancreatic ductal adenocarcinoma |
| PET | positron emission tomography |
| PSMA | prostate-specific membrane antigen |
| SATA | N-succinimidyl–S-acetylthioacetate |
| SMCC | succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate |
| SPAAC | strain-promoted alkyne–azide cycloaddition |
| SPECT | single photon emission computed tomography |
| TCEP | Tris(2-carboxyethyl)phosphine |
| TCO | transcyclooctene |
| TFP | tetrafluorophenol |
| VEGF | vascular endothelial growth factor |
Notes
The authors declare the following competing financial interest(s): Franck Denat is a scientific advisor in Chematech.
References
- Borjesson P. K.; Jauw Y. W.; Boellaard R.; de Bree R.; Comans E. F.; Roos J. C.; Castelijns J. A.; Vosjan M. J.; Kummer J. A.; Leemans C. R.; et al. (2006) Performance of immuno-positron emission tomography with zirconium-89-labeled chimeric monoclonal antibody U36 in the detection of lymph node metastases in head and neck cancer patients. Clin. Cancer Res. 12, 2133–2140. 10.1158/1078-0432.CCR-05-2137. [Abstract] [CrossRef] [Google Scholar]
- Dijkers E. C.; Oude Munnink T. H.; Kosterink J. G.; Brouwers A. H.; Jager P. L.; de Jong J. R.; van Dongen G. A.; Schroder C. P.; Lub-de Hooge M. N.; de Vries E. G. (2010) Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 87, 586–592. 10.1038/clpt.2010.12. [Abstract] [CrossRef] [Google Scholar]
- Pandit-Taskar N.; O’Donoghue J. A.; Durack J. C.; Lyashchenko S. K.; Cheal S. M.; Beylergil V.; Lefkowitz R. A.; Carrasquillo J. A.; Martinez D. F.; Fung A. M.; et al. (2015) A Phase I/II Study for Analytic Validation of 89Zr-J591 ImmunoPET as a Molecular Imaging Agent for Metastatic Prostate Cancer. Clin. Cancer Res. 21, 5277–5285. 10.1158/1078-0432.CCR-15-0552. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- van Es S. C.; Brouwers A. H.; Mahesh S. V. K.; Leliveld-Kors A. M.; de Jong I. J.; Lub-de Hooge M. N.; de Vries E. G. E.; Gietema J. A.; Oosting S. F. (2017) 89Zr-Bevacizumab PET: Potential Early Indicator of Everolimus Efficacy in Patients with Metastatic Renal Cell Carcinoma. J. Nucl. Med. 58, 905–910. 10.2967/jnumed.116.183475. [Abstract] [CrossRef] [Google Scholar]
- Gaykema S. B.; Brouwers A. H.; Hovenga S.; Lub-de Hooge M. N.; de Vries E. G.; Schroder C. P. (2012) Zirconium-89-trastuzumab positron emission tomography as a tool to solve a clinical dilemma in a patient with breast cancer. J. Clin. Oncol. 30, e74–75. 10.1200/JCO.2011.38.0204. [Abstract] [CrossRef] [Google Scholar]
- Gebhart G.; Lamberts L. E.; Wimana Z.; Garcia C.; Emonts P.; Ameye L.; Stroobants S.; Huizing M.; Aftimos P.; Tol J.; et al. (2016) Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann. Oncol. 27, 619–624. 10.1093/annonc/mdv577. [Abstract] [CrossRef] [Google Scholar]
- Desar I. M.; Stillebroer A. B.; Oosterwijk E.; Leenders W. P.; van Herpen C. M.; van der Graaf W. T.; Boerman O. C.; Mulders P. F.; Oyen W. J. (2010) 111In-bevacizumab imaging of renal cell cancer and evaluation of neoadjuvant treatment with the vascular endothelial growth factor receptor inhibitor sorafenib. J. Nucl. Med. 51, 1707–1715. 10.2967/jnumed.110.078030. [Abstract] [CrossRef] [Google Scholar]
- Heskamp S.; Boerman O. C.; Molkenboer-Kuenen J. D.; Oyen W. J.; van der Graaf W. T.; van Laarhoven H. W. (2013) Bevacizumab reduces tumor targeting of anti-epidermal growth factor and anti-insulin-like growth factor 1 receptor antibodies. Int. J. Cancer 133, 307.10.1002/ijc.28046. [Abstract] [CrossRef] [Google Scholar]
- Pastuskovas C. V.; Mundo E. E.; Williams S. P.; Nayak T. K.; Ho J.; Ulufatu S.; Clark S.; Ross S.; Cheng E.; Parsons-Reponte K.; et al. (2012) Effects of anti-VEGF on pharmacokinetics, biodistribution and tumor penetration of trastuzumab in a preclinical breast cancer model. Mol. Cancer Ther. 11, 752.10.1158/1535-7163.MCT-11-0742-T. [Abstract] [CrossRef] [Google Scholar]
- Perk L. R.; Visser G. W.; Vosjan M. J.; Stigter-van W. M.; Tijink B. M.; Leemans C. R.; van Dongen G. A. (2005) (89)Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals (90)Y and (177)Lu in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J. Nucl. Med. 46, 1898–1906. [Abstract] [Google Scholar]
- Rizvi S. N.; Visser O. J.; Vosjan M. J.; van Lingen A.; Hoekstra O. S.; Zijlstra J. M.; Huijgens P. C.; van Dongen G. A.; Lubberink M. (2012) Biodistribution, radiation dosimetry and scouting of 90Y-ibritumomab tiuxetan therapy in patients with relapsed B-cell non-Hodgkin’s lymphoma using 89Zr-ibritumomab tiuxetan and PET. Eur. J. Nucl. Med. Mol. Imaging 39, 512–520. 10.1007/s00259-011-2008-5. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Verel I.; Visser G. W. M.; Boerman O. C.; van Eerd J. E. M.; Finn R.; Boellaard R.; Vosjan M. J. W. D.; Stigter-van Walsum M.; Snow G. B.; van Dongen G. E. M. (2003) Long-Lived Positron Emitters Zirconium-89 and Iodine-124 for Scouting of Therapeutic Radioimmunoconjugates with PET. Cancer Biother.Radiopharm. 18, 655–661. 10.1089/108497803322287745. [Abstract] [CrossRef] [Google Scholar]
- Meijs W. E.; Haisma H. J.; Klok R. P.; van Gog F. B.; Kievit E.; Pinedo H. M.; Herscheid J. D. (1997) Zirconium-labeled monoclonal antibodies and their distribution in tumor-bearing nude mice. J. Nucl. Med. 38, 112–118. [Abstract] [Google Scholar]
- Jauw Y. W.; Menke-van der Houven van Oordt C. W.; Hoekstra O. S.; Hendrikse N. H.; Vugts D. J.; Zijlstra J. M.; Huisman M. C.; van Dongen G. A. (2016) Immuno-Positron Emission Tomography with Zirconium-89-Labeled Monoclonal Antibodies in Oncology: What Can We Learn from Initial Clinical Trials?. Front. Pharmacol. 7, 131.10.3389/fphar.2016.00131. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Deri M. A.; Zeglis B. M.; Francesconi L. C.; Lewis J. S. (2013) PET imaging with 89Zr: From radiochemistry to the clinic. Nucl. Med. Biol. 40, 3–14. 10.1016/j.nucmedbio.2012.08.004. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gaykema S. B.; Schroder C. P.; Vitfell-Rasmussen J.; Chua S.; Oude Munnink T. H.; Brouwers A. H.; Bongaerts A. H.; Akimov M.; Fernandez-Ibarra C.; Lub-de Hooge M. N.; et al. (2014) 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin. Cancer Res. 20, 3945–3954. 10.1158/1078-0432.CCR-14-0491. [Abstract] [CrossRef] [Google Scholar]
- van de Watering F. C.; Rijpkema M.; Perk L.; Brinkmann U.; Oyen W. J.; Boerman O. C. (2014) Zirconium-89 labeled antibodies: a new tool for molecular imaging in cancer patients. BioMed Res. Int. 2014, 203601.10.1155/2014/203601. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lamberts L. E.; Williams S. P.; Terwisscha van Scheltinga A. G.; Lub-de Hooge M. N.; Schroder C. P.; Gietema J. A.; Brouwers A. H.; de Vries E. G. (2015) Antibody positron emission tomography imaging in anticancer drug development. J. Clin. Oncol. 33, 1491–1504. 10.1200/JCO.2014.57.8278. [Abstract] [CrossRef] [Google Scholar]
- Dijkers E. C.; Kosterink J. G.; Rademaker A. P.; Perk L. R.; van Dongen G. A.; Bart J.; de Jong J. R.; de Vries E. G.; Lub-de Hooge M. N. (2009) Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J. Nucl. Med. 50, 974–981. 10.2967/jnumed.108.060392. [Abstract] [CrossRef] [Google Scholar]
- Heskamp S.; van Laarhoven H. W.; Molkenboer-Kuenen J. D.; Franssen G. M.; Versleijen-Jonkers Y. M.; Oyen W. J.; van der Graaf W. T.; Boerman O. C. (2010) ImmunoSPECT and immunoPET of IGF-1R expression with the radiolabeled antibody R1507 in a triple-negative breast cancer model. J. Nucl. Med. 51, 1565–1572. 10.2967/jnumed.110.075648. [Abstract] [CrossRef] [Google Scholar]
- Holland J. P.; Divilov V.; Bander N. H.; Smith-Jones P. M.; Larson S. M.; Lewis J. S. (2010) 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J. Nucl. Med. 51, 1293–1300. 10.2967/jnumed.110.076174. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Laverman P.; van der Geest T.; Terry S. Y.; Gerrits D.; Walgreen B.; Helsen M. M.; Nayak T. K.; Freimoser-Grundschober A.; Waldhauer I.; Hosse R. J.; et al. (2015) Immuno-PET and Immuno-SPECT of Rheumatoid Arthritis with Radiolabeled Anti-Fibroblast Activation Protein Antibody Correlates with Severity of Arthritis. J. Nucl. Med. 56, 778–783. 10.2967/jnumed.114.152959. [Abstract] [CrossRef] [Google Scholar]
- Price T. W.; Greenman J.; Stasiuk G. J. (2016) Current advances in ligand design for inorganic positron emission tomography tracers 68Ga, 64Cu, 89Zr and 44Sc. Dalton Trans. 45, 15702–15724. 10.1039/C5DT04706D. [Abstract] [CrossRef] [Google Scholar]
- Brasse D.; Nonat A. (2015) Radiometals: towards a new success story in nuclear imaging?. Dalton Trans. 44, 4845–4858. 10.1039/C4DT02911A. [Abstract] [CrossRef] [Google Scholar]
- Zeglis B. M.; Houghton J. L.; Evans M. J.; Viola-Villegas N.; Lewis J. S. (2014) Underscoring the Influence of Inorganic Chemistry on Nuclear Imaging with Radiometals. Inorg. Chem. 53, 1880–1899. 10.1021/ic401607z. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Price E. W.; Orvig C. (2014) Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 43, 260–290. 10.1039/C3CS60304K. [Abstract] [CrossRef] [Google Scholar]
- Adumeau P.; Sharma S. K.; Brent C.; Zeglis B. M. (2016) Site-Specifically Labeled Immunoconjugates for Molecular Imaging--Part 2: Peptide Tags and Unnatural Amino Acids. Mol. Imaging Biol. 18, 153–165. 10.1007/s11307-015-0920-y. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Adumeau P.; Sharma S. K.; Brent C.; Zeglis B. M. (2016) Site-Specifically Labeled Immunoconjugates for Molecular Imaging--Part 1: Cysteine Residues and Glycans. Mol. Imaging Biol. 18, 1–17. 10.1007/s11307-015-0919-4. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Vugts D. J.; Visser G. W. M.; van Dongen G. A. M. S. (2013) 89Zr-PET Radiochemistry in the Development and Application of Therapeutic Monoclonal Antibodies and Other Biologicals. Curr. Top. Med. Chem. 13, 446–457. 10.2174/1568026611313040005. [Abstract] [CrossRef] [Google Scholar]
- Fischer G.; Seibold U.; Schirrmacher R.; Wängler B.; Wängler C. (2013) 89Zr, a Radiometal Nuclide with High Potential for Molecular Imaging with PET: Chemistry, Applications and Remaining Challenges. Molecules 18, 6469–6490. 10.3390/molecules18066469. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Meijs W. E.; Herscheid J. D. M.; Haisma H. J.; Pinedo H. M. (1992) Evaluation of desferal as a bifunctional chelating agent for labeling antibodies with Zr-89. Int. J. Rad Appl. Instrum A 43, 1443–1447. 10.1016/0883-2889(92)90170-J. [Abstract] [CrossRef] [Google Scholar]
- Verel I.; Visser G. W.; Boellaard R.; Stigter-van W. M.; Snow G. B.; van Dongen G. A. (2003) 89Zr immuno-PET: comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J. Nucl. Med. 44, 1271–1281. [Abstract] [Google Scholar]
- Perk L. R.; Vosjan M. J. W. D.; Visser G. W. M.; Budde M.; Jurek P.; Kiefer G. E.; van Dongen G. A. M. S. (2010) p-Isothiocyanatobenzyl-desferrioxamine: a new bifunctional chelate for facile radiolabeling of monoclonal antibodies with zirconium-89 for immuno-PET imaging. Eur. J. Nucl. Med. Mol. Imaging 37, 250–259. 10.1007/s00259-009-1263-1. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rudd S. E.; Roselt P.; Cullinane C.; Hicks R. J.; Donnelly P. S. (2016) A desferrioxamine B squaramide ester for the incorporation of zirconium-89 into antibodies. Chem. Commun. 52, 11889–11892. 10.1039/C6CC05961A. [Abstract] [CrossRef] [Google Scholar]
- Sijbrandi N. J.; Merkul E.; Muns J. A.; Waalboer D. C. J.; Adamzek K.; Bolijn M.; Montserrat V.; Somsen G. W.; Haselberg R.; Steverink P. J. G. M.; et al. (2017) A Novel Platinum(II)–Based Bifunctional ADC Linker Benchmarked Using 89Zr-Desferal and Auristatin F–Conjugated Trastuzumab. Cancer Res. 77, 257.10.1158/0008-5472.CAN-16-1900. [Abstract] [CrossRef] [Google Scholar]
- Zeglis B. M.; Mohindra P.; Weissmann G. I.; Divilov V.; Hilderbrand S. A.; Weissleder R.; Lewis J. S. (2011) Modular Strategy for the Construction of Radiometalated Antibodies for Positron Emission Tomography Based on Inverse Electron Demand Diels–Alder Click Chemistry. Bioconjugate Chem. 22, 2048–2059. 10.1021/bc200288d. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zeglis B. M.; Emmetiere F.; Pillarsetty N.; Weissleder R.; Lewis J. S.; Reiner T. (2014) Building Blocks for the Construction of Bioorthogonally Reactive Peptides via Solid-Phase Peptide Synthesis. ChemistryOpen 3, 48–53. 10.1002/open.201402000. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Vugts D. J.; Vervoort A.; Stigter-van Walsum M.; Visser G. W.; Robillard M. S.; Versteegen R. M.; Vulders R. C.; Herscheid J. K.; van Dongen G. A. (2011) Synthesis of phosphine and antibody-azide probes for in vivo Staudinger ligation in a pretargeted imaging and therapy approach. Bioconjugate Chem. 22, 2072–2081. 10.1021/bc200298v. [Abstract] [CrossRef] [Google Scholar]
- Tinianow J. N.; Gill H. S.; Ogasawara A.; Flores J. E.; Vanderbilt A. N.; Luis E.; Vandlen R.; Darwish M.; Junutula J. R.; Williams S.-P.; et al. (2010) Site-specifically 89Zr-labeled monoclonal antibodies for ImmunoPET. Nucl. Med. Biol. 37, 289–297. 10.1016/j.nucmedbio.2009.11.010. [Abstract] [CrossRef] [Google Scholar]
- Ma M. T.; Meszaros L. K.; Paterson B. M.; Berry D. J.; Cooper M. S.; Ma Y.; Hider R. C.; Blower P. J. (2015) Tripodal tris(hydroxypyridinone) ligands for immunoconjugate PET imaging with 89Zr4+: comparison with desferrioxamine-B. Dalton Trans. 44, 4884–4900. 10.1039/C4DT02978J. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zeglis B. M.; Davis C. B.; Aggeler R.; Kang H. C.; Chen A.; Agnew B. J.; Lewis J. S. (2013) Enzyme-Mediated Methodology for the Site-Specific Radiolabeling of Antibodies Based on Catalyst-Free Click Chemistry. Bioconjugate Chem. 24, 1057–1067. 10.1021/bc400122c. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Vosjan M. J. W. D.; Perk L. R.; Visser G. W. M.; Budde M.; Jurek P.; Kiefer G. E.; van Dongen G. A. M. S. (2010) Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat. Protoc. 5, 739–743. 10.1038/nprot.2010.13. [Abstract] [CrossRef] [Google Scholar]
- Meyer J.-P.; Adumeau P.; Lewis J. S.; Zeglis B. M. (2016) Click Chemistry and Radiochemistry: The First 10 Years. Bioconjugate Chem. 27, 2791–2807. 10.1021/acs.bioconjchem.6b00561. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Meimetis L. G.; Boros E.; Carlson J. C.; Ran C.; Caravan P.; Weissleder R. (2016) Bioorthogonal Fluorophore Linked DFO—Technology Enabling Facile Chelator Quantification and Multimodal Imaging of Antibodies. Bioconjugate Chem. 27, 257–263. 10.1021/acs.bioconjchem.5b00630. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Agarwal P.; Bertozzi C. R. (2015) Site-Specific Antibody–Drug Conjugates: The Nexus of Bioorthogonal Chemistry, Protein Engineering, and Drug Development. Bioconjugate Chem. 26, 176–192. 10.1021/bc5004982. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Merten H.; Brandl F.; Plückthun A.; Zangemeister-Wittke U. (2015) Antibody–Drug Conjugates for Tumor Targeting—Novel Conjugation Chemistries and the Promise of non-IgG Binding Proteins. Bioconjugate Chem. 26, 2176–2185. 10.1021/acs.bioconjchem.5b00260. [Abstract] [CrossRef] [Google Scholar]
- Jeger S.; Zimmermann K.; Blanc A.; Grünberg J.; Honer M.; Hunziker P.; Struthers H.; Schibli R. (2010) Site-Specific and Stoichiometric Modification of Antibodies by Bacterial Transglutaminase. Angew. Chem., Int. Ed. 49, 9995–9997. 10.1002/anie.201004243. [Abstract] [CrossRef] [Google Scholar]
- van Geel R.; Wijdeven M. A.; Heesbeen R.; Verkade J. M. M.; Wasiel A. A.; van Berkel S. S.; van Delft F. L. (2015) Chemoenzymatic Conjugation of Toxic Payloads to the Globally Conserved N-Glycan of Native mAbs Provides Homogeneous and Highly Efficacious Antibody–Drug Conjugates. Bioconjugate Chem. 26, 2233–2242. 10.1021/acs.bioconjchem.5b00224. [Abstract] [CrossRef] [Google Scholar]
- Zeglis B. M.; Davis C. B.; Abdel-Atti D.; Carlin S. D.; Chen A.; Aggeler R.; Agnew B. J.; Lewis J. S. (2014) Chemoenzymatic Strategy for the Synthesis of Site-Specifically Labeled Immunoconjugates for Multimodal PET and Optical Imaging. Bioconjugate Chem. 25, 2123–2128. 10.1021/bc500499h. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Houghton J. L.; Zeglis B. M.; Abdel-Atti D.; Aggeler R.; Sawada R.; Agnew B. J.; Scholz W. W.; Lewis J. S. (2015) Site-specifically labeled CA19.9-targeted immunoconjugates for the PET, NIRF, and multimodal PET/NIRF imaging of pancreatic cancer. Proc. Natl. Acad. Sci. U. S. A. 112, 15850–15855. 10.1073/pnas.1506542112. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Guerard F.; Lee Y.-S.; Tripier R.; Szajek L. P.; Deschamps J. R.; Brechbiel M. W. (2013) Investigation of Zr(iv) and 89Zr(iv) complexation with hydroxamates: progress towards designing a better chelator than desferrioxamine B for immuno-PET imaging. Chem. Commun. 49, 1002–1004. 10.1039/C2CC37549D. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Patra M.; Bauman A.; Mari C.; Fischer C. A.; Blacque O.; Haussinger D.; Gasser G.; Mindt T. L. (2014) An octadentate bifunctional chelating agent for the development of stable zirconium-89 based molecular imaging probes. Chem. Commun. 50, 11523–11525. 10.1039/C4CC05558F. [Abstract] [CrossRef] [Google Scholar]
- Vugts D. J.; Klaver C.; Sewing C.; Poot A. J.; Adamzek K.; Huegli S.; Mari C.; Visser G. W.; Valverde I. E.; Gasser G.; et al. (2017) Comparison of the octadentate bifunctional chelator DFO*-pPhe-NCS and the clinically used hexadentate bifunctional chelator DFO-pPhe-NCS for 89Zr-immuno-PET. Eur. J. Nucl. Med. Mol. Imaging 44, 286–295. 10.1007/s00259-016-3499-x. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Guérard F.; Lee Y.-S.; Brechbiel M. W. (2014) Rational Design, Synthesis, and Evaluation of Tetrahydroxamic Acid Chelators for Stable Complexation of Zirconium(IV). Chem. - Eur. J. 20, 5584–5591. 10.1002/chem.201304115. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhai C.; Summer D.; Rangger C.; Franssen G. M.; Laverman P.; Haas H.; Petrik M.; Haubner R.; Decristoforo C. (2015) Novel Bifunctional Cyclic Chelator for 89Zr Labeling–Radiolabeling and Targeting Properties of RGD Conjugates. Mol. Pharmaceutics 12, 2142–2150. 10.1021/acs.molpharmaceut.5b00128. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Boros E.; Holland J. P.; Kenton N.; Rotile N.; Caravan P. (2016) Macrocycle-Based Hydroxamate Ligands for Complexation and Immunoconjugation of 89Zirconium for Positron Emission Tomography (PET) Imaging. ChemPlusChem 81, 274–281. 10.1002/cplu.201600003. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Deri M. A.; Ponnala S.; Zeglis B. M.; Pohl G.; Dannenberg J. J.; Lewis J. S.; Francesconi L. C. (2014) Alternative Chelator for 89Zr Radiopharmaceuticals: Radiolabeling and Evaluation of 3,4,3-(LI-1,2-HOPO). J. Med. Chem. 57, 4849–4860. 10.1021/jm500389b. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Deri M. A.; Ponnala S.; Kozlowski P.; Burton-Pye B. P.; Cicek H. T.; Hu C.; Lewis J. S.; Francesconi L. C. (2015) p-SCN-Bn-HOPO: A Superior Bifunctional Chelator for 89Zr ImmunoPET. Bioconjugate Chem. 26, 2579–2591. 10.1021/acs.bioconjchem.5b00572. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tinianow J. N.; Pandya D. N.; Pailloux S. L.; Ogasawara A.; Vanderbilt A. N.; Gill H. S.; Williams S. P.; Wadas T. J.; Magda D.; Marik J. (2016) Evaluation of a 3-hydroxypyridin-2-one (2,3-HOPO) Based Macrocyclic Chelator for 89Zr4+ and Its Use for ImmunoPET Imaging of HER2 Positive Model of Ovarian Carcinoma in Mice. Theranostics 6, 511–521. 10.7150/thno.14261. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pandya D. N.; Pailloux S.; Tatum D.; Magda D.; Wadas T. J. (2015) Di-macrocyclic terephthalamide ligands as chelators for the PET radionuclide zirconium-89. Chem. Commun. 51, 2301–2303. 10.1039/C4CC09256B. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Perk L. R.; Visser O. J.; Stigter-van Walsum M.; Vosjan M. J. W. D.; Visser G. W. M.; Zijlstra J. M.; Huijgens P. C.; van Dongen G. A. M. S. (2006) Preparation and evaluation of 89Zr-Zevalin for monitoring of 90Y-Zevalin biodistribution with positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 33, 1337–1345. 10.1007/s00259-006-0160-0. [Abstract] [CrossRef] [Google Scholar]
- Price E. W.; Cawthray J. F.; Bailey G. A.; Ferreira C. L.; Boros E.; Adam M. J.; Orvig C. (2012) H4octapa: An Acyclic Chelator for 111In Radiopharmaceuticals. J. Am. Chem. Soc. 134, 8670–8683. 10.1021/ja3024725. [Abstract] [CrossRef] [Google Scholar]
- Price E. W.; Zeglis B. M.; Lewis J. S.; Adam M. J.; Orvig C. (2014) H6phospa-trastuzumab: bifunctional methylenephosphonate-based chelator with 89Zr, 111In and 177Lu. Dalton Trans. 43, 119–131. 10.1039/C3DT51940F. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pandya D. N.; Bhatt N.; Yuan H.; Day C. S.; Ehrmann B. M.; Wright M.; Bierbach U.; Wadas T. J. (2017) Zirconium tetraazamacrocycle complexes display extraordinary stability and provide a new strategy for zirconium-89-based radiopharmaceutical development. Chem. Sci. 8, 2309–2314. 10.1039/C6SC04128K. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gorden A. E. V.; Xu J.; Raymond K. N.; Durbin P. (2003) Rational Design of Sequestering Agents for Plutonium and Other Actinides. Chem. Rev. 103, 4207–4282. 10.1021/cr990114x. [Abstract] [CrossRef] [Google Scholar]
- Wängler C.; Schäfer M.; Schirrmacher R.; Bartenstein P.; Wängler B. (2011) DOTA derivatives for site-specific biomolecule-modification via click chemistry: Synthesis and comparison of reaction characteristics. Bioorg. Med. Chem. 19, 3864–3874. 10.1016/j.bmc.2010.12.047. [Abstract] [CrossRef] [Google Scholar]
- Moreau M.; Raguin O.; Vrigneaud J.-M.; Collin B.; Bernhard C.; Tizon X.; Boschetti F.; Duchamp O.; Brunotte F.; Denat F. (2012) DOTAGA-Trastuzumab. A New Antibody Conjugate Targeting HER2/Neu Antigen for Diagnostic Purposes. Bioconjugate Chem. 23, 1181–1188. 10.1021/bc200680x. [Abstract] [CrossRef] [Google Scholar]
- Bernhard C.; Moreau M.; Lhenry D.; Goze C.; Boschetti F.; Rousselin Y.; Brunotte F.; Denat F. (2012) DOTAGA–Anhydride: A Valuable Building Block for the Preparation of DOTA-Like Chelating Agents. Chem. - Eur. J. 18, 7834–7841. 10.1002/chem.201200132. [Abstract] [CrossRef] [Google Scholar]
- Stasiuk G. J.; Long N. J. (2013) The ubiquitous DOTA and its derivatives: the impact of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid on biomedical imaging. Chem. Commun. 49, 2732–2746. 10.1039/c3cc38507h. [Abstract] [CrossRef] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1021/acs.bioconjchem.7b00325
Read article for free, from open access legal sources, via Unpaywall:
https://pubs.acs.org/doi/pdf/10.1021/acs.bioconjchem.7b00325
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1021/acs.bioconjchem.7b00325
Article citations
Zirconium 89 and Copper 64 for ImmunoPET: From Antibody Bioconjugation and Radiolabeling to Molecular Imaging.
Pharmaceutics, 16(7):882, 30 Jun 2024
Cited by: 0 articles | PMID: 39065579 | PMCID: PMC11279968
Review Free full text in Europe PMC
Automated synthesis of [89Zr]ZrCl4, [89Zr]ZrDFOSquaramide-bisPh(PSMA) and [89Zr]ZrDFOSquaramide-TATE.
EJNMMI Radiopharm Chem, 9(1):39, 08 May 2024
Cited by: 0 articles | PMID: 38717578 | PMCID: PMC11078908
Approaches to Reducing Normal Tissue Radiation from Radiolabeled Antibodies.
Pharmaceuticals (Basel), 17(4):508, 16 Apr 2024
Cited by: 2 articles | PMID: 38675468 | PMCID: PMC11053530
Review Free full text in Europe PMC
Good practices for 89Zr radiopharmaceutical production and quality control.
EJNMMI Radiopharm Chem, 9(1):40, 11 May 2024
Cited by: 3 articles | PMID: 38733556 | PMCID: PMC11088613
Review Free full text in Europe PMC
Interrogating the Theranostic Capacity of a MUC16-Targeted Antibody for Ovarian Cancer.
J Nucl Med, 65(4):580-585, 01 Apr 2024
Cited by: 2 articles | PMID: 38485271 | PMCID: PMC10995531
Go to all (72) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
p-SCN-Bn-HOPO: A Superior Bifunctional Chelator for (89)Zr ImmunoPET.
Bioconjug Chem, 26(12):2579-2591, 25 Nov 2015
Cited by: 52 articles | PMID: 26550847 | PMCID: PMC4962612
Comparison of the octadentate bifunctional chelator DFO*-pPhe-NCS and the clinically used hexadentate bifunctional chelator DFO-pPhe-NCS for 89Zr-immuno-PET.
Eur J Nucl Med Mol Imaging, 44(2):286-295, 30 Aug 2016
Cited by: 63 articles | PMID: 27573793 | PMCID: PMC5215071
Total-Body PET and Highly Stable Chelators Together Enable Meaningful 89Zr-Antibody PET Studies up to 30 Days After Injection.
J Nucl Med, 61(3):453-460, 27 Sep 2019
Cited by: 48 articles | PMID: 31562219 | PMCID: PMC7067524
Recent Advances in Zirconium-89 Chelator Development.
Molecules, 23(3):E638, 12 Mar 2018
Cited by: 31 articles | PMID: 29534538 | PMCID: PMC6017441
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Dutch Research Council (NWO) (1)
Grant ID: 91617039
H2020 Health (1)
Grant ID: 116106