Abstract
Importance
Determining factors associated with endothelial cell loss after Descemet stripping automated endothelial keratoplasty (DSAEK) could improve long-term graft survival.Objective
To evaluate the associations of donor, recipient, and operative factors with endothelial cell density (ECD) 3 years after DSAEK in the Cornea Preservation Time Study.Design, setting, and participants
This cohort study was a secondary analysis of data collected in a multicenter, double-masked, randomized clinical trial. Forty US clinical sites with 70 surgeons participated, with donor corneas provided by 23 US eye banks. Individuals undergoing DSAEK for Fuchs dystrophy or pseudophakic/aphakic corneal edema were included.Interventions
The DSAEK procedure, with random assignment of a donor cornea with a preservation time of 0 to 7 days or 8 to 14 days.Main outcomes and measures
Endothelial cell density at 3 years as determined by a reading center from eye bank and clinical specular or confocal central endothelial images.Results
The study included 1090 participants (median age, 70 years) with 1330 affected eyes (240 bilateral cases [22.0%]), who underwent DSAEK for Fuchs dystrophy (1255 eyes [94.4%]) or pseudophakic/aphakic corneal edema (PACE) (75 eyes [5.6%]). Of these, 801 eyes (60.2%) belonged to women and 1207 (90.8%) to white individuals. A total of 749 participants (913 eyes; 164 [21.9%] bilateral cases) had functioning grafts with acceptable endothelial images preoperatively and at 3 years postoperatively and were included in this analysis. Factors associated with a lower ECD at 3 years (estimated effect with 99% CI) in the final multivariable model included donors with diabetes (-103 [-196 to -9] cells/mm2), lower screening ECD (-234 [-331 to -137] per 500 cells/mm2), recipient diagnosis of PACE (-257 [-483 to -31] in cells/mm2), and operative complications (-324 [-516 to -133] in cells/mm2). Endothelial cell loss (ECL) from a preoperative measurement to a 3-year postoperative measurement was 47% (99% CI, 42%-52%) for participants receiving tissue from donors with diabetes vs 43% (99% CI, 39%-48%) without diabetes; it was 53% (99% CI, 44%-62%) for participants diagnosed with PACE vs 44% (99% CI, 39%-49%) for those diagnosed with Fuchs dystrophy, and 55% (99% CI, 48%-63%) in participants who experienced operative complications vs 44% (99% CI, 39%-48%) in those who did not. No other donor, recipient, or operative factors were significantly associated with 3-year ECD.Conclusions and relevance
Donor diabetes, lower screening ECD, a PACE diagnosis in the recipient, and operative complications were associated with lower ECD at 3 years after DSAEK surgery and may be associated with long-term graft success. While causation cannot be inferred, further studies on the association of donor diabetes and PACE in recipients with lower 3-year ECD warrant further study.Free full text

Donor, Recipient, and Operative Factors Associated With Increased Endothelial Cell Loss in the Cornea Preservation Time Study
Associated Data
Key Points
Question
What donor, recipient, and operative factors are associated with endothelial cell density 3 years after Descemet stripping automated endothelial keratoplasty (DSAEK) in the Cornea Preservation Time Study cohort?
Findings
In this study, donors with diabetes, diagnosis of pseudophakic/aphakic corneal edema in the recipient, and operative complications were associated with lower endothelial cell density at 3 years.
Meaning
Optimizing donor selection and minimizing surgical trauma could help minimize endothelial cell loss and improve graft survival after DSAEK. Mechanisms whereby corneal tissue from diabetes in donors and pseudophakic/aphakic corneal edema in recipients affect endothelial cell density warrant further study.
Abstract
Importance
Determining factors associated with endothelial cell loss after Descemet stripping automated endothelial keratoplasty (DSAEK) could improve long-term graft survival.
Objective
To evaluate the associations of donor, recipient, and operative factors with endothelial cell density (ECD) 3 years after DSAEK in the Cornea Preservation Time Study.
Design, Setting, and Participants
This cohort study was a secondary analysis of data collected in a multicenter, double-masked, randomized clinical trial. Forty US clinical sites with 70 surgeons participated, with donor corneas provided by 23 US eye banks. Individuals undergoing DSAEK for Fuchs dystrophy or pseudophakic/aphakic corneal edema were included.
Interventions
The DSAEK procedure, with random assignment of a donor cornea with a preservation time of 0 to 7 days or 8 to 14 days.
Main Outcomes and Measures
Endothelial cell density at 3 years as determined by a reading center from eye bank and clinical specular or confocal central endothelial images.
Results
The study included 1090 participants (median age, 70 years) with 1330 affected eyes (240 bilateral cases [22.0%]), who underwent DSAEK for Fuchs dystrophy (1255 eyes [94.4%]) or pseudophakic/aphakic corneal edema (PACE) (75 eyes [5.6%]). Of these, 801 eyes (60.2%) belonged to women and 1207 (90.8%) to white individuals. A total of 749 participants (913 eyes; 164 [21.9%] bilateral cases) had functioning grafts with acceptable endothelial images preoperatively and at 3 years postoperatively and were included in this analysis. Factors associated with a lower ECD at 3 years (estimated effect with 99% CI) in the final multivariable model included donors with diabetes (−103 [−196 to −9] cells/mm2), lower screening ECD (−234 [−331 to −137] per 500 cells/mm2), recipient diagnosis of PACE (−257 [−483 to −31] in cells/mm2), and operative complications (−324 [−516 to −133] in cells/mm2). Endothelial cell loss (ECL) from a preoperative measurement to a 3-year postoperative measurement was 47% (99% CI, 42%-52%) for participants receiving tissue from donors with diabetes vs 43% (99% CI, 39%-48%) without diabetes; it was 53% (99% CI, 44%-62%) for participants diagnosed with PACE vs 44% (99% CI, 39%-49%) for those diagnosed with Fuchs dystrophy, and 55% (99% CI, 48%-63%) in participants who experienced operative complications vs 44% (99% CI, 39%-48%) in those who did not. No other donor, recipient, or operative factors were significantly associated with 3-year ECD.
Conclusions and Relevance
Donor diabetes, lower screening ECD, a PACE diagnosis in the recipient, and operative complications were associated with lower ECD at 3 years after DSAEK surgery and may be associated with long-term graft success. While causation cannot be inferred, further studies on the association of donor diabetes and PACE in recipients with lower 3-year ECD warrant further study.
Introduction
Risk factors for endothelial cell loss (ECL) have been described after penetrating keratoplasty (PK) in the Specular Microscopy Ancillary Study1,2,3 and other large studies,4,5 as well as after Descemet Stripping Automated Endothelial Keratoplasty (DSAEK) in single-center studies.6,7 Given key differences in eye bank and surgical methods for DSAEK compared with PK, understanding the main factors that influence ECL after DSAEK is important for prolonging the health of the participants with these grafts through appropriate donor selection, understanding the effects of recipient preoperative diagnosis, and optimizing surgical techniques.
The Cornea Preservation Time Study (CPTS) was a prospective, randomized clinical trial designed to determine the effect of donor preservation time (PT) on graft survival and endothelial cell loss after DSAEK.8 At 3 years after DSAEK, a donor PT of 12 to 14 days was associated with a lower graft survival rate, but differences were small when PT was shorter than 12 days.9 There was a small but significant difference in ECL at 3 years between corneas preserved for 0 to 7 days (37%) and those preserved for 8 to 14 days (40%).10 In this study, we evaluate other donor factors, as well as recipient and operative factors, associated with endothelial cell density (ECD) during the first 3 years after DSAEK, complementing a recent study examining the association of these factors with graft success.11
Methods
Details of the CPTS protocol and primary results have been published previously.8,9,10 Briefly, participants undergoing DSAEK were randomized to receive tissue preserved for 0 to 7 days or 8 to 14 days with a primary outcome of graft survival at 3 years. The protocol was approved by institutional review boards governing each investigational site, and individual participants gave written informed consent to participate in the study. The study adhered to the tenets of the Declaration of Helsinki and was registered as a clinical trial (NCT01537393).
Participants were enrolled between April 2012 and February 2014; eligible participants had corneal endothelial dysfunction, including Fuchs endothelial corneal dystrophy (FECD) and pseudophakic/aphakic corneal edema (PACE). Donors had a minimum eye bank–measured central ECD of 2300 cells/mm2, and donor corneas were stored at 2° C to 8° C in either Optisol GS (Bausch & Lomb) or Life 4° C (Numedis, Inc).8
We have recently defined and assessed multiple donor, recipient, and operative factors influencing graft success in the CPTS.11 These same parameters were assessed in this analysis. In brief, donor information was gathered and reported by the 23 Eye Bank Association of America–accredited eye banks in the CPTS. Using methods consistent with standard eye bank practice, the eye banks recorded donor data, including donor age, sex, race/ethnicity, cause of death, and history of diabetes (yes/no) determined from medical records and/or next of kin, as was done in the Cornea Donor Study.12,13 Additional parameters involving procurement information, tissue preparation, recipient data (including sex, race/ethnicity, diabetes [historically determined as in the Cornea Donor Study12,13], ocular history and medications, and preoperative recipient diagnosis), operative details, and surgical complications were collected prospectively and analyzed in this study.
Endothelial Imaging and Image Analysis
The Corneal Image Analysis Reading Center (CIARC) at Case Western Reserve University and University Hospitals Eye Institute (Cleveland, Ohio), was the reading center determining ECD and was responsible for quality control measures at the eye banks and clinical sites, including image quality, certification, and calibration procedures.8,10,14,15 Eye banks obtained 1 to 3 initial specular image(s) of the central endothelium and then determined ECD by their usual analysis method (referred to as screening ECD). This assessment was performed prior to randomization and was not standardized. If a donor cornea was CPTS assigned, eye banks also obtained 3 study images, termed preoperative, either after lamellar dissection, or, if the donor cornea was to be surgeon prepared, prior to shipment. Postoperatively, 3 specular or confocal microscopic images of the central corneal endothelium of the graft were obtained at 6, 12, 24, and 36 months, depending on how long a participant remained in the follow-up cohort without graft failure or regraft. All preoperative donor and postoperative clinical images were transferred to CIARC by the Data Management and Analysis Center (Jaeb Center for Health Research, Tampa, Florida) in a deidentified manner with the study number and date of acquisition.
Endothelial Cell Density Determination
Details of CIARC procedures have been previously described with variable frame analysis for ECD determination by 2 masked independent readers, and adjudication, if indicated.8,10,14 In brief, the ECD of all analyzable images was independently determined by 2 readers using the variable frame analysis method.15 If the ECDs determined by the 2 readers differed by 5.0% or more, a third independent determination of ECD was made by an adjudicator. Final ECD was the average of all ECDs that were within 5% of each other.
Statistical Analysis
The analysis was restricted to eyes with a functioning graft at 3 years after DSAEK and analyzable preoperative and 3-year endothelial images. The preoperative ECD was considered the baseline for these analyses. Associations between donor, recipient, and operative factors, including complications9 and 3-year ECD (in cells/mm2), were evaluated in multivariable linear mixed models. All candidate factors that were considered are listed in the Table and eTable 1 in the Supplement. Continuous covariates were included in all models in continuous form but were categorized for display and ease of interpretation in the Table and eTable 1 in the Supplement. Missing data were treated as a separate category for discrete factors, and a missing indicator was created for continuous factors.
Table.
| Characteristic | No. (N = = 913) 913) | Base Modela | Multivariable Modelb | Mean Loss, % (99%CI) b,c | ||
|---|---|---|---|---|---|---|
| Mean ECD at 3 y (99% CI), Cells/mm2 | P Value | Mean ECD at 3 y (99% CI), Cells/mm2 | P Value | |||
| Significant donor factors | ||||||
| Screening ECD (eye bank–determined), cells/mm2 | ||||||
| <2500 | 196 | 1356 (1200-1512) | <.001 | 1374 (1218-1530) | <.001 | 50 (44-55) |
| 2500-2749 | 336 | 1443 (1307-1580) | 1461 (1322-1599) | 46 (41-51) | ||
| 2750-2999 | 212 | 1555 (1408-1701) | 1572 (1424-1719) | 42 (37-48) | ||
| ≥3000 | 169 | 1765 (1602-1928) | 1754 (1592-1916) | 37 (31-43) | ||
| Preoperative ECD (Cornea Image Analysis Reading Center determined), cells/mm2 | ||||||
| <2500 | 216 | 1206 (1058-1353) | <.001 | 1318 (1168-1469) | <.001 | 43 (37-48) |
| 2500-2749 | 252 | 1502 (1360-1644) | 1542 (1401-1683) | 41 (35-46) | ||
| 2750-2999 | 236 | 1577 (1433-1720) | 1553 (1410-1696) | 45 (40-51) | ||
| ≥3000 | 209 | 1764 (1616-1911) | 1664 (1511-1817) | 49 (43-54) | ||
| Diabetic donor | ||||||
| No | 674 | 1543 (1417-1668) | .003 | 1550 (1423-1677) | .005 | 43 (30-48) |
| Yes | 239 | 1431 (1288-1573) | 1447 (1305-1589) | 47 (42-52) | ||
| Significant recipient factors | ||||||
| Recipient diagnosis | ||||||
| Pseudophakic/aphakic corneal edema only | 36 | 1281 (1028-1533) | .006 | 1276 (1032-1520) | .003 | 53 (43-62) |
| Fuchs endothelial corneal dystrophy | 877 | 1532 (1410-1653) | 1533 (1407-1659) | 44 (39-49) | ||
| Baseline intraocular pressure, mm Hgd | ||||||
| 8-19 | 839 | 1503 (1381-1625) | .005 | 1511 (1386-1636) | .004 | 45 (40-49) |
| 20-27 | 74 | 1666 (1472-1861) | 1667 (1477-1856) | 39 (32-46) | ||
| Significant operative factors | ||||||
| Operative complicationse | ||||||
| No | 867 | 1537 (1413-1662) | <.001 | 1539 (1414-1665) | <.001 | 44 (39-48) |
| Yes | 46 | 1188 (964-1411) | 1215 (999-1431) | 55 (48-63) | ||
| Nonsignificant donor factors | ||||||
| Donor age, y | ||||||
| ≤30 | 52 | 1731 (1513-1948) | .002 | 1643 (1430-1856) | .08 | 41 (34-49) |
| 31-50 | 144 | 1552 (1395-1710) | 1553 (1397-1709) | 43 (37-49) | ||
| 51-60 | 232 | 1524 (1382-1667) | 1521 (1378-1663) | 44 (39-50) | ||
| 61-70 | 352 | 1456 (1322-1589) | 1481 (1347-1616) | 46 (41-51) | ||
| 71-75 | 133 | 1533 (1372-1695) | 1560 (1401-1720) | 43 (37-49) | ||
| Storage solution | ||||||
| Life 4°C (Numedic) | 35 | 1189 (836-1541) | .01 | 1206 (863-1550) | .01 | 55 (43-68) |
| Optisol GS (Bausch & Lomb) | 878 | 1531 (1412-1651) | 1539 (1416-1662) | 44 (39-48) | ||
| Change in solutionf | ||||||
| Original | 256 | 1617 (1408-1827) | .02 | 1617 (1403-1831) | .03 | 41 (33-49) |
| Fresh | 388 | 1449 (1313-1584) | 1463 (1324-1601) | 46 (41-52) | ||
| Surgeon dissected tissue | 269 | 1639 (1446-1832) | 1636 (1444-1827) | 40 (33-47) | ||
| Descemet stripping automated endothelial keratoplasty lenticule thickness, μmg | ||||||
| <100 | 138 | 1463 (1288-1638) | .05 | 1459 (1289-1630) | .06 | 46 (40-53) |
| 100-<125 | 266 | 1546 (1402-1691) | 1560 (1416-1703) | 43 (38-48) | ||
| 125-<150 | 244 | 1523 (1376-1670) | 1546 (1400-1692) | 44 (38-49) | ||
| ≥150 | 231 | 1474 (1321-1627) | 1482 (1330-1633) | 46 (41-52) | ||
| Any eye bank observationsh | ||||||
| No | 522 | 1505 (1381-1629) | .06 | 1511 (1384-1638) | .10 | 45 (40-49) |
| Yes | 122 | 1451 (1282-1620) | 1477 (1310-1644) | 46 (40-52) | ||
| Surgeon dissected tissue | 269 | 1646 (1451-1840) | 1643 (1450-1835) | 40 (33-47) | ||
| Nonsignificant recipient factors | ||||||
| Recipient age, y | ||||||
| 42-65 | 254 | 1553 (1410-1696) | .03 | 1556 (1414-1699) | .12 | 43 (38-48) |
| 66-75 | 405 | 1528 (1395-1661) | 1520 (1386-1654) | 44 (39-49) | ||
| 76-91 | 254 | 1460 (1319-1601) | 1494 (1352-1635) | 46 (40-51) | ||
| Nonsignificant operative factors | ||||||
| Complete air fill duration, mini | ||||||
| <10 | 23 | 1655 (1316-1993) | .08 | 1629 (1302-1956) | .03 | 40 (28-52) |
| 10-19 | 667 | 1523 (1384-1661) | 1531 (1391-1671) | 44 (39-49) | ||
| ≥20 | 218 | 1488 (1318-1659) | 1500 (1330-1671) | 46 (39-52) | ||
| Injection of viscoelastic | ||||||
| No | 143 | 1371 (1185-1558) | .01 | 1418 (1231-1605) | .06 | 48 (41-55) |
| Yes | 770 | 1562 (1435-1689) | 1557 (1426-1687) | 43 (30-48) | ||
| Concomitant cataract surgery | ||||||
| No | 416 | 1467 (1337-1598) | .01 | 1491 (1358-1624) | .09 | 46 (41-51) |
| Yes | 497 | 1565 (1434-1697) | 1555 (1422-1688) | 43 (38-48) | ||
Abbreviation: ECD, endothelial cell density.
 −
− Baseline ECD)/Baseline ECD]
Baseline ECD)/Baseline ECD] ×
× 100.
100.The base model for evaluating candidate factors included PT, because of its influence on ECD in the CPTS,10 and preoperative ECD measured by the CIARC to account for differing initial ECDs among donor tissues. All models included surgeon and recipient as random effects to account for possible correlation between grafts performed by the same surgeon (which is called a surgeon effect) and fellow eyes of the same recipient. Each factor was evaluated first by adding the factor to the base model. Candidate factors were selected for inclusion in a final multivariable model in 2 stages. In stage 1, baseline donor and recipient factors with P values less than .10 were included in a backward multivariable model selection procedure.16 In stage 2, all factors selected in stage 1 were retained and operative factors with P values less than .10 were included in another backward multivariable model selection procedure to yield a final model. To provide additional information, effects adjusted for the factors in the final model were provided for each factor discarded during variable selection. Owing to the multiple comparisons, only donor and recipient factors with P values less than .01 at the first stage and operative factors with P values less than .01 at the second stage were considered statistically significant and remained in the final model.
The effect of categorical factors on ECD at 3 years was reported as the estimate of the mean difference in ECD from the reference category with 99% CI. For ease of interpretation, the effect of continuous factors on ECD at 3 years was expressed as the estimate of the mean difference in ECD associated with a clinically relevant difference in the continuous factor and corresponding 99% CI. All reported P values are 2-sided. The data were analyzed using SAS version 9.4 (SAS Institute) and R version 3.4 (R Foundation for Statistical Computing)17 from September 2017 to September 2018.
Results
A total of 1090 individuals with 1330 eyes (including 240 individuals [22.0%] with bilateral cases) were enrolled. Participants were 42 to 90 years old (median age, 70 years) at the time of enrollment between April 2012 and February 2014. Of the 1330 eyes, 801 (60.2%) belonged to women, and 1207 [90.8%) belonged to white individuals. A total of 1255 eyes (94.4%) had FECD, and 75 eyes (5.6%) had PACE. This analysis includes 913 clear grafts of the 1330 eyes included in the CPTS (68.5%) in 749 participants with available screening, preoperative, and 3-year images.
Donors contributing corneas were 12 to 75 years old (median age, 61 years). Those in the 0-day to 7-day PT group had a median PT of 6 days; those in the 8-day to 14-day PT group had a median of 11 days. The recipient and donor baseline characteristics of this subset were similar to the characteristics of the full CPTS cohort.8,10 Of those excluded from this analysis, 32 eyes lacked CIARC-determined preoperative ECD values. The CIARC did not obtain 16 of these 32 images from the eye banks, while the other 16 were attained but not analyzable.
The 3-year ECD adjusted mean estimates are shown in the Table and eTable 1 in the Supplement for each candidate factor. Factors with a corresponding P value of less than .10 when included in the base model are included in the Table. After accounting for PT and preoperative ECD, the final multivariable model showed the donor factors independently associated with a lower ECD at 3 years included lower screening ECD (−234 [99% CI, −331 to −137] cells/mm2 at 3 years for every 500 cells/mm2 difference in the screening ECD) and donors with diabetes (−103 [99% CI, −196 to −9] cells/mm2) compared with those without diabetes. The recipient factors associated with lower ECD at 3 years were a recipient diagnosis of PACE (−257 [99% CI, −483 to −31] cells/mm2) vs FECD and lower baseline intraocular pressure (IOP) (−80 [99% CI, −151 to −9] cells/mm2 for every 5–mm Hg difference in baseline IOP level). Notably, baseline IOP level was not significant after excluding eyes that were treated for glaucoma medically or surgically before DSAEK. Finally, the sole operative factor associated with lower ECD at 3 years was operative complications (−324 [99% CI, −516 to −133]) (Table). The small numbers of eyes with intraoperative complications in subgroups, as listed in the Table, precluded analysis of the influence of each of these complications on ECD at 3 years.
The associations between screening and preoperative ECD and 3-year ECD are also shown as scatterplots in Figure 1 and Figure 2. Although eye bank screening ECD and CIARC preoperative ECD were correlated (ρ =
= 0.53), each provided independent predictive information on 3-year ECD.
0.53), each provided independent predictive information on 3-year ECD.
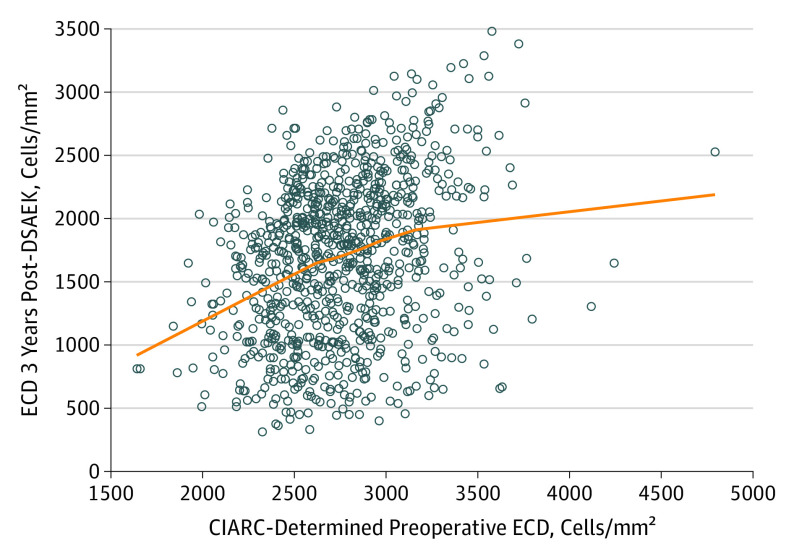
The line represents a LOESS curve fitted to the data points (0.628; ρ =
= 0.30).
0.30).
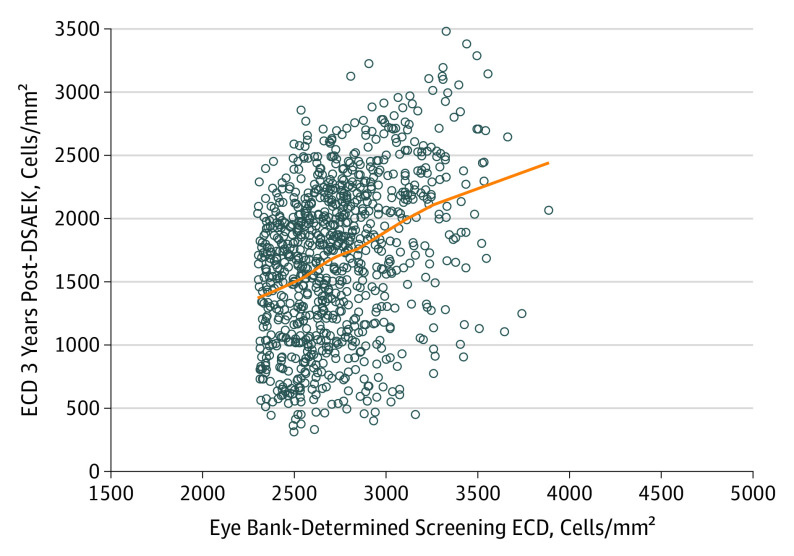
The line represents a LOESS curve fitted to the data points (0.605; ρ =
= 0.36).
0.36).
Donor factors (age, storage solution, replacement of storage solution [if performed], lenticule thickness, eye bank observations), recipient age, and operative factors (complete air fill duration, viscoelastic injection, and concomitant cataract surgery) were included in the model selection, but were not independent risk factors (ie, P ≥
≥ .01) after adjusting for the factors included in the final model (Table). Mean percentage of ECL was 47% (99% CI, 42%-52%) for participants receiving tissue from donors with diabetes vs 43% (99% CI, 39%-48%) for those receiving tissue from donors without diabetes, 53% (99% CI, 44%-62%) for participants with PACE vs 44% (99% CI, 39%-49%) for participants with FECD, and 55% (99% CI, 48%-63%) when operative complications occurred vs 44% (99% CI, 39%-48%) when operative complications did not occur (Table). No other preoperative donor, recipient, or operative factors were included in the model selection procedure (base model P value >.10; eTable 1 in the Supplement). Neither the method of insertion, whether protected or not protected (eTable 1 in the Supplement), nor wound incision size were statistically significant. eTable 2 in the Supplement shows mean ECD over time for select candidate factors.
.01) after adjusting for the factors included in the final model (Table). Mean percentage of ECL was 47% (99% CI, 42%-52%) for participants receiving tissue from donors with diabetes vs 43% (99% CI, 39%-48%) for those receiving tissue from donors without diabetes, 53% (99% CI, 44%-62%) for participants with PACE vs 44% (99% CI, 39%-49%) for participants with FECD, and 55% (99% CI, 48%-63%) when operative complications occurred vs 44% (99% CI, 39%-48%) when operative complications did not occur (Table). No other preoperative donor, recipient, or operative factors were included in the model selection procedure (base model P value >.10; eTable 1 in the Supplement). Neither the method of insertion, whether protected or not protected (eTable 1 in the Supplement), nor wound incision size were statistically significant. eTable 2 in the Supplement shows mean ECD over time for select candidate factors.
Discussion
The CPTS was primarily designed to determine the effect of donor PT on graft success and ECL. Longer PT was associated with greater ECL at 3 years after DSAEK surgery, although much of the effect was attributed to the very longest PTs, which were from 12 to 14 days.10 In this prespecified secondary analysis, lower ECD at 3 years was also associated with the additional factors of diabetes in the donor, recipient diagnosis, and operative complications.
Donor Factors
Diabetes
Donors with diabetes were associated with lower ECD at 3 years after DSAEK, including adjustment for the preoperative ECD difference between donors without and with diabetes (66 cells/mm2; eTable 2 in the Supplement). These findings support the known deleterious effects of diabetes on the corneal endothelium biochemically,18,19 morphologically,20,21,22,23 and functionally,20,21,24,25 in mitochondrial damage26 and Descemet membrane strength.27 Although previous studies did not find an effect of donor diabetes on ECL after PK2,12 and Descemet membrane endothelial keratoplasty (DMEK),28 these studies were limited by historical diabetes characterization. Notably, we have also reported a significant association between diabetes in the donor and graft failure after DSAEK, particularly primary or early failures,11 unlike previous studies of PK,12,13 DSAEK,29 and DMEK,28 which did not find a deleterious effect. These studies were also limited by less rigorous historical diabetes characterization.
ECD
Lower screening and preoperative ECD were both associated with lower postoperative ECD at 3 years; however, ECD preoperatively may not be as important as the rate of ECL postoperatively, especially in the short term, and graft survival.30,31 The association of screening with preoperative ECD, postoperative ECD, and graft failure in the CPTS will be examined in future analyses performed similarly in the Specular Microscopy Ancillary Study.31
Other Donor Factors
Donor factors that were not associated with greater ECL included cause of death, time from death to preservation, lenticule thickness, eye bank-dissected vs surgeon-dissected donor lenticule tissue, and donor sex. Female donors were associated with lower ECL at 5 years in the Cornea Donor Study,2 but not in the CPTS, adding to the controversy regarding sex mismatch and graft success.32,33 A trend toward higher ECL with older donor age was observed, although the association became nonsignificant in the final statistical model. Older donor age (66 to 75 years, compared with 12 to 65 years) is known to be associated with a higher rate of ECL after PK.3 Death to refrigeration time, refrigeration to preservation time, and death to preservation time were not significant risk factors for ECL, although the range of each of these times was limited (≤10, 18, and 22 hours, respectively). Notably, eye bank slitlamp observations did not have an association with 3-year ECD, in contrast with surgeon-reported operative complications. This can be explained by the eye bank criteria that were in place for selecting appropriate donor tissue according to the CPTS protocol.
Recipient Factors
Diagnosis
Recipient diagnosis was the main recipient factor that affected ECL at 3 years. Endothelial cell loss was greater for participants with PACE compared with participants with FECD, paralleling our graft survival findings11 and others.6 The differing percentage of ECL at 3 years by recipient diagnosis in the CPTS (53% for participants with PACE vs 44% for participants with FECD) could be explained by peripheral host corneal endothelial health, with higher ECL associated with diminished peripheral ECD in individuals with PACE. In individuals with FECD, peripheral-to-central endothelial cell migration may occur after removing the central endothelium without34 or with the topical ρ-kinase inhibitor ripasudil,35 resulting in clear corneas, whereas similar success has not been reported in participants with PACE who have poor peripheral ECD. Similarly, after successful DSAEK procedures, healthier peripheral endothelial cells could help sustain central ECD in participants with FECD compared with participants with PACE.
Preoperative IOP
Lower preoperative IOP was also associated with greater ECL 3 years after DSAEK procedures; however, this was no longer significant after excluding participants whose eyes were treated for glaucoma medically or surgically before DSAEK. Greater complication and failure rates of DSAEK owing to endothelial failure after trabeculectomy and shunt tube placement have been reported.36,37 In the participants in the CPTS whose eyes maintained normal anterior chamber anatomy and were medically well controlled, glaucoma history did not seem to influence long-term ECD.
Operative Factors
Operative Complications
The only operative factor associated with greater ECL was a surgeon-reported operative complication. The measured ECL at 3 years was 55% when a surgeon-reported operative complication occurred, compared with 44% when the surgeon did not report such complication. This parallels the findings on graft success11 and is therefore potentially one of the most important modifiable risk factors that affects graft outcome and long-term ECL. High early ECL after PK was associated with late graft failure from nonimmunologic endothelial attrition in 1 long-term study,30 and early ECL is much higher with DSAEK than PK.39
Insertion Methods and Incision Size
Insertion method was not associated with 3-year ECD in this study. Although the model-based mean 3-year ECD for the glide-pull method was markedly lower (1355 cells/mm2) than in other methods (ranging from a mean of 1415 cells/mm2 for the Sheets glide/shovel method to 1676 cells/mm2 for the Busin glide method), there were only 21 cases with this method, far fewer than the next least frequent method (ie, the 92 cases via the pull-through method). The lack of significance for insertion methods may be associated with the number of cases in each group compared with other studies,40,41,42,43,44 or it may indicate that when the technique is successfully performed, it does not affect ECL. Incision size was also not a statistically significant factor for lower 3-year ECD, as long as insertion was protected for smaller length incisions (4.0 mm and shorter), unlike a previous report with a smaller incision (ie, 3.2 mm) and unprotected insertion.45 Finally, after PK, the Cornea Donor Study found that larger diameter grafts were associated with higher ECD postoperatively,2 while the CPTS2 and Terry et al46 did not observe an effect for DSAEK, even though larger mean diameter grafts were used compared with the Cornea Donor Study.3
Limitations
The CPTS prospective study design, large sample size, inclusion of many surgeons and eye banks, centralized endothelial cell analysis, and very low percentage of nonanalyzable images preoperatively (2%) and at 3 years (2%)10 enable the data from this study to be generally applied to DSAEK procedures that use cold storage of donor corneas. However, the endothelial cell data reported are only from surviving recipients and grafts at 3 years postoperatively. The issue of drop out of participants with graft failure has been approached by mathematical modeling,6,47,48,49 but the relatively short follow-up time of 3 years is not suitable for this statistical approach. Excluding failed grafts could introduce bias,10 but the magnitude of any bias is likely small in our analysis, since only 34 of the 79 failures were not primary or early failures.9 Of the original 1330 eyes in this study, 913 (68.5%) were available for analysis, resulting in meaningful conclusions similar to our studies in the Specular Microscopy Ancillary Study.2 In addition, the risk factors reported in this study do not include postoperative events, such as graft dislocation or rejection, that could also be important for ECL and graft survival after DSAEK compared with PK; these risk factors will be reported separately (R. Doyle Stulting, MD, written communication, October 1, 2018).
Having shown the effect of diabetes in the donor on both ECL and lower graft success11 compared with the previous literature,12,28,29 a randomized clinical trial seems warranted based on rigorous assessment of diabetes status of the donor. To provide greater precision in such a trial, diabetes should be better characterized in the donor as well as the recipient, by not only historical means, but also via diabetes severity grading,50 as well as hemoglobin A1c testing51 to enable the detection of undiagnosed diabetes.52
Conclusions
The CPTS has shown that, in addition to PT, donor diabetes, PACE, and operative complications are associated with lower 3-year ECD; however, with the putative exception of PT, causation cannot be inferred from these associations. Optimizing donor selection and minimizing surgical trauma and operative complications could help minimize ECL and improve graft survival after DSAEK with findings that may be applicable to DMEK with a similar ECL pattern.53,54,55
Notes
Supplement.
eTable 1. Factors Not Associated with 3-year Endothelial Cell Density in the Cornea Preservation Time Study
eTable 2. Mean Endothelial Cell Density Over Time by Select Recipient, Donor, and Operative Factors in the Cornea Preservation Time Study
References
Full text links
Read article at publisher's site: https://doi.org/10.1001/jamaophthalmol.2018.5669
Read article for free, from open access legal sources, via Unpaywall:
https://jamanetwork.com/journals/jamaophthalmology/articlepdf/2711252/jamaophthalmology_lass_2018_oi_180100.pdf
Citations & impact
Impact metrics
Article citations
Safety of intraoperative autologous plasma incubation of corneal grafts for reducing endothelial cell loss: a pilot study.
Front Med (Lausanne), 11:1368117, 12 Aug 2024
Cited by: 0 articles | PMID: 39188872 | PMCID: PMC11345243
Squishy matters - Corneal mechanobiology in health and disease.
Prog Retin Eye Res, 99:101234, 02 Jan 2024
Cited by: 1 article | PMID: 38176611 | PMCID: PMC11193890
Review Free full text in Europe PMC
Fusogenic liposome-coated nanoparticles for rapid internalization into donor corneal endothelial tissue to enable prophylaxis before transplantation.
Nanoscale Adv, 5(23):6410-6422, 10 Nov 2023
Cited by: 2 articles | PMID: 38024318 | PMCID: PMC10662038
Type II Diabetes Mellitus Causes Extracellular Matrix Alterations in the Posterior Cornea That Increase Graft Thickness and Rigidity.
Invest Ophthalmol Vis Sci, 64(7):26, 01 Jun 2023
Cited by: 3 articles | PMID: 37326594 | PMCID: PMC10281062
Donor-Related Risk Factors for Graft Decompensation Following Descemet's Stripping Automated Endothelial Keratoplasty.
Front Med (Lausanne), 9:810536, 04 Feb 2022
Cited by: 2 articles | PMID: 35252249 | PMCID: PMC8889573
Go to all (21) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT01537393
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Corneal Endothelial Cell Loss 3 Years After Successful Descemet Stripping Automated Endothelial Keratoplasty in the Cornea Preservation Time Study: A Randomized Clinical Trial.
JAMA Ophthalmol, 135(12):1394-1400, 01 Dec 2017
Cited by: 33 articles | PMID: 29127432 | PMCID: PMC6583548
Donor, Recipient, and Operative Factors Associated with Graft Success in the Cornea Preservation Time Study.
Ophthalmology, 125(11):1700-1709, 09 Aug 2018
Cited by: 34 articles | PMID: 30098353 | PMCID: PMC6196643
Effect of Graft Attachment Status and Intraocular Pressure on Descemet Stripping Automated Endothelial Keratoplasty Outcomes in the Cornea Preservation Time Study.
Am J Ophthalmol, 203:78-88, 06 Mar 2019
Cited by: 6 articles | PMID: 30849341 | PMCID: PMC6612575
Eye bank issues: II. Preservation techniques: warm versus cold storage.
Int Ophthalmol, 28(3):155-163, 01 Jun 2008
Cited by: 53 articles | PMID: 17505780 | PMCID: PMC2359829
Review Free full text in Europe PMC
 1
1





