Abstract
Free full text

Contribution of non-neutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared to multigenic vaccines
Abstract
Previously, chronic phase protection against SHIV89.6P challenge was significantly greater in macaques primed with replicating adenovirus type 5 host range mutant (Ad5hr)-recombinants encoding HIVtat and env and boosted with Tat and Env protein compared to macaques primed with multigenic Ad-recombinants (HIVtat, HIVenv, SIVgag, SIVnef) and boosted with Tat, Env and Nef proteins. The greater protection was correlated with Tat and Env binding antibodies. As the macaques lacked SHIV89.6P neutralizing activity pre-challenge, we investigated whether antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cell mediated viral inhibition (ADCVI) might exert a protective effect. We clearly show that Tat can serve as an ADCC target, although the Tat-specific activity elicited did not correlate with better protection. However, Env-specific ADCC activity was consistently higher in the Tat/Env group, with sustained cell killing post-challenge exhibited at higher levels (p < 0.00001) for a longer duration (p = 0.0002) compared to the multigenic group. ADCVI was similarly higher in the Tat/Env group, and significantly correlated with reduced acute phase viremia at weeks 2 and 4 post-challenge (p = 0.046 and 0.011, respectively). Viral specific IgG and IgA antibodies in mucosal secretions were elicited but did not influence the outcome of the intravenous SHIV89.6P challenge. The higher ADCC and ADCVI activities seen in the Tat/Env group provide a plausible mechanism responsible for the greater chronic phase protection. As Tat is known to enhance cell-mediated immunity to co-administered antigens, further studies should explore its impact on antibody induction so that it may be optimally incorporated into HIV vaccine regimens.
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI) publisher of The JI, holds the copyright to this manuscript. This manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found at “www.jimmunol.org.”
Introduction
The HIV/AIDS pandemic remains a major public health concern and development of an efficacious vaccine is still the best option to combat the disease. To date, at least 33.2 million people worldwide are infected with HIV and on average, 2.5 million new infections occur every year (www.who.int/mediacentre/news/releases/2007/pr61/en/index.html). One of the strategies being evaluated for AIDS vaccine development uses viral vectors to deliver viral subunits to the immune system. Our vaccine approach is based on replication-competent Ad recombinants (1) which have been shown to elicit better and more persistent cellular immune responses and prime higher titered antibodies compared to non-replicating Ad recombinants encoding the same HIV gene products (2). The antibodies induced were able to neutralize primary isolates and showed antibody- dependent cellular cytotoxicity (ADCC) activity across HIV clades (3). In the rhesus macaque system, when replicating Ad-SIV recombinant priming was followed by envelope subunit protein boosting, the combination approach elicited potent protection against intrarectal challenge with virulent SIVmac251 (4). This protection was durable a year later without intervening vaccination (5).
The ultimate goal of AIDS vaccine research is to provide sterilizing immunity, presumably by induction of neutralizing antibodies (6) by candidate HIV envelope vaccines, thereby completely preventing HIV infection. Passive transfer studies utilizing monoclonal or polyclonal HIV neutralizing antibodies have provided complete or partial protection against homologous viral challenge in non-human primate models (7, 8). However, in clinical trials, neutralizing antibody responses against HIV gp120 have not provided sufficient protective efficacy, primarily due to the inherent variability of the HIV envelope. Structural studies of the HIV envelope may yet reveal an envelope design able to elicit broadly reactive antibodies able to neutralize across multiple HIV clades.
In the meantime, vaccine strategies are focused on limiting the initial viral burden during the acute phase of infection and lowering the viral set point during the chronic infection phase, thus reducing virus transmissibility and retarding disease progression. This strategy relies on induction of both humoral and cellular immune responses to a spectrum of HIV antigens. In addition to anti-envelope responses, antibodies to early HIV regulatory gene products, including Tat, Rev, and Nef, might also be expected to impact HIV acute infection. These antigens, in addition to Env and other viral structural proteins such as Gag, can also elicit cellular immune responses believed to exert greater control post-acute infection.
Tat, an early gene product, is a potent transactivator of HIV gene expression and is essential for viral infectivity and pathogenesis (9-12). Additionally, the Tat released from infected cells and taken up by other infected or uninfected cells is capable of multiple functions (13). It can promote viral replication, transactivate tat-defective or latent proviruses, modulate cellular gene expression, upregulate HIV co-receptors and induce or inhibit apoptosis (13-20). Thus, inhibition of Tat functions may be a good strategy to control viral replication. In fact, anti-Tat antibodies can inhibit uptake of extracellular Tat by surrounding cells, thus limiting HIV replication and transmission (21, 22). In natural infection, the presence of Tat-specific CTLs and/or anti-Tat antibody were associated with control of viral replication and slow progression to AIDS (22-28). These findings suggest that immunization with Tat-based vaccines may impact both the acute and chronic phases of subsequent HIV infection (29). Notably, a HIV Tat vaccine has been successfully tested in a human therapeutic phase I trial, providing the basis for moving forward with phase II studies (30).
Recently, we investigated an Ad5 host range mutant (Ad5hr)-HIVtat recombinant as a vaccine candidate in rhesus macaques (31), alone or in combination with Ad5hr-HIVenv, or together in a multigenic mixture including Ad5hr-HIVenv, SIV239gag and SIV239nef. After boosting with HIV Env, HIV Tat, and SIV Nef proteins, the animals were challenged intravenously with SHIV89.6P. The protocol design was based on the hypothesis that the envelope components, compared to a solely Tat-based vaccine, would enhance acute phase protection resulting from antibody induction, while the multigenic vaccine would enhance protection during the chronic phase due primarily to cellular immune responses. Unexpectedly, the macaques immunized with the Tat/Env regimen displayed the most potent protection, with a significant 4 log reduction in chronic viremia compared to controls, and a significant 1 log greater reduction in chronic viremia compared to the multigenic group. While the vaccines elicited cellular immunity, the significantly greater reduction of chronic phase viral load in the Tat/Env group compared to the multigenic group was correlated with Tat and Env binding antibodies (31).
Here, we investigated anti-envelope and anti-Tat antibody responses in greater depth in order to elucidate a possible basis for the better protection in the previous study. The pre-challenge anti-envelope antibodies did not neutralize the SHIV89.6P challenge virus. Neutralizing antibodies only developed post-challenge, exhibiting similar levels in both the Tat/Env and multigenic groups. Therefore, we examined other non-neutralizing, functional activities of envelope antibodies, including antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cell mediated viral inhibition (ADCVI). We also investigated whether anti-Tat antibodies mediate ADCC activity.
Materials and Methods
Vaccines, macaques and immunization and challenge regimen
This study makes use of sera, plasma, and secretory samples obtained from previously reported immunized and challenged macaques (31). The vaccine immunogens used for priming included Ad5 host range mutant (Ad5hr) recombinants separately encoding HIV89.6Pgp140ΔCFI (32), HIVIIIB tat(33), SIV239gag (34), and SIV239 nefΔ1-13 (35). The empty Ad5hrΔ E3 vector was used as control. Protein boosts included native HIVIIIB Tat (Advanced BioScience Laboratories, Inc. (ABL), Kensington, MD) HIV89.6P gp140ΔCFI protein, and SIV239 Nef (ABL).
Nineteen juvenile Mamu A*01-negative Indian rhesus macaques (Macaca mulatta) were housed at the Washington National Primate Research Center (WaNPRC, Seattle, WA) and maintained following the guidelines and protocols of the Animal Care and Use Committee, WaNPRC, University of Washington (Seattle,WA). Eight macaques were included in each immunization group: 4 females and 4 males in the Tat/Env group, and 5 females and 3 males in the multigenic group. The control group was made up of 2 females and one male. The previously published immunization and challenge schedule, vaccine dosages and routes of administration (31) are outlined in Fig. 1A
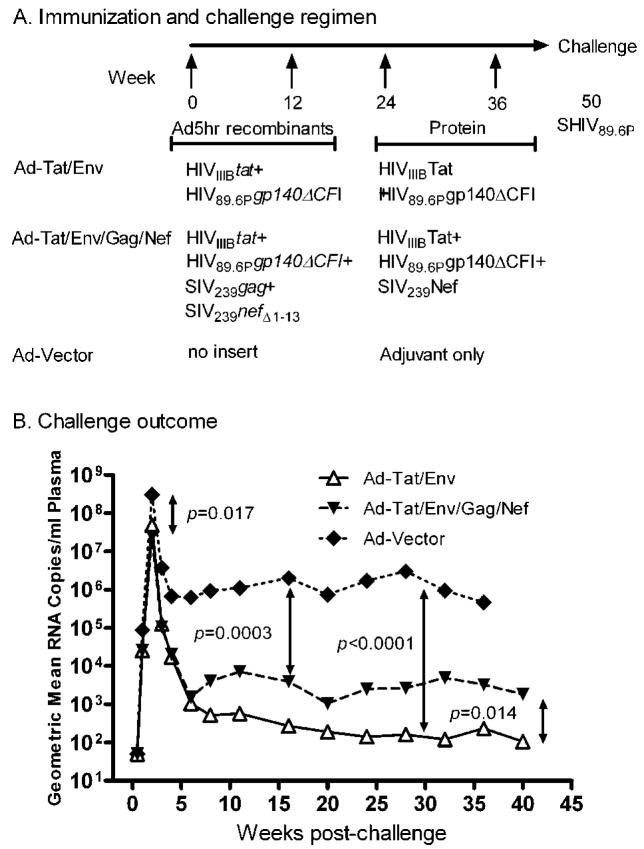
(A). Ad5hr recombinant dose: 5×108 PFU each, made up to a total Ad5hr dose of 2×109 PFU with Ad5hrΔ3 vector as necessary; week 0 given intranasally; week 12 given intratracheally; HIVIIIB Tat, 10 μg administered subcutaneously in alum; HIV89.6P gp140ΔCFI protein, 100 μg administered intramuscularly in MPL-SE; SIV239 Nef, 20 μg administered intramuscularly in MPL-SE. SHIV89.6P, 30 50% monkey infective doses administered intravenously in PBS. (B) Geometric mean plasma viremia following intravenous challenge with SHIV89.6P for the three immunization groups.
Sample collection
Plasma and sera were collected before and after challenge and stored at -70°C. Prior to use in functional assays, the samples were thawed at room temperature, diluted 10-fold with R-10 medium (RPMI 1640 containing 10% fetal calf serum, 2 mM L-glutamine and antibiotics), and heat inactivated at 56°C for 30 minutes.
Rectal and vaginal secretions were obtained as previously described (36) by gently swabbing the mucous membrane surfaces with cotton-tipped applicator sticks, after which the applicators were stored at -70°C in phosphate-buffered saline containing 0.1% BSA, 0.01% thimerosal, and 750 Kallikrein inhibitor units of aprotinin/ml. Any sample which contained blood was not analyzed.
Systemic binding antibody
Serum antibodies to HIV Tat, SHIV89.6P gp140, and SIV Nef were assessed by enzyme-linked immunosorbent assay (ELISA) as described previously (36). The titer of the antibody was defined as the serum dilution at which the optical density of the test serum was two times greater than that of the negative control naïve rhesus macaque serum diluted 1:50.
Mucosal binding antibody
Specific IgG and IgA antibodies in rectal and vaginal secretions were determined using a fluorescent bead-based flow cytometric assay (37). Briefly, 4 to 10 μg of each viral protein: HIV89.6P gp140, SIV239 Gag, SIV239 Nef, and HIVIIIB Tat, were covalently coupled to a specific carboxylated microsphere (bead) set (Bio-Rad Laboratories Inc. Hercules, CA; Luminex Corporation, Austin, TX). Each bead set is internally dyed with different ratios of two spectrally distinct fluorophores, making each bead set distinguishable by its fluorescent emission when excited by a laser. A mixture of the conjugated beads was deposited into wells of a 96-well filter plate. Following washing with PBS containing 0.1% Tween-20 (PBS-T), pre-diluted samples in 5% Blotto (5 gm nonfat dry milk in 100 ml PBS plus 0.05% Tween) were added to the wells and allowed to react for 1.5 hrs on a plate shaker in the dark at room temperature (RT). After incubation, the beads were washed 3 times in PBS-T and reacted with a reporter fluorescent antibody for 40 minutes on a plate shaker at RT while protected from light. After further washing and resuspension in PBS, the samples were read on a Bio-Plex array reader. Antibody titer is defined as the reciprocal of the sample dilution at which the mean fluorescent intensity (MFI) of 100 counted beads in the test sample was greater than that of the negative control sample (pre-immunization sample) diluted 1:40.
Total IgG and IgA antibodies in rectal and vaginal secretions were determined by enzyme-linked immunosorbent assay (ELISA) (36) with modifications as described here. Briefly, Maxisorp plates (Nalge Nunc, Rochester, NY) were coated with 100 μL of a 10 μg/mL solution of either affinity purified goat anti-monkey IgG or IgA (Kirkegaard and Perry Laboratories (KPL), Gaithersburg, MD) for one hour at 37°C in 5% CO2. Plates were washed five times with wash solution (KPL) and subsequently blocked with a 1:10 dilution of milk blocking solution (KPL). Serially diluted mucosal samples were added to the plate and incubated at 37°C with 5% CO2 for one hour. After washing, the plates were reacted with the corresponding secondary antibodies (KPL). Following intensive washes, the plates were developed for 15 min with 100 μL of TMB 2-component Microwell Peroxidase Substrate (KPL). The reaction was stopped using 100 μL 1M phosphoric acid and the absorbance was read at 630nm. Sets of monkey IgG or human secretory IgA standards were included on each plate. Concentrations of total IgG and IgA in test samples were determined based on standard curves of known antibody concentrations in the standards measured in the same plate.
The specific activity of rectal and vaginal IgG and IgA antigen-specific antibodies was calculated by dividing the antibody titer by the total IgG or IgA concentration. Results are reported as fold increase in specific activity compared to specific activity of antibody in the pre-immunization sample. Fold increases greater than 2 are considered positive.
ADCC
CEM-NKR cells (AIDS Research and Reference Reagent Program, NIAID) coated with HIV89.6P gp140 were used as targets for the rapid fluorometric ADCC (RFADCC) assay as described (37). To evaluate ADCC mediated by anti-Tat antibody, the optimal concentration of HIVIIIB Tat protein bound to the surface of CEM-NKR cells was first determined by immunofluorescence assay as described below. Subsequently, 10 μg of purified oxidized HIVIIIB Tat protein were coated onto 1×106 CEM-NKR cells. The cells were double stained with a membrane dye, PKH-26 (Sigma-Aldrich) and a viability dye, CFSE (Molecular Probes) prior to the addition of pre-diluted macaque plasma and human effector cells at a ratio of 50:1 E:T. A duplicate of fifty thousand non-gated events were acquired within 24 hours of the ADCC assay using a FACSCalibur flow cytometer (Beckton Dickinson) and CellQuest Software, setting FL1 as the CFSE emission channel and FL2 as the PKH-26 emission channel. Percent killing was determined by back-gating on the PKH-26 high population of targets and reported as the percentage of cells that lost the CFSE viability dye. Data analysis was done using WINMDI 2.9 software. Controls included were non-stained and single stained target cells. ADCC titers are defined as the reciprocal dilution at which the percent ADCC killing was greater than the mean percent killing of the negative controls plus 3 SDs.
ADCC mediated by Nef-specific antibodies was similarly evaluated using vaccinia-Nef-infected H9 cells. Briefly, H9 cells were infected with 1 MOI of Vaccinia-Nef (vNef157) (39), kindly provided by Tilahun Yilma, UC Davis, and incubated for an hour at 37°C in 5% CO2 with gentle agitation every 15 minutes. The cells were washed twice with R-10 and incubated for 18-24 hours under the same conditions before using as targets. Cell viability was confirmed using trypan blue staining, and SIV Nef surface expression was examined by immunofluorescence staining as described below.
Immunofluorescence microscopy
Viable CEM-NKR cells were enriched by Ficoll-Hypaque density centrifugation and washed with PBS. Cells (1×106) were coated with varying amounts of purified, oxidized HIVIIIB Tat protein, 10 μg of HIVIIIB gp120 or mock coated with R-10 medium for one hour at RT with gentle agitation every 15 minutes. Coated cells were washed twice with PBS, cytospun on Poly-L-lysine coated microscope slides (D Tekdon Inc., Myakka City, FL), fixed with 4% (vol/vol) paraformaldehyde in PBS for 15 minutes, washed with PBS and stained with rabbit anti-HIV Tat polyclonal serum (Cat. No. 705, NIH AIDS Research and Reference Reagent Program) or Tat monoclonal antibody, 1D9 (Cat. No. 7377; NIH AIDS Research and Reference Reagent Program). After 30 minutes incubation at RT and three washes with PBS, the cells were reacted for 30 minutes at RT with corresponding secondary antibodies conjugated to FITC ( goat anti-Rabbit IgG for polyclonal anti-Tat (Zymed Lab, California, USA; goat-anti-Mouse IgG for monoclonal anti-Tat (ImmunoPure, PierceBio) and with phalloidin-conjugated to Texas red (Molecular Probes, Invitrogen) for actin staining . Slides were washed and Vectashield with DAPI (Molecular Probes) was used as the mounting medium. Cells were imaged by confocal microscopy.
H9 cells infected for 24 hours with 0.1, 0.5 and 1 MOI of recombinant vaccinia-Nef were also analyzed for Nef surface expression. The infected cells were reacted with SIVmac251Nef monoclonal antibody 17.2 (AIDS Research and Reference Reagent Program, National Institutes of Health), washed with PBS and subsequently reacted with goat anti-Mouse IgG conjugated with FITC (ImmunoPure, PierceBio) and phalloidin-conjugated to Texas red. Cells were mounted and examined as above.
ADCVI
The ADCVI assay was based on methods previously described (40, 41). Briefly, human peripheral blood mononuclear cells (huPBMCs) which served as target cells were first stimulated with phytohemagglutinin (PHA, 2 μg/ml) and recombinant interleukin-2 (IL-2, 0.5 ng/ml) for 72 h, then washed and infected with 200 TCID50 of SHIV89.6P. After adsorption for 1 h, the huPBMCs were washed and incubated further at 37°C in 5% CO2 for 48 h in R-10. Infected target cells (5×104) were next plated onto wells of a 96-well round-bottom microtiter plate, and 1:100 dilutions of test plasma were added to the target cells along with huPBMC effector cells at an E:T ratio of 20:1. Plasma in the absence of effector cells was also tested. Target cells without plasma and effector cells were used as control. After 7 days incubation at 37°C in 5% CO2, supernatant fluids were collected and assayed for p27 by ELISA (ABL, Kensington, MD). Virus inhibition due to ADCVI was calculated as follows: percent inhibition = 100 × {[1 - ([p27E+]/[p27c])] - [1 - ([p27E-]/[p27c])]}, where [p27c] is the p27 concentration of control, [p27E+] and [p27E-] are the p27 concentration in the presence or absence of effector cells, respectively.
Statistical analysis
The Wilcoxon rank-sum test and Wei-Johnson method were used to analyze differences between binding, ADCC and ADCVI titers in experimental and control groups at all time points evaluated. Analyses of % ADCC killing and of differences in specific IgG and IgA antibodies in mucosal secretions of experimental and control macaques were conducted using repeated measures analysis of variance. Durations of enhanced ADCC killing in the two immunization groups following challenge were compared using the Cochran-Armitage test. The Spearman rank correlation coefficient test was used to determine the relationship between ADCVI activity and viral burden.
Results
Systemic envelope-specific ADCC
Previously, we reported that anti-envelope and anti-Tat antibodies were correlated with the better protection seen in macaques immunized with a Tat/Env vaccine regimen compared to a multigenic regimen (31). The vaccine-induced binding antibodies seen against SHIV89.6P envelope are summarized in Figure 2A. At week 48, two weeks prior to challenge, the Tat/Env immunization group exhibited significantly higher titers against SHIV89.6P gp140 (p = 0.020) compared to the multigenic group. Additionally, anti-envelope binding antibodies were also higher in the Tat/Env group over weeks 6 - 11 post-challenge compared to the multigenic group (p = 0.0044). While neutralizing antibodies against SHIV89.6P were not elicited prior to the intravenous challenge, a neutralizing response did appear quickly post-challenge in the immunized macaques. However, as previously reported, no significant differences in titer were observed between the two immunization groups (30). Therefore, here we explored other functional antibody activities as a possible basis for the better protection seen in the Tat/Env group. As we have previously seen a correlation of antibodies mediating ADCC with protection (42), we explored this functional activity first.
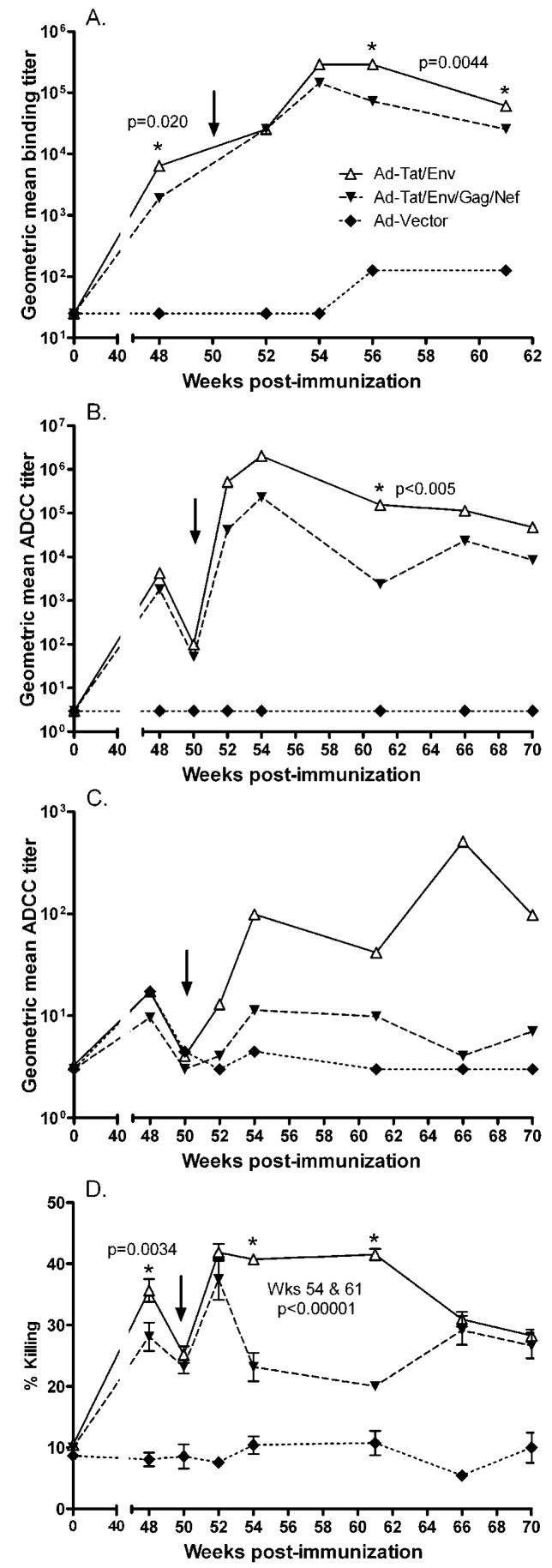
(A) Vaccine-induced binding antibody responses to HIV89.6P gp140 before and after challenge. Geometric mean titers for each immunization group are shown (from ref. 30). The arrow indicates time of intravenous SHIV89.6P challenge at week 50. (B) ADCC-mediating antibody titers in sequential plasma samples from immunized and control macaques against HIV89.6P gp140-coated targets. (C) ADCC-mediating antibodies in sequential plasma specimens from immunized and control macaques against H9 target cells infected with HIVIIIB. (D) Percent ADCC killing mediated by antibodies in plasma of immunized and control macaques using HIV89.6P gp140-coated targets.
ADCC activity of macaque plasma using SHIV89.6P gp140 coated targets was evaluated at week 48, 2 weeks prior to challenge, and at several time points out to 20 weeks following challenge (Fig. 2B). Both the Tat/Env and multigenic groups exhibited equivalent ADCC titers before and at the time of challenge, however, the Tat/Env group displayed a more pronounced anamnestic response 2 weeks post challenge. Higher ADCC titers were consistently seen in the Tat/Env group post-challenge from week 52 to week 70, although a statistically significant difference only occurred at week 61, 11 weeks post-challenge (p < 0.005 after correction for multiple times tested). Compared to the controls which consistently had undetectable ADCC activity, ADCC titers of the two vaccinated groups were significantly elevated at all time points evaluated (p <= 0.012 for the Tat/Env group and p <= 0.042 for the multigenic group by the Wilcoxon rank-sum test).
ADCC activity was also evaluated using heterologous HIVIIIB infected targets (Fig. 2C). A similar pattern was observed, although ADCC titers were much lower. The Tat/Env group maintained consistently higher ADCC titers compared to the multigenic group over weeks 52-70 (2 to 20 weeks post-challenge), although significant differences were not reached.
In addition to the titer of antibodies mediating ADCC activity, we also investigated the level of cell lysis (Fig. 2D). Using SHIV gp140 coated targets, the mean % ADCC killing at week 48 prior to challenge was significantly higher in the Tat/Env group (36%) compared to the multigenic group (28%) (p=0.0034; Fig. 2D). Equivalent anamnestic responses were seen 2 weeks post challenge (week 52) in both groups. However, elevated percent killing persisted in the Tat/Env group compared to the multigenic group, with significant differences in killing levels seen at weeks 54 and 61 (p<0.00001 after correction for multiplicity). The duration of the elevated response, defined by the time to return to percent killing levels at time of challenge, was significantly longer in the Tat/Env group (week 70 or later) than the multigenic group (week 61), p = 0.0002.
Systemic Tat-specific ADCC
All macaques in both immunized groups developed strong binding antibodies to Tat as published previously (30), and shown here (Fig. 3A). At two weeks prior to challenge (week 48), macaques in the Tat/Env group exhibited significantly higher Tat-specific binding antibodies compared to the multigenic group (p=0.028). Tat is expressed on the surface of about 6% of peripheral blood mononuclear cells (PBMC) obtained from HIV infected patients, as shown by immunostaining and flowcytometric analysis, and is also released and can be taken up by neighboring uninfected cells (43). Therefore we investigated whether anti-Tat antibodies induced by the vaccine regimens could mediate ADCC activity.

(A) Vaccine-induced binding antibody responses to HIV Tat before and after challenge (from ref. 30). The arrows indicate time of intravenous SHIV89.6P challenge at week 50. (B) Titers of ADCC-mediating antibodies in sequential plasma specimens from immunized and control macaques using HIV Tat-coated CEM-NKR cells as targets. (C). Percent ADCC killing mediated by antibodies in plasma of immunized and control macaques using HIV Tat-coated targets.
Initially, we explored whether H9 cells infected in vitro with HIVIIIB would serve as appropriate target cells. We observed a low percentage of cells expressing Tat protein on the cell surface by indirect immunofluorescence assay (IFA), similar to that reported for cells in HIV-infected individuals (432, and data not shown). As this low-level of positive cells would not provide sufficient sensitivity in the in vitro ADCC assay, we coated CEM-NKR cells with purified oxidized HIVIIIB Tat protein, ranging from 10 ng to 10 μg, and examined them by IFA using both monoclonal antibody 1D9 and a polyclonal anti-Tat antibody. A dose-dependent result was observed, with Tat cell surface expression varying from 0-1% (10 ng) to 99-100% (10 μg) with both antibodies (Table 1A). Mock coated control CEM-NKR cells were negative for Tat cell surface expression at all concentrations tested. Representative IFA results showing monoclonal antibody 1D9 staining of CEM-NKR cells coated with 10 μg HIVIIIB Tat or mock coated with R-10 alone are shown in Figure 4A-B. CEM-NKR cells coated with HIVIIIB gp120 and stained with monoclonal antibody 1D9 were also negative (data not shown).
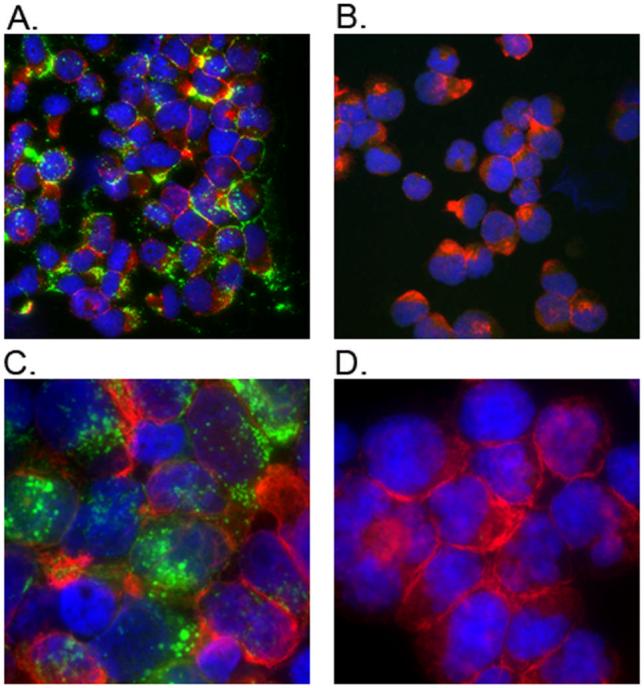
(A) CEM-NKR cells coated with 10 μg of HIVIIIB purified oxidized Tat protein, and (B) CEM-NKR cells mock coated with R-10, both stained with 1D9 anti-Tat monoclonal antibody. (C) H9 cells infected with vNef157, and (D) Mock infected H9 cells, both stained with 17.2 anti-Nef monoclonal antibody. Presence of Tat or Nef on the surface of the cells is shown in green (FITC); actin is shown in red (Texas red) and cell nuclei are shown in blue (DAPI).
Table I
Dose-dependent surface expression of HIV Tat-coated and vaccinia-SIV Nef-infected cells.
| Percent positive cells | |||||||
|---|---|---|---|---|---|---|---|
| A. Tat Protein | 10 ng | 100 ng | 500 ng | 1 μg | 5 μg | 10 μg | Mock |
| Tat MoAb 1D9 | 0-1% | 12-14% | 26-31% | 42-52% | 66-70% | 99-100% | Neg |
| Tat polyclonal serum | 0-1% | 10-14% | 32-36% | 47-58% | 59-62% | 100% | Neg |
| B. Vaccinia-Nef | 0.1 MOI | 0.5 MOI | 1.0 MOI | Mock | |||
| Nef MoAb 17.2 | 40-50% | 70-80% | 95-100% | Neg | |||
Using CEM-NKR cells coated with 10 μg Tat as targets, we analyzed plasma samples collected before and after challenge for Tat-specific ADCC activity (Fig. 3B). Results showed high titer ADCC activity for both the Tat/Env and multigenic groups two weeks after the second protein boost (week 38). The titer was maintained for the Tat/Env group until week 48, at which time a significant difference was observed compared to the multigenic group (p = 0.011) as well as to the controls (p = 0.0061). Both immunization groups exhibited low level Tat-specific ADCC activity at the time of challenge. The Ad-multigenic group displayed a rebound in Tat-specific ADCC titer at week 4 post-challenge (week 54), significantly higher than the Tat/Env group (p=0.014). Subsequent time points showed comparable ADCC titers between both immunization groups with no significant differences.
Similarly to the Env-specific ADCC activity, we also investigated whether differences existed in levels of cell lysis resulting from ADCC activity. As shown in Figure 3C, overall Tat-specific percent killing was lower in comparison to Env-specific ADCC percent killing (see Fig. 2D). Further, no significant differences were seen between the immunization groups at any time point except week 54 (4 weeks post-challenge), where the modestly elevated percent killing by the multigenic group reached a marginal significant difference compared to the Tat/Env group (p=0.047 after correction for multiplicity). This result coincided with the elevated ADCC titer exhibited by the multigenic group at the same time point (Fig. 3B).
Systemic Nef-specific ADCC
HIV Nef has been reported to be partially expressed on the surface of HIV-infected cells and to serve as a target for ADCC (44-47). Therefore, we established a system to explore Nef-specific ADCC activity. H9 cells were infected with Vaccinia-Nef (vNef157) (39). Eighteen to twenty-four hours later, the cells which were 90-95% viable by trypan blue staining were assessed for surface expression of Nef by IFA. A dose effect dependent on the vaccinia-Nef MOI was observed (Table 1B). Representative IFA results showing staining with Mab 17.2 of H9 cells infected with vNef157 with an MOI of 1 and mock infected H9 cells are shown in Fig. 4C, D. Subsequently, using 1 MOI of vaccinia-Nef to produce target cells, we analyzed the plasma of macaques from the Ad-multigenic group which showed Nef-specific binding antibodies (31). All samples were negative for ADCC activity at a 1:10 dilution, the lowest dilution tested (data not shown). The Tat/Env group was not immunized with Nef, and did not show detectable Nef binding antibodies as expected, so plasma from these macaques were not included in the analysis.
Systemic ADCVI
To explore possible effects of another non-neutralizing antibody function in the two immunization groups, we evaluated ADCVI activity in macaque plasma before and after challenge (Fig. 5A). A consistently higher ADCVI activity in the Tat/Env group compared to the multigenic group was seen at all time points analyzed. Nevertheless, only differences between the two groups at weeks 61 and 70 approached statistical significance (p values of 0.065 and 0.10, respectively). Notably, however, the Tat/Env immunization group exhibited a significant inverse correlation between ADCVI activity at the time of challenge (week 50) and peak acute viremia which occurred at week 2 post-challenge (r = -0.74, p = 0.046; (Fig. 5B). A stronger inverse correlation was also observed for week 50 ADCVI activity of the Tat/Env group and acute viremia 4 weeks post-challenge (r = -0.86, p = 0.011) (Figure 5C). The inverse correlation between the Tat/Env group ADCVI activity at time of challenge (week 50) and acute plasma viremia did not persist at later time points (weeks 58-70). No significant inverse correlation was observed between ADCVI activity and level of viremia in either the acute or chronic phase of infection for the multigenic group.

(A) Mean % ADCVI ± standard error of the mean. (B) Significant negative correlation between ADCVI activity at challenge (week 50) and week 2 viremia in the Ad-Tat/Env group. (C) Significant negative correlation between ADCVI activity at challenge (week 50) and week 4 viremia in the Tat/Env group.
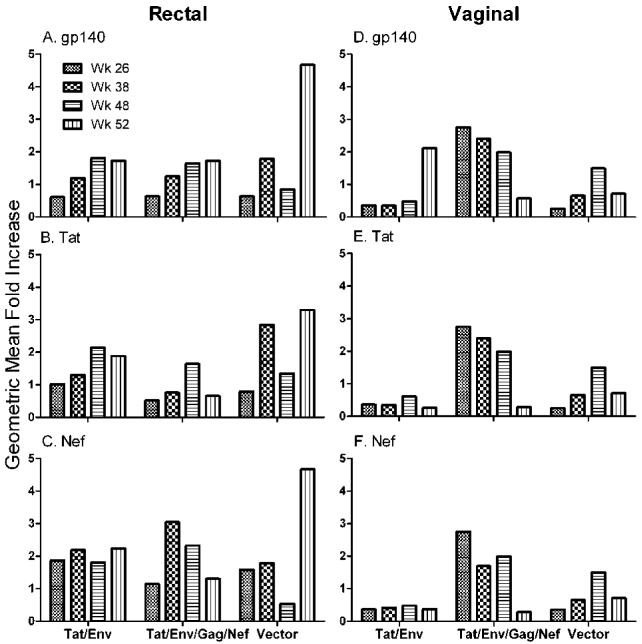
Mucosal humoral immune responses
Although in this study the SHIV89.6P challenge was administered intravenously, we considered that vaccine-elicited mucosal antibodies might have contributed to control of viral spread within the gastrointestinal tract. Therefore, we evaluated induction of mucosal antibodies by the vaccination regimens. IgG binding antibodies were first assayed in rectal secretions, as well as in vaginal secretions of the female macaques (Fig. 6). Induction of HIV gp140-specific IgG was initially detected in both the Tat/Env and multigenic groups at week 26 after the first protein boost and was strongly enhanced by week 38 after the second protein boost in both rectal and vaginal secretions (Fig. 6A, D). Negligible levels were seen shortly before challenge (week 48), but good anamnestic responses were observed two weeks post challenge (week 52). Significant differences in antibody levels were not observed at any time point between the two immunization groups. Anti-envelope IgG antibodies were not seen in secretions of control macaques.

Titers of each sample are normalized to total IgG present in the sample. Results are expressed as fold increases in antibody specific activity compared to the specific activity of a pre-immunization sample from the same animal.
The HIV Tat-specific IgG antibodies exhibited the same pattern prior to challenge, although the IgG level detected in the vaginal secretions was higher than that seen in the rectal secretions of both immunized groups (Fig. 6B, E). No difference in IgG level was observed between the vaccinated groups at all time points analyzed, and again, secretions of control macaques were antibody negative. In contrast to the strong envelope-specific anamnestic response observed in secretions of both immunization groups, only low-level increases in Tat antibody were detected post-challenge (week 52).
For completeness, we also evaluated mucosal secretions for Nef and Gag-specific IgG antibodies. As expected since only the multigenic group received Nef immunogens, SIV Nef-specific antibodies of IgG isotype were observed only in rectal and vaginal secretions of the multigenic group (Fig. 6C, F). As with the envelope specific responses, a boost in specific antibody was observed at week 38 after the second protein immunization. Further, a strong anamnestic Nef-specific response was observed post-challenge. The secretions of control macaques were negative. Neither the Tat/Env nor the multigenic group was boosted with Gag protein. Consequently, rectal and vaginal secretions of all macaques at the time points evaluated were negative for anti-Gag responses (data not shown).
The level of specific antibodies of IgA isotype induced in rectal and vaginal secretions of all the macaques rarely reached the positive cut-off value of a two-fold increase, and no significant differences were observed between the Tat/Env and multigenic immunization groups. (Fig. 7A-F). The strongest antibody responses were seen post-challenge in the control group which exhibited Env, Tat, and Nef-specific IgA antibodies.
Titers of each sample are normalized to total IgA present in the sample. Results are expressed as fold increases in antibody specific activity compared to the specific activity of a pre-immunization sample from the same animal.
Overall, as significant differences between the Tat/Env and multigenic groups were not observed in either IgG or IgA mucosal binding antibodies, further studies for functional activity were not conducted.
Discussion
Antibodies play a pivotal role in protecting against viral infections and if present as a result of prior infection or vaccination act as a rapid first line of defense against invading pathogens. Neutralizing antibodies can block viral entry, thus preventing infection, and can limit the spread of infection by blocking cell-to-cell viral transmission. In the macaques studied here, no neutralizing activity to SHIV89.6P was detected in sera of the immunized macaques prior to challenge (31). Further, the post-challenge neutralizing antibodies that developed exhibited similar levels between immunization groups. Therefore, here we examined other non-neutralizing, functional antibody activities in order to elucidate the basis for the correlation of binding antibody with the significantly better protection exhibited by macaques in the Tat/Env group.
ADCC is a potent immune mechanism that bridges innate and adaptive immune responses. It can eliminate cells expressing viral antigens on the cell surface via FcγR-bearing effector cells, targeted by specific antibody bound to the FcγR. Antibodies mediating ADCC are one of the first immune responses to appear following HIV/SIV infection, often preceding neutralizing antibody (48, 49). In HIV-infected patients and SIV-infected rhesus macaques, ADCC activity has been associated with reduced viremia, slow disease progression and better clinical outcome (42, 450-54). Among patients with progressive or late stage disease, declining or low ADCC titer has been associated with low CD4 counts (55-58). A protective role for ADCC activity has not been established, however. Some HIV patients have not shown any correlation between ADCC activity and disease progression (59, 60). Further, passive transfer of non-neutralizing, ADCC-mediating IgG to neonatal rhesus macaques did not protect against SIVmac251 oral challenge, although several variables might have affected the outcome (61).
Here, envelope-specific ADCC titers exhibited by the Tat/Env group were consistently higher compared to the multigenic group at all time points evaluated using gp140 coated targets. Moreover, the ADCC titer was significantly elevated at week 11 post challenge (p < 0.005). ADCC titers against heterologous HIV-1 IIIB infected target cells were also higher compared to the multigenic group at all post-challenge time points evaluated. Importantly, when ADCC activity was evaluated based on level of cell lysis rather than titer, the Tat/Env group exhibited significantly greater killing just prior to challenge (p = 0.0034) and over weeks 4 to 11 post-challenge (p < 0.00001) compared to the multigenic group. (Fig. 2D). Further, the elevated killing level of the Tat/Env group was maintained longer post-challenge compared to the multigenic group (p = 0.0002). These results are consistent with the higher Envelope-specific binding antibody titers induced in the Tat/Env macaques prior to and post challenge (Fig. 2A). Further, they suggest a mechanistic basis for the significantly better chronic-phase protection (Fig. 1B) displayed by the Tat/Env-immunized macaques.
To date, HIV antigens reported to serve as targets for ADCC include the transmembrane (gp41) and extracellular (gp120) envelope proteins (62, 63), and Nef (44-47). Here, Nef-specific antibodies did not mediate killing of target cells infected with vaccinia-Nef. It may be that the Nef epitope expressed on the surface of HIV-infected cells is not present on the surface of cells infected with vaccinia-Nef. Unfortunately, Nef was not expressed on the surface of a sufficiently high percentage of SIV-infected cells to allow their use in the RFADCC assay.
Tat has also been reported to be expressed on the cell surface (43). Further, Tat antibodies have been associated with control of viral replication and slow progression to AIDS (22, 24, 26, 28, 64, 65) though others have reported no correlation between Tat antibodies and disease progression (66). Here we investigated whether anti-Tat antibodies induced in the immunized macaques could mediate killing of Tat-expressing target cells by the ADCC mechanism. Our results show clearly for the first time that Tat serves as target for ADCC and that vaccine induced Tat-specific antibodies in the macaque plasma mediate ADCC killing. The Tat/Env group showed higher Tat specific ADCC activity prior to challenge (week 48, p= 0.011) compared to the multigenic group, but this was not maintained post-challenge, and thus likely did not contribute to better chronic phase protection of the Tat/Env group. The higher Tat-specific ADCC anamnestic response at week 54 of macaques in the multigenic group compared to Tat/Env group (p=0.014) was not maintained at later time points. Based on the timing of this response, it did not seem to influence differences in acute or chronic phase viremia between the two immunization groups.
Whether Tat-mediated ADCC activity impacts natural infection is not known. Although the low level of Tat cell surface expression on infected cells is not sufficient for detection of ADCC activity in the RFADCC assay in vitro, they might nevertheless serve as in vivo targets. Further, in natural infection, the soluble Tat released from infected cells that binds to neighboring infected and uninfected cells may facilitate Tat-specific ADCC-mediated “bystander killing”. This potentially deleterious effect of anti-Tat antibodies could be prevented if high-titer vaccine induced anti-Tat antibodies were present prior to infection and able to eliminate newly-infected, Tat-expressing cells. Alternatively, the anti-Tat antibodies might simply bind the released Tat and clear it, preventing its absorption to bystander cells. These possibilities should be further explored.
ADCVI, like ADCC, depends on interactions between antibody and FcγR-bearing effector cells. However, the readout is not target cell lysis but inhibition of viral replication, mediated not only by ADCC but also soluble antiviral factors secreted by activated NK cells (40, 67) and other cell types, or by FcγR-mediated phagocytosis (68). ADCVI activity has been associated with reduction in viremia during acute HIV infection, a reduced rate of HIV infection, and protection in neonatal rhesus macaques passively infused with non-neutralizing antibody (40, 69, 70). Here, antibody titers mediating ADCVI activity of the Tat/Env group were consistently higher, though not significantly so, than those of the multigenic group at all time points analyzed pre- and post-challenge. Notably, ADCVI activity of the Tat/Env group at the time of challenge (week 50) was inversely correlated with acute viremia at weeks 2 (r = -0. 74; p = 0.046) and 4 (r = -0.86; p = 0.011) post-challenge (Fig. 5B, C), but not at later time points. Although this ADCVI activity was not directly associated with the stronger protection seen in the Tat/Env group during chronic infection, its impact on the initial viral burden perhaps facilitated chronic phase viremia control by other mechanisms. Overall, correlations of viral loads with ADCC killing were weaker than with ADCVI (not shown). This may reflect the several mechanisms, including ADCC, which can contribute to ADCVI activity, perhaps providing a greater impact on acute viremia levels. The significant differences in both % ADCC killing and viral loads between the two immunization groups were seen during the chronic phase of infection when additional immune responses such as viral-specific CD8 T cell activity likely impacted viremia control. In this circumstance, a direct correlation between viral loads and ADCC killing would be difficult to discern, in spite of the significantly higher, sustained ADCC activity in the Tat/Env group.
Taken together, our data indicate that non-neutralizing antibody activities had a significant impact on the challenge outcome, resulting in better chronic phase protection of the macaques immunized by the Tat/Env regimen. Anti-envelope antibody played a direct role by mediating Env-specific ADCC which exhibited sustained, higher titered activity during the chronic phase in the Tat/Env immunized animals. ADCVI was also enhanced in the Tat/Env immunization group, and correlated significantly with better acute phase protection. However, it cannot be attributed to any particular antigen, although Env is certainly involved. These results are consistent with the overall higher antibody titers elicited in the Tat/Env immunized macaques. What remains to be determined is the mechanism leading to improved antibody induction in this group of macaques. Tat is known to enhance and broaden cellular immune responses to co-administered antigens including Gag and Env (33, 71). Whether it similarly enhances antibody induction is unknown. Here, as both immunization groups included Tat, perhaps competition in the multigenic group diminished overall antibody responses. Whether antibody avidity was somehow increased in the Tat/Env group as compared to the multigenic group is not known. As this parameter could have affected ADCC and ADCVI activities, it should be explored in the future. It will be important to investigate all such possibilities, in order to make the best use of the Tat immunogen in vaccine strategies.
Acknowledgments
We thank Tilahun Yilma, University of California, Davis, for providing the vaccinia-Nef construct, vNef157 and James McNally and Tatiana Karpova , NCI, NIH, Bethesda, MD, for assistance with imaging. The following reagents were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: CEM-NKr cells from Dr. Peter Cresswell; Rabbit anti-HIV Tat polyclonal serum, #705, from Dr. Bryan Cullen; Tat monoclonal antibody 1D9, #7377, from Dr. Dag E. Helland; SIV Nef monoclonal antibody 17.2 from Quattromed Ltd.
This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute and by NIH, NIAID, Simian Vaccine Evaluation contract N01-AI-15431 to the University of Washington.
Abbreviations used in this paper
| ADCC | Ab-dependent cellular cytotoxicity |
| ADCVI | Ab-dependent cell-mediated virus inhibition |
| Ad | adenovirus |
| Ad5hr | Ad type 5 host range mutant |
| RFADCC | rapid fluorometric ADCC assay |
| MOI | multiplicity of infection |
References
Full text links
Read article at publisher's site: https://doi.org/10.4049/jimmunol.0803115
Read article for free, from open access legal sources, via Unpaywall:
https://journals.aai.org/jimmunol/article-pdf/182/6/3718/1277046/zim00609003718.pdf
Free after 12 months at www.jimmunol.org
http://www.jimmunol.org/cgi/content/full/182/6/3718
Free to read at www.jimmunol.org
http://www.jimmunol.org/cgi/content/abstract/182/6/3718
Free after 12 months at www.jimmunol.org
http://www.jimmunol.org/cgi/reprint/182/6/3718
Citations & impact
Impact metrics
Citations of article over time
Article citations
An Adenovirus-Based Recombinant Herpes Simplex Virus 2 (HSV-2) Therapeutic Vaccine Is Highly Protective against Acute and Recurrent HSV-2 Disease in a Guinea Pig Model.
Viruses, 15(1):219, 13 Jan 2023
Cited by: 3 articles | PMID: 36680259 | PMCID: PMC9861952
Decoding human-macaque interspecies differences in Fc-effector functions: The structural basis for CD16-dependent effector function in Rhesus macaques.
Front Immunol, 13:960411, 05 Sep 2022
Cited by: 10 articles | PMID: 36131913 | PMCID: PMC9484259
Broadly binding and functional antibodies and persisting memory B cells elicited by HIV vaccine PDPHV.
NPJ Vaccines, 7(1):18, 09 Feb 2022
Cited by: 2 articles | PMID: 35140230 | PMCID: PMC8828892
Distinct antibody profiles in HLA-B∗57+, HLA-B∗57- HIV controllers and chronic progressors.
AIDS, 36(4):487-499, 01 Mar 2022
Cited by: 2 articles | PMID: 34581307 | PMCID: PMC8876439
ADCC-mediating non-neutralizing antibodies can exert immune pressure in early HIV-1 infection.
PLoS Pathog, 17(11):e1010046, 17 Nov 2021
Cited by: 9 articles | PMID: 34788337 | PMCID: PMC8598021
Go to all (106) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques.
J Virol, 84(14):7161-7173, 05 May 2010
Cited by: 129 articles | PMID: 20444898 | PMCID: PMC2898229
A replication-competent adenovirus-human immunodeficiency virus (Ad-HIV) tat and Ad-HIV env priming/Tat and envelope protein boosting regimen elicits enhanced protective efficacy against simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques.
J Virol, 81(7):3414-3427, 17 Jan 2007
Cited by: 69 articles | PMID: 17229693 | PMCID: PMC1866031
Vectored Gag and Env but not Tat show efficacy against simian-human immunodeficiency virus 89.6P challenge in Mamu-A*01-negative rhesus monkeys.
J Virol, 79(19):12321-12331, 01 Oct 2005
Cited by: 56 articles | PMID: 16160159 | PMCID: PMC1211517
Combination Adenovirus and Protein Vaccines Prevent Infection or Reduce Viral Burden after Heterologous Clade C Simian-Human Immunodeficiency Virus Mucosal Challenge.
J Virol, 92(2):e01092-17, 02 Jan 2018
Cited by: 20 articles | PMID: 29093095 | PMCID: PMC5752948
Funding
Funders who supported this work.
Intramural NIH HHS (2)
Grant ID: Z01 BC005536-21
Grant ID: Z99 CA999999
NIAID NIH HHS (1)
Grant ID: N01AI15431




