Abstract
Free full text

Long COVID: major findings, mechanisms and recommendations
Abstract
Long COVID is an often debilitating illness that occurs in at least 10% of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. More than 200 symptoms have been identified with impacts on multiple organ systems. At least 65 million individuals worldwide are estimated to have long COVID, with cases increasing daily. Biomedical research has made substantial progress in identifying various pathophysiological changes and risk factors and in characterizing the illness; further, similarities with other viral-onset illnesses such as myalgic encephalomyelitis/chronic fatigue syndrome and postural orthostatic tachycardia syndrome have laid the groundwork for research in the field. In this Review, we explore the current literature and highlight key findings, the overlap with other conditions, the variable onset of symptoms, long COVID in children and the impact of vaccinations. Although these key findings are critical to understanding long COVID, current diagnostic and treatment options are insufficient, and clinical trials must be prioritized that address leading hypotheses. Additionally, to strengthen long COVID research, future studies must account for biases and SARS-CoV-2 testing issues, build on viral-onset research, be inclusive of marginalized populations and meaningfully engage patients throughout the research process.
Introduction
Long COVID (sometimes referred to as ‘post-acute sequelae of COVID-19’) is a multisystemic condition comprising often severe symptoms that follow a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. At least 65 million individuals around the world have long COVID, based on a conservative estimated incidence of 10% of infected people and more than 651 million documented COVID-19 cases worldwide1; the number is likely much higher due to many undocumented cases. The incidence is estimated at 10–30% of non-hospitalized cases, 50–70% of hospitalized cases2,3 and 10–12% of vaccinated cases4,5. Long COVID is associated with all ages and acute phase disease severities, with the highest percentage of diagnoses between the ages of 36 and 50 years, and most long COVID cases are in non-hospitalized patients with a mild acute illness6, as this population represents the majority of overall COVID-19 cases. There are many research challenges, as outlined in this Review, and many open questions, particularly relating to pathophysiology, effective treatments and risk factors.
Hundreds of biomedical findings have been documented, with many patients experiencing dozens of symptoms across multiple organ systems7 (Fig. 1). Long COVID encompasses multiple adverse outcomes, with common new-onset conditions including cardiovascular, thrombotic and cerebrovascular disease8, type 2 diabetes9, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)10,11 and dysautonomia, especially postural orthostatic tachycardia syndrome (POTS)12 (Fig. 2). Symptoms can last for years13, and particularly in cases of new-onset ME/CFS and dysautonomia are expected to be lifelong14. With significant proportions of individuals with long COVID unable to return to work7, the scale of newly disabled individuals is contributing to labour shortages15. There are currently no validated effective treatments.
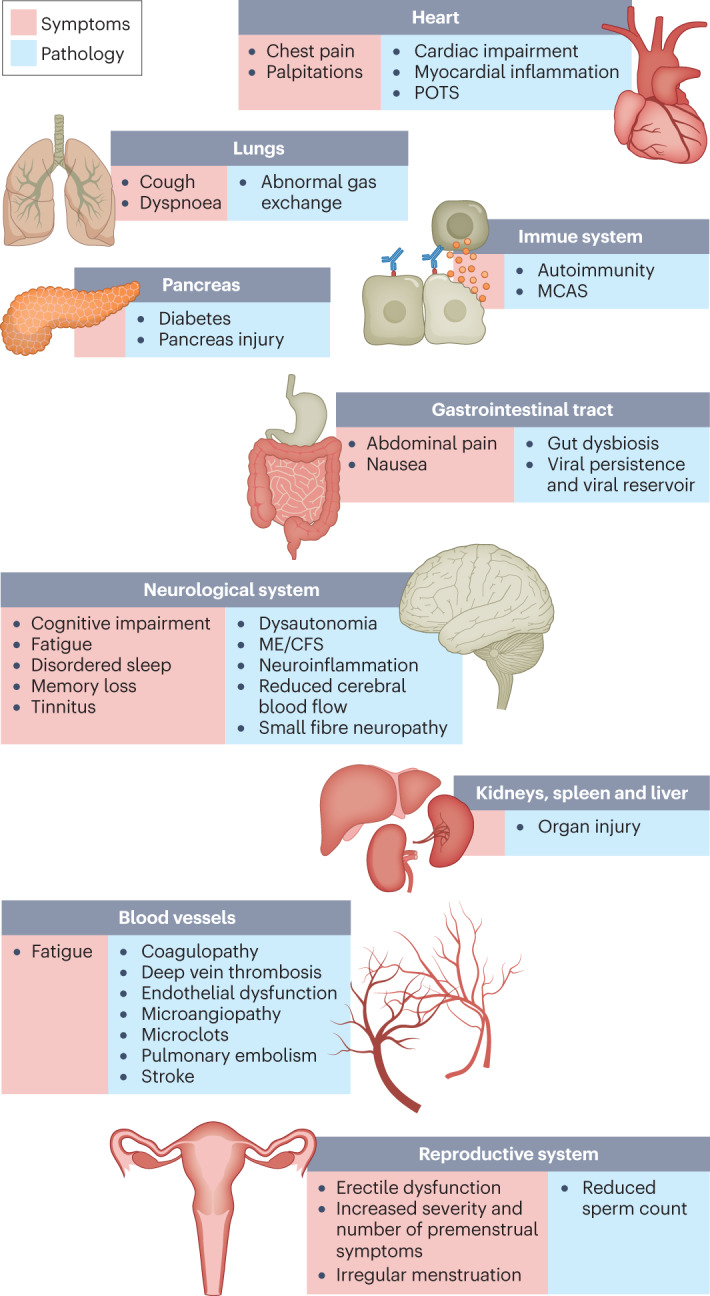
The impacts of long COVID on numerous organs with a wide variety of pathology are shown. The presentation of pathologies is often overlapping, which can exacerbate management challenges. MCAS, mast cell activation syndrome; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome; POTS, postural orthostatic tachycardia syndrome.
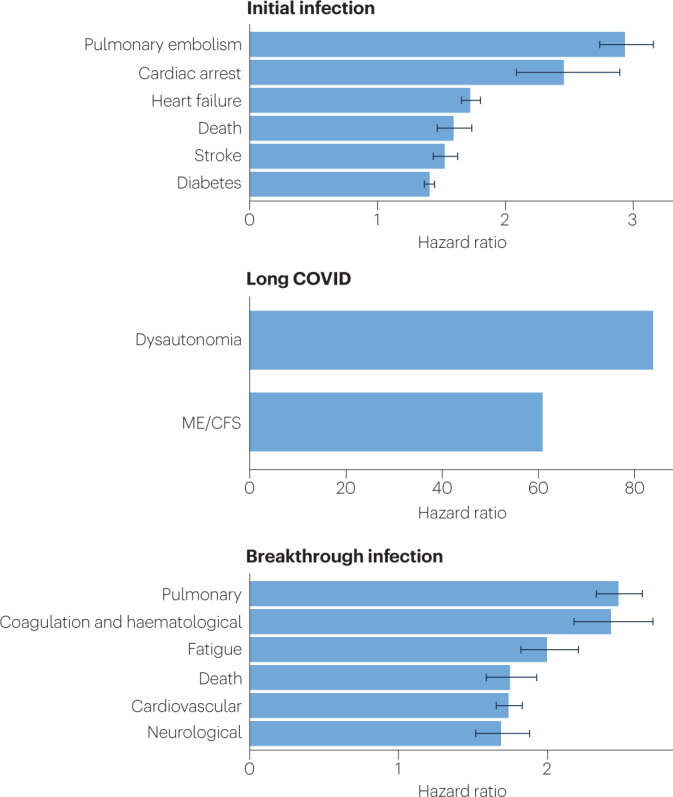
Because diagnosis-specific data on large populations with long COVID are sparse, outcomes from general infections are included and a large proportion of medical conditions are expected to result from long COVID, although the precise proportion cannot be determined. One year after the initial infection, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections increased the risk of cardiac arrest, death, diabetes, heart failure, pulmonary embolism and stroke, as studied with use of US Department of Veterans Affairs databases. Additionally, there is clear increased risk of developing myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia. Six months after breakthrough infection, increased risks were observed for cardiovascular conditions, coagulation and haematological conditions, death, fatigue, neurological conditions and pulmonary conditions in the same cohort. The hazard ratio is the ratio of how often an event occurs in one group relative to another; in this case people who have had COVID-19 compared with those who have not. Data sources are as follows: diabetes9, cardiovascular outcomes8, dysautonomia12,201, ME/CFS10,202 and breakthrough infections4.
There are likely multiple, potentially overlapping, causes of long COVID. Several hypotheses for its pathogenesis have been suggested, including persisting reservoirs of SARS-CoV-2 in tissues16,17; immune dysregulation17–20 with or without reactivation of underlying pathogens, including herpesviruses such as Epstein–Barr virus (EBV) and human herpesvirus 6 (HHV-6) among others17,18,21,22; impacts of SARS-CoV-2 on the microbiota, including the virome17,23–25; autoimmunity17,26–28 and priming of the immune system from molecular mimicry17; microvascular blood clotting with endothelial dysfunction17,29–31; and dysfunctional signalling in the brainstem and/or vagus nerve17,32 (Fig. 3). Mechanistic studies are generally at an early stage, and although work that builds on existing research from postviral illnesses such as ME/CFS has advanced some theories, many questions remain and are a priority to address. Risk factors potentially include female sex, type 2 diabetes, EBV reactivation, the presence of specific autoantibodies27, connective tissue disorders33, attention deficit hyperactivity disorder, chronic urticaria and allergic rhinitis34, although a third of people with long COVID have no identified pre-existing conditions6. A higher prevalence of long Covid has been reported in certain ethnicities, including people with Hispanic or Latino heritage35. Socio-economic risk factors include lower income and an inability to adequately rest in the early weeks after developing COVID-19 (refs. 36,37). Before the emergence of SARS-CoV-2, multiple viral and bacterial infections were known to cause postinfectious illnesses such as ME/CFS17,38, and there are indications that long COVID shares their mechanistic and phenotypic characteristics17,39. Further, dysautonomia has been observed in other postviral illnesses and is frequently observed in long COVID7.
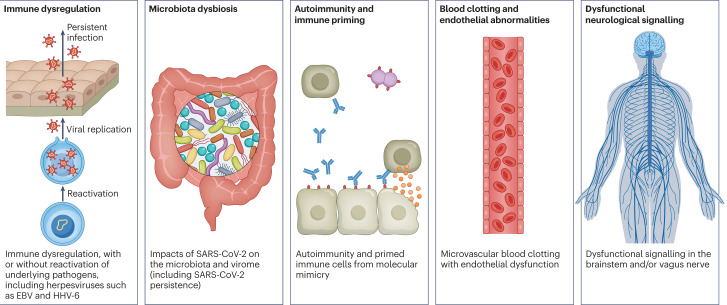
There are several hypothesized mechanisms for long COVID pathogenesis, including immune dysregulation, microbiota disruption, autoimmunity, clotting and endothelial abnormality, and dysfunctional neurological signalling. EBV, Epstein–Barr virus; HHV-6, human herpesvirus 6; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In this Review, we explore the current knowledge base of long COVID as well as misconceptions surrounding long COVID and areas where additional research is needed. Because most patients with long COVID were not hospitalized for their initial SARS-CoV-2 infection6, we focus on research that includes patients with mild acute COVID-19 (meaning not hospitalized and without evidence of respiratory disease). Most of the studies we discuss refer to adults, except for those in Box 1.
Major findings
Immunology and virology
Studies looking at immune dysregulation in individuals with long COVID who had mild acute COVID-19 have found T cell alterations, including exhausted T cells18, reduced CD4+ and CD8+ effector memory cell numbers18,19 and elevated PD1 expression on central memory cells, persisting for at least 13 months19. Studies have also reported highly activated innate immune cells, a lack of naive T and B cells and elevated expression of type I and type III interferons (interferon-β (IFNβ) and IFNλ1), persisting for at least 8 months20. A comprehensive study comparing patients with long COVID with uninfected individuals and infected individuals without long COVID found increases in the numbers of non-classical monocytes, activated B cells, double-negative B cells, and IL-4- and IL-6-secreting CD4+ T cells and decreases in the numbers of conventional dendritic cells and exhausted T cells and low cortisol levels in individuals with long COVID at a median of 14 months after infection18. The expansion of cytotoxic T cells has been found to be associated with the gastrointestinal presentation of long COVID27. Additional studies have found elevated levels of cytokines, particularly IL-1β, IL-6, TNF and IP10 (refs. 40,41), and a recent preprint has reported persistent elevation of the level of CCL11, which is associated with cognitive dysfunction42. It remains to be seen whether the pattern of cytokines in ME/CFS, where the levels of certain cytokines are elevated in the first 2–3 years of illness but decrease over time without a corresponding decrease in symptoms43, is similar in long COVID.
Multiple studies have found elevated levels of autoantibodies in long COVID27, including autoantibodies to ACE2 (ref. 28) (the receptor for SARS-CoV-2 entry), β2-adrenoceptor, muscarinic M2 receptor, angiotensin II AT1 receptor and the angiotensin 1–7 MAS receptor26. High levels of other autoantibodies have been found in some patients with COVID-19 more generally, including autoantibodies that target the tissue (such as connective tissue, extracellular matrix components, vascular endothelium, coagulation factors and platelets), organ systems (including the lung, central nervous system, skin and gastrointestinal tract), immunomodulatory proteins (cytokines, chemokines, complement components and cell-surface proteins)44. A major comprehensive study, however, did not find autoantibodies to be a major component of long COVID18.
Reactivated viruses, including EBV and HHV-6, have been found in patients with long COVID18,21,22,27 (and have been identified in ME/CFS45), and lead to mitochondrial fragmentation and severely affect energy metabolism46. A recent preprint has reported that EBV reactivation is associated with fatigue and neurocognitive dysfunction in patients with long COVID22.
Several studies have shown low or no SARS-CoV-2 antibody production and other insufficient immune responses in the acute stage of COVID-19 to be predictive of long COVID at 6–7 months, in both hospitalized patients and non-hospitalized patients47,48. These insufficient immune responses include a low baseline level of IgG48, low levels of receptor-binding domain and spike-specific memory B cells, low levels of nucleocapsid IgG49 and low peaks of spike-specific IgG47. In a recent preprint, low or absent CD4+ T cell and CD8+ T cell responses were noted in patients with severe long COVID49, and a separate study found lower levels of CD8+ T cells expressing CD107a and a decline in nucleocapsid-specific interferon-γ-producing CD8+ T cells in patients with long COVID compared with infected controls without long COVID50. High levels of autoantibodies in long COVID have been found to be inversely correlated with protective COVID-19 antibodies, suggesting that patients with high autoantibody levels may be more likely to have breakthrough infections27. SARS-CoV-2 viral rebound in the gut, possibly resulting from viral persistence, has also been associated with lower levels and slower production of receptor-binding domain IgA and IgG antibodies51. There are major differences in antibody creation, seroreversion and antibody titre levels across the sexes, with women being less likely to seroconvert, being more likely to serorevert and having lower antibody levels overall52,53, even affecting antibody waning after vaccination54.
Several reports have pointed towards possible viral persistence as a driver of long COVID symptoms; viral proteins and/or RNA has been found in the reproductive system, cardiovascular system, brain, muscles, eyes, lymph nodes, appendix, breast tissue, hepatic tissue, lung tissue, plasma, stool and urine55–60. In one study, circulating SARS-CoV-2 spike antigen was found in 60% of a cohort of 37 patients with long COVID up to 12 months after diagnosis compared with 0% of 26 SARS-CoV-2-infected individuals, likely implying a reservoir of active virus or components of the virus16. Indeed, multiple reports following gastrointestinal biopsies have indicated the presence of virus, suggestive of a persistent reservoir in some patients58,61.
Vascular issues and organ damage
Although COVID-19 was initially recognized as a respiratory illness, SARS-CoV-2 has capability to damage many organ systems. The damage that has been demonstrated across diverse tissues has predominantly been attributed to immune-mediated response and inflammation, rather than direct infection of cells by the virus. Circulatory system disruption includes endothelial dysfunction and subsequent downstream effects, and increased risks of deep vein thrombosis, pulmonary embolism and bleeding events29,30,62. Microclots detected in both acute COVID-19 and long COVID contribute to thrombosis63 and are an attractive diagnostic and therapeutic target. Long-term changes to the size and stiffness of blood cells have also been found in long COVID, with the potential to affect oxygen delivery64. A long-lasting reduction in vascular density, specifically affecting small capillaries, was found in patients with long COVID compared with controls, 18 months after infection65. A study finding elevated levels of vascular transformation blood biomarkers in long COVID also found that the angiogenesis markers ANG1 and P-selectin both had high sensitivity and specificity for predicting long COVID status66.
An analysis of the US Department of Veterans Affairs databases (VA data) including more than 150,000 individuals 1 year after SARS-CoV-2 infection indicated a significantly increased risk of a variety of cardiovascular diseases, including heart failure, dysrhythmias and stroke, independent of the severity of initial COVID-19 presentation8 (Fig. 2). Cardiac MRI studies revealed cardiac impairment in 78% of 100 individuals who had a prior COVID-19 episode (investigated an average of 71 days after infection67) and in 58% of participants with long COVID (studied 12 months after infection68), reinforcing the durability of cardiac abnormalities.
Multiple studies have revealed multi-organ damage associated with COVID-19. One prospective study of low-risk individuals, looking at the heart, lungs, liver, kidneys, pancreas and spleen, noted that 70% of 201 patients had damage to at least one organ and 29% had multi-organ damage69. In a 1-year follow-up study, conducted by the same research group with 536 participants, the study authors found that 59% had single-organ damage and 27% multi-organ damage70. A dedicated kidney study of VA data including more than 89,000 individuals who had COVID-19 noted an increased risk of numerous adverse kidney outcomes71. Another VA data analysis, including more than 181,000 individuals who had COVID-19, found that infection also increases the risk of type 2 diabetes9 (Fig. 2). The organ damage experienced by patients with long COVID appears durable, and long-term effects remain unknown.
Neurological and cognitive systems
Neurological and cognitive symptoms are a major feature of long COVID, including sensorimotor symptoms, memory loss, cognitive impairment, paresthesia, dizziness and balance issues, sensitivity to light and noise, loss of (or phantom) smell or taste, and autonomic dysfunction, often impacting activities of daily living7,32. Audiovestibular manifestations of long COVID include tinnitus, hearing loss and vertigo7,72.
In a meta-analysis, fatigue was found in 32% and cognitive impairment was found in 22% of patients with COVID-19 at 12 weeks after infection3. Cognitive impairments in long COVID are debilitating, at the same magnitude as intoxication at the UK drink driving limit or 10 years of cognitive ageing73, and may increase over time, with one study finding occurrence in 16% of patients at 2 months after infection and 26% of patients at 12 months after infection74. Activation of the kynurenine pathway, particularly the presence of the metabolites quinolinic acid, 3-hydroxyanthranilic acid and kynurenine, has been identified in long COVID, and is associated with cognitive impairment74. Cognitive impairment has also been found in individuals who recovered from COVID-19 (ref. 75), and at higher rates when objective versus subjective measures were used3, suggesting that a subset of those with cognitive impairment may not recognize and/or report their impairment. Cognitive impairment is a feature that manifests itself independently of mental health conditions such as anxiety and depression74,76, and occurs at similar rates in hospitalized and non-hospitalized patients74,76. A report of more than 1.3 million people who had COVID-19 showed mental health conditions such as anxiety and depression returned to normal over time, but increased risks of cognitive impairment (brain fog), seizures, dementia, psychosis and other neurocognitive conditions persisted for at least 2 years77.
Possible mechanisms for these neuropathologies include neuroinflammation, damage to blood vessels by coagulopathy and endothelial dysfunction, and injury to neurons32. Studies have found Alzheimer disease-like signalling in patients with long COVID78, peptides that self-assemble into amyloid clumps which are toxic to neurons79, widespread neuroinflammation80, brain and brainstem hypometabolism correlated with specific symptoms81,82 and abnormal cerebrospinal fluid findings in non-hospitalized individuals with long COVID along with an association between younger age and a delayed onset of neurological symptoms83. Multilineage cellular dysregulation and myelin loss were reported in a recent preprint in patients with long COVID who had mild infections, with microglial reactivity similar to that seen in chemotherapy, known as ‘chemo-brain’42. A study from the UK Biobank, including brain imaging in the same patients before and after COVID-19 as well as control individuals, showed a reduction in grey matter thickness in the orbitofrontal cortex and parahippocampal gyrus (markers of tissue damage in areas connected to the primary olfactory cortex), an overall reduction in brain size and greater cognitive decline in patients after COVID-19 compared with controls, even in non-hospitalized patients. Although that study looked at individuals with COVID-19 compared with controls, not specifically long COVID, it may have an implication for the cognitive component of long COVID84. Abnormal levels of mitochondrial proteins as well as SARS-CoV-2 spike and nucleocapsid proteins have been found in the central nervous system85. Tetrahydrobiopterin deficiencies and oxidative stress are found in long COVID as well86.
In the eyes, corneal small nerve fibre loss and increased dendritic cell density have been found in long COVID87,88, as well as significantly altered pupillary light responses89 and impaired retinal microcirculation90. SARS-CoV-2 can infect and replicate in retinal59 and brain91 organoids. Other manifestations of long COVID include retinal haemorrhages, cotton wool spots and retinal vein occlusion92.
Mouse models of mild SARS-CoV-2 infection demonstrated microglial reactivity and elevated levels of CCL11, which is associated with cognitive dysfunction and impaired neurogenesis42. Hamster models exhibited an ongoing inflammatory state, involving T cell and myeloid activation, production of pro-inflammatory cytokines and an interferon response that was correlated with anxiety and depression-like behaviours in the hamsters, with similar transcriptional signatures found in the tissue of humans who had recovered from COVID-19 (ref. 93). Infected non-human primates with mild illness showed neuroinflammation, neuronal injury and apoptosis, brain microhaemorrhages, and chronic hypoxaemia and brain hypoxia94.
Recent reports indicate low blood cortisol levels in patients with long COVID as compared with control individuals, more than 1 year into symptom duration18,27. Low cortisol production by the adrenal gland should be compensated by an increase in adrenocorticotropic hormone (ACTH) production by the pituitary gland, but this was not the case, supporting hypothalamus–pituitary–adrenal axis dysfunction18. This may also reflect an underlying neuroinflammatory process. Low cortisol levels have previously been documented in individuals with ME/CFS.
ME/CFS, dysautonomia and related conditions
ME/CFS is a multisystem neuroimmune illness with onset often following a viral or bacterial infection. Criteria include a “substantial reduction or impairment in the ability to engage in pre-illness levels of occupational, educational, social, or personal activities” for at least 6 months, accompanied by a profound fatigue that is not alleviated by rest, along with postexertional malaise, unrefreshing sleep and cognitive impairment or orthostatic intolerance (or both)95. Up to 75% of people with ME/CFS cannot work full-time and 25% have severe ME/CFS, which often means they are bed-bound, have extreme sensitivity to sensory input and are dependent on others for care96. There is a vast collection of biomedical findings in ME/CFS97,98, although these are not well known to researchers and clinicians in other fields.
Many researchers have commented on the similarity between ME/CFS and long COVID99; around half of individuals with long COVID are estimated to meet the criteria for ME/CFS10,11,29,100, and in studies where the cardinal ME/CFS symptom of postexertional malaise is measured, a majority of individuals with long COVID report experiencing postexertional malaise7,100. A study of orthostatic stress in individuals with long COVID and individuals with ME/CFS found similar haemodynamic, symptomatic and cognitive abnormalities in both groups compared with healthy individuals101. Importantly, it is not surprising that ME/CFS should stem from SARS-CoV-2 infection as 27.1% of SARS-CoV infection survivors in one study met the criteria for ME/CFS diagnosis 4 years after onset102. A wide range of pathogens cause ME/CFS onset, including EBV, Coxiella burnetii (which causes Q fever), Ross River virus and West Nile virus38.
Consistent abnormal findings in ME/CFS include diminished natural killer cell function, T cell exhaustion and other T cell abnormalities, mitochondrial dysfunction, and vascular and endothelial abnormalities, including deformed red blood cells and reduced blood volume. Other abnormalities include exercise intolerance, impaired oxygen consumption and a reduced anaerobic threshold, and abnormal metabolic profiles, including altered usage of fatty acids and amino acids. Altered neurological functions have also been observed, including neuroinflammation, reduced cerebral blood flow, brainstem abnormalities and elevated ventricular lactate level, as well as abnormal eye and vision findings. Reactivated herpesviruses (including EBV, HHV-6, HHV-7 and human cytomegalovirus) are also associated with ME/CFS97,98,103,104.
Many of these findings have been observed in long COVID studies in both adults and children (Box 1). Long COVID research has found mitochondrial dysfunction including loss of mitochondrial membrane potential105 and possible dysfunctional mitochondrial metabolism106, altered fatty acid metabolism and dysfunctional mitochondrion-dependent lipid catabolism consistent with mitochondrial dysfunction in exercise intolerance107, redox imbalance108, and exercise intolerance and impaired oxygen extraction100,109,110. Studies have also found endothelial dysfunction29, cerebral blood flow abnormalities and metabolic changes81,111–113 (even in individuals with long COVID whose POTS symptoms abate114), extensive neuroinflammation42,80, reactivated herpesviruses18,21,27, deformed red blood cells64 and many findings discussed elsewhere. Microclots and hyperactivated platelets are found not only in individuals with long COVID but also in individuals with ME/CFS115.
Dysautonomia, particularly POTS, is commonly comorbid with ME/CFS116 and also often has a viral onset117. POTS is associated with G protein-coupled adrenergic receptor and muscarinic acetylcholine receptor autoantibodies, platelet storage pool deficiency, small fibre neuropathy and other neuropathologies118. Both POTS and small fibre neuropathy are commonly found in long COVID111,119, with one study finding POTS in 67% of a cohort with long COVID120.
Mast cell activation syndrome is also commonly comorbid with ME/CFS. The number and severity of mast cell activation syndrome symptoms substantially increased in patients with long COVID compared with pre-COVID and control individuals121, with histamine receptor antagonists resulting in improvements in the majority of patients19.
Other conditions that are commonly comorbid with ME/CFS include connective tissue disorders including Ehlers–Danlos syndrome and hypermobility, neuro-orthopaedic spinal and skull conditions, and endometriosis33,122,123. Evidence is indicating these conditions may be comorbid with long COVID as well. The overlap of postviral conditions with these conditions should be explored further.
Reproductive system
Impacts on the reproductive system are often reported in long COVID, although little research has been done to document the extent of the impact and sex-specific pathophysiology. Menstrual alterations are more likely to occur in women and people who menstruate with long COVID than in women and people who menstruate with no history of COVID and those who had COVID-19 but not long COVID124. Menstruation and the week before menstruation have been identified by patients as triggers for relapses of long COVID symptoms7. Declined ovarian reserve and reproductive endocrine disorder have been observed in people with COVID-19 (ref. 125), and initial theories suggest that SARS-CoV-2 infection affects ovary hormone production and/or the endometrial response due to the abundance of ACE2 receptors on ovarian and endometrial tissue126. Individuals with both COVID-19 and menstrual changes were more likely to experience fatigue, headache, body ache and pain, and shortness of breath than those who did not have menstrual changes, and the most common menstrual changes were irregular menstruation, increased premenstrual symptoms and infrequent menstruation127.
Research on ME/CFS shows associations between ME/CFS and premenstrual dysphoric disorder, polycystic ovarian syndrome, menstrual cycle abnormalities, ovarian cysts, early menopause and endometriosis128–130. Pregnancy, postpartum changes, perimenopause and menstrual cycle fluctuations affect ME/CFS and influence metabolic and immune system changes129. Long COVID research should focus on these relationships to better understand the pathophysiology.
Viral persistence in the penile tissue has been documented, as has an increased risk of erectile dysfunction, likely resulting from endothelial dysfunction131. In one study, impairments to sperm count, semen volume, motility, sperm morphology and sperm concentration were reported in individuals with long COVID compared with control individuals, and were correlated with elevated levels of cytokines and the presence of caspase 8, caspase 9 and caspase 3 in seminal fluid132.
Respiratory system
Respiratory conditions are a common phenotype in long COVID, and in one study occurred twice as often in COVID-19 survivors as in the general population2. Shortness of breath and cough are the most common respiratory symptoms, and persisted for at least 7 months in 40% and 20% of patients with long COVID, respectively7. Several imaging studies that included non-hospitalized individuals with long COVID demonstrated pulmonary abnormalities including in air trapping and lung perfusion133,134. An immunological and proteomic study of patients 3–6 months after infection indicated apoptosis and epithelial damage in the airway but not in blood samples135. Further immunological characterization comparing individuals with long COVID with individuals who had recovered from COVID-19 noted a correlation between decreased lung function, systemic inflammation and SARS-CoV-2-specific T cells136.
Gastrointestinal system
Long COVID gastrointestinal symptoms include nausea, abdominal pain, loss of appetite, heartburn and constipation137. The gut microbiota composition is significantly altered in patients with COVID-19 (ref. 23), and gut microbiota dysbiosis is also a key component of ME/CFS138. Higher levels of Ruminococcus gnavus and Bacteroides vulgatus and lower levels of Faecalibacterium prausnitzii have been found in people with long COVID compared with non-COVID-19 controls (from before the pandemic), with gut dysbiosis lasting at least 14 months; low levels of butyrate-producing bacteria are strongly correlated with long COVID at 6 months24. Persisting respiratory and neurological symptoms are each associated with specific gut pathogens24. Additionally, SARS-CoV-2 RNA is present in stool samples of patients with COVID-19 (ref. 139), with one study indicating persistence in the faeces of 12.7% of participants 4 months after diagnosis of COVID-19 and in 3.8% of participants at 7 months after diagnosis61. Most patients with long COVID symptoms and inflammatory bowel disease 7 months after infection had antigen persistence in the gut mucosa140. Higher levels of fungal translocation, from the gut and/or lung epithelium, have been found in the plasma of patients with long COVID compared with those without long COVID or SARS-CoV-2-negative controls, possibly inducing cytokine production141. Transferring gut bacteria from patients with long COVID to healthy mice resulted in lost cognitive functioning and impaired lung defences in the mice, who were partially treated with the commensal probiotic bacterium Bifidobacterium longum25.
Timelines
The onset and time course of symptoms differ across individuals and by symptom type. Neurological symptoms often have a delayed onset of weeks to months: among participants with cognitive symptoms, 43% reported a delayed onset of cognitive symptoms at least 1 month after COVID-19, with the delay associated with younger age83. Several neurocognitive symptoms worsen over time and tend to persist longer, whereas gastrointestinal and respiratory symptoms are more likely to resolve7,74,142. Additionally, pain in joints, bones, ears, neck and back are more common at 1 year than at 2 months, as is paresthesia, hair loss, blurry vision and swelling of the legs, hands and feet143. Parosmia has an average onset of 3 months after the initial infection144; unlike other neurocognitive symptoms, it often decreases over time143.
Few people with long COVID demonstrate full recovery, with one study finding that 85% of patients who had symptoms 2 months after the initial infection reported symptoms 1 year after symptom onset143. Future prognosis is uncertain, although diagnoses of ME/CFS and dysautonomia are generally lifelong.
Diagnostic tools and treatments
Although diagnostic tools exist for some components of long COVID (for example, tilt table tests for POTS145 and MRI scans to detect cardiovascular impairment68), diagnostic tools for long COVID are mostly in development, including imaging to detect microclots63, corneal microscopy to identify small fibre neuropathy87, new fragmentation of QRS complex on electrocardiograms as indicative of cardiac injury146 and use of hyperpolarized MRI to detect pulmonary gas exchange abnormalities147. On the basis of the tests that are offered as standard care, the results for patients with long COVID are often normal; many providers are unaware of the symptom-specific testing and diagnostic recommendations from the ME/CFS community148. Early research into biomarkers suggests that levels of extracellular vesicles85 and/or immune markers indicating high cytotoxicity149 could be indicative of long COVID. Intriguingly, dogs can identify individuals with long COVID on the basis of sweat samples150. Biomarker research in ME/CFS may also be applicable to long COVID, including electrical impedance blood tests, saliva tests, erythrocyte deformation, sex-specific plasma lipid profiles and variables related to isocapnic buffering151–154. The importance of developing and validating biomarkers that can be used for the diagnosis of long COVID cannot be adequately emphasized — they will not only be helpful in establishing the diagnosis but will also be helpful for objectively defining treatment responses.
Although there are currently no broadly effective treatments for long COVID, treatments for certain components have been effective for subsets of populations (Table 1). Many strategies for ME/CFS are effective for individuals with long COVID, including pacing7,37 and symptom-specific pharmacological options (for example, β-blockers for POTS, low-dose naltrexone for neuroinflammation155 and intravenous immunoglobulin for immune dysfunction) and non-pharmacological options (including increasing salt intake for POTS, cognitive pacing for cognitive dysfunction and elimination diets for gastrointestinal symptoms)96. Low-dose naltrexone has been used in many diseases, including ME/CFS155, and has also shown promise in treating long COVID156. H1 and H2 antihistamines, often following protocols for mast cell activation syndrome and particularly involving famotidine, are used to alleviate a wide range of symptoms19,157, although they are not a cure. Another drug, BC007, potentially addresses autoimmunity by neutralizing G protein-coupled receptor autoantibody levels158. Anticoagulant regimens are a promising way to address abnormal clotting159; in one study, resolution of symptoms was seen in all 24 patients receiving triple anticoagulant therapy31. Apheresis has also shown promise to alleviate long COVID symptoms; it has been theorized to help remove microclots160 and has been shown to reduce autoantibodies in ME/CFS161. However, it is quite expensive, and its benefits are uncertain. Some supplements have shown promise in treating both long COVID and ME/CFS, including coenzyme Q10 and d-ribose162, and may deserve further study.
Table 1
Summary of candidate treatments and supporting evidence
| Symptoms and/or biological mechanism | Treatments | Supporting evidence | Comments |
|---|---|---|---|
| Postexertional malaise | Pacing | ME/CFS literature | Exercise, cognitive behavioural therapy and graded exercise therapy are contraindicated |
| POTS | Pharmacological: β-blockers, pyridostigmine, fludrocortisone, midodrine | POTS and ME/CFS literature | Options can be prioritized on the basis of a specific constellation of symptoms |
| Non-pharmacological: increase salt and fluid intake, intravenously administered salt, compression stockings | POTS and ME/CFS literature | – | |
| Immune dysfunction | Intravenous immunoglobulin | ME/CFS literature | Consider consulting an immunologist on implementation |
| Cognitive dysfunction | Cognitive pacing | ME/CFS literature | Consider implementation alongside pacing physical exertion |
| Cognitive dysfunction | Postconcussion syndrome protocols | ME/CFS and postconcussion syndrome literature | – |
| Fatigue | Coenzyme Q10, d-ribose | ME/CFS literature | – |
| Pain, fatigue, neurological symptoms | Low-dose naltrexone | ME/CFS and other literature | Substantial anecdotal reports of success within the patient community |
| Fatigue, unrefreshing sleep, brain fog | Low-dose aripiprazole | ME/CFS literature | – |
| Autoimmunity | BC007 | Long COVID case report | Neutralizes G protein-coupled receptor autoantibodies |
| Abnormal clotting | Anticoagulants | Long COVID pilot study | Additional trials in progress |
| Abnormal clotting | Apheresis | ME/CFS literature, long COVID pilot study | – |
| Viral persistence and antivirals (COVID-19) | Paxlovid | Long COVID case reports | No active trials, despite strong evidence for viral persistence |
| Viral persistence and antivirals (reactivations such as of EBV, HCMV and VZV) | Valaciclovir, famciclovir, valganciclovir and other antivirals | ME/CFS literature | – |
| Endothelial dysfunction | Sulodexide | Long COVID pilot study | – |
| Gastrointestinal symptoms | Probiotics | Long COVID pilot study | Resolved gastrointestinal and other symptoms |
| Dysautonomia | Stellate ganglion block | Long COVID case report | Effects may wane over time and require repeated procedures |
| Endothelial function, microcirculation, inflammatory markers and oxidative stress | Pycnogenol | COVID-19 pilot study | – |
| MCAS | H1 and H2 antihistamines, particularly famotidine | Long COVID case reports, MCAS literature | Expected to treat symptoms, not underlying mechanism |
| Autonomic dysfunction | Transcutaneous vagal stimulation | Long COVID pilot study | – |
EBV, Epstein–Barr virus; HCMV, human cytomegalovirus; MCAS, mast cell activation syndrome; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome; POTS, postural orthostatic tachycardia syndrome; VZV, varicella zoster virus.
Of note, exercise is harmful for patients with long COVID who have ME/CFS or postexertional malaise110,163 and should not be used as a treatment164–166; one study of people with long COVID noted that physical activity worsened the condition of 75% of patients, and less than 1% saw improvement109.
Pilot studies and case reports have revealed additional treatment options worth exploring. A case report noted resolution of long COVID following treatment with the antiviral Paxlovid167, and a study investigating the treatment of acute COVID-19 with Paxlovid showed a 25% reduction in the incidence of long COVID168; Paxlovid should be investigated further for prevention and treatment of long COVID. A small trial of sulodexide in individuals with endothelial dysfunction saw a reduction in symptom severity169. Pilot studies of probiotics indicated potential in alleviating gastrointestinal and non-gastrointestinal symptoms170,171. Two patients with long COVID experienced substantial alleviation of dysautonomia symptoms following stellate ganglion block172. An early study noted that Pycnogenol statistically significantly improved physiological measurements (for example, reduction in oxidative stress) and quality of life (indicated by higher Karnofsky Performance Scale Index scores)173,174, as hypothesized on the basis of success in other clinical studies.
Taken together, the current treatment options are based on small-scale pilot studies in long COVID or what has been effective in other diseases; several additional trials are in progress175. There is a wide range of possible treatment options from ME/CFS covering various mechanisms, including improving natural killer cell function, removing autoantibodies, immunosuppressants, antivirals for reactivated herpesviruses, antioxidants, mitochondrial support and mitochondrial energy generation176,177; most need to be clinically trialled, which should happen urgently. Many newer treatment options remain underexplored, including anticoagulants and SARS-CoV-2-specific antivirals, and a lack of funding is a significant limitation to robust trials.
Impact of vaccines, variants and reinfections
The impact of vaccination on the incidence of long COVID differs across studies, in part because of differing study methods, time since vaccination and definitions of long COVID. One study indicated no significant difference in the development of long COVID between vaccinated individuals and unvaccinated individuals178; other studies indicate that vaccines provide partial protection, with a reduced risk of long COVID between 15% and 41%4,5, with long COVID continuing to impact 9% of people with COVID-19.
The different SARS-CoV-2 variants and level of (and time since) vaccination may impact the development of long COVID. The UK’s Office for National Statistics found that long COVID was 50% less common in double-vaccinated participants with Omicron BA.1 than in double-vaccinated participants Delta, but that there was no significant difference between triple-vaccinated participants; it also found long COVID was more common after Omicron BA.2 infection than after BA.1 infection in triple-vaccinated participants, with 9.3% developing long COVID from infection with the BA.2 variant179.
The impact of vaccination on long COVID symptoms in people who had already developed long COVID differs among patients, with 16.7% of patients experiencing a relief of symptoms, 21.4% experiencing a worsening of symptoms and the remainder experiencing unchanged symptoms180.
Reinfections are increasingly common181. The impact of multiple instances of COVID-19, including the rate of long COVID in those who recovered from a first infection but developed long COVID following reinfection, and the impact of reinfection on those with pre-existing long COVID is crucial to understand to inform future policy decisions. Early research shows an increasing risk of long COVID sequelae after the second and third infection, even in double-vaccinated and triple-vaccinated people182. Existing literature suggests multiple infections may cause additional harm or susceptibility to the ME/CFS-type presentation33,183.
There is also early evidence that certain immune responses in people with long COVID, including low levels of protective antibodies and elevated levels of autoantibodies, may suggest an increased susceptibility to reinfection27.
Challenges and recommendations
Issues with PCR and antibody testing throughout the pandemic, inaccurate pandemic narratives and widespread lack of postviral knowledge have caused downstream issues and biases in long COVID research and care.
Testing issues
Most patients with COVID-19 from the first waves did not have laboratory-confirmed infection, with PCR tests being difficult to access unless individuals were hospitalized. Only 1–3% of cases to March 2020 were likely detected184, and the CDC estimates that only 25% of cases in the USA were reported from February 2020 to September 2021 (ref. 185); that percentage has likely decreased with the rise in use of at-home rapid tests.
Although PCR tests are our best tool for detecting SARS-CoV-2 infections, their false negative rates are still high186. Further bias is caused by false negative rates being higher in women and adults younger than 40 years187, those with a low viral load188 and children (Box 1), with several studies showing 52–90% of cases in children missed by PCR tests189,190. The high false negative PCR rate results in symptomatic patients with COVID-19, who seek a COVID-19 test but receive a false negative result, being included as a control in many studies. Those who have a positive PCR test result (who are more likely to be included in research) are more likely to be male or have a higher viral load. Additionally, the lack of test accessibility as well as the false negative rates has created a significant barrier to care, as many long COVID clinics require PCR tests for admission.
Similarly, there is a broad misconception that everyone makes and retains SARS-CoV-2 antibodies, and many clinicians and researchers are unaware of the limited utility of antibody tests to determine prior infection. Between 22% and 36% of people infected with SARS-CoV-2 do not seroconvert, and many others lose their antibodies over the first few months, with both non-seroconversion and seroreversion being more likely in women, children and individuals with mild infections52,53,191–193. At 4 and 8 months after infection, 19% and 61% of patients, respectively, who had mild infections and developed antibodies were found to have seroreverted, compared with 2% and 29% of patients, respectively who had severe infections191. Still, many clinicians and researchers use antibody tests to include or exclude patients with COVID-19 from control groups.
Furthermore, during periods of test inaccessibility, tests were given on the basis of patients having COVID-19-specific symptoms such as loss of smell and taste, fever, and respiratory symptoms, resulting in a bias towards people with those symptoms.
Misinformation on PCR and antibody tests has resulted in the categorization of patients with long COVID into non-COVID-19 control groups, biasing the research output. Because low or no antibody levels and viral load may be related to long COVID pathophysiology, including a clinically diagnosed cohort will strengthen the research.
Important miscues
The narrative that COVID-19 had only respiratory sequelae led to a delayed realization of the neurological, cardiovascular and other multisystem impacts of COVID-19. Many long COVID clinics and providers still disproportionately focus on respiratory rehabilitation, which results in skewed electronic health record data. Electronic health record data are also more comprehensive for those who were hospitalized with COVID-19 than for those who were in community care, leading to a bias towards the more traditional severe respiratory presentation and less focus on non-hospitalized patients, who tend to have neurological and/or ME/CFS-type presentations.
The narrative that initially mild COVID-19 cases, generally defined as not requiring hospitalization in the acute phase, would not have long-term consequences has also had downstream effects on research. These so-called mild cases that result in long COVID often have an underlying biology different from acute severe cases, but the same types of tests are being used to evaluate patients. This is despite basic tests such as D-dimer, C-reactive protein (CRP) and antinuclear antibody tests and complete blood count being known to often return normal results in patients with long COVID. Tests that return abnormal results in patients with ME/CFS and dysautonomia, such as total immunoglobulin tests, natural killer cell function tests, the tilt table or NASA lean test, the four-point salivary cortisol test, reactivated herpesvirus panels, small fibre neuropathy biopsy, and tests looking for abnormal brain perfusion96, should instead be prioritized. Other recurring issues include studies failing to include the full range of symptoms, particularly neurological and reproductive system symptoms, and not asking patients about symptom frequency, severity and disability. Cardinal symptoms such as postexertional malaise are not widely known, and therefore are rarely included in study designs.
Widespread lack of postviral knowledge and misinformation
The widespread lack of knowledge of viral-onset illnesses, especially ME/CFS and dysautonomia, as well as often imperfect coding, prevents these conditions from being identified and documented by clinicians; this means that they are frequently absent from electronic health record data. Further, because ME/CFS and dysautonomia research is not widely known or comprehensively taught in medical schools194, long COVID research is often not built on past findings, and tends to repeat old hypotheses. Additionally, long COVID research studies and medical histories tend to document only the risk factors for severe acute COVID-19, which are different from the risk factors for conditions that overlap with long COVID such as ME/CFS and dysautonomia (for example, connective tissue disorders such as Ehlers–Danlos syndrome, prior illnesses such as infectious mononucleosis and mast cell involvement)33,195,196.
Clinicians who are not familiar with ME/CFS and dysautonomia often misdiagnose mental health disorders in patients; four in five patients with POTS receive a diagnosis with a psychiatric or psychological condition before receiving a POTS diagnosis, with only 37% continuing to have the psychiatric or psychological diagnosis once they have received their POTS diagnosis117. Researchers who are unfamiliar with ME/CFS and dysautonomia often do not know to use specific validated tools when conducting mental health testing, as anxiety scales often include autonomic symptoms such as tachycardia, and depression scales often include symptoms such as fatigue, both of which overestimate mental health disorder prevalence in these conditions197,198.
Recommendations
Although research into long COVID has been expansive and has accelerated, the existing research is not enough to improve outcomes for people with long COVID. To ensure an adequate response to the long COVID crisis, we need research that builds on existing knowledge and is inclusive of the patient experience, training and education for the health-care and research workforce, a public communication campaign, and robust policies and funding to support research and care in long COVID.
Research
We need a comprehensive long COVID research agenda that builds on the existing knowledge from ME/CFS, dysautonomia and other viral-onset conditions, including but not limited to brain and brainstem inflammation, appropriate neuroimaging techniques, neuroimmunology, metabolic profiling, impaired endothelial function, mitochondrial fragmentation, antiviral and metabolic phenotypes, hypoperfusion/cerebral blood flow, nanoneedle diagnostic testing, overlaps with connective tissue disorders, autoimmunity and autoantibodies, viral/microbial persistence, intracranial hypertension, hypermobility, craniocervical obstructions, altered T and B cells, metabolomics and proteomics, elevated blood lactate level, herpesvirus reactivations, immune changes in the early versus late postviral years, and changes to the gut microbiota. The mechanisms of and overlaps between long COVID and connective tissue involvement, mast cells and inflammatory conditions such as endometriosis are particularly understudied and should be focused on. Because of the high prevalence of ME/CFS, POTS and other postinfectious illnesses in patients with long COVID, long COVID research should include people who developed ME/CFS and other postinfectious illnesses from a trigger other than SARS-CoV-2 in comparator groups to improve understanding of the onset and pathophysiology of these illnesses113. Additionally, there is a known immune exhaustion process that occurs between the second and third year of illness in ME/CFS, with test results for cytokines being different between patients who have been sick for shorter durations (less than 2 years) than for those who have been sick for longer durations43. Because of this, studies should implement subanalyses based on the length of time participants have been ill. Because ME/CFS and dysautonomia research is not widely known across the biomedical field, long COVID research should be led by experts from these areas to build on existing research and create new diagnostic and imaging tools.
Robust clinical trials must be a priority moving forward as patients currently have few treatment options. In the absence of validated treatment options, patients and physicians conduct individual experiments, which result in the duplication of efforts without generalizable knowledge and pose undue risks to patients. Robust study design and knowledge sharing must be prioritized by both funding institutions and clinician-researchers.
It is critical that research on long COVID be representative of (or oversample) the populations who had COVID-19 and are developing long COVID at high rates, which is disproportionately people of colour35. Medical research has historically under-represented these populations, and over-representation of white and socio-economically privileged patients has been common in long COVID research. Researchers must work within communities of colour, LGBTQ+ communities and low-income communities to build trust and conduct culturally competent studies that will provide insights and treatments for long COVID for marginalized populations.
As a subset of patients will improve over time, and others will have episodic symptoms, care should be taken to incorporate the possibility of alleviation of symptoms into the study design, and care should be taken not to ascribe improvement to a particular cause without proper modelling.
Finally, it is critical that communities of patients with long COVID and associated conditions are meaningfully engaged in long COVID research and clinical trials. The knowledge of those who experience an illness is crucial in identifying proper study design and key research questions and solutions, improving the speed and direction of research.
Training and education of the health-care and research workforce
To prepare the next generation of health-care providers and researchers, medical schools must improve their education on pandemics, viruses and infection-initiated illnesses such as long COVID and ME/CFS, and competency evaluations should include these illnesses. As of 2013, only 6% of medical schools fully cover ME/CFS across the domains of treatment, research and curricula, which has created obstacles to care, accurate diagnosis, research and treatment194. To ensure people with long COVID and associated conditions can receive adequate care now, professional societies and government agencies must educate the health-care and research workforce on these illnesses, including the history of and current best practices for ME/CFS to not repeat mistakes of the past, which have worsened patients’ prognoses. The research community has made a misstep in its efforts to treat ME/CFS199, and some physicians, poorly educated in the aetiology and pathophysiology of the disorder, still advise patients to pursue harmful interventions such as graded exercise therapy and cognitive behavioural therapy, despite the injury that these interventions cause200 and the fact that they are explicitly not advised as treatments163,164,166.
Public communications campaign
In addition to providing education on long COVID to the biomedical community, we need a public communications campaign that informs the public about the risks and outcomes of long COVID.
Policies and funding
Finally, we need policies and funding that will sustain long COVID research and enable people with long COVID to receive adequate care and support. For instance, in the USA, the creation of a national institute for complex chronic conditions within the NIH would go a long way in providing a durable funding mechanism and a robust research agenda. Further, we need to create and fund centres of excellence, which would provide inclusive, historically informed and culturally competent care, as well as conduct research and provide medical education to primary care providers. Additionally, research and clinical care do not exist in silos. It is critical to push forward policies that address both the social determinants of health and the social support that is needed for disabled people.
Conclusions
Long COVID is a multisystemic illness encompassing ME/CFS, dysautonomia, impacts on multiple organ systems, and vascular and clotting abnormalities. It has already debilitated millions of individuals worldwide, and that number is continuing to grow. On the basis of more than 2 years of research on long COVID and decades of research on conditions such as ME/CFS, a significant proportion of individuals with long COVID may have lifelong disabilities if no action is taken. Diagnostic and treatment options are currently insufficient, and many clinical trials are urgently needed to rigorously test treatments that address hypothesized underlying biological mechanisms, including viral persistence, neuroinflammation, excessive blood clotting and autoimmunity.
Acknowledgements
We would like to thank the long COVID and associated conditions patient and research community and the entire team at Patient-Led Research Collaborative. E.J.T. was supported by National Center for Advancing Translational Sciences (NCATS) grant UL1TR002550.
Peer review
Peer review information
Nature Reviews Microbiology thanks Akiko Iwasaki, Ziyad Al-Aly and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/17/2023
A Correction to this paper has been published: 10.1038/s41579-023-00896-0
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/s41579-022-00846-2
Read article for free, from open access legal sources, via Unpaywall:
https://www.nature.com/articles/s41579-022-00846-2.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/141252064
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/s41579-022-00846-2
Article citations
Cost-effectiveness of an online supervised group physical and mental health rehabilitation programme for adults with post-COVID-19 condition after hospitalisation for COVID-19: the REGAIN RCT.
BMC Health Serv Res, 24(1):1326, 31 Oct 2024
Cited by: 0 articles | PMID: 39482691 | PMCID: PMC11528998
A worldwide look into long COVID-19 management: an END-COVID survey.
ERJ Open Res, 10(6):96-2024, 11 Nov 2024
Cited by: 0 articles | PMID: 39534773 | PMCID: PMC11551856
Overlapping conditions in Long COVID at a multisite academic center.
Front Neurol, 15:1482917, 25 Oct 2024
Cited by: 0 articles | PMID: 39524912
Is there a rationale for hyperbaric oxygen therapy in the patients with Post COVID syndrome? : A critical review.
Eur Arch Psychiatry Clin Neurosci, 15 Nov 2024
Cited by: 0 articles | PMID: 39545965
Review
A worldwide perspective of long COVID management: how can we END-COVID?
ERJ Open Res, 10(6):500-2024, 11 Nov 2024
Cited by: 0 articles | PMID: 39534771 | PMCID: PMC11551858
Go to all (1,105) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Orthostatic Symptoms and Reductions in Cerebral Blood Flow in Long-Haul COVID-19 Patients: Similarities with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.
Medicina (Kaunas), 58(1):28, 24 Dec 2021
Cited by: 38 articles | PMID: 35056336 | PMCID: PMC8778312
Long COVID in pediatrics-epidemiology, diagnosis, and management.
Eur J Pediatr, 183(4):1543-1553, 27 Jan 2024
Cited by: 5 articles | PMID: 38279014 | PMCID: PMC11001657
Review Free full text in Europe PMC
Long COVID: mechanisms, risk factors and recovery.
Exp Physiol, 108(1):12-27, 22 Nov 2022
Cited by: 78 articles | PMID: 36412084 | PMCID: PMC10103775
Review Free full text in Europe PMC
Analysis of post COVID-19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome.
J Adv Res, 40:179-196, 26 Nov 2021
Cited by: 53 articles | PMID: 36100326 | PMCID: PMC8619886
Review Free full text in Europe PMC

 3
3


