Abstract
Free full text

Transmembrane mucins as novel therapeutic targets
Abstract
Membrane-tethered mucin glycoproteins are abundantly expressed at the apical surfaces of simple epithelia, where they play important roles in lubricating and protecting tissues from pathogens and enzymatic attack. Notable examples of these mucins are MUC1, MUC4 and MUC16 (also known as cancer antigen 125). In adenocarcinomas, apical mucin restriction is lost and overall expression is often highly increased. High-level mucin expression protects tumors from killing by the host immune system, as well as by chemotherapeutic agents, and affords protection from apoptosis. Mucin expression can increase as the result of gene duplication and/or in response to hormones, cytokines and growth factors prevalent in the tumor milieu. Rises in the normally low levels of mucin fragments in serum have been used as markers of disease, such as tumor burden, for many years. Currently, several approaches are being examined that target mucins for immunization or nanomedicine using mucin-specific antibodies.
Apical mucosal surfaces are dominated by a thick forest of high-molecular-weight, heavily glycosylated proteins collectively referred to as mucins. Mucin glycoproteins are characterized as proteins heavily substituted with O-linked oligosaccharides, most often in tandem repeat domains rich in serine, threonine and proline residues. The resulting molecules are extremely hydrophilic with physically extended structures supported by the large hydration sphere of the oligosaccharides as well as the structural rigidity conferred by proline residues. At least 21 mucin genes exist in humans [1]. Mucins can be subdivided into two classes: secreted or gel-forming, and cell surface or trans-membrane [2]. The strict classification of the latter is complicated by the occurrence of splice variants that generate secreted forms [3,4], as well as post-translational processing events that cleave the protein core, separating the mucin domains from their membrane anchors [5–9]. Nonetheless, it appears that the membrane-anchored forms of these gene products are the predominant forms in most situations. The current article will focus on three complex, transmembrane mucins, MUC1, MUC4 and MUC16. The unique features and functions of each mucin will be discussed, as well as the opportunities these molecules present as novel therapeutic targets.
Mucin structure & synthesis
The generic structures of transmembrane mucins consist of a very large extracellular domain dominated by tandem repeat motifs of sequences that function as acceptors of O-linked oligosaccharides, a single, short transmembrane domain and a relatively small cytoplasmic tail. Furthermore, as discussed later, the MUC4 and MUC16 extracellular domains have a number of other structural features. Mucin mRNAs encode N-terminal signal sequences and are translated in the rough endoplasmic reticulum where core N-glycosylation occurs; however, most of the glycosylation reactions of mucin-type oligosaccharides occur in the Golgi apparatus [10,11]. Mucin-type oligosaccharides vary in size, being as small as one saccharide unit (approximately 200 MW) or substantially larger (Figure 1C). Mucin ectodomains typically contain hundreds of these oligosaccharides, giving them a very large and heterogeneous size distribution. The oligosaccharides hydrate and contribute to the lubricating and generally anti-adhesive functions of these molecules. Nonetheless, certain sialic acid and sulfation modifications can occur that generate siglec and selectin ligands, thereby creating adhesion-promoting motifs [12,13]. The oligosaccharides may also serve as binding sites for bacteria [14,15]. It is not clear if different mucin glycoproteins expressed by the same cell express distinct profiles of mucin oligosaccharides; however, there is no evidence to indicate that the tandem motifs present in different mucin core proteins are differentially glycosylated. The glycomic analyses done to date focus on total glycan profiles from a given mucin or mucin fragment. It remains possible that glycan structural differences occur at specific sites or in response to physiologic stimuli, such as hormones or cytokines.
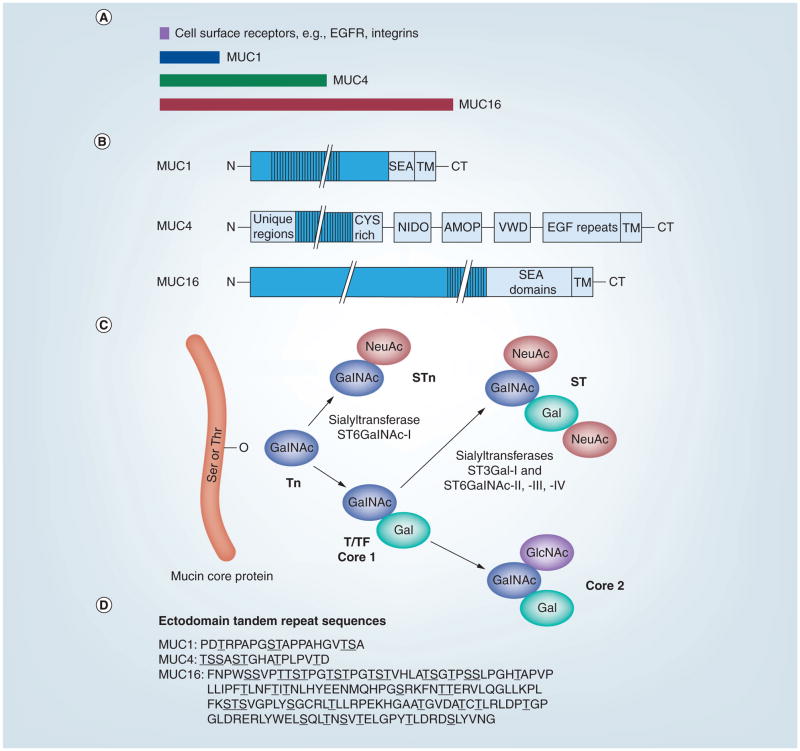
(A) The sizes of typical cell surface receptors, such as intergrins and EGFR, relative to those of MUC1, MUC4 and MUC16 are shown. (B) Domain organization of MUC1, MUC4 and MUC16. (C) Cancer-associated antigens (Tn, STn and T/TF) result from aberrant O-glycosylation on the ectodomains of MUC1, 4 and 16. O-linked glycosylation is initiated by adding GalNAc to the protein backbone, resulting in the Tn antigen. NeuAc of the GalNAc moiety results in the formation of the STn antigen. Alternatively, core 1 structures, T and TF, are synthesized by the addition of Gal to the Tn antigen. Non-cancer-associated antigens are formed from either the sialylation of the core 1 structures, resulting in the mono- or disialyl-T antigen, or the continued branching/elongation by adding GlcNAc to form the core 2 structures. Further elongation of the core 2 structure forms more complex peripheral antigens, such as the variations of Lewis and Sialyl–Lewis. (D) O-glycosylation occurs on either a Ser or Thr residue within the tandem repeat sequences of the mucin ectodomains. The consensus sequences for the tandem repeats in human MUC1, MUC4 and MUC16 are shown using the single letter amino acid code. Serine and threonine residues are underlined. Note the large number of proline residues in these motifs.
AMOP: Adhesion-associated domain; CT: Cytoplasmic tail; CYS rich: Cysteine-rich domain; EGF repeats: EGF-like repeat region; EGFR: EGF receptor; Gal: Galactose; GalNAc: N-acetylgalactosamine; GlcNAc: N-acetylglucosamine; MUC: Mucin; NeuAc: Sialylation; NIDO: Nidogen-like domain; SEA: Sea urchin sperm receptor enterokinase-agrin domain; Ser: Serine; ST: Disialyl-T; STn: Sialyl-Tn; T/TF: Thomsen–Friedenreich antigen; Thr: Threonine; TM: Transmembrane domain; Tn: Precursor to T antigen; VWD: von Willebrand factor type D domain.
Excellent, detailed reviews on the membrane-anchored mucins are available [2,16]. A comparison of the relative sizes of mucin core proteins and major structural features is shown in Figures 1A & 1B. The majority of MUC1’s extracellular domain consists of a series of 20–21-amino-acid tandem repeats. In humans, the number of these repeats varies (25–125) in different MUC1 alleles, accounting for allelic polymorphism and different sizes of the mature glycoproteins ranging from 250 kDA to >1 MDa [17]. In addition, splice variants and differential glycosylation contribute to polymorphisms in mature MUC1 [18]. Other regions of the ecto-domain are also heavily O-glycosylated. Near the transmembrane region is a sperm protein, enterokinase and agrin domain containing an autocleavage site. Although this autocleavage appears to be quite efficient, the two resulting MUC1 subunits remain tightly associated [5,6]. MUC1’s C-terminal region consists of a highly conserved 72-amino-acid cytoplasmic tail region with many proposed functions (see Table 1 and discussion later).
Table 1
Membrane-bound mucins.
| Function | MUC1 | MUC4 | MUC16 | Ref. |
|---|---|---|---|---|
| Ectodomain | ||||
| Anti-adhesive | + | + | ? | [20–21] |
| Proadhesive | + | ? | + | [22,24–25] |
| Lubrication | + | + | + | Reviewed in [2] |
| ERBB signaling | + | + | ? | Reviewed in [16,26,38] |
|
| ||||
| Cytoplasmic tail | ||||
| Phosphorylation sites | + | Potential | Potential | Reviewed in |
| Signal transduction | + | ? | ? | [16,43] |
| Transcriptional regulation | + | ? | ? | |
+: Known to be present; ?: Unknown.
MUC4 is substantially larger and more complex than MUC1, consisting of a complex of two subunits generated from a single polypeptide precursor [7]. The N-terminal α subunit ranges in size from 3000 to 7300 amino acids and contains a series of imperfect repeats, an O-glycosylated region of 145–395 variable number tandem 16 amino acid repeats, a unique sequence, a cysteine-rich domain, a nidogen homology domain (NIDO), an adhesion-associated domain (AMOP) and a von Willebrand factor D sequence (VWD) prior to its proteolytic cleavage site. The C-terminal β subunit following the proteolytic cleavage site contains 1156 amino acids and consists of an N-glycosylated region containing three EGF-like domains, a single transmembrane spanning sequence and a 22-amino-acid cytoplasmic tail.
MUC16 is by far the largest of the group, with an overall core protein size of approximately 22,000 amino acids (ca. 2.5 MDa). The N-terminal region consists of a very large, heavily O-glycosylated region, a region of more than 60–156 amino acid tandem repeats carrying both N- and O-linked oligosaccharides, and a membrane proximal region containing 16 sperm protein, enterokinase and agrin domains. Characterization of the N- and O-linked oligo-saccharides associated with MUC16 (cancer antigen [CA] 125) isolated from an ovarian cancer cell line has been reported, with both classes containing some interesting structural features [19]. The much smaller C-terminal region contains a single transmembrane region followed by a 32-amino-acid cytoplasmic tail.
Functions
A major, shared function of the ectodomains of these membrane-tethered mucins is to hydrate and lubricate cell surfaces. The ecto-domains of MUC1 and MUC4 are also anti-adhesive, a function that requires a critical, minimal number of tandem repeats to afford sufficient steric hindrance to cell surface receptors [20,21]. As mentioned previously, MUC1 can also carry selectin ligands and, therefore, may support cell attachment in some instances [22]. An anti-adhesive function for MUC16 also seems likely since like MUC1, MUC16 inhibits synapse formation between natural killer cells and their targets [23]. Interestingly, MUC16 is proposed to bind both Siglec-9 [24] and mesothelin [25] and, therefore, may support cell attachment in certain scenarios. Other functions are presented in Table 1 and the discussion after.
MUC1 and MUC4 also complex with members of the ERBB family of receptors and modulate signal transduction mediated by these receptors (reviewed in [16,26]). In MUC1, this interaction requires the presence of the cytoplasmic tail, which becomes tyro-sine phosphorylated upon ERBB ligand addition. In MUC4, the interaction appears to involve its EGF-like domains acting like chaperones as well as weak receptor ligands. The cytoplasmic tail of MUC1 is also involved in a large array of other signal transduc-tion events including modulation of gene transcription, complexing with various cytoplasmic signaling proteins and protection of cells from apoptosis (Table 1; reviewed in [27]).
Interesting, yet still poorly understood, are the effects of trans-membrane mucins and their shed fragments on the immune system. Vasir et al. demonstrated that MUC1 is not only induced in T cells by IL-7, but also polarizes at the site of T cell–dendritic cell synapses [28]. In other studies, these mucins inhibit immune recognition [28–30], apparently by sterically inhibiting access to the cell surface, and/or can be immunosuppressive [31,32]. Thus, these mucins contribute to complex, protective functions with regard to the immune system that, in the context of tumors, can protect the tumor cells from host immune surveillance.
MUC1-null mice have been created with phenotypes including slower growth of T-antigen-induced mammary tumors and increased susceptibility to infection/inflammation [33,34], although at least some of the latter responses are dependent on the genetic background of the mice used [35]. MUC16-null mice were recently created with no obvious phenotype, at least with regard to fertility and embryo/fetal development [36]. Interestingly, some reduction in MUC1 mRNA was evident in MUC16-null uteri, indicating that increased expression of MUC1 did not compensate for loss of MUC16. Mice double null for MUC1 and MUC16 have not been reported, and neither have MUC4-null mice.
Regulation of mucin expression
Membrane-tethered mucins demonstrate a tissue-specific distribution, suggesting a controlled regulation of their expression. Most epithelial cells produce mucins, and relative amounts of MUC1, MUC4 and MUC16 may vary depending on cell and tissue type [16,37].
Tissue distribution
MUC1 is expressed on the apical surface of nearly all simple epithelial tissues, as well as tumor cells. MUC1 expression is associated with epithelial cell differentiation in the stomach, pancreas, lung, trachea, kidney, salivary and mammary glands and the female reproductive tract [16,38,39]. MUC1 is also expressed on certain nonepithelial cells such as hematopoietic cells, activated T cells and sperm [40–43]. MUC4 is also expressed in many normal epithelial tissues, both during development and in adults, including those of the eye, oral cavity, lacrimal glands, salivary gland, female reproductive tract, prostate gland, stomach, colon, lung, trachea and mammary gland. In lungs, MUC4 is the first mucin to be expressed [16,44]. MUC16, widely known as the serum marker of ovarian cancer (CA 125), is expressed on the epithelial cells of the eye, respiratory tract, female reproductive tract and the mesothelium of the abdominal cavity [45–48]. Of the three membrane mucins discussed, MUC16 is the least well characterized. While a variety of monoclonal antibodies to MUC16 (CA 125) have been described, their reactivities differ significantly [49] and may be influenced by glycosylation.
MUC1, MUC4 and MUC16 expression in cancer progression and metastasis is characterized by increased levels, altered gly-cosylation and aberrant surface distribution patterns. Aberrant expression of MUC1 in several adenocarcinomas, including breast, pancreas, colon, lung and endometrial cancer, is well established. However, in the case of prostate cancer, MUC1 expression is a poor marker of progression [50]. MUC4 is aberrantly expressed in ovarian tumors and premalignant and malignant pancreatic lesions. In prostate carcinomas, MUC4 expression is significantly downregulated [51]. MUC16 expression has been well investigated in ovarian carcinoma, and it is highly expressed in both ovarian and endometrial cancers [52,53]. A more detailed discussion on mucins in cancer is provided in the following sections.
Transcriptional regulation
Understanding the molecular controls over mucin gene expression may provide new opportunities to therapeutically intervene to manipulate mucin levels to enhance or reduce their function. Transcriptional regulation of MUC1 and MUC4 has been reviewed in detail [44,54]. Based on the epithelial-specific expression of human MUC1 in transgenic mice, it was determined that the regulatory elements necessary for normal patterns are within the proximal 1.4-kb region of the MUC1 promoter. The highly active region of the MUC1 promoter is encompassed within the −600 +1 base pair region of the transcriptional start site and has multiple response elements including those for Sp1, an E-box, signal transducers and activators of transcription (STATs) and NF-κB family members, a P-responsive region (PRE) and peroxisome proliferator-activated receptor (PPAR) responsive region [54]. Proinflammatory cytokines greatly stimulate MUC1 expression in various cellular contexts [54]. Estrogen (E2) and progesterone (P) regulate mucin gene expression in the female reproductive tract of humans and rodents. Steroid hormonal influences on MUC1 gene expression has been reviewed in detail by Carson et al. [54]. Rodent MUC4 expression increases with high levels of E2 and decreases when P levels are dominant [55,56]. Similar to murine MUC1 regulation, E2 stimulates rodent MUC4 expression [56,57]. MUC4 expression is also reduced at time of implantation [58] in rats. Both MUC4 and MUC16 expression have been profiled during the menstrual cycle; however, intensive studies to understand regulation by steroid hormones are yet to be investigated [59,60]. Loss of MUC16 also may aid blasctocyst adherence [60]. In addition, P receptor (PR) regulates human MUC1 gene expression in a PR isoform-specific fashion [61]. Progesterone-stimulated MUC1 expression is antagonized by PPAR-γ [62] in both uterine epithelial and cancer cells. MUC1 gene expression is also modulated by a dynamic interplay among cytokine-activated transcription factors, PR isoforms and transcriptional coregulators such as p300 and SRC-3 [63].
Characterization of the proximal 3.7 kb of the MUC4 5′-flank-ing region revealed four transcriptional start sites, three in the distal region and one in the proximal region. The proximal region is TATA-less, while the distal promoter is characterized by a TATA box [64]. Numerous transcription factor binding sites important for initiation of transcription are located within the proximal promoter. Transcriptional regulation of MUC4 has been examined in some detail in pancreatic cancer cells. IFN-γ activates MUC4 expression via STAT-1 upregulation [65]. TGF-β also activates MUC4 expression [66]. Retinoic acid (RA) increases MUC4 expression via RAR-α [67]. IFN-γ and RA synergize at the transcriptional level to induce MUC4 expression in pancreatic cancer cells [68]. IFN-γ and TNF-α activate MUC4 transcription via STAT and NF-κB family members [64]. Interleukins (IL-4 and IL-9) also regulate MUC4 transcription. Other transcription factors that regulate MUC4 expression are hepatocyte nuclear factors (HNF-1/-4), forkhead box A (FOX A1/A2), GATA (-4/-5/-6) and caudal-related homeobox (CDX -1/-2) [69,70].
In human corneal epithelial cells, IFN-γ and TNF-α modulate MUC16 protein expression and ectodomain release [71]. Nonetheless, regulation of MUC16 gene expression and functional analyses of the MUC16 promoter have yet to be examined. Putative binding sites for transcription factors, such as ER, STAT1, STAT3, CRE-BP1 (ATF2) and NF-κB, are evident by sequence inspection [72]; however, functional studies have not been performed to determine a true role for these elements. Recent developments have been made in understanding epigenetic control of mucin transcription in cancer, as reviewed by Van Seuningen and Vincent, for enabling new therapeutic approaches [73].
Post-transcriptional regulation
The full-length splice variant of MUC1 is also known as MUC1/REP [74]. Alternate forms of MUC1 mRNA give rise to MUC1 isoforms lacking the transmembrane and cytoplasmic domains, MUC1/SEC [74] or the tandem repeat region, MUC1/Y and MUC1/X [75,76]. MUC1/X was also reported as MUC1/Z [77]. Another splice variant of MUC1 is MUC1/ZD, which lacks the tandem repeat leading to a frameshift in the cytoplasmic tail sequence [78]. Although these truncated forms are coexpressed with the full length MUC1, the full-length form is predominantly expressed.
Alternative splicing of the MUC4 transcript generates a large series of splice variants [79]. A total of 24 different MUC4 splice variants (sv0 [full length] to sv21-MUC4; MUC4X and MUC4/Y) have been categorized into three distinct families: a secreted family with 12 variants, a membrane-bound family with five variants and others that include the membrane-bound form, but lack the tandem repeat domain (MUC4/X and MUC4/Y) [3,79,80]. MUC4 splice variants have been studied primarily in pancreatic carcinoma cell lines; however, their expression is not detected in the normal pancreas [3,79,80]. The functional significance of these mRNA splice variants is unknown because of the lack of corresponding protein expression data. Although CA 125, the secreted form of MUC16, has been widely accepted as a marker for ovarian cancer, little is known regarding its transcriptional or post-transcriptional regulation.
Mucins in cancer
Both secreted and membrane-bound mucins are well established as tumor markers [81,82], as they are commonly overexpressed, underglycosylated [2,83,84] and associated with a poor prognosis in many carcinomas [85–94]. Their barrier function potentially creates a local microenvironment, physically and chemically protecting metastatic cells from possible unfavorable conditions during invasion and tumor growth. The steric and charged properties of this barrier can facilitate or inhibit molecular diffusion, increasing the capture of growth factors or cytokines that contribute to tumor growth, protect tumor cells from immune system attacks, and inhibit uptake of many hydrophobic chemotherapeutic drugs [2,95,96]. Moreover, this barrier also reduces cell–cell and cell–extracellular matrix adhesion through both steric hindrance and by binding and occupying adhesion molecules [97,98], further promoting metastatic activity [2,28,99–101]. In tumor cells, membrane-bound mucins lose their normal restriction to the apical cell surface and instead are found distributed over the entire cell surface, allowing interactions with growth factor receptors normally sequestered to the basolateral membrane. MUC1’s cytoplasmic tail also participates in multiple signal transduction processes in cancer cells (Table 1).
Unlike MUC1, MUC4 contains an EGF-like motif within its juxtamembrane ectodomain and is capable of interacting with ErbB2 to enhance oncogenic pathways in cancer cells [2]. In polarized cells expressing MUC4, this complex segregates ErbB2 in the apical cell membrane, away from ErbB3 in the lateral membrane. However, when polarity is lost, these two ErbB family members are permitted to interact, driving cell proliferation, motility and inhibition of apoptosis [102]. Similar to MUC1, MUC4 inactivates proapoptotic Bad and stabilizes anti-apoptotic Bcl-xL [103].
The biological significance of MUC16 overexpression in many cancers is poorly understood and studies have mostly focused on clinical applications. Although the MUC16 cytoplasmic domain contains potential phosphorylation sites [16], there is minimal evidence of cell signaling involvement. Recently, MUC16 demonstrated a possible role in ovarian cancer cell epithelial–mesen-chymal transition, presumably through the modulation of EGFR phosphorylation [104]. Extracellularly, MUC16 has been shown to contribute to ovarian cancer metastasis [25,105] and host immune response suppression [106].
Aberrant glycosylation of mucins in cancer
The characteristic patterns of reduced O-linked glycosylation expose and alter the conformation of the variable number tandem repeats (VNTRs) within the ectodomains of mucins. The exposed cancer-related epitopes can be tissue- and cancer-grade-specific. Altered glycosyltransferase expression in cancer cells leads to truncated O-linked glycan structures and high mannose and bisecting type N-linked glycans. This aberrant glycosylation can form several well-known core oligosaccharide antigens associated with tumors – Tn, sialyl-Tn (STn), Thomsen–Friedenreich (T or TF) and sialyl-T (ST) (Figure 1C). Moreover, complex peripheral O-linked oligosaccharide antigens can also form from further glycosylation of the core 2 antigen, for example, the variations of Lewis and Sialyl–Lewis antigens [19,107–109]. Clinicians and researchers have taken advantage of these antigens, using monoclonal antibodies specific towards these unique carbohydrate targets and exposed protein core to distinguish cancerous from healthy tissue [36,110–112]. Interestingly, the overexpression of sialyltransferases and subsequential increased sialylation is in itself believed to be important in the progression of many types of cancer [113–120]; however, a mechanism has not been proposed.
Cancer diagnosis & prognosis
Abnormal mucin expression and/or glycosylation has been observed in the sera and tumors of various cancers, including lung, colon, pancreatic, ovarian and breast [109,121,122]. Heterogeneous mucin expression within types of cancer overlapping patterns among different types of cancer, and increased serum levels observed in benign conditions limit their use as diagnostic markers in determining the primary sites of metastatic disease [123].
An example of this is seen in breast cancer, where overexpression of all three of these mucins has been observed [85,121,122,124]. Although increased serum MUC1 in breast cancer patients is associated with higher tumor burden [125,126], no correlation has been shown towards tumorigenicity or metastasis [127]. However, increased levels of aberrantly glycosylated MUC1 has been shown to be a predictor of poor chemotherapeutic response [128] and an almost twice as low 5-year survival rate [129]. In addition, an accumulation of cytoplasmic MUC1 is also associated with a lower breast cancer survival rate [85]. Increased MUC4 staining has a positive correlation with higher tumor grades [85,130,131], but it is unclear if this can be associated with patient prognosis [85]. MUC16 stained positive in invasive micropapillary carcinoma of the breast in one study, but its prognostic correlation was not examined [132].
In prostate cancer, there is no consensus supporting MUC1 expression in normal and tumorigenic tissue, or an association with higher Gleason grades [50,85,111,133,134]. The lack of consensus can be attributed to the variability of alternate O-linked glyco-sylation and antibody specificity. However, in assessment of prostate cancer tissue using antibodies that can detect forms of MUC1 independent of glycosylation patterns (CT1 and 214D4 [135,136]), only 41% of normal prostate tissue samples were positive for MUC1, while it was only present in 26% of prostate tumor samples [137]. For MUC4, one study showed expression levels were downregulated in prostate cancer tissue, with 84.2% of tissue from normal cases stained positive for MUC4 compared with only 26.3% of the cancer cases [51].
MUC16 cancer-associated glycoforms (Tn and STn) and elevated serum levels are detected in more than 80% of epithelial ovarian cancer patients, making it useful for early disease detection, diagnostic staging and determining therapeutic treatment success [138–142]. However, aberrant MUC16 expression has not been correlated with patient prognosis [86].
Although both MUC1 and MUC4 are expressed in normal and cancerous lung tissue [143], MUC4 expression is reduced in non-small-cell carcinomas (NSCLC), whereas MUC1 expression remains constant [144]. Detection of MUC1 with glycosylation-independent antibodies [145–147] and depolarized localization are both considered poor prognostic markers [93]. Interestingly, one study found detection of MUC1 using a TF or Tn antigen-spe-cific antibody correlated with a more favorable prognosis [148]. Similarly, a possible association between elevated levels of MUC4 and longer survival rates for stages I and II NSCLC adeno-carcinoma patients has been suggested [149]. Conversely, in small-sized lung adenocarcinoma patients, high MUC4 expression has a lower survival rate [94,144].
Transmembrane mucins as therapeutic targets
Given their abundance and overexpression in many adeno-carcinomas, and the post-translational alteration in their glyco-sylation patterns, it is not surprising that transmembrane mucins have been investigated as potential targets for therapies. While there are currently no mucin-targeted therapies in clinical use, several MUC1 therapies are in clinical trials. Potential applications using nanotechnology-based therapies targeting mucins are also being examined in this regard (see Figure 2).
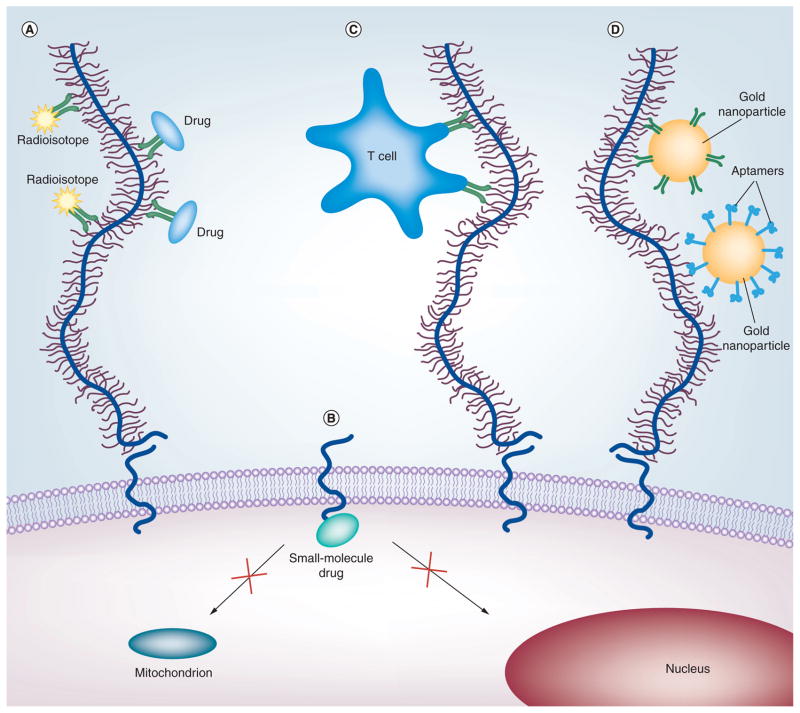
(A) Antibody-based therapies include radioisotopes (yellow star shapes) and drugs (blue ovals) conjugated to antibodies (green Y-shapes) recognizing the ectodomains of mucins. (B) Peptides and small-molecule drugs (turquoise oval) can block the active cytoplasmic domain of MUC1, thus preventing its localization to the mitochondria or nucleus. (C) Immunotherapies designed to stimulate the body’s immune response and induce T cells (blue amoeboid shape) that target mucins. (D) Targeted nanotherapies use antibodies or aptamers (blue hook shapes) that recognize the ectodomains of mucins to deliver gold nanoparticles (large orange circles) or quantum dots. For details, see text.
Antibody targeting
Antibodies targeting receptors that have prominent roles in cancer have been developed and US FDA approved for clinical use (e.g., Her2 [Herceptin®] and EGFR [cetuximab]). A number of antibodies have been raised against MUC1, and over 200 references in the literature describe anti-MUC1 antibodies. The VNTR region is extremely immunogenic, especially the APDTR region [150], and therefore leads to the development of a series of monoclonal antibodies targeting the N-terminus that are reactive with various epitopes of the domain [151].
While promising in animal models and preclinical studies, no MUC1 antibody therapy has successfully completed all phases of clinical trials. One humanized antibody, HMFG1, which recognizes the PDTR motif within the VNTR region, has been formulated in various treatments (in combination with cyto-toxic drugs) by the company Antisoma plc for the treatment of breast cancer [152]. Radiotherapies conjugated to HMFG1 [153], among other antibodies [154], have had disappointing outcomes, with no improved survival of the treated patients compared with the untreated patients. A Phase III clinical trial with Y90-labeled HMFG1 revealed that leakage of the antibody from the peritoneum and limited radiation treatment within the single dose were potential reasons for the failure of their trial for the treatment of ovarian cancer [155]. Promising early clinical studies have been reported recently by Immunomedics, Inc. for a combination of 90Y-PAM4 antibody to MUC1 (clivatuzumab) treatment with gemcitabine for advanced pancreatic cancer [156], although previous studies using this antibody were unsuccessful [157]. In addition, PAM4 has been shown to be effective in a blood assay for diagnosing early-stage pancreatic cancer [158].
Mucin antibodies have also been investigated for targeted drug delivery. Two MUC16 antibodies, one recognizing a non-repeat epitope in the extracellular domain and one recognizing the VNTR region, were conjugated to cytotoxic drugs. The antibody–drug conjugate targeted to the repeat region was more effi-cacious in a tumor mouse model than its non-repeat-targeting counterpart [159]. An antibody specific to the α/β junction of MUC1, the site of autocatalytic cleavage, was conjugated to an exotoxin that was only effective when it is internalized. In vivo results in a mouse model with human breast cancer cell lines show tumor suppression, indicating the antibody–toxin conjugates were intracellularized for the exotoxin to take effect [160].
Most of the antibody-targeted therapies have focused on the N-terminus of mucins, primarily because they are physically accessible to the therapy, and for their ability to target the altered glycoforms with an array of antibodies to choose from. However, these therapies must overcome the large pool of circulating shed N-terminal subunits for antibodies to reach the surface of carcinoma cells [83]. Furthermore, tumors are heterogenous tissues, as are the populations of MUC1 glycoforms present in these cancerous cells. Targeting more than one membrane-bound mucin and/or multiple epitopes of the same mucin at one time may prove to be a more effective therapeutic approach. In addition, antibodies recognizing the carboxy-terminal of mucins, which are unaffected by specific glycoform epitopes, may also hold promise for diagnostic and therapeutic applications [161].
Drug targets
The cytoplasmic domain (CD) of MUC1 plays an important role in cell signaling during cancer progression. MUC1-CD binds β-catenin, while also inhibiting the degradation of the EGFR. A peptide, MUC1 inhibitory peptide, designed to block the intracellular interactions between MUC1/β-catenin and MUC1/EGFR by acting as a binding decoy was used in vivo in mice. Mouse models treated with peptide, MUC1 inhibitory peptide displayed tumor regression and significant inhibition of tumor growth rate [162].
In cancer cells where MUC1 is overexpressed, MUC1-CD accumulates in the cytosol where it can localize to the mitochondria and nucleus [163]. Another peptide, GO-201, was designed to block the region where MUC1-CD is believed to dimerize/oligomerize and thus prevent its translocation. Treatment with GO-201 resulted in growth arrest and death of human breast cancer cells in a xenograft mouse model [164]. Furthermore, this peptide has shown high efficacy in a xenograft prostate cancer mouse model based on the MUC1-expressing prostate cancer cells, demonstrating complete tumor regression and prolonged lack of recurrence [165]. When non-MUC1-expressing prostate cancer cells were treated with GO-201, there was no evidence of cell death in vitro, as was the case for MUC1-positive cells, underlining the targeting specificity of the peptide [165]. This therapy is currently in Phase I trials in patients with advanced solid tumors [201].
Interestingly, small molecules found in nature appear to be effective in blocking MUC1-CD dimerization. Apigenin, a flavanoid found in leafy plants and vegetables, has been shown to interfere with the dimerization of the C-terminus of MUC1, thus blocking its localization to the nucleus. Apigenin-induced suppression of MUC1 C-terminal expression increased apoptosis and reduced clonogenic survival [166]. Similarly, the small molecule thymoqui-none, found in the seeds of Nigella sativa, reduces MUC4 expression in vitro. The decrease in MUC4 expression correlated with an increase in apoptosis, decreased motility and decreased migration in pancreatic cancer cells [167]. These small molecules are part of a larger class of natural products, which have been investigated for their anticancer properties.
Vaccines & immunotherapy
Of all the MUC1-targeted therapies in clinical trials, only two are in Phase III trials. Both are vaccines. The L-BLP25 liposome vaccine is designed to stimulate the body’s immune system and induce T cells that target cells expressing MUC1. The vaccine consists of a 25-amino-acid peptide containing the VNTR region and a palmi-toyl lysine residue at the carboxy terminal to enhance the incorporation of the lipopeptide into the liposome particle. Currently, it is in Phase III trials for NSCLC, which represents 80% of all lung cancers. A Phase IIb trial for stage IIIb/IV demonstrated that NSCLC patients who received the vaccine had a median survival of 30.6 months, versus 13.3 months [168]. L-BLP25 also shows promise for prostate cancer. The mean prostate-specific antigen doubling time in patients increased from 8.9 months at the onset of the trial to 12.2 months at the end of the study [169]. In addition, L-BLP25 is in Phase III trials for hormone-sensitive breast cancer [1].
The vaccine TG4010 (modified vaccinia virus Ankara-MUC1-IL2), a modified vaccinia virus Ankara strain based on a recombinant vaccinia virus expressing MUC1 antigen and the human cytokine IL-2, was also tested on patients with NSCLC [170]. During a Phase IIB trial, the 6-month survival rate increased to 17.1 months versus 11.3 months for patients who had normal levels of activated natural killer cells.
Another virus-based vaccine, PANVAC, which targeted carcino-embryonic antigen and MUC1, showed promise early on in clinical trials. In Phase I, patients with advanced pancreatic cancer who generated vaccine-specific T-cell responses had a median survival of 15.1 months compared with those without T-cell reactivity (3.9 months) [171]. A pilot study of PANVAC also showed promise for ovarian and breast cancer [172]. However, a Phase III randomized, controlled clinical trial of PANVAC for the treatment of patients with advanced pancreatic cancer did not meet its primary efficacy end point of improving overall survival compared with palliative chemotherapy or best supportive care [202]. PANVAC is one of many MUC1 immunotherapies to fail in a human clinical trial. Unfortunately, most of these attempts fail to translate from preclinical success into clinical efficacy [173].
Nanotherapies
Biological events, including those involved in cancer, occur on the nanoscale. If these processes can be detected or targeted as they happen, cancer could be diagnosed and treated at earlier stages. In addition, with this molecular precision, tumors can be targeted and destroyed with minimal harm to surrounding cells. As of yet, there are few studies using nanoscale entities that target membrane-bound mucins for diagnosis or treatment. One study [174] combined an imaging agent, quantum dots (QDs), conjugated to a DNA aptamer that was specific to the MUC1 glycoforms found on cancer cells [175]. This QD conjugate was also bound to doxorubicin via a pH-sensitive hydrazone bond, allowing the drug to be released after being intracellularized. In vivo mouse studies showed the QD-MUC1 aptamer accumulated selectively in tumor cells versus QD alone, indicating an active tumor-targeting mechanism in addition to the enhanced permeation and retention effect that allows most nanoparticles to passively target tumors through leaky vasculature. The release of doxorubicin, which quenches the fluorescence of QDs, was monitored in vitro via fluorescence under acidic conditions. This could provide a useful in vivo monitoring tool to measure doxorubicin release over time.
Another study used MUC1 to target pancreatic cancer cell xenografts in mouse models using gold nanoparticles conjugated to an anti-MUC1, PAM4. Radiofrequency radiation was used to trigger a hyperthermic effect from the gold particles, causing the cancer cells, which had a selectively higher uptake of gold nanoparticles than healthy cells, to be destroyed [176].
Recently, MUC1-expressing cells have been selectively killed in vitro by targeted plasmonic nanobubbles (PNBs) [177]. PNBs are transient lethal events generated by the laser irradiation of clusters of gold nanoparticles. The mechanical impact of these bubbles disrupts the cellular membrane and results in cell death. MUC1-targeted gold nanoparticles specifically bind to MUC1-expressing cells over cells that do not express MUC1. PNBs generated from anti-MUC1-gold particles were large enough to be cytotoxic. By comparison, PNBs targeting EGFR were also studied and resulted in smaller, less damaging bubbles, indicating that MUC1 was a superior targeting agent.
Expert commentary
Membrane-tethered mucins are extremely large cell surface components that play protective roles at the apical surfaces of normal epithelia and include MUC1, MUC4 and MUC16. Much is known about the normal patterns of distribution of these glycoproteins. Membrane-tethered mucins are often over-expressed in many cancers, but the heavy reliance on the use of glycoform-specific mucin antibodies in many studies may confuse interpretation. With regard to MUC1, much has been learned about gene regulation expression, including control by various cytokine-stimulated transcription factors and steroid hormone receptor family members. Information on MUC4 regulation is more limited and virtually nothing is known about MUC16 in this regard. More than one mucin can be expressed by a given cell in both normal and pathological contexts. Thus, it is important to understand if these genes are regulated coordinately and can be targeted as a group, or require individual therapeutic approaches.
Five-year view
There are potential medical benefits associated with being able to control membrane-tethered mucin expression. Increasing expression should improve mucosal barrier functions in individuals at risk of infections, or enhance other protective functions afforded by these glycoproteins. By contrast, reduction of mucin expression would be desirable in other instances, for example, in cancer therapies. Developing a more comprehensive understanding of the regulation of the major membrane-tethered mucin genes should reveal if they can be controlled as a group pharmacologically. Mucin-reducing therapies are likely to make tumors more susceptible to therapies with cytotoxic agents currently employed in cancer treatments. Few candidate agents to reduce expression of membrane-tethered mucins exist, although PPAR-γ agonists show some promise in this regard. Epitopes within the tandem repeat domains have been employed as agents to stimulate the host immune system to respond to mucin-expressing tumors; however, in spite of promising results in animal studies, human trials using this approach have, to date, been disappointing. More work is necessary to determine why the responses vary so much among species. Finally, available mucin-specific antibodies are currently being used to direct nanoparticles with cytotoxic payloads or cytolytic capabilities to selectively destroy mucin overexpressing tumors. These studies are at a primitive stage, but are certainly worth exploring. Systemic delivery of mucin-directed nanoparticles may be problematic for various reasons. Nonetheless, it should be possible in certain instances to deliver these agents directly to the tumor sites, for example, endome-trial carcinomas. The high accessibility of membrane-tethered mucins at the cell surface makes them excellent candidates for such targeting approaches.
Acknowledgments
The authors thank Mary C Farach-Carson and members of the Carson and Farach-Carson laboratories for many helpful discussions. We are deeply appreciative of the excellent secretarial assistance of Sharron Kingston.
Footnotes
For reprint orders, please contact moc.sweiver-trepxe@stnirper
Financial & competing interests disclosure
The authors were supported by NIH grant R01HD029963 (to Daniel D Carson), NIH grant P50CA098258 NCI Uterine SPORE Pilot Project (to Daniel D Carson) and NIH grant U54 CA151668 Pilot Project (to Pamela Constantinou). The authors have no other relevant affiliations or financial in involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Websites
Full text links
Read article at publisher's site: https://doi.org/10.1586/eem.11.70
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3245640?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Potential mechanisms of traditional Chinese medicine in the treatment of liver cirrhosis: a focus on gut microbiota.
Front Microbiol, 15:1407991, 21 Aug 2024
Cited by: 0 articles | PMID: 39234554 | PMCID: PMC11371771
Review Free full text in Europe PMC
Targeting Siglec-Sialylated MUC1 Immune Axis in Cancer.
Cancers (Basel), 16(7):1334, 29 Mar 2024
Cited by: 0 articles | PMID: 38611013 | PMCID: PMC11011055
Review Free full text in Europe PMC
In vitro characterisation of [177Lu]Lu-DOTA-C595 as a novel radioimmunotherapy for MUC1-CE positive pancreatic cancer.
EJNMMI Radiopharm Chem, 8(1):18, 14 Aug 2023
Cited by: 1 article | PMID: 37578571 | PMCID: PMC10425306
Preliminary Development and Testing of C595 Radioimmunoconjugates for Targeting MUC1 Cancer Epitopes in Pancreatic Ductal Adenocarcinoma.
Cells, 11(19):2983, 24 Sep 2022
Cited by: 3 articles | PMID: 36230945 | PMCID: PMC9563759
Hyperpolarized MRI with silicon micro and nanoparticles: Principles and applications.
Wiley Interdiscip Rev Nanomed Nanobiotechnol, 13(6):e1722, 13 May 2021
Cited by: 3 articles | PMID: 33982426 | PMCID: PMC9352437
Review Free full text in Europe PMC
Go to all (19) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Differential regulation of membrane-associated mucins in the human ocular surface epithelium.
Invest Ophthalmol Vis Sci, 45(1):114-122, 01 Jan 2004
Cited by: 53 articles | PMID: 14691162
MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope.
Invest Ophthalmol Vis Sci, 44(6):2487-2495, 01 Jun 2003
Cited by: 129 articles | PMID: 12766047
Distinct evolution of the human carcinoma-associated transmembrane mucins, MUC1, MUC4 AND MUC16.
Gene, 373:28-34, 24 Feb 2006
Cited by: 70 articles | PMID: 16500040
Mucins and mucin binding proteins in colorectal cancer.
Cancer Metastasis Rev, 23(1-2):77-99, 01 Jan 2004
Cited by: 295 articles | PMID: 15000151
Review
Funding
Funders who supported this work.
NCI NIH HHS (4)
Grant ID: U54 CA151668
Grant ID: U54 CA151668-03
Grant ID: P50 CA098258
Grant ID: P50 CA098258-08
NICHD NIH HHS (2)
Grant ID: R01 HD029963-20
Grant ID: R01 HD029963




