Abstract
Free full text

An SRY-related gene expressed during spermatogenesis in the mouse encodes a sequence-specific DNA-binding protein.
Abstract
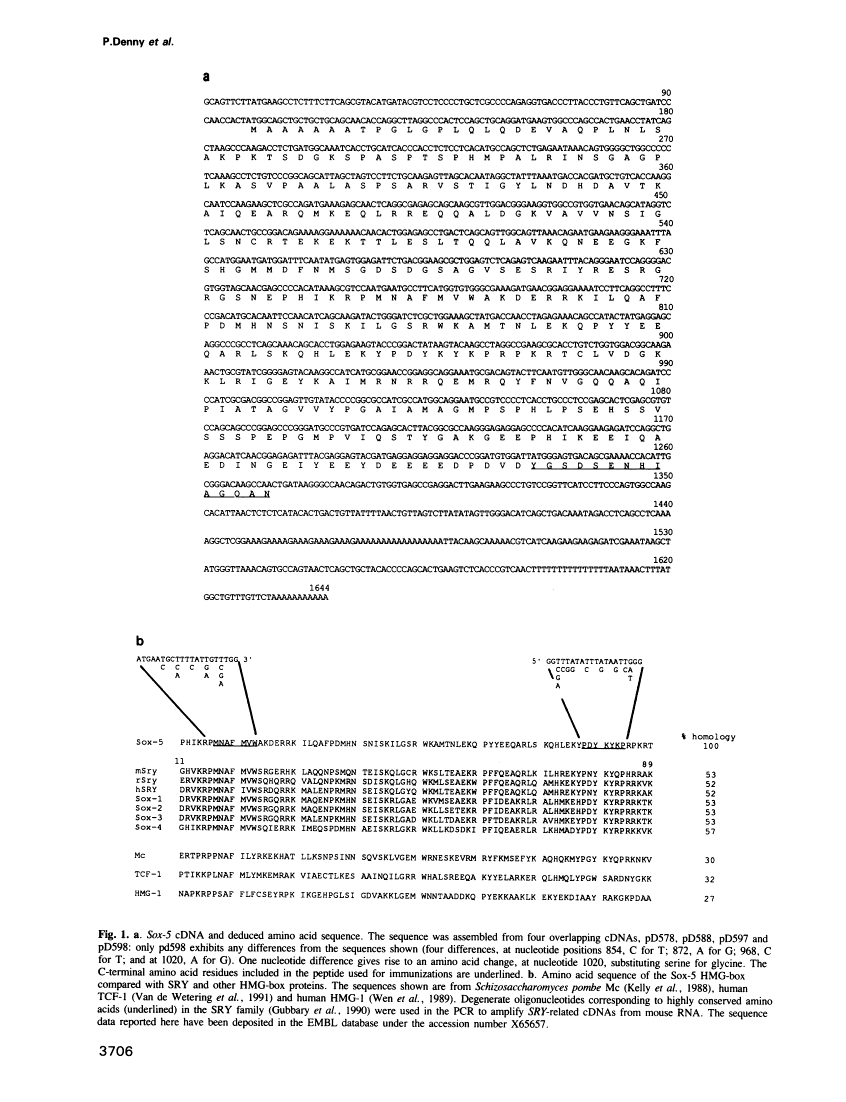
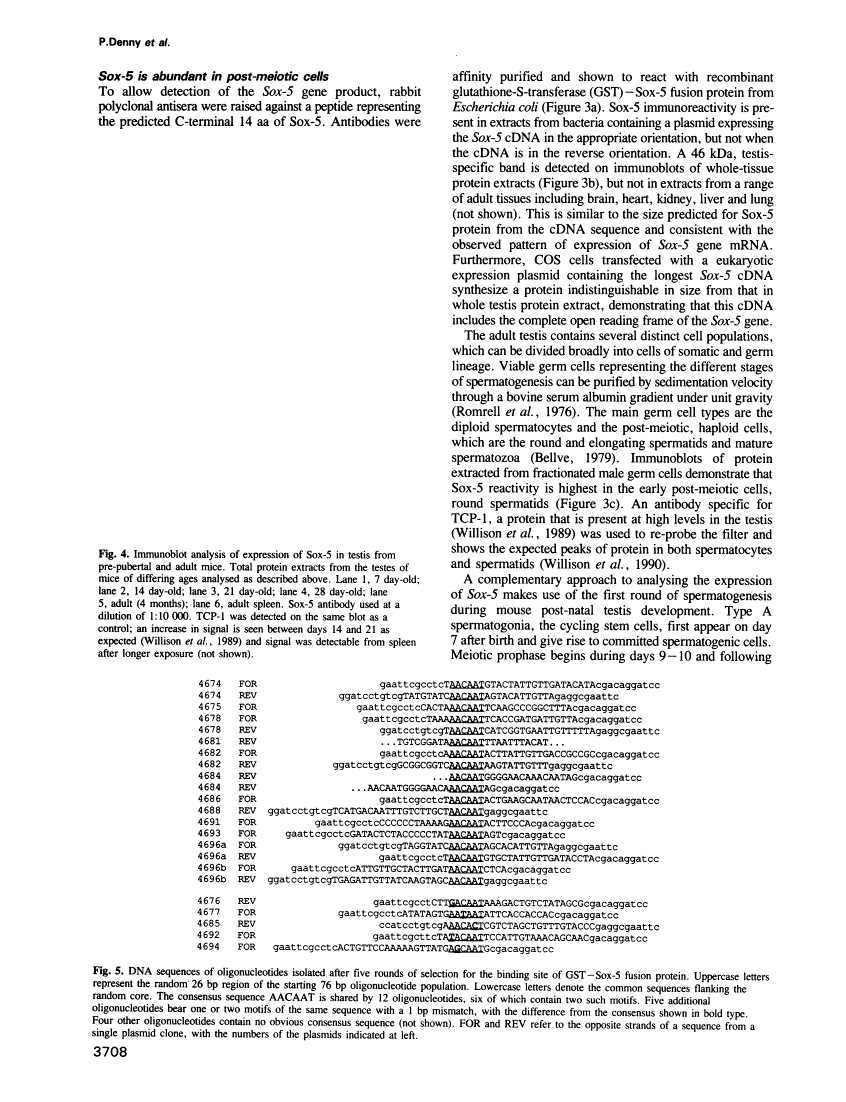
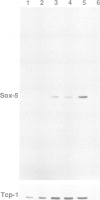
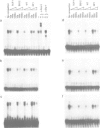
SRY, the testis determining gene, encodes a member of a family of DNA binding proteins characterized by an amino acid sequence motif known as the HMG box. Using degenerate primers and the polymerase chain reaction, we have isolated SRY-related cDNAs from adult murine testis RNA. One of these, Sox-5, encodes a 43 kDa HMG-box protein with similarities to transcription activating proteins. Anti-Sox-5 antibody was used to analyse expression of Sox-5 in pre-pubertal testis and in fractionated spermatogenic cells. Sox-5 is restricted to post-meiotic germ cells, being found at highest levels in round spermatids. Sox-5 is a DNA binding protein and binding site selection assays suggest that it can bind specifically to oligonucleotides containing the consensus motif AACAAT. Sry can also bind to this motif, indicating that the Sry family may have overlapping sequence specificities.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.7M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachvarov D, Moss T. The RNA polymerase I transcription factor xUBF contains 5 tandemly repeated HMG homology boxes. Nucleic Acids Res. 1991 May 11;19(9):2331–2335. [Europe PMC free article] [Abstract] [Google Scholar]
- Burgoyne PS, Buehr M, Koopman P, Rossant J, McLaren A. Cell-autonomous action of the testis-determining gene: Sertoli cells are exclusively XY in XX----XY chimaeric mouse testes. Development. 1988 Feb;102(2):443–450. [Abstract] [Google Scholar]
- Castrop J, van Norren K, Clevers H. A gene family of HMG-box transcription factors with homology to TCF-1. Nucleic Acids Res. 1992 Feb 11;20(3):611–611. [Europe PMC free article] [Abstract] [Google Scholar]
- Chittenden T, Livingston DM, Kaelin WG., Jr The T/E1A-binding domain of the retinoblastoma product can interact selectively with a sequence-specific DNA-binding protein. Cell. 1991 Jun 14;65(6):1073–1082. [Abstract] [Google Scholar]
- Chu G, Hayakawa H, Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. [Europe PMC free article] [Abstract] [Google Scholar]
- Denny P, Ashworth A. A zinc finger protein-encoding gene expressed in the post-meiotic phase of spermatogenesis. Gene. 1991 Oct 15;106(2):221–227. [Abstract] [Google Scholar]
- Denny P, Swift S, Brand N, Dabhade N, Barton P, Ashworth A. A conserved family of genes related to the testis determining gene, SRY. Nucleic Acids Res. 1992 Jun 11;20(11):2887–2887. [Europe PMC free article] [Abstract] [Google Scholar]
- Garnier J, Osguthorpe DJ, Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. [Abstract] [Google Scholar]
- Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Münsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990 Jul 19;346(6281):245–250. [Abstract] [Google Scholar]
- Harley VR, Jackson DI, Hextall PJ, Hawkins JR, Berkovitz GD, Sockanathan S, Lovell-Badge R, Goodfellow PN. DNA binding activity of recombinant SRY from normal males and XY females. Science. 1992 Jan 24;255(5043):453–456. [Abstract] [Google Scholar]
- Jantzen HM, Admon A, Bell SP, Tjian R. Nucleolar transcription factor hUBF contains a DNA-binding motif with homology to HMG proteins. Nature. 1990 Apr 26;344(6269):830–836. [Abstract] [Google Scholar]
- Jones N. Structure and function of transcription factors. Semin Cancer Biol. 1990 Feb;1(1):5–17. [Abstract] [Google Scholar]
- Kelly M, Burke J, Smith M, Klar A, Beach D. Four mating-type genes control sexual differentiation in the fission yeast. EMBO J. 1988 May;7(5):1537–1547. [Europe PMC free article] [Abstract] [Google Scholar]
- Koopman P, Münsterberg A, Capel B, Vivian N, Lovell-Badge R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature. 1990 Nov 29;348(6300):450–452. [Abstract] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991 May 9;351(6322):117–121. [Abstract] [Google Scholar]
- McLaren A. Sex determination in mammals. Trends Genet. 1988 Jun;4(6):153–157. [Abstract] [Google Scholar]
- Mermod N, O'Neill EA, Kelly TJ, Tjian R. The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell. 1989 Aug 25;58(4):741–753. [Abstract] [Google Scholar]
- Nasrin N, Buggs C, Kong XF, Carnazza J, Goebl M, Alexander-Bridges M. DNA-binding properties of the product of the testis-determining gene and a related protein. Nature. 1991 Nov 28;354(6351):317–320. [Abstract] [Google Scholar]
- Parisi MA, Clayton DA. Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science. 1991 May 17;252(5008):965–969. [Abstract] [Google Scholar]
- Pelletier J, Schalling M, Buckler AJ, Rogers A, Haber DA, Housman D. Expression of the Wilms' tumor gene WT1 in the murine urogenital system. Genes Dev. 1991 Aug;5(8):1345–1356. [Abstract] [Google Scholar]
- Pollock R, Treisman R. A sensitive method for the determination of protein-DNA binding specificities. Nucleic Acids Res. 1990 Nov 11;18(21):6197–6204. [Europe PMC free article] [Abstract] [Google Scholar]
- Romrell LJ, Bellvé AR, Fawcett DW. Separation of mouse spermatogenic cells by sedimentation velocity. A morphological characterization. Dev Biol. 1976 Mar;49(1):119–131. [Abstract] [Google Scholar]
- Rubin MR, Toth LE, Patel MD, D'Eustachio P, Nguyen-Huu MC. A mouse homeo box gene is expressed in spermatocytes and embryos. Science. 1986 Aug 8;233(4764):663–667. [Abstract] [Google Scholar]
- Shackleford GM, Varmus HE. Expression of the proto-oncogene int-1 is restricted to postmeiotic male germ cells and the neural tube of mid-gestational embryos. Cell. 1987 Jul 3;50(1):89–95. [Abstract] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990 Jul 19;346(6281):240–244. [Abstract] [Google Scholar]
- Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. [Abstract] [Google Scholar]
- Studier FW. Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. J Mol Biol. 1991 May 5;219(1):37–44. [Abstract] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. [Europe PMC free article] [Abstract] [Google Scholar]
- Treacy MN, He X, Rosenfeld MG. I-POU: a POU-domain protein that inhibits neuron-specific gene activation. Nature. 1991 Apr 18;350(6319):577–584. [Abstract] [Google Scholar]
- Travis A, Amsterdam A, Belanger C, Grosschedl R. LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor alpha enhancer function [corrected]. Genes Dev. 1991 May;5(5):880–894. [Abstract] [Google Scholar]
- van de Wetering M, Oosterwegel M, Dooijes D, Clevers H. Identification and cloning of TCF-1, a T lymphocyte-specific transcription factor containing a sequence-specific HMG box. EMBO J. 1991 Jan;10(1):123–132. [Europe PMC free article] [Abstract] [Google Scholar]
- Waterman ML, Fischer WH, Jones KA. A thymus-specific member of the HMG protein family regulates the human T cell receptor C alpha enhancer. Genes Dev. 1991 Apr;5(4):656–669. [Abstract] [Google Scholar]
- Wen L, Huang JK, Johnson BH, Reeck GR. A human placental cDNA clone that encodes nonhistone chromosomal protein HMG-1. Nucleic Acids Res. 1989 Feb 11;17(3):1197–1214. [Europe PMC free article] [Abstract] [Google Scholar]
- Willison K, Lewis V, Zuckerman KS, Cordell J, Dean C, Miller K, Lyon MF, Marsh M. The t complex polypeptide 1 (TCP-1) is associated with the cytoplasmic aspect of Golgi membranes. Cell. 1989 May 19;57(4):621–632. [Abstract] [Google Scholar]
- Willison KR, Hynes G, Davies P, Goldsborough A, Lewis VA. Expression of three t-complex genes, Tcp-1, D17Leh117c3, and D17Leh66, in purified murine spermatogenic cell populations. Genet Res. 1990 Oct-Dec;56(2-3):193–201. [Abstract] [Google Scholar]
- Wolgemuth DJ, Viviano CM, Gizang-Ginsberg E, Frohman MA, Joyner AL, Martin GR. Differential expression of the mouse homeobox-containing gene Hox-1.4 during male germ cell differentiation and embryonic development. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5813–5817. [Europe PMC free article] [Abstract] [Google Scholar]
- Giese K, Cox J, Grosschedl R. The HMG domain of lymphoid enhancer factor 1 bends DNA and facilitates assembly of functional nucleoprotein structures. Cell. 1992 Apr 3;69(1):185–195. [Abstract] [Google Scholar]
Associated Data
Articles from The EMBO Journal are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1002/j.1460-2075.1992.tb05455.x
Read article for free, from open access legal sources, via Unpaywall:
https://www.onlinelibrary.wiley.com/doi/pdfdirect/10.1002/j.1460-2075.1992.tb05455.x
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1002/j.1460-2075.1992.tb05455.x
Article citations
Biological functions and therapeutic potential of SRY related high mobility group box 5 in human cancer.
Front Oncol, 14:1332148, 21 May 2024
Cited by: 0 articles | PMID: 38835366 | PMCID: PMC11148273
Review Free full text in Europe PMC
mRNA expression profile reveals differentially expressed genes in splenocytes of experimental autoimmune encephalomyelitis model.
Int J Exp Pathol, 104(5):247-257, 10 Jul 2023
Cited by: 3 articles | PMID: 37427716
Coordinated microRNA/mRNA Expression Profiles Reveal Unique Skin Color Regulatory Mechanisms in Chinese Giant Salamander (Andrias davidianus).
Animals (Basel), 13(7):1181, 28 Mar 2023
Cited by: 1 article | PMID: 37048437 | PMCID: PMC10093658
Functional analysis of a SoxE gene in the oriental freshwater prawn, Macrobrachium nipponense by molecular cloning, expression pattern analysis, and in situ hybridization (de Haan, 1849).
3 Biotech, 10(1):10, 04 Dec 2019
Cited by: 6 articles | PMID: 31857938 | PMCID: PMC6892990
De novo gonad transcriptome analysis of the common littoral shrimp Palaemon serratus: novel insights into sex-related genes.
BMC Genomics, 20(1):757, 22 Oct 2019
Cited by: 9 articles | PMID: 31640556 | PMCID: PMC6805652
Go to all (169) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A gene that is related to SRY and is expressed in the testes encodes a leucine zipper-containing protein.
Mol Cell Biol, 15(7):3759-3766, 01 Jul 1995
Cited by: 46 articles | PMID: 7791783 | PMCID: PMC230614
Identification of two Sox17 messenger RNA isoforms, with and without the high mobility group box region, and their differential expression in mouse spermatogenesis.
J Cell Biol, 133(3):667-681, 01 May 1996
Cited by: 127 articles | PMID: 8636240 | PMCID: PMC2120827
A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2.
Development, 122(2):509-520, 01 Feb 1996
Cited by: 267 articles | PMID: 8625802
The molecular action and regulation of the testis-determining factors, SRY (sex-determining region on the Y chromosome) and SOX9 [SRY-related high-mobility group (HMG) box 9].
Endocr Rev, 24(4):466-487, 01 Aug 2003
Cited by: 110 articles | PMID: 12920151
Review
Funding
Funders who supported this work.