Abstract
Free full text

Critical contribution of liver natural killer T cells to a murine model of hepatitis
Abstract
Natural killer T (NKT) cells constitute a distinct subpopulation of T cells with a unique antigen specificity, prompt effector functions, and an unusual tissue distribution. NKT cells are especially abundant in the liver, but their physiological function in this organ remains unclear. In the present study, we examined the possible contribution of NKT cells to a murine model of hepatitis induced by i.v. injection of Con A. CD1-deficient mice lacking NKT cells were highly resistant to Con A-induced hepatitis. Adoptive transfer of hepatic NKT cells isolated from wild-type mice, but not from FasL-deficient gld mice, sensitized CD1-deficient mice to Con A-induced hepatitis. Furthermore, adoptive transfer of hepatic mononuclear cells from wild-type mice, but not from CD1-deficient mice, sensitized gld mice to Con A-induced hepatitis. Upon Con A administration, hepatic NKT cells rapidly up-regulated cell surface FasL expression and FasL-mediated cytotoxicity. At the same time, NKT cells underwent apoptosis leading to their rapid disappearance in the liver. These results implicated FasL expression on liver NKT cells in the pathogenesis of Con A-induced hepatitis, suggesting a similar pathogenic role in human liver diseases such as autoimmune hepatitis.
Natural killer T (NKT) cells constitute a distinctive subpopulation of mature T cells that coexpress the NK1.1 Ag in mice (1–3). Recent studies have shown that these cells exhibit unique features with regard to antigen specificity, effector functions, and tissue distribution (1–3). NKT cells express T cell antigen receptor (TCR) composed of a single invariant TCR-α chain (Vα14-Jα281) and a highly skewed TCR β-chain (Vβ8, Vβ7, or Vβ2) (1–3), which recognizes glycolipid Ags or particular hydrophobic peptide presented by the MHC class Ib molecule CD1d (4–7). Although the physiological ligand for this TCR has not been identified, the development of NKT cells is strictly restricted by CD1d, and NKT cells do not develop in CD1d-deficient mice (8–10). NKT cells have been shown to exert various effector functions (1–3, 11–19). It has been reported that NKT cells secrete a large amount of IL-4 and IFN-γ promptly after stimulation with anti-CD3 mAb or with the glycolipid ligand, α-galactosylceramide (α-GalCer) (11–13). It also was shown that NKT cells exhibited perforin- and/or FasL-mediated cytotoxicity, especially when activated by α-GalCer or IL-12, resulting in potent antitumor activity (14–18). Moreover, it recently was reported that stimulation of NKT cells by α-GalCer rapidly induced activations of innate (NK cells) and adaptive (T cells and B cells) immune responses (19). NKT cells have an unusual tissue distribution. They are abundant in the liver, bone marrow, and thymus but are relatively rare in lymph nodes and spleen (1–3). Although the physiological function of NKT cells in tissues where these cells are abundant remains unclear, we previously have suggested a critical contribution of NKT cells to hepatic injury caused by the generalized Shwartzman reaction (20).
In addition to the Shwartzman reaction, several murine models have been used to investigate the pathogenesis of hepatitis, which include d-galactosamine/lipopolysaccharide-induced hepatitis and Con A-induced hepatitis (21–24). It has been suggested that the former represents an experimental model for fulminant hepatitis in sepsis, and that the latter is more relevant to autoimmune hepatitis (25, 26). Recent studies have shown that Fas/FasL-mediated cytotoxicity is critically important for hepatic injury in these models, although the effector cells expressing FasL remain unclear (27–30). In the present study, we explored the possible contribution of liver NKT cells to the pathogenesis of Con A-induced hepatitis by using CD1-deficient mice lacking NKT cells and adoptive transfer of liver NKT cells. We found a critical role of FasL expressed on NKT cells upon Con A administration, which not only induced hepatic injury but also led to apoptotic elimination of activated NKT cells in the liver. The pathophysiological relevance of these findings is discussed.
Materials and Methods
Mice.
Male C57BL/6 (B6) mice, 8 wk of age, were purchased from Clear Japan (Tokyo). B6 gld/gld (gld) mice and perforin-deficient mice originally were purchased from The Jackson Laboratory and maintained in our animal facility. B6 CD1d-deficient mice were generated as described (10) and used at 8 wk of age. All mice were maintained under specific pathogen-free conditions.
Reagents.
A neutralizing anti-mouse FasL mAb (MFL3) was obtained from PharMingen. Concanamycin A (CMA), which inhibits perforin-mediated cytotoxicity (31), was purchased from Wako Pure Chemical (Osaka).
Induction of Con A-Induced Hepatitis.
Con A (Sigma) was dissolved in pyrogen-free PBS and i.v. injected to mice through the tail vein at a dose of 15 mg/kg (29). Sera from individual mice were obtained 20 h after Con A injection. Serum aminotransferase [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)] activities were measured by the standard photometric method using Hitachi type 7350 automatic analyzer (Tokyo).
Flow Cytometric Analysis.
Surface phenotype of the cells was characterized by three-color flow cytometry as described (32, 33). To avoid the nonspecific binding of antibodies to FcγR, the cells were preincubated with anti-mouse CD16/32 (2.4G2) mAb before staining, which diminished the binding of isotype control. Then, the cells were incubated with a saturating amount of FITC-conjugated anti-mouse CD3 (145–2C11) mAb and phycoerythin (PE)-conjugated anti-NK1.1 (PK 136) mAb. In some experiments, the cells were incubated with a saturating amount of biotinylated anti-FasL (MFL3) mAb or anti-Fas (Jo2) mAb, before incubation with FITC-conjugated anti-mouse NK1.1 mAb, Cy-chrome-conjugated anti-CD3 mAb, and PE-conjugated streptavidin. All staining reagents were obtained from PharMingen. After washing with PBS twice, the stained cells were analyzed on a FACScan (Becton Dickinson), and data were processed by the cell quest program (Becton Dickinson). Detection of apoptotic cells by annexin V binding was performed by using an annexin V-FITC apoptosis detection kit (PharMingen) according to the manufacturer's instruction.
(145–2C11) mAb and phycoerythin (PE)-conjugated anti-NK1.1 (PK 136) mAb. In some experiments, the cells were incubated with a saturating amount of biotinylated anti-FasL (MFL3) mAb or anti-Fas (Jo2) mAb, before incubation with FITC-conjugated anti-mouse NK1.1 mAb, Cy-chrome-conjugated anti-CD3 mAb, and PE-conjugated streptavidin. All staining reagents were obtained from PharMingen. After washing with PBS twice, the stained cells were analyzed on a FACScan (Becton Dickinson), and data were processed by the cell quest program (Becton Dickinson). Detection of apoptotic cells by annexin V binding was performed by using an annexin V-FITC apoptosis detection kit (PharMingen) according to the manufacturer's instruction.
Mononuclear Cell (MNC) Preparation and Adoptive Transfer.
Hepatic MNCs were prepared as described (15). T cells, NKT cells and NK cells were isolated from naive B6 hepatic MNCs by a panning method using anti-CD5 mAb and anti-DX5 mAb (PharMingen) to avoid the stimulation of these cells by cross-linking with mAbs. Briefly, B cells were removed by adherence on plates coated with affinity-purified goat anti-mouse IgG + IgM Ab (Caltag, South San Francisco, CA) twice. NK cells were obtained by positive selection on anti-DX5 mAb-coated plates. CD5-positive cells, including both T and NKT cells, were positively selected on anti-CD5 mAb-coated plates. Then, DX5+CD5+ NKT cells were positively separated from DX5−CD5+ T cells on anti-DX5 mAb-coated plates. Purity of the respective cell fractions was verified by two-color flow cytometry analysis using FITC-conjugated anti-mouse CD3 and PE-conjugated anti-NK1.1 mAbs. The NK cell fraction contained >95% CD3−NK1.1+ NK cells and <5% CD3+ T or CD3+NK1.1+ NKT cells. The NKT cell fraction contained >95% CD3+NK1.1+ NKT cells and <5% CD3+NK1.1− T cells or CD3−NK1.1+ NK cells. The T cell fraction contained >90% CD3+NK1.1− T cells, approximately 5% CD3+NK1.1+ NKT cells, and <5% CD3−NK1.1+ NK cells. Whole hepatic MNCs (5 × 106 cells) or the isolated subpopulation (2 × 106 cells) were mixed with 15 mg/kg Con A in 50 μl of pyrogen-free PBS immediately before injection into the liver of recipient mice. Under ether anesthesia, 50 μl of the suspension was injected into the lateral left lobe of liver at a rate of 10 μl/sec by using a 29-gauge needle attached to a 1-ml syringe under direct visualization. Sera were obtained from individual mice 6 h later, and serum AST and ALT levels were determined as described above.
and PE-conjugated anti-NK1.1 mAbs. The NK cell fraction contained >95% CD3−NK1.1+ NK cells and <5% CD3+ T or CD3+NK1.1+ NKT cells. The NKT cell fraction contained >95% CD3+NK1.1+ NKT cells and <5% CD3+NK1.1− T cells or CD3−NK1.1+ NK cells. The T cell fraction contained >90% CD3+NK1.1− T cells, approximately 5% CD3+NK1.1+ NKT cells, and <5% CD3−NK1.1+ NK cells. Whole hepatic MNCs (5 × 106 cells) or the isolated subpopulation (2 × 106 cells) were mixed with 15 mg/kg Con A in 50 μl of pyrogen-free PBS immediately before injection into the liver of recipient mice. Under ether anesthesia, 50 μl of the suspension was injected into the lateral left lobe of liver at a rate of 10 μl/sec by using a 29-gauge needle attached to a 1-ml syringe under direct visualization. Sera were obtained from individual mice 6 h later, and serum AST and ALT levels were determined as described above.
Histological Examination.
Hematoxylin/eosin staining of paraffin-embedded liver sections was performed as described (29).
Cytotoxic Assay.
Cytolytic activity of hepatic MNCs was tested against human Fas cDNA-transfected WR19L (WR/F) cells by a standard 4-h 51Cr release assay as described (15). WR/F was kindly provided by S. Yonehara (Kyoto University, Kyoto, Japan) and cultured in RPMI medium 1640 containing 10% FCS and 2 mM l-glutamine (34). 51Cr-labeled target cells (104/well) were incubated in a total volume of 200 μl with the effector cells at the indicated effector-to-target ratios in 10% FCS-RPMI 1640 in 96-well round-bottom microtiter plates. After 4 h of incubation, the supernatant was harvested and counted in a gamma counter. Specific cytotoxicity was calculated as described (15). In some experiments, cytotoxic assay was performed in the presence of anti-FasL (MFL-3) mAb (10 μg/ml) and/or CMA (50 nM).
Statistical Analysis.
Statistical analysis between each group was performed by two-sample t test. P values less than 0.05 were considered significant.
Results
Impaired Con A-Induced Hepatitis in CD1-Deficient Mice.
To explore the possible contribution of NKT cells to the pathogenesis of Con A-induced hepatitis, we i.v. injected Con A into CD1-deficient mice that selectively lack NKT cells and measured serum AST and ALT levels 20 h after injection. In preliminary experiments, elevated serum levels of AST and ALT were first observed 6–8 h after injection and peaked at 20 h after injection (data not shown). As shown in Fig. Fig.11A, serum AST and ALT levels were markedly reduced in CD1-deficient mice as compared with wild-type mice when either 30 mg/kg or 15 mg/kg of Con A was injected. Histological findings of degenerative changes in the livers were clearly correlated with the serum aminotransferase levels (data not shown). These results indicated that NKT cells play a critical role in the induction of Con A hepatitis.
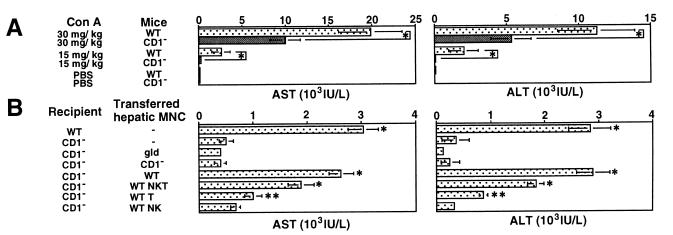
Critical contribution of NKT cells to Con A-induced hepatitis. (A) Impairment of Con A-induced hepatitis in CD1-deficient mice. Wild-type B6 mice (WT) and CD1-deficient B6 mice (CD1−) were i.v. injected with 30 mg/kg or 15 mg/kg Con A or with PBS only. Sera from individual mice were obtained 20 h later, and AST and ALT levels were measured. Data are presented as mean ± SD of 10 mice in each group. Similar results were obtained in three independent experiments. *, P < 0.01. (B) Adoptive transfer of hepatic NKT cells isolated from wild-type (WT) mice sensitizes CD1-deficient (CD1−) mice to Con A-induced hepatitis. As control, 15 mg/kg Con A was injected into the liver of WT or CD1− mice without adoptive transfer of hepatic MNCs. The other groups of CD1− mice were injected with 15 mg/kg Con A along with total hepatic MNCs (5 × 106 cells) isolated from the indicated mice or the indicated hepatic MNC subpopulation (2 × 106 cells) isolated from WT mice. Sera from individual mice were obtained 6 h later, and AST and ALT levels were measured. Data are shown as the mean ± SD of 10 mice in each group. Similar results were obtained in three independent experiments. *, P < 0.01 and **, P < 0.05 as compared with Con A-injected CD1− mice.
Adoptive Transfer of Hepatic NKT Cells Sensitizes CD1-Deficient Mice to Con A-Induced Hepatitis.
To provide further evidence for the contribution of NKT cells to Con A-induced hepatitis, we tested whether adoptive transfer of NKT cells could sensitize CD1-deficient mice to Con A-induced hepatitis. The intrahepatic injection of 15 mg/kg of Con A along with hepatic MNCs (5 × 106) from wild-type mice that contained 15% NKT cells elevated the serum AST and ALT levels in CD1-deficient mice, whereas the injection of Con A together with hepatic MNCs from CD1-deficient mice that contained less than 2% NKT cells did not (Fig. (Fig.11B). Among the hepatic MNC subpopulations from wild-type mice, NKT cells were more efficient than T cells to induce Con A hepatitis in CD1-deficient mice, whereas NK cells were not effective (Fig. (Fig.11B). In these adoptive transfer experiments, serum AST and ALT levels peaked at 6–8 h after Con A injection into the liver in each group (data not shown). Histological examination showed a diffuse and massive degenerative change in the liver of CD1-deficiemt mice when NKT cells and Con A were coinjected (Fig. (Fig.22D), which was comparable to that observed in i.v. Con A-injected wild-type mice (Fig. (Fig.22A). In contrast, only a focal and mild injury was observed in the liver of CD1-deficient mice when Con A was injected alone (Fig. (Fig.22B) or when hepatic MNC from CD1-deficient mice and Con A were coinjected (Fig. (Fig.22C). These results indicated that hepatic NKT cells play a pivotal role in the Con A-induced hepatitis.
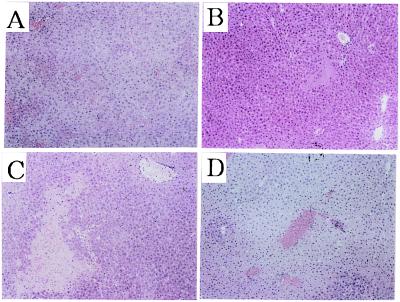
Histological examination of the Con A- and NKT-induced hepatitis in CD1-deficient mice. The livers were removed from i.v. Con A-injected wild-type mice 20 h later (A), intrahepatic Con A-injected CD1-deficient mice (B), intrahepatic Con A- and CD1-deficient hepatic MNC-injected CD1-deficient mice (C), and intrahepatic Con A and wild-type NKT cells-injected CD1-deficient mice (D) 6 h later. Paraffin sections were stained with hematoxylin/eosin. (Original magnifications: ×200.)
Critical Contribution of FasL to NKT Cell-Mediated Con A Hepatitis.
It has been shown that FasL-deficient gld mice are resistant to Con A-induced hepatitis, suggesting a critical contribution of FasL (29, 30). However, the effector cells expressing FasL remain unclear. Consistent with these experiments, the injection of Con A together with hepatic MNCs from wild-type mice substantially elevated the serum AST and ALT levels in gld mice, whereas the injection of Con A together with hepatic MNCs from gld mice that contained 15% NKT cells did not (Fig. (Fig.33A). These findings indicate a requirement of functional FasL expression on hepatic MNCs. To identify the critical cell population, hepatic MNCs from CD1-deficient mice or hepatic MNC subpopulations isolated from wild-type mice were injected into the liver of gld mice with 15 mg/kg of Con A. As shown in Fig. Fig.33B, the adoptive transfer of wild-type hepatic MNCs, but not CD1-deficient hepatic MNCs lacking NKT cells, sensitized gld mice to Con A hepatitis. Conversely, the adoptive transfer of gld hepatic MNC, which contained 15% NKT cells, did not sensitize CD1-deficient mice to Con A hepatitis (Fig. (Fig.11B). Among the hepatic MNC subpopulations from wild-type mice, NKT cells were more efficient than T cells to sensitize gld mice to Con A hepatitis (Fig. (Fig.33B). These results indicated that functional FasL expression on hepatic NKT cells plays a critical role in the Con A-induced hepatitis.
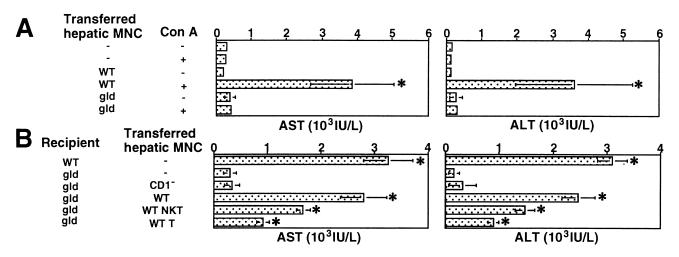
Critical contribution of FasL on NKT cells for Con A-induced hepatitis. (A) Adoptive transfer of hepatic MNC isolated from wild-type (WT) mice sensitizes gld mice to Con A-induced hepatitis. Total hepatic MNCs (5 × 106 cells) isolated from the indicated mice and/or 15 mg/kg Con A were injected into the liver of gld mice. Sera from individual mice were obtained 6 h after injection, and AST and ALT levels were measured. Data are shown as the mean ± SD of 10 mice in each group. Similar results were obtained in three independent experiments. *, P < 0.01 compared with Con A-injected gld mice. (B) Adoptive transfer of hepatic MNC from CD1-deficient (CD1−) mice does not sensitize gld mice to Con A-induced hepatitis. Total hepatic MNCs (5 × 106 cells) isolated from the indicated mice or the indicated hepatic MNC subpopulation (2 × 106 cells) isolated from wild-type (WT) mice were injected into the liver of gld mice along with 15 mg/kg Con A. Sera from individual mice were obtained 6 h later, and AST and ALT levels were measured. Data are shown as the mean ± SD of 10 mice in each group. Similar results were obtained in three independent experiments. *, P < 0.01 compared with Con A-injected gld mice.
FasL Expression on NKT Cells After Con A Injection.
We next verified the expression of FasL on hepatic MNC subpopulations by flow cytometry after Con A injection into wild-type B6 mice. As shown in Fig. Fig.4,4, FasL expression on hepatic NKT cells was markedly elevated 2 h after Con A injection, whereas FasL expression on T cells or NK cells was only slightly enhanced. We also examined the expression of Fas on these cells (Fig. (Fig.4).4). Hepatic NKT cells and T cells constitutively expressed Fas at high level, whereas NK cells expressed a lower level of Fas. The Fas expression on these cells was not elevated 2 h after Con A injection.
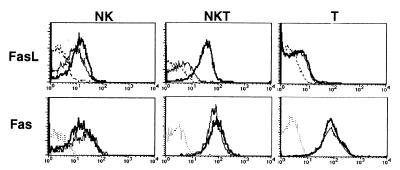
Expression of FasL and Fas on hepatic MNC subpopulations with or without Con A injection. Wild-type B6 mice were i.v. injected with 15 mg/kg Con A or PBS only. Hepatic MNC were isolated 2 h later, and then stained with biotinylated anti-FasL mAb or anti-Fas mAb followed by FITC-conjugated anti-mouse NK1.1 mAb, Cy-chrome-conjugated anti-CD3 mAb, and PE-conjugated streptavidin. Expression of FasL or Fas was analyzed on electronically gated NK1.1+CD3− (NK), NK1.1+CD3+ (NKT), or NK1.1−CD3+ (T) cells. Bold lines indicate the staining of Con A-injected hepatic MNCs, thin lines indicate the staining of PBS-injected hepatic MNCs, and broken lines indicate the background staining with isotype-matched control IgG. Similar results were obtained in three independent experiments.
FasL-Mediated Cytotoxic Activity of Hepatic NKT Cells After Con A Injection.
To further substantiate the functional expression of FasL on NKT cells, we next examined the cytotoxic activity of hepatic MNCs against FasL-susceptible WR/F target cells 2 h after Con A injection into wild-type and CD1-deficient mice. As shown in Fig. Fig.55A, Con A-induced cytotoxicity was significantly impaired in hepatic MNCs from CD1-deficient mice as compared with wild-type mice (P < 0.01), indicating that the Con A-induced cytotoxicity is mediated mainly by NKT cells. Moreover, the Con A-induced cytotoxic activity of wild-type hepatic MNCs was inhibited to the level of CD1-deficient hepatic MNCs by addition of a neutralizing anti-FasL mAb, whereas the residual cytotoxicity was abrogated by combination with a perforin inhibitor, CMA (Fig. (Fig.55B). These results indicated that NKT cells are mainly responsible for the FasL-mediated cytotoxicity induced in hepatic MNCs after Con A administration.
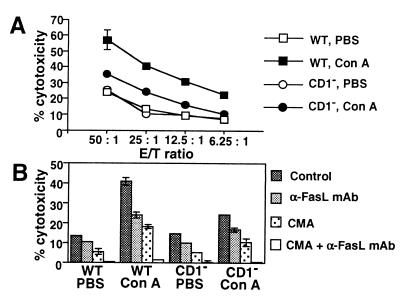
FasL-mediated cytotoxic activity of hepatic NKT cells after Con A injection. (A) Impairment of Con A-induced cytotoxicity in CD1-deficient (CD1−) mice. Wild-type (WT) and CD1− mice were i.v. injected with 15 mg/kg of Con A or PBS only. Hepatic MNCs were isolated 2 h later, and their cytotoxic activity was tested against WR/F target cells by 4 h 51Cr release assay at the indicated effector-to-target (E/T) ratios. Data are represented as the mean ± SD of triplicate wells. Similar results were obtained in three independent experiments. (B) FasL- and perforin-mediated cytotoxicity induced by Con A injection. Hepatic MNCa were prepared as described above, and the cytotoxicity against WR/F was tested in the presence or absence of α-FasL mAb (10 μg/ml) and/or CMA (50 nM) at an effector-to-target ratio of 25. Data are represented as the mean ± SD of triplicate wells. Similar results were obtained in three independent experiments.
Selective Depletion of Hepatic NKT Cells After Con A Injection.
When we examined the phenotype of hepatic MNCs after Con A injection, we found that NKT cells were rapidly and selectively depleted from hepatic MNCs. As shown in Fig. Fig.66A, a marked depletion of NKT cells was observed 4–20 h after i.v. injection of 15 mg/kg Con A into wild-type B6 mice. In contrast, the numbers of NK cells and T cells were not significantly changed during these periods. Hepatic NKT cells began to repopulate 36 h after Con A injection and were almost completely restored within 3 days (data not shown). The yielded cell numbers of hepatic MNCs were not significantly affected by Con A injection (data not shown). These results indicated that NKT cells are rapidly and selectively depleted from the liver after Con A administration, while they act as predominant effector cells in Con A-induced hepatitis.

Apoptotic elimination of hepatic NKT cells after Con A injection. (A) Selective depletion of hepatic NKT cells upon administration of Con A. Wild-type B6 mice were i.v. injected with 15 mg/kg Con A. After the indicated period, hepatic MNCs were isolated and stained with FITC-conjugated anti-CD3 mAb and PE-conjugated anti-NK1.1 mAb. The percentage of NKT cells (boxed) indicated in each panel represents mean ± SD of five mice at each time point. Similar results were obtained in three independent experiments. (B) Delayed depletion of hepatic NKT cells in gld mice. Perforin-deficient mice and gld mice were i.v. injected with 15 mg/kg of Con A. After the indicated period, hepatic MNCs were isolated and stained with FITC-conjugated anti-CD3 mAb and PE-conjugated anti-NK1.1 mAb. The percentage of NKT cells (boxed) indicated in each panel represents the mean ± SD of five mice at each time point. Similar results were obtained in two independent experiments. (C) FasL-mediated apoptosis of hepatic NKT cells. Wild-type (WT) and gld mice were i.v. injected with 15 mg/kg Con A or PBS only. Hepatic MNC were isolated 2 h later and stained with PE-conjugated anti-NK1.1 mAb and Cy-chrome-conjugated anti-CD3 mAb. Then, annexin V binding assay was performed by using annexin V-FITC apoptosis detection kit. Annexin V binding was analyzed on electronically gated NK1.1+CD3− (NK), NK1.1+CD3+ (NKT), or NK1.1−CD3+ (T) cells. Bold lines indicate the annexin V binding to Con A-injected hepatic MNCs, and thin lines indicate the annexin V binding to PBS-injected hepatic MNCs. Similar results were obtained in two independent experiments.
FasL-Mediated Apoptosis Is at Least Partly Responsible for Con A-Induced Depletion of NKT Cells.
Because we observed that hepatic NKT cells constitutively expressed Fas at a high level and also up-regulated FasL expression rapidly after Con A administration (Fig. (Fig.4),4), we next addressed the possibility that the rapid and selective depletion of hepatic NKT cells may be caused by Fas/FasL-mediated suicide. Thus, we i.v. injected Con A into FasL-deficient gld mice and followed the depletion of NKT cells in hepatic MNCs by flow cytometry. We also used perforin-deficient mice to address the possible contribution of perforin-mediated killing. As shown in Fig. Fig.66B, NKT cells were not depleted 4 h after Con A injection into gld mice, but they eventually were depleted 8 h later. In contrast, perforin-deficient mice showed a similar kinetics of NKT cell depletion to that observed in wild-type mice. These results suggested that the Fas/FasL-mediated suicide is, at least in part, responsible for the rapid depletion of hepatic NKT cells after Con A administration. To confirm the FasL-mediated apoptosis of NKT cells early after Con A administration, we analyzed annexin V binding to hepatic MNC subpopulations 2 h after Con A injection into wild-type and gld mice. As shown in Fig. Fig.66C, hepatic NKT cells isolated from wild-type mice 2 h after Con A injection exhibited substantially increased annexin V binding as compared with NKT cells isolated from PBS-injected mice, whereas hepatic NKT cells from gld mice 2 h after Con A injection did not. The annexin V binding to hepatic NK cells or T cells was not affected by Con A injection in either wild-type or gld mice. These results indicated that the FasL-mediated apoptosis occurred in hepatic NKT cells early after Con A administration.
Discussion
We previously investigated a possible contribution of NKT cells to Con A-induced hepatitis and observed that depletion of NK1.1+ NK and NKT cells, but not asialo GM1+ NK cells, from wild-type B6 mice ameliorated Con A-induced hepatitis and that β2m-deficient mice lacking CD8+ T and NKT cells were rather resistant to Con A-induced hepatitis (35). These observations suggested that NKT cells might play a critical role in Con A-induced hepatitis. We also previously investigated the involvement of FasL in Con A-induced hepatitis (29), but the effector cells expressing FasL have not been identified in that study or by others (27–30). In this study, we demonstrated that CD1-deficient mice that specifically lack NKT cells were highly resistant to Con A-induced hepatitis and that adoptive transfer of hepatic NKT cells from wild-type mice can sensitize CD1-deficient mice to Con A-induced hepatitis, indicating a critical contribution of NKT cells. Because adoptive transfer of hepatic MNCs from FasL-deficient gld mice did not sensitize CD1-deficient mice, functional FasL expression on NKT cells was required. Consistently, hepatic NKT cells up-regulated cell surface FasL expression and FasL-mediated cytotoxic activity rapidly after Con A administration. These results clearly indicated that Con A-induced hepatitis, which is a murine model of autoimmune hepatitis, is primarily mediated by FasL expressed on hepatic NKT cells. Although the possible contribution of NKT cells to other hepatitis models remains to be determined in further studies, it previously was demonstrated that NKT cells critically contribute to liver injury caused by the generalized Shwartzman reaction and by Salmonella infections (20, 36), suggesting a general contribution of NKT cells to immune-mediated hepatitis. Therefore, NKT cells appear to play crucial roles in the induction of hepatic injury by cooperating with conventional T cells (28, 37) and macrophages (38), and through the effector mechanisms involving the Fas/FasL system (27–30), perforin/granzyme system (39), and IFN-γ (40), IL-4 (35, 39) and/or TNF-α-mediated system (35, 41).
NKT cells are abundant in the liver (1–3), but their physiological or pathological role in this organ remains unclear. In our preliminary experiments to establish the adoptive transfer system, we observed that injection of 5 × 106 wild-type hepatic MNCs into the tail vein or portal vein did not sensitize CD1-deficient mice to Con A hepatitis, possibly because of poor migration or lodging of injected NKT cells into the liver. To support this notion, we could not detect NKT cells or CD1+ MNCs in the liver after i.v. or intraportal injection of CD1+ hepatic MNCs from wild-type mice into CD1-deficient mice (data not shown). In contrast, direct injection of hepatic MNCs from wild type, but not CD1-deficient or gld mice, into the liver lobule along with Con A induced a robust hepatitis, indicating a critical contribution of liver-resident NKT cells. It has been shown that hepatocytes express CD1d, which may be involved in the selection and/or expansion of NKT cells in the liver (7, 42). In our present system, there was no requirement for CD1 expression in the liver, because adoptive transfer of NKT cells along with Con A induced hepatitis in the liver of CD1-deficient mice. This finding is not surprising, because Con A can activate NKT cells independently of their TCR-mediated recognition of a specific antigen, which is also true for conventional T cells. However, in a physiological situation hepatic NKT cells may be activated by CD1-restricted antigens. In this respect, it is noteworthy that NKT cells appear to respond to glycosylphosphatidylinositol (GPI)-anchored antigens of Plasmodium and Trypanosoma in a CD1d-restricted manner and to oligomannosylated GPI of Mycobacterium (43–46). Thus, NKT cells may contribute to the innate immune response against diverse pathogens in the liver, and this NKT cell activation by microbial ligands would conduct subsequent activation of innate and adoptive effector lymphocytes, as demonstrated after α-galactosylceramide treatment (19).
We here showed that hepatic NKT cells, but not T cells, rapidly up-regulated cell surface FasL expression upon Con A administration, which not only induced hepatitis but also led to apoptotic elimination of NKT cells in the liver. We previously showed that hepatic NKT cells, but not T cells, constitutively express FasL mRNA (17). Therefore, the rapid appearance of FasL on the surface of Con A-stimulated NKT cells may be predominantly mediated by the delivery of intracellularly stored FasL to the cell surface, as recently reported for cytotoxic T lymphocyte clones (47). The rapid depletion of activated NKT cells in the liver appears to underlie the transient nature of Con A hepatitis and to be physiological relevant to limit liver injury by these cells, because the continued presence of activated NKT cells expressing FasL would lead to fatal liver damage, as observed in mice injected with anti-Fas mAb (27). Therefore, hepatic NKT cells may need to be eliminated quickly after they accomplish their effector functions in the liver. Consistent with this notion, it has been reported recently that NKT cells were rapidly depleted after i.v. injection of anti-CD3 mAb or IL-12 and then repopulated organs within 2 days (33). Our findings further substantiate the rapid turnover of activated NKT cells in the liver.
Although the molecular mechanism for the apoptotic elimination of activated NKT cells in the liver has not been determined, our findings that NKT cells are not significantly depleted 4 h after Con A injection into gld mice demonstrated that Fas/FasL-mediated suicide is primarily responsible for the rapid depletion of hepatic NKT cells. However, at 8 h after Con A injection, hepatic NKT cells eventually were depleted in gld mice, suggesting other, more slowly acting mechanisms. It has been reported that some members of the TNF family other than FasL, including TNF and TRAIL, are involved in activation-induced cell death (AICD) of preactivated T cells (48, 49). Therefore, these molecules may be involved in the AICD of NKT cells. Further analysis of these mechanisms that deplete activated NKT cells in the liver may lead to strategies to inhibit the massive and chronic hepatitis in pathological conditions.
Acknowledgments
This work was supported by a grant-in-aid for Scientific Research from the Ministry of Education, Science, and Culture, Japan.
Abbreviations
| MNC | mononuclear cell |
| NK | natural killer |
| NKT | natural killer T |
| CMA | concanamycin A |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| TCR | T cell antigen receptor |
| PE | phycoerythin |
| WR/F | Fas cDNA-transfected WR19L |
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040566697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040566697
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.040566697
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc25857?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Rhoifolin Attenuates Concanavalin A-Induced Autoimmune Hepatitis in Mice via JAKs/STATs Mediated Immune and Apoptotic Processes.
ACS Omega, 9(42):43233-43251, 11 Oct 2024
Cited by: 0 articles | PMID: 39464476 | PMCID: PMC11500133
Microcirculatory disturbance in acute liver injury is triggered by IFNγ-CD40 axis.
J Inflamm (Lond), 21(1):23, 21 Jun 2024
Cited by: 0 articles | PMID: 38907339 | PMCID: PMC11191181
Early infiltrating NKT lymphocytes attenuate bone regeneration through secretion of CXCL2.
Sci Adv, 10(20):eadl6343, 17 May 2024
Cited by: 2 articles | PMID: 38758783 | PMCID: PMC11100573
Specific and shared biological functions of PARP2 - is PARP2 really a lil' brother of PARP1?
Expert Rev Mol Med, 26:e13, 03 May 2024
Cited by: 1 article | PMID: 38698556 | PMCID: PMC11140550
Review Free full text in Europe PMC
Tissue damage from chronic liver injury inhibits peripheral NK cell abundance and proinflammatory function.
J Leukoc Biol, 115(6):1042-1052, 01 May 2024
Cited by: 0 articles | PMID: 38315633 | PMCID: PMC11135618
Go to all (388) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Mice deficient in Vbeta8+NKT cells are resistant to experimental hepatitis but are partially susceptible to generalised Shwartzman reaction.
Clin Exp Med, 7(1):30-38, 01 Mar 2007
Cited by: 6 articles | PMID: 17380303
Contribution of CD1d-unrestricted hepatic DX5+ NKT cells to liver injury in Plasmodium berghei-parasitized erythrocyte-injected mice.
Int Immunol, 16(6):787-798, 13 Apr 2004
Cited by: 21 articles | PMID: 15096477
Enhancement of the synthetic ligand-mediated function of liver NK1.1Ag+ T cells in mice by interleukin-12 pretreatment.
Immunology, 113(1):35-43, 01 Sep 2004
Cited by: 20 articles | PMID: 15312134
Experimental hepatitis and role of cytokines.
Acta Gastroenterol Belg, 60(2):176-179, 01 Apr 1997
Cited by: 46 articles | PMID: 9260332
Review





