Abstract
Free full text

Therapeutic targeting of trained immunity
Abstract
Immunotherapy is revolutionizing the treatment of diseases in which dysregulated immune responses have an important role. However, most of the immunotherapy strategies currently being developed engage the adaptive immune system. In the past decade, both myeloid (monocytes, macrophages and dendritic cells) and lymphoid (natural killer cells and innate lymphoid cells) cell populations of the innate immune system have been shown to display long-term changes in their functional programme through metabolic and epigenetic programming. Such reprogramming causes these cells to be either hyperresponsive or hyporesponsive, resulting in a changed immune response to secondary stimuli. This de facto innate immune memory, which has been termed ‘trained immunity’, provides a powerful ‘targeting framework’ to regulate the delicate balance of immune homeostasis, priming, training and tolerance. In this Opinion article, we set out our vision of how to target innate immune cells and regulate trained immunity to achieve long-term therapeutic benefits in a range of immune-related diseases. These include conditions characterized by excessive trained immunity, such as inflammatory and autoimmune disorders, allergies and cardiovascular disease and conditions driven by defective trained immunity, such as cancer and certain infections.
Immunotherapy is one of the most exciting therapeutic promises of the 21st century1,2. Its foundation was laid in the late 19th century by William Coley3, a bone surgeon at New York Cancer Hospital. He discovered cases of spontaneous cancer regression following infection, which inspired the development of what today is considered the first immunotherapeutic approach in a non-infectious disease4. His method involved injecting streptococcal organisms into a patient’s tumour5. Although Coley successfully treated several patients6, his work was met with a lot of criticism and scepticism because of the unpredictability of the approach. Coley’s immunotherapeutic method quickly fell out of fashion after the introduction of radiotherapy and chemotherapy, modalities with much more predictable and consistent outcomes.
In recent decades, our knowledge of the immune system has yielded several promising immunotherapeutic approaches that provide great benefits to patients. Current clinically relevant immunotherapies engage either effector molecules, such as cytokines, or the cellular stage of adaptive immunity. In autoimmune and autoinflammatory diseases, anti-cytokine therapies can successfully neutralize bioactive cytokines7, whereas the most intensely used immunotherapy in cancer patients comprises the application of checkpoint-inhibitor drugs8. These drugs take the brake off T cells, enabling them to eliminate tumour cells9–12. Specific antibodies against cytotoxic T lymphocyte-associated antigen 4 (CTLA4), as well as antibodies against programmed cell death 1 (PD-1) and its ligand PD-L1, are the most advanced in terms of clinical application13. Alternatively, adoptive T cell therapies involve collecting these cells from a patient, expanding their number in culture and re-introducing them into the body14,15. In culture, T cells can also be genetically modified to increase their affinity for tumour cells16. Dendritic cell therapy is another therapeutic modality that has gained a lot of traction17. It involves presenting tumour-specific antigens to dendritic cells, either ex vivo or in vivo, to induce a tumour-specific T cell response18.
Whereas the aforementioned immunotherapeutic approaches mostly focus on T lymphocytes, which are cells from the adaptive immune system, Coley’s approach can retrospectively be considered a modality that engages the innate immune system. The Bacille Calmette-Guérin (BCG) vaccine, a weakened version of Mycobacterium bovis, which causes tuberculosis in cattle, is its actual successor. In 1959, Lloyd Old and colleagues reported the use of the BCG vaccine as an immunotherapeutic to treat cancer19. Today, the BCG vaccine is a US Food and Drug Administration-approved treatment modality for bladder cancer20, and other malignancies such as lymphoma21 and melanoma22 also reportedly respond to the BCG vaccine.
Importantly, the heterologous effects of BCG vaccination also served as the basis for the discovery of ‘trained immunity’23,24. Trained immunity is a de facto immune memory of the innate immune system25–27 and involves the epigenetic programming of myeloid lineage cells, which results in changes in their metabolic and phenotypical behaviour that enable a stronger immune response to secondary stimuli26. Interestingly, Buffen and colleagues identified trained immunity to be the therapeutic mechanism by which BCG exerts its protective effects in bladder cancer28.
In this Opinion article, we detail the latest insights on the mechanisms responsible for the induction of trained immunity, including all its relevant molecular, cellular and systems machineries, and lay out a strategy for its exploitation as a novel immunotherapeutic target for immune system rebalancing. We provide a framework for developing targeted approaches to regulate trained immunity and for exploring their potential to treat a range of immune-related diseases. These include conditions characterized by excessive trained immunity, such as inflammatory and autoimmune disorders, allergies and cardiovascular disease, as well as conditions driven by defective trained immunity, such as cancer and certain infections (FIG. 1). Finally, we provide a vision of how to use trained-immunity-regulating therapeutics in synergy with existing immunotherapies.
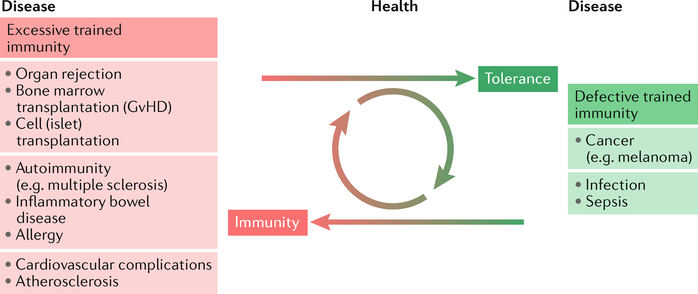
Conditions that are characterized by excessive trained immunity, including organ rejection, cardiovascular diseases and autoimmune diseases, and conditions in which defective trained immunity facilitates disease progression, such as cancer, represent two sides of the ‘same immunological coin’. Therefore, regulating trained immunity can be developed into a therapeutic avenue to treat such diseases. Therapeutically engaging trained immunity is compelling as it allows for durable responses, yet these are reversible. GvHD, graft versus host disease.
Trained immunity
Conventionally, immune systems in vertebrate animals are subdivided into two parts29. The first part, innate immunity, provides an initial response to an infection within minutes to hours and is relatively nonspecific30. Its cellular component comprises natural killer (NK) cells, innate lymphoid cells (ILCs) and phagocytes such as monocytes, macrophages and neutrophils31. This latter group of cells can engulf bacteria and particles. The complement system, along with a large array of defensins, chemokines and cytokines, comprise the innate immune system’s humoral (non-cellular) component32. It provides a host defence mechanism through the activation of a complex machinery of precursor proteins. Upon this activation, an amplification cascade of protein cleaving results in complement fixation, which can nonspecifically induce the rupture of an intruded bacterial cell wall and, through opsonization (the binding of proteins on a particle), facilitate phagocytes to clear foreign and damaged materials.
The second stage of the response to infection involves the immune system’s second part — the adaptive response — in which T and B lymphocytes specifically recognize a pathogen, proliferate and become activated against that pathogen. These cells also build immunological memory of that specific infection33. The specificity of the adaptive immune system response is mediated by recombination of the immunoglobulin genes at the lymphocyte level. Immunological memory results in a quicker and quantitatively better immune response (as compared with the primary response alone) against a previously encountered antigen.
In the past decade, emerging evidence has shown long-term adaptation25,27 of the innate immune system through epigenetic and metabolic programming of myeloid cells26, resulting in hyperresponsiveness upon re-stimulation in these cells. Through hyperresponsiveness, trained immunity also engages the adaptive immune system via adjuvant-increased co-stimulatory molecules and cytokine production34. An integrated view of immune memory, involving both innate and adaptive immunity, captures immune system function and how it protects against (re-)infection much better35.
Following the observation that monocytes from test subjects who were vaccinated with BCG strongly responded to the non-related stimulus Candida albicans, and in conjunction with previously reported nonspecific BCG protection in epidemiological studies, a hypothesis emerged about the existence of a de facto innate, more primitive, immune memory. This innate immune memory was first suggested by earlier studies in mice that were deficient in functional T and B cells and were exposed to a mild C. albicans infection36,37. It was subsequently validated in Rag1−/− mice38 — which lack mature T and B cells — as they were protected against a C. albicans re-infection by the increased responsiveness of monocytes and macrophages.
Building on this ground-breaking work on trained immunity, a series of studies unravelled the mechanisms by which myeloid cells preserve their ability to respond to an insult more quickly and strongly. First and foremost, trained immunity is regulated by epigenetic and metabolic modifications that account for the ability of myeloid cells to increase the production of specific inflammatory cytokines. Exposure of human monocytes to either C. albicans or β-glucan in vitro showed genome-wide changes in epigenetic marks, including histone H3 lysine 4 monomethylation (H3K4me1), trimethylation (H3K4me3) and H3 lysine 27 acetylation (H3K27ac) (FIG. 2, top). Other studies identified BCG and peptidoglycans as inducers of these trained-immunity-associated epigenetic modifications, albeit through the nucleotide-binding oligomerization domain-containing protein 2 (NOD2)-dependent pathway. In addition to these epigenetic modifications, cellular metabolism pathways are simultaneously upregulated. In fact, these metabolic changes enhance the capacity of the cell to modulate the function of certain epigenetic enzymes. Upon β-glucan exposure, the axis involving dectin 1 (encoded by CLEC7A and known as a β-glucan receptor), AKT, mechanistic target of rapamycin (mTOR) and hypoxia-inducible factor 1α (HIFlα) switches cellular metabolism from oxidative phosphorylation to glycolysis39, which is associated with a reduced basal respiration rate, increased glucose consumption and higher lactate production.
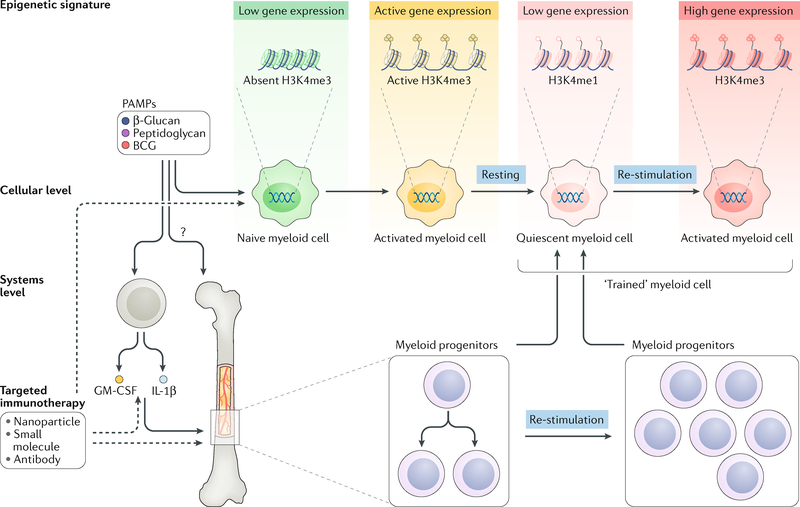
Trained immunity is regulated by metabolic and epigenetic rewiring of innate immune cells. Although the exact histone modifications that occur in this rewiring are still the topic of intense investigations, the histone mark H3K4 trimethylation (H3K4me3) has been identified to correlate well with Bacille Calmette-Guérin (BCG)-induced and β-glucan-induced trained immunity. Whereas naive cells (green) respond relatively mildly to an insult, ‘trained’ cells (red) respond much more strongly to the same stimulus. The fungal pathogen-associated molecular pattern (PAMP) β-glucan, bacterial PAMP BCG and other molecular structures such as peptidoglycans and their derivatives have been identified to induce trained immunity. At the cellular level, myeloid cells that are exposed to the aforementioned PAMPs undergo epigenetic and metabolic rewiring, resulting in a stronger response upon restimulation. At a systems level, involving the full haematopoietic system in mammals, bone marrow progenitors can be stimulated to produce ‘trained’ myeloid cells for a prolonged period of time, thereby providing a compelling framework for durable therapeutic interventions. GM-CSF, granulocyte-macrophage colony-stimulating factor; H3K4me1, H3K4 monomethylation; IL, interleukin.
Although these epigenetic and metabolic changes that underlie the increased response to a secondary insult of an individual myeloid cell are well known, the regulation of trained immunity on a systems level was only first described in early 2018 (REFS40,41). Monocytes have a lifespan of only a few days42, but trained immunity’s protective function is preserved for much longer, up to several months or almost a year in patients43, through functional changes in haematopoietic stem and progenitor cells (FIG. 2, bottom). In mice, Mitroulis and colleagues observed more myeloid-biased multipotent progenitors and long-term haematopoietic stem cells (LT-HSCs) in the bone marrow after administering β-glucan40. Various cell proliferation-associated pathways — including cell cycle genes, cholesterol biosynthesis and glycolysis — were upregulated and were identified to be dependent on interleukin (IL)-1β and granulocyte-macrophage colony-stimulating factor (GM-CSF). The longevity of these effects was found to persist for up to a month, and similar observations have been made after administering BCG44.
In addition to pharmacologically induced trained immunity, a recent study found that in experimental myocardial infarction, myeloid-biased progenitor cells in the bone marrow are distally stimulated to produce neutrophils and monocytes through GM-CSF45. In ischaemic heart disease, Christ and colleagues observed innate immune reprogramming in an atherosclerosis mouse model, in which mice lack the low-density lipoprotein receptor (Ldlr−/−). These mice lack LDLR expression on liver cells and cannot properly process cholesterol. When fed a Western and high-fat diet, long-lived transcriptional and epigenetic reprogramming of myeloid progenitor cells produced inflammatory monocytes in this mouse model41. These epigenetic modifications, which were associated with activation of the NLRP3-dependent inflammasome (a multiprotein complex responsible for inflammatory processes) and associated IL-1β secretion, persisted after the mice were switched back to a regular chow diet.
The trained-immunity-associated epigenetic, cellular and systems processes depicted in FIG. 2 provide ample possibilities for highly specific immunotherapeutic interventions. Blockade of IL-1β7 and GM-CSF46 are clinically available treatment modalities that most likely also target trained immunity. In turn, small-molecule inhibitors of epigenetic pathways may directly intervene in immune cell function47.
Pathways in trained immunity
Trained immunity cell types
Thus far, the research focus on trained immunity has been mainly on monocytes, macrophages and NK cells, but other innate immune cell types, such as ILCs, can also display trained immunity characteristics. Some of the first evidence that macrophages have adaptive features was derived from studies that show lipopolysaccharide (LPS)-induced gene-specific chromatin modifications48. Moreover, exposure of monocytes and/or macrophages to C. albicans or β-glucan enhanced their subsequent response to stimulation with unrelated pathogens or pathogen-associated molecular patterns (PAMPs)38, which was accompanied by significant reprogramming of chromatin marks26,38,48. In addition, infection with parasitic49 and viral50 pathogens can induce trained-immunity-like responses.
NK cells can also have a stronger response after a previous challenge51,52 and undergo expansion during virus infection. NK cell activation through cytomegalovirus infection may provide protection against re-infection by rapidly degranulating and producing cytokines in a T cell-independent manner53. Adoptive transfer experiments have demonstrated that activated NK cells can proliferate in vivo and protect naive recipient mice against virus infection, which indicates the protective immunological memory role that these cells have. The nonspecific protective effects of BCG infection have also been linked to NK cell activation. In BCG-vaccinated individuals, NK cells have enhanced pro-inflammatory cytokine production in response to mycobacteria and other unrelated pathogens, and BCG NK cells are at least partially responsible for the nonspecific protection against C. albicans in mice54.
ILCs bridge innate and adaptive immunity55,56 and can acquire trained immunity properties. For example, group 2 ILCs (ILC2s) do not express antigen receptors but are activated by cytokines and have the potential to ‘remember’ this activation status. Inhaled allergens in the lung stimulate ILC2s to synthesize cytokines such as IL-5 and IL-13 (REF.57). Subsequently, a population of allergen-trained ILC2s persists in the lung and lymph nodes. These cells display a more robust secondary response upon challenge with unrelated allergens, indicative of the non-antigen-specific character of ILC2 memory58.
Trained immunity signalling events
The induction of trained immunity by microbial ligands is facilitated by specific receptor signalling pathways that subsequently activate metabolic, epigenetic and transcriptional events (FIG. 3).
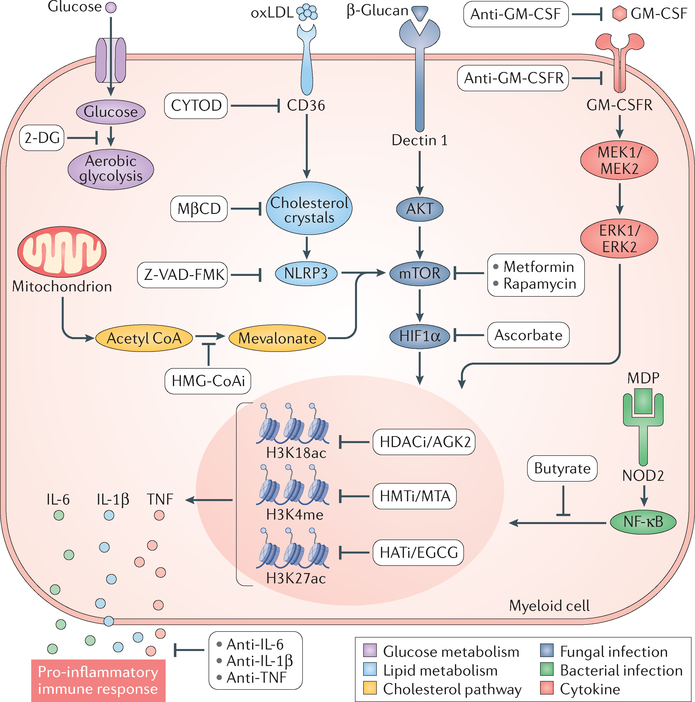
Changes in glucose (purple pathway) or lipid (light blue pathway) metabolism may both lead to epigenetic modifications underlying cytokine expression. Metabolic switching towards aerobic glycolysis results in epigenetic modifications in innate immune cells and enhanced secretion of pro-inflammatory cytokines73. The role of glycolysis as a pathway that drives the induction of trained immunity in monocytes is demonstrated by the blockade of glycolysis by incubation of cells with 2-deoxy-d-giucose (2-DG). The pharmacological modulation of rate-limiting glycolysis enzymes with 2-DG inhibits the generation of histone marks underlying trained immunity74. Oxidized low-density lipoprotein (oxLDL) induces trained immunity72 (light blue pathway). OxLDL-dependent activation of NLRP3 leads to trained immunity41. CD36 internalization, cholesterol crystal formation and NLRP3 activation can be inhibited by cytochalasin D (CYTOD), methyl-β-cyclodextrin (MβCD) and Z-VAD-FMK, respectively68. The cholesterol synthesis pathway (yellow), through mevalonate, is linked to the induction of trained immunity. Inhibition of cholesterol synthesis with fluvastatin downregulates H3K4 trimethylation (H3K4me3) and prevents the induction of trained immunity and the production of pro-inflammatory cytokines75. Mevalonate induces trained immunity by epigenetic reprograming of macrophages76, which is prevented by inhibitors of enzymes downstream of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase (HMG-CoAi). In trained immunity, the most widely studied pattern recognition receptors (PRRs) are the C-type lectin receptor dectin 1 (REF.59) (dark blue pathway), which is involved in antifungal immunity and can be activated by β-glucan, and nucleotide-binding oligomerization domain-containing protein 2 (NOD2; green pathway), which recognizes bacterial molecules, such as muramyl dipeptide (MDP). Dectin 1-mediated macrophage activation induces specific epigenetic marks that lead to trained immunity39. This pathway is inhibited by metformin, rapamycin and ascorbate, which target AKT, mechanistic target of rapamycin (mTOR) and hypoxia-inducible factor 1α (HIFlα), respectively39. Peptidoglycan (PepG) is a pathogen-associated molecular pattern (PAMP) that synergizes with endotoxin to cause the release of inflammatory cytokines64,65. MDP is the smallest PepG-derived molecular structure that can engage NOD2 (REF.66). NOD2 activation and signalling through nuclear factor (NF)-κB stimulates epigenetic rewiring of macrophages and induces trained immunity23. This activation of macrophages is inhibited by butyrate156, which prevents histone acetylation157. Finally, certain cytokines, such as granulocyte-macrophage colony-stimulating factor (GM-CSF), can induce trained immunity, resulting in increased tumour necrosis factor (TNF) production upon subsequent lipopolysaccharide (LPS) stimulation. This process is dependent on MAPKs, ERK1 and ERK2 (REF.158). H3K18ac, H3K18 acetylation; H3K27ac, H3K27 acetylation; HATi, histone acetyl transferase inhibitor; HDACi, histone deacetylase inhibitor; HMTi, histone methyltransferase inhibitor; IL, interleukin.
Dectin 1-dependent fungal pathway
Present in the fungal cell wall, β-glucans are polysaccharides that are rich in β1,3-linked or both β1,3-linked and β1,6-linked glucose and that are recognized by macrophages as PAMPs through dectin 1, a C-type transmembrane lectin receptor59. Macrophage activation via dectin 1 induces specific epigenetic marks, including the histone marks H3K4me1, H3K4me3 and H3K27Ac, that lead to trained immunity39 (FIG. 3, red pathway) and elicit nonspecific immune responses to exogenous pathogens. This activation pathway can be exploited for therapeutic interventions in fungal infections, for example, by non-lethal infection with C. albicans, which protects mice against lethal candidiasis through monocyte-dependent trained immunity38. On the basis of this approach tested in murine experimental models, human experimental therapies with purified β-glucans have been developed and are currently under evaluation in clinical trials as immunostimulants in several types of malignancy, including neuroblastoma and breast, lung and colorectal cancers60–63.
NOD2-dependent bacterial pathway
Peptidoglycan is a PAMP component of the cell wall of both Gram-positive and Gram-negative bacteria that synergizes with other bacterial components such as lipopeptides or endotoxins to cause inflammatory cytokine release64,65. The peptidoglycan minimal bioactive motif common to all bacteria is muramyl dipeptide (MDP). Innate immune cell activation by MDP involves the cytoplasmic pattern recognition receptor (PRR) NOD2 (REF.66). NOD2 activation and signalling through nuclear factor (NF)-κB stimulates epigenetic rewiring of macrophages and induces trained immunity23 (FIG. 3, green pathway).
Oxidized low-density lipoprotein
Products of lipid metabolism may also lead to the induction of trained immunity. Oxidized low-density lipoprotein (oxLDL) is a damage-associated molecular pattern (DAMP) that binds to the cell surface receptor CD36 of myeloid cells67. Once internalized and released into the cytoplasm, oxLDL may lead to the formation of cholesterol crystals, which activate the NLRP3 inflammasome68,60 that results in a long-lasting inflammatory response characterized by release of IL-1β, and subsequently other pro-inflammatory cytokines (FIG. 3, blue pathway). NLRP3 activation induced by a Western diet in Ldlr−/− mice established a mechanistic link between oxLDL-induced trained immunity and cardiovascular diseases through the activation of the inflammasome41. Whereas oxLDL induces a long-lasting pro-inflammatory phenotype in monocytes and accelerates atherosclerosis, the histone methyltransferase inhibitor 5′-deoxy-5′-methylthioadenosine70,71 (MTA) completely abolishes the trained immunity phenotype induced by oxLDL by reversing the methylation of histones necessary for the change in chromatin architecture that ensures increased gene transcription72.
Metabolic and epigenetic rewiring
Among the mechanisms that regulate trained immunity, one of the most important processes is metabolic rewiring of innate immune cells. A key part to this rewiring is the switch from oxidative phosphorylation towards aerobic glycolysis, which results in innate immune cell activation and pro-inflammatory cytokine secretion73. C. albicans and β-glucan induce this specific metabolic process through the AKT-mTOR-HIF1α pathway. In addition, BCG vaccination induces immunometabolic activation and epigenetic remodelling30, whereas inhibition of glycolysis by 2-deoxyglucose (2-DG) during BCG-induced training nullifies the increased cytokine production (FIG. 3, purple pathway). Conversely, pharmacological modulation of rate-limiting glycolysis enzymes with rapamycin or metformin inhibits the formation of histone marks H3K4me3 and H3K9me3 underlying both β-glucan-induced and BCG-induced trained immunity74,75.
Another important metabolic event in trained monocytes is the anabolic repurposing of the Krebs cycle towards synthesizing cholesterol and phospholipids from citrate and acetyl CoA. β-Glucan exposure upregulates cholesterol synthesis75, whereas the 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase fluvastatin prevents trained immunity through downregulating H3K4me3 and blocking pro-inflammatory cytokine production. Mevalonate synthesis has a central role in this process76 (FIG. 3, yellow pathway). Inhibition of glycolysis with 2-DG, of mTOR with rapamycin and of histone methylation with methyltioadenosine (MTA; a methyltransferase inhibitor) prevents mevalonate-induced trained immunity, indicating a delicate balance between molecular, metabolic and epigenetic control of trained macrophages76.
The Krebs cycle is replenished by glutaminolysis. Interestingly, glutaminolysis leads to accumulated succinate and especially fumarate, which are cofactors for important epigenetic enzyme families. In this context, succinate curbs lysine-specific demethylase 6 (KDM6; also known as JMJD3), leading to enhanced H3K27 trimethylation of particular genes, such as those that characterize alternatively activated macrophages, associated with an anti-inflammatory phenotype77. However, JMJD3 enzyme expression does not differ in trained monocytes. By contrast, fumarate inhibits KDM5 histone demethylases; both the expression and function of KDM5 have been shown to be blocked in trained monocytes75. Because KDM5 is an H3K4 demethylase, its suppression enables long-term stability of this important mark of open chromatin and thus facilitates gene transcription.
Trained immunity in disease
In most animal lineages, trained immunity most likely evolved as a primitive form of immune memory to protect the host more effectively against re-infection, with beneficial effects for survival27. However, dysregulated activation of trained immunity can lead to either hyperinflammation or immunodepression, depending on whether trained immunity induction is exacerbated or dampened.
Innate immune cell reprogramming has a likely beneficial role in maintaining a relatively high threshold of cellular activation in organs in which LPS naturally occurs at physiological levels (for example, in the gastrointestinal tract)48. By contrast, in sepsis, LPS-induced tolerance of innate immune cells can contribute to immune paralysis, placing the individual at greater risk of opportunistic infections78. Persistent silencing of important host defence genes due to epigenetic mechanisms mediates these effects79,80.
Similar to sepsis, a defective myeloid cell activation programme may also occur in cancer. In this context, the incidence of immunosuppressive myeloid cells has high clinical relevance. Particularly relevant are the myeloid-derived suppressor cells (MDSCs), which derive from either neutrophilic or monocyte precursors. MDSCs are a heterogeneous cell population composed of progenitors and precursors of myeloid cells that exert immunosuppressive effects, facilitating tumour development81. The function of MDSCs is determined by their epigenetic programme, including DNA methylation, histone modifications and modulatory non-coding RNA82. A similar pattern occurs when monocytes infiltrate tumours and differentiate into tumour-associated macrophages (TAMs) that promote tumour growth and suppress antitumoural immune responses83. Epigenetic reprogramming is a central feature of TAM differentiation, as long-term histone modifications, such as changes in H3K4me3 and H3K9me, underlie and induce a pro-tumorigenic profile in these cells84. Rewiring the epigenetic and functional programmes of MDSCs and/or TAMs by inducing trained immunity may be a compelling target for immunotherapy in cancer85.
Other clinical conditions are associated with excessive or inappropriate induction of trained immunity. Although trained immunity is an adaptive response that facilitates the long-lasting capacity to respond more strongly to stimuli, this type of high-alert immune state, when inappropriately activated, can also exacerbate tissue damage during chronic inflammatory conditions. One clear example of exaggerated trained immunity induction occurs in hyper-IgD syndrome (HIDS), an autoinflammatory disorder caused by defects in mevalonate kinase and characterized by attacks of sterile inflammation (fever, rash, joint and abdominal pain). In patients with HIDS, accumulated mevalonate amplifies the AKT-mTOR pathway, which in turn induces HIFlα activation and a shift from oxidative phosphorylation to glycolysis. This response results in circulating monocytes with a trained immunity phenotype76.
The key part played by IL-1β in the induction of trained immunity suggests its importance in other autoinflammatory disorders such as familial fever syndromes, which are characterized by inflammasome activation86, as well as gout and inflammatory bowel disease87. A study by Wendeln et al. that investigated innate immune memory in neurological diseases showed that inflammatory stimuli induce acute immune training and tolerance of brain-resident macrophages (microglia) through epigenetic reprogramming in mouse models of Alzheimer disease and stroke88. Importantly, these observations open new avenues for treating neurological disorders at the level of the immune system, which do not require therapeutics to cross the blood-brain barrier.
There is strong epidemiological evidence that patients with autoimmunity or chronic inflammatory conditions, such as rheumatoid arthritis, are more susceptible to atherosclerosis89. It is tempting to speculate that this increased risk could be caused by the underlying chronic inflammatory condition that triggers a maladaptive state in innate immune cells that changes their local responsiveness in atherosclerotic lesions90. In strong support of this hypothesis, trained immunity can be induced by oxLDL or lipoprotein a (Lpa) in human monocytes via epigenetic reprogramming72. Interestingly, a Western-type diet can induce epigenetic and metabolic reprogramming in myeloid cell precursors in the bone marrow, a process practically identical to the induction of trained immunity41.
This inappropriate activation of trained immunity mechanisms that can lead to innate immune cell maladaptation may be involved in the pathogenesis of other inflammatory diseases such as type 2 diabetes and systemic lupus erythematosus91, which are prevalent in Western societies. Interestingly, even relatively short-term hyperglycaemia in diabetes can generate long-term vascular deleterious effects92. This process has been termed ‘hyperglycaemic memory’ and is accompanied by sustained NF-κB activation by increased H3K4me1 and decreased H3K9me3 at selected genes93. In rheumatoid arthritis, innate immune cells are responsible for the inflammation that has tissue-damaging effects, with macrophages as the main producers of pro-inflammatory cytokines. Importantly, PI3K-mTOR and MAPK signalling pathways are activated in the monocytes isolated from patients with rheumatoid arthritis94, and inhibiting mTOR reduced synovial osteoclast formation and protected against local bone erosions and cartilage loss95. In addition, the balance between the biological activity of histone acetyl transferases (HATs) and histone deacetylases (HDACs) moves in the direction of histone acetylation in rheumatoid arthritis synovial tissue96. Tumour necrosis factor (TNF) is one of the main pro-inflammatory cytokines secreted by the inflamed synovium in rheumatoid arthritis, and biological therapies using inhibitory antibodies against cytokines have proved to be efficacious both in murine models and clinically97,98. Interestingly, the TNF blockers etanercept and adalimumab downregulate trimethylation of H3K4, H3K27, H3K36 and H3K79, as well as acetylation of H3 and H4 at the promoter site of CC-chemokine ligand 2 (CCL2; also known as MCP1) in monocytes, all of which are changes that are correlated with rheumatoid arthritis disease activity99.
Additional rheumatological disorders in which myeloid cells are epigenetically reprogrammed are Sjogren syndrome, Behçet disease, granulomatosis with polyangiitis (formerly known as Wegener granulomatosis) and systemic sclerosis47,100. The mechanisms leading to these epigenetic and functional changes are still not fully understood, but the information already available argues that trained immunity mechanisms are a rational therapeutic target in these disorders.
In conclusion, although the adaptive ability of innate immune cells to tune their responses to changing environments has evolved to prepare these cells for unpredictable events, such as invading pathogens, the epigenetic mechanisms that control the memory of the environmental trigger may also lead to persistent disease-associated phenotypes. Hence, altering the changed epigenetic landscape by pharmacological means or behavioural changes could be a promising strategy to restore homeostatic immune status.
Targeting trained immunity
As described in the previous sections, regulating trained immunity involves both its induction by PAMPs and DAMPs and its suppression by molecular inhibitors of numerous processes. Induction can be achieved through different trained-immunity-promoting pathways, which can be engaged by bacterial, fungal or metabolic ‘trainers’ as well as oxLDL or certain cytokines. Alternatively, preventing trained immunity induction can be achieved by modulating these pathways upstream. Furthermore, induction of trained immunity can also be reduced by inhibiting glycolysis39,75, and we anticipate that suppressing epigenetic changes with histone or DNA methylation inhibitors will have the same effect. However, inhibiting trained immunity might trigger numerous adverse effects with different severity levels depending on the inhibitor that has been used. Therefore, we foresee methods, such as using nanocarriers101–104, that can deliver these types of compound to the desired immune cells and their progenitors more precisely to be the way forward. In this section, we discuss the molecules that, in principle, can be exploited to regulate trained immunity. Different trained-immunity-regulating molecular structures are provided in FIG. 4.
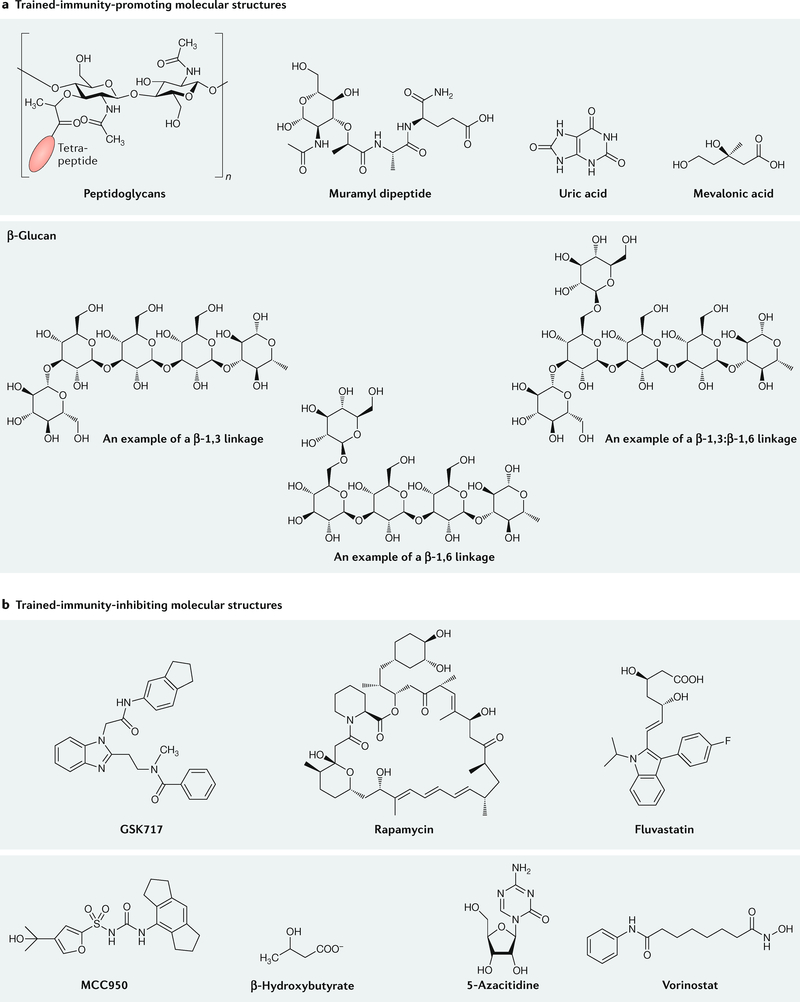
a | Peptidoglycans, molecular derivatives of peptidoglycans, β-glucans and small molecules that promote trained immunity. b | Examples of small-molecule inhibitors of metabolic and epigenetic pathways that regulate trained immunity.
Promoting trained immunity
The smallest molecular structure capable of inducing an NOD2-dependent immune response is MDP. MDP is a synthetic peptide conjugate comprising N-acetyl muramic acid and the short amino acid chain of L-alanine D-isoglutamine dipeptide105,106. In principle, any peptide, or molecular structures containing this peptide, should be exploitable for the induction of trained immunity.
Alternatively, trained immunity can be induced by fungal pathogens through the dectin 1 pathway107. Some dectin 1-activating polysaccharides, including a liposomal formulation, were extensively studied by Palma and colleagues108, who found that 1,3-linked glucose oligomers, with a minimum length of 10-mers or 11-mers, were required for dectin 1 binding. Consequently, and unlike NOD2 binding, a small-molecule ligand cannot induce dectin 1-dependent trained immunity.
In addition to PAMP-related mechanisms, metabolic ‘trainers’, such as uric acid87 and oxLDL41,72, can induce trained immunity through mTOR signalling and phosphorylation of AKT, which implies that uric acid itself can be used to induce trained immunity. Finally, Bekkering and colleagues found that mevalonate induces immunity training via activation of the insulin growth factor 1 (IGF1) receptor (IGF1R) and mTOR and subsequent histone modifications76. Mevalonic acid, which accumulates after addition of 6-fluoromevalonate, can therefore be used to induce trained immunity pharmacologically. Future studies will likely identify currently unknown pathways and molecular structures, including other bacterial and fungal derivatives as well as viral PAMPs, that promote trained immunity.
Inhibiting trained immunity
NOD2 and dectin 1 activation by PAMPs are, thus far, the most extensively studied and best understood pathways that induce trained immunity. NOD2 activation can be pharmacologically suppressed using the small-molecule inhibitor GSK669 and its more potent analogue GSK717 (REF.109). Upstream dectin 1 inhibition can be achieved with agents blocking this receptor, such as antibodies110 or laminarin111.
Regarding inhibition of metabolic pathways that regulate trained immunity, mTOR inhibitors, such as rapamycin, and most likely other rapalogues112, effectively inhibit trained immunity in vitro; however, in vivo, although they effectively suppress T cell proliferation, these drugs have little impact on innate immune cells113. In terms of inhibiting cholesterol synthesis, blocking the rate-limiting enzyme HMG-CoA reductase with fluvastatin can blunt trained immunity’s induction by β-glucan in vitro76. Delivery of nanoparticles with the HMG-CoA reductase inhibitor simvastatin to plaque macrophages in atherosclerotic apolipoprotein E (Apoe−/−) mice resulted in rapidly reduced vessel wall inflammation114. Although trained immunity was not the focus of this study, we do anticipate that nanotechnology-facilitated ‘statin repurposing’ could be employed towards this goal.
Inhibiting the NLRP3 inflammasome may be another route to suppress trained immunity. The small-molecule inhibitor MCC950, a diarylsulfonylurea-containing compound, and the ketone metabolite β-hydroxybutyrate can act on the inflammasome to blunt IL-1β-release and inflammation115–118.
Finally, as trained immunity is regulated by epigenetic rewiring, its suppression may also be achieved by restricting epigenetic changes with compounds such as histone or DNA methylation inhibitors. An excellent overview on epigenetic drug discovery for immune-related diseases was recently published in this journal47. Epigenetic modifications that can be inhibited in this context include those mediated by DNA methyltransferases (DNMTs), lysine methyltransferases, HDACs and the BET bromodomains. DNMT inhibitors include azacytidine and decitabine; although there are 20 mammalian proteins that can methylate lysines119, all of these should theoretically be inhibitable by small molecules. HDACs are widely studied and can be inhibited by trichostatin A or vorinostat. Several other small-molecule compounds that have been developed for inhibiting HDAC and BET bromodomains are described by Tough et al.47.
Regulating trained immunity
As discussed in the previous sections, a range of PRRs, including TLRs, NOD2, dectin 1 and the inflammasome, can be engaged to promote trained immunity. In vitro studies demonstrate that, in addition to BCG, several other PAMPs and DAMPs, including peptidoglycans and β-glucan, can be therapeutically exploited as trained-immunity-promoting agents. However, in vivo therapeutic exploitation of molecules that inhibit trained immunity is hampered by toxicity, immune-related adverse effects and poor bioavailability to target the relevant myeloid cells and their progenitors. As innovative alternatives to oral, subcutaneous, intraperitoneal or intravenous administration of compounds that regulate trained immunity, we propose the use of antibodies, RNA interference (RNAi) therapeutics and nano-immunotherapeutic approaches. We envision that nano-immunotherapy in particular can be devised to exhibit avidity for myeloid-biased progenitors in the bone marrow, which facilitates the induction of durable, reliable and specific responses without severe immune-related adverse effects.
Antibodies and RNAi therapeutics
As IL-1β and GM-CSF are key regulators of trained immunity induced in myeloid-biased progenitors40, antibodies against both molecules should be effective in inhibiting trained immunity. In fact, the effects of trained immunity were actively suppressed with a therapeutic monoclonal antibody targeting IL-1β in patients with cardiovascular disease in the recent CANTOS trial120. In addition to reducing recurrent atherothrombotic events, which may be linked to the prevention of ‘training’ induced by the primary myocardial infarction, other conditions in which the macrophage NLRP3 inflammasome plays a part can be treated with this IL-1β antibody. In fact, before the CANTOS trial, anti-IL-1β was proved effective in clinical trials in rheumatoid arthritis, gout, type 2 diabetes and several other diseases7. Monoclonal antibodies against GM-CSF are in advanced stages of clinical testing for conditions ranging from rheumatoid arthritis to multiple sclerosis and asthma46,121.
An emerging alternative modality for therapeutically blocking specific pathways or the expression of molecules related to trained immunity is RNAi122. Although RNAi has been successfully applied in a preclinical setting to reduce immune cell recruitment after myocardial infarction, there are few studies describing the use of RNAi therapeutics to downregulate pathways with direct relevance to trained immunity. IL-1β silencing mediated by RNAi has been achieved in vitro, but in the absence of compelling in vivo preclinical data, this technology’s clinical translation is not yet within reach123,124.
Nano-immunotherapy
Traditionally, using nanoparticles in medicine, also referred to as nanomedicine, has focused on improving drug delivery, for instance, to tumours125 or sites of inflammation126. Nanoparticle delivery can enhance the percentage of a drug reaching its intended target and improve its toxicity profile127. Moreover, nanoparticle delivery may facilitate cellular internalization of the drug, which is particularly relevant for nucleotide therapeutics128, which have to act in the cytoplasm or cell nucleus. Moreover, nanoparticles can protect drugs from being prematurely metabolized or degraded129.
We previously ventured into applying nanomaterials to engage immune cells, particularly myeloid cells, in cardiovascular disease104,114,130–132, cancer133,134 and graft transplantation113. In these studies, we used so-called high-density lipoprotein (HDL) nanobiologics as immunotherapeutic agents to exert therapeutic benefits both systematically and locally, for example, at an atherosclerotic lesion101. In a mouse allograft model, we show that a trained-immunity-inhibiting nanobiologic rebalances the immune system tolerance, as was evident from increased blood levels of tolerogenic Ly6Clo monocytes113. Importantly, we found that these nanobiologics accumulated not only in the heart allograft but also, to a strong degree, in myeloid cells and their progenitors in the bone marrow. Linking this observation to the recent discovery that trained immunity is a property of certain myeloid-biased progenitors in the bone marrow, we propose developing nanomaterials that engage these cells.
The ability of nanomaterials to accumulate in the bone marrow relies on a combination of their physicochemical features and (specific) engagement of immune cells and their progenitors. This dual process can be accomplished by designing nanoparticles with an inherent biodistribution that skews towards bone marrow uptake, which may be additionally surface functionalized with ligands that target certain immune receptors on progenitor and stem cells135. However, recent advances in nanomaterial production allow the creation of so-called nanoparticle libraries, which contain nanomaterials that vary in composition, size and surface chemistry101. Screening such a library in vivo using a combination of imaging and immunological techniques131 (BOX 1) can identify nanomaterials that display avidity towards the bone marrow and progenitor cells relevant to trained immunity (FIG. 5).
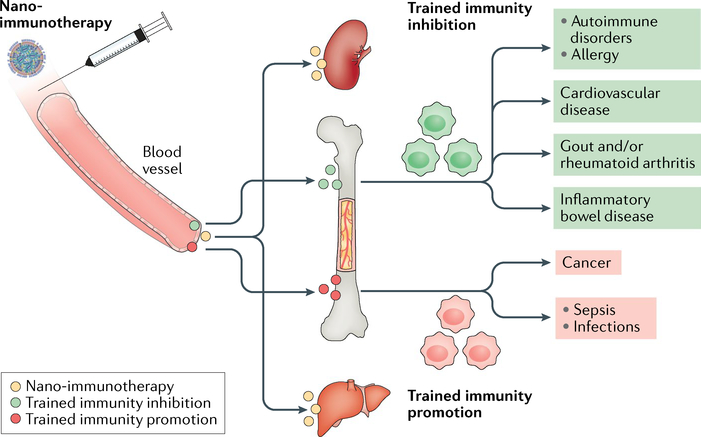
Long-term therapeutic benefits can theoretically be achieved by the intravenous administration of nanomaterials that engage myeloid cells and their stem and progenitor cells in the bone marrow. Intravenously administrable nanomaterials (yellow circles) typically accumulate in the liver and spleen but can be designed to exhibit bone marrow proclivity. Induction of trained immunity can be prevented by functionalizing these nanomaterials with molecular structures that inhibit epigenetic and metabolic pathways that regulate trained immunity (green circles). The resulting ‘green’ cells have an alternatively activated phenotype. Conversely, by incorporating molecular structures derived from PAMPs that activate dectin 1 or nucleotide-binding oligomerization domain-containing protein 2 (NOD2), nanomaterials (red circles) can be applied to promote trained immunity. These ‘red’ cells have an inflammatory phenotype. Systemically inhibiting trained immunity using this nanotechnology-based approach may be employed to treat a variety of conditions, ranging from cardiovascular disease and its clinical consequences myocardial infarction and stroke to autoimmune disorders. Therapeutically inducing trained immunity may find use in overcoming immunoparalysis in sepsis and infections and in treating cancers.
Nanomaterials with favourable biodistribution and immune-cell-engaging properties can, in principle, be chemically functionalized with trained-immunity-inhibiting or immunity-promoting agents (FIG. 4). Trained-immunity-promoting (red) and trained-immunity-inhibiting (green) nanoparticles can be intravenously applied to induce epigenetic and metabolic changes in myeloid-biased progenitor cells. These changes stimulate these cells to proliferate and produce ‘trained’ myeloid cells — particularly inflammatory monocytes — that can exert therapeutic benefits in conditions in which defective, or excessive, trained immunity drives disease progression. This situation is particularly relevant to cancer, sepsis and certain infections. Alternatively, trained-immunity-inhibiting nanoparticles can be used to treat conditions in which excessive trained immunity incentivizes inflammatory atherosclerosis and its clinical consequences, namely, myocardial infarction and stroke, or autoimmune disorders such as rheumatoid arthritis and inflammatory bowel disease.
Even optimized bone-marrowavid nanomaterials will accumulate in the spleen and liver and may also end up at inflammatory sites or tumours. Although liver uptake may cause unwanted side effects, the accumulation of trained-immunity-regulating nanoparticles in the spleen and at diseased sites can be beneficial. Swirski et al. identified a reservoir of splenic monocytes that are deployed to sites of inflammation136. Nanoparticle training of these splenic monocytes, before their deployment, may enable their therapeutic exploitation137. Directly targeting immune cells at disease sites may not result in long-term trained immunity benefits but does have the potential to skew tissue myeloid cells towards a phenotype that helps resolve inflammation, for instance, in atherosclerotic plaques114. Alternatively, directly inducing trained immunity in TAMs may decrease tumour cell dissemination and reduce cancer invasiveness. A summary of our view on the application of nanomaterials as trained-immunity-regulating agents is provided in FIG. 5.
Combinations to add specificity
Targeted regulation of trained immunity, as described in the previous sections, provides nonspecific protection against immune-related diseases. At the same time, trained immunity is based on durable, yet temporary, epigenetic and metabolic reprogramming of myeloid cells and their bone marrow progenitors40. Given these premises, how can trained immunity be best therapeutically exploited, how can specificity be introduced and how can durability be regulated? In this section, we discuss possible combination strategies that either synergize with trained-immunity-targeted therapeutics or elevate their therapeutic specificity.
Enhancing specificity
Applying trained-immunity-inhibiting nanobiologics to promote allograft acceptance in a heart transplantation mouse model provided a compelling framework for synergistically enhancing therapeutic efficacy of a trained-immunity-inhibiting nanotherapy113. The nanobiologic immunotherapy, consisting of an HDL nanoparticle incorporating an mTOR inhibitor (mTORi-HDL), effectively blunted β-glucan training of human monocytes in vitro113. Intravenous administration of mTORi-HDL to mice on the day they received a heart allograft, as well as 2 and 5 days post-transplantation, markedly increased survival from 8 to 60 days, and a subset of mice survived up to 100 days. However, a combination treatment of co-injected mTORi-HDL and a nanobiologic (tumour necrosis factor receptor-associated factor 6 inhibitor (TRAF6i)-HDL)132 that impairs CD40 co-stimulation further increased allograft survival to 90 days. The immune response underlying organ rejection involves T cell activation through a cascade that includes alloantigen presentation (signal 1), co-stimulation (signal 2) and soluble cytokine secretion (signal 3). Whereas mT ORi-HDL trained-immunity-inhibiting nano-immunotherapy primarily regulates cytokine secretion (signal 3), TRAF6i-HDL blunts co-stimulation (signal 2) (FIG. 6a). The specificity of this synergistic approach could be improved through an additional immunotherapy that focuses on antigen presentation (signal 1), which has been achieved by Maldonado et al., who functionalized poly lactic-co-glycolic acid (PLGA) nanoparticles with both an antigen and rapamycin103. Intravenous and subcutaneous injection of these nanoparticles leads to CD4+ T cell tolerance in mice. Therefore, unlike the above-described study with mTORi-HDL, the observed effects are through lymphocytes and not innate immune cells. However, in both studies these effects are dependent on formulating rapamycin into nanoparticles and are not observed for the free drug. Alternatively, nanomaterials may exhibit an inherent capacity for immune cell polarization, as has been demonstrated for iron oxide nanoparticles that enhance the accumulation of pro-inflammatory macrophages in tumours138.
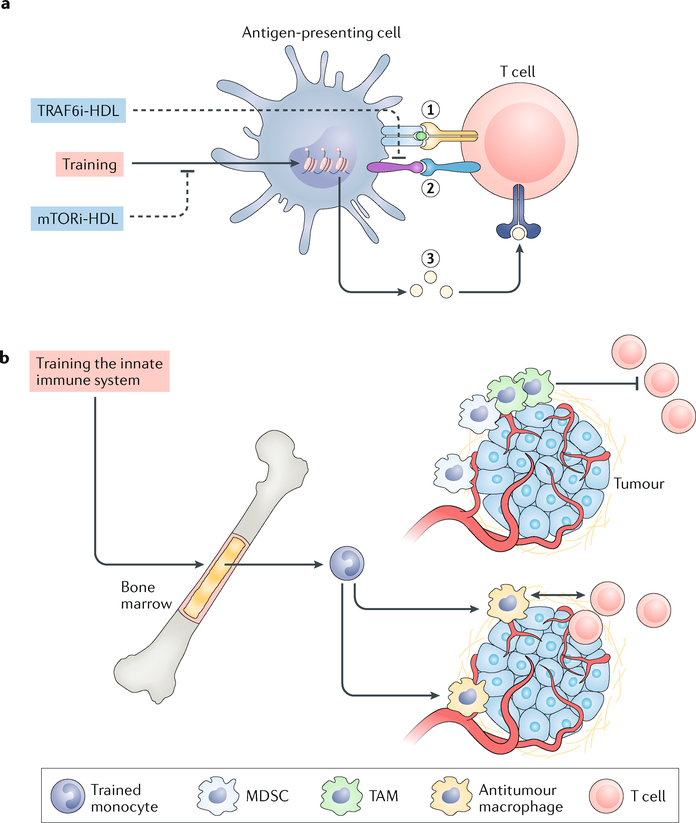
a | Antigen-presenting cells can induce immune tolerance by targeted suppression of trained immunity through inhibition of the mechanistic target of rapamycin (mTOR) pathway with a nanobiologic, resulting in the expansion of regulatory T (Treg) cells. These Treg cells maintain tolerance to self-antigens, prevent autoimmune disease and promote allograft acceptance. This process can be amplified by synergistically blocking the interaction between CD40 and tumour necrosis factor receptor-associated factor 6 (TRAF6) in monocytes and macrophages, which blunts CD40 ligand-dependent T cell activation. b | Impaired antitumour immunity is caused by immunosuppressive cell infiltration and macrophages that are programmed to drive immune suppression, leading to cytotoxic T cell exclusion. The induction of trained immunity results in enhanced ‘trained’ monocyte numbers that differentiate into antitumour macrophages and facilitate T cell activation. HDL, high-density lipoprotein; MDSC, myeloid-derived suppressor cell; mTORi-HDL, HDL nanoparticle incorporating an mTOR inhibitor; TAM, tumour-associated macrophage; TRAF6i-HDL, HDL nanoparticle incorporating a TRAF6 inhibitor.
On the basis of our initial experience modulating trained immunity therapeutically113, we advocate combining it with immunotherapeutic strategies that target different pathways in the immune response. For example, it is increasingly evident that even for susceptible tumour types, such as melanoma, checkpoint blockade benefits only a subset of patients10,139. The pooled analysis of the KEYNOTE-001 (REF.140) trial found that approximately 34% of patients with late-stage melanoma had an objective response, whereas only 6% of the patients were full responders141. Additionally, in a variety of other malignancies, including prostate142,143 and ovarian cancer144, checkpoint-inhibitor drugs exert very little therapeutic benefit. Recent work on peripheral blood from patients has uncovered — using high-dimensional single-cell mass cytometry and a bioinformatics pipeline — that the frequency of classically activated (by pro-inflammatory stimuli) monocytes predicts therapeutic response in melanoma patients145. However, high levels of immunosuppressive myeloid cells lead to T cell dysfunction and failure to respond to checkpoint blockade immunotherapy145. We foresee that trained-immunity-promoting therapies can promote systemic and tumour-accumulated classically activated monocytes, thereby overcoming the immunosuppressive tumour microenvironment146. This enhances the ability of T cells to kill tumour cells and may increase the immune system’s susceptibility to checkpoint-inhibitor drugs (FIG. 6b).
Many other combinations can be considered. For example, the therapeutic efficacy of dendritic cell therapy can potentially be synergistically enhanced with trained-immunity-promoting agents. Alternatively, treating autoimmune diseases with IL-1β blockers can be complemented with agents that inhibit trained immunity.
Modulating durability
Although trained immunity is durable, it is not a permanent immune memory40. This impermanence is both a challenge and an opportunity. Temporarily inducing trained immunity pharmacologically could combat events such as immunoparalysis in sepsis147. At the same time, after the infection has been fought off, the risk of autoimmune disorders should be mitigated by subduing exogenously induced trained immunity. The opposite holds true for conditions that benefit from trained immunity suppression. When a therapeutic benefit is achieved, the treatment should be terminated. Therefore, considerable research effort should focus on investigating different regimens that involve the induction of trained immunity followed by induced inhibition and vice versa. Because epigenetic modifications underlie trained immunity, hindering epigenetic deactivation with compounds such as DNMT and HDAC inhibitors47 could potentially extend artificially induced trained immunity.
Conclusions
Trained immunity provides a compelling framework for regulating myeloid cell function. Its induction may provide therapeutic benefits for a range of conditions that are characterized by defective trained immunity, including cancer and sepsis. In addition, autoimmune disorders and cardiovascular diseases can potentially be treated by actively suppressing trained immunity. Moreover, remnant epigenetic activity following a pathological process is often the underlying cause of increased susceptibility to recurrent events. Although the mechanisms are poorly understood, compelling evidence of their existence was recently provided in the context of preclinical atherosclerosis induced by a high-fat diet41.
Although trained immunity is a nonspecific immune memory, the specificity of various trained immunity programmes can likely be therapeutically exploited. For example, the trained immunity programme for atherosclerosis will be different from that for rheumatoid arthritis. In addition, further research is needed to assess the various epigenetic and metabolic changes in myeloid and NK cells. A precise description of these mechanisms and cells will serve as the developmental groundwork for specific therapies, and, accordingly, specific trained immunity processes will need to be regulated in specific cell populations and their progenitors. Highly efficient targeting will most likely be the most elegant strategy to achieve this. In addition to nanoparticles, polymeric materials and supramolecular systems may be employed to design trained-immunity-regulating therapeutics.
Regarding clinical translation, a vast uncharted preclinical territory must still be explored. Fortuitously, extensive experience with numerous trained-immunity-regulating drugs and delivery platforms should expedite the development of innovative pathways. In the years ahead, we expect these trained-immunity-targeted therapeutics to come to fruition as both monotherapies and companion therapies that prime the immune system and increase therapeutic susceptibility and efficacy.
Acknowledgements
This work was supported by National Institutes of Health (NIH) grants R01 CA220234, R01 HL144072, P01 HL131478, and Netherlands Organization for Scientific Research (NWO) grant ZonMW Vici 91818622 (all to W.J.M.M.), as well as NIH grants R01 HL143814 and P01HL131478 (both to Z.A.F.). J.O. is supported by R01 AI139623, as well as SAF2013-48834-R and SAF2016-80031-R grants from the Spanish Government. L.A.B.J. is supported by a Competitiveness Operational Programme grant of the Romanian Ministry of European Funds (HINT, P_37_762). M.G.N. is supported by a European Research Council (ERC) Consolidator Grant (#310372) and an NWO Spinoza Prize. The authors thank K. Joyes for editing the manuscript.
Footnotes
Competing interests
The authors declare that they are scientific founders of Trained Therapeutics Discovery.
Contributor Information
Willem J. M. Mulder, Translational and Molecular Imaging Institute, Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, USA. Department of Oncological Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, USA. Laboratory of Chemical Biology, Department of Biomedical Engineering and Institute for Complex Molecular Systems, Eindhoven University of Technology, Eindhoven, Netherlands. Department of Medical Biochemistry, Amsterdam University Medical Centers, Academic Medical Center, Amsterdam, Netherlands.
Jordi Ochando, Department of Oncological Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, USA. Transplant Immunology Unit, National Centre of Microbiology, Instituto de Salud Carlos III, Madrid, Spain.
Leo A. B. Joosten, Department of Internal Medicine and Radboud Center for Infectious Diseases, Radboud University Medical Center, Nijmegen, Netherlands. Department of Medical Genetics, Iuliu Haţieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania.
Zahi A. Fayad, Translational and Molecular Imaging Institute, Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Mihai G. Netea, Department of Internal Medicine and Radboud Center for Infectious Diseases, Radboud University Medical Center, Nijmegen, Netherlands. Department for Genomics and Immunoregulation, Life and Medical Sciences Institute (LIMES), University of Bonn, Bonn, Germany.
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/s41573-019-0025-4
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7069501
Citations & impact
Impact metrics
Article citations
Advanced technologies for the development of infectious disease vaccines.
Nat Rev Drug Discov, 21 Oct 2024
Cited by: 0 articles | PMID: 39433939
Review
Effective xanthine oxidase inhibitor urate lowering therapy in gout is linked to an emergent serum protein interactome of complement and inflammation modulators.
Sci Rep, 14(1):24598, 19 Oct 2024
Cited by: 0 articles | PMID: 39426967 | PMCID: PMC11490615
Trained immunity of synovial macrophages is associated with exacerbated joint inflammation and damage after Staphylococcus aureus infection.
Inflamm Res, 73(11):1995-2008, 28 Sep 2024
Cited by: 0 articles | PMID: 39340660
Trial watch: anticancer vaccination with dendritic cells.
Oncoimmunology, 13(1):2412876, 09 Oct 2024
Cited by: 0 articles | PMID: 39398476 | PMCID: PMC11469433
Review Free full text in Europe PMC
Quantitative characterization of immune cells by measuring cellular signal transduction pathway activity.
Sci Rep, 14(1):24487, 18 Oct 2024
Cited by: 0 articles | PMID: 39424625 | PMCID: PMC11489675
Go to all (180) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Defining trained immunity and its role in health and disease.
Nat Rev Immunol, 20(6):375-388, 04 Mar 2020
Cited by: 1008 articles | PMID: 32132681 | PMCID: PMC7186935
Review Free full text in Europe PMC
The role of the interleukin-1 family in trained immunity.
Immunol Rev, 281(1):28-39, 01 Jan 2018
Cited by: 58 articles | PMID: 29248003
Review
Trained immunity: A program of innate immune memory in health and disease.
Science, 352(6284):aaf1098, 21 Apr 2016
Cited by: 1189 articles | PMID: 27102489 | PMCID: PMC5087274
Review Free full text in Europe PMC
Trained immunity: General and emerging concepts.
Immunol Rev, 323(1):164-185, 29 Mar 2024
Cited by: 4 articles | PMID: 38551324
Review
Funding
Funders who supported this work.
European Research Council (1)
The interaction landscape between microbial colonization and functional genome of the host: a systems biology approach in fungal infections (SysBioFun)
Prof Mihai Netea, Radboud University Nijmegen
Grant ID: 310372
NCI NIH HHS (1)
Grant ID: R01 CA220234
NHLBI NIH HHS (3)
Grant ID: R01 HL143814
Grant ID: P01 HL131478
Grant ID: R01 HL144072
NIAID NIH HHS (1)
Grant ID: R01 AI139623
ZonMw (1)
Nanobiologic training of innate immunity to treat disease
prof. Mulder, University of Amsterdam
Grant ID: 91818622





