Abstract
Free full text

Mapping the Human Memory B Cell and Serum Neutralizing Antibody Responses to Dengue Virus Serotype 4 Infection and Vaccination
ABSTRACT
The four dengue virus (DENV) serotypes are mosquito-borne flaviviruses responsible for dengue fever and dengue hemorrhagic fever. People exposed to DENV develop antibodies (Abs) that strongly neutralize the serotype responsible for infection. Historically, infection with DENV serotype 4 (DENV4) has been less common and less studied than infections with the other three serotypes. However, DENV4 has been responsible for recent large and sustained epidemics in Asia and Latin America. The neutralizing antibody responses and the epitopes targeted against DENV4 have not been characterized in human infection. In this study, we mapped and characterized epitopes on DENV4 recognized by neutralizing antibodies in people previously exposed to DENV4 infections or to a live attenuated DENV4 vaccine. To study the fine specificity of DENV4 neutralizing human antibodies, B cells from two people exposed to DENV4 were immortalized and screened to identify DENV-specific clones. Two human monoclonal antibodies (MAbs) that neutralized DENV4 were isolated, and their epitopes were finely mapped using recombinant viruses and alanine scan mutation array techniques. Both antibodies bound to quaternary structure epitopes near the hinge region between envelope protein domain I (EDI) and EDII. In parallel, to characterize the serum neutralizing antibody responses, convalescence-phase serum samples from people previously exposed to primary DENV4 natural infections or a monovalent DENV4 vaccine were analyzed. Natural infection and vaccination also induced serum-neutralizing antibodies that targeted similar epitope domains at the EDI/II hinge region. These studies defined a target of neutralizing antigenic site on DENV4 targeted by human antibodies following natural infection or vaccination.
IMPORTANCE The four serotypes of dengue virus are the causative agents of dengue fever and dengue hemorrhagic fever. People exposed to primary DENV infections develop long-term neutralizing antibody responses, but these principally recognize only the infecting serotype. An effective vaccine against dengue should elicit long-lasting protective antibody responses to all four serotypes simultaneously. We and others have defined antigenic sites on the envelope (E) protein of viruses of dengue virus serotypes 1, 2, and 3 targeted by human neutralizing antibodies. The epitopes on DENV4 E protein targeted by the human neutralizing antibodies and the mechanisms of serotype 4 neutralization are poorly understood. Here, we report the properties of human antibodies that neutralize dengue virus serotype 4. People exposed to serotype 4 infections or a live attenuated serotype 4 vaccine developed neutralizing antibodies that bound to similar sites on the viral E protein. These studies have provided a foundation for developing and evaluating DENV4 vaccines.
INTRODUCTION
Dengue virus (DENV) is a mosquito-borne positive-sense RNA virus belonging to the Flaviviridae family (1). Dengue is transmitted to people by Aedes aegypti or Aedes albopictus mosquitoes (2, 3). Recent estimates indicate that nearly 400 million people are infected worldwide each year, which makes dengue the most common and serious vector-borne disease of humans (4).
While the majority of DENV infections are asymptomatic, symptomatic infections can cause disease in a spectrum ranging from mild dengue fever to severe dengue hemorrhagic fever and dengue shock syndrome (5). A primary DENV infection provides lifelong protection against disease caused by the infecting homologous serotype in most subjects (6). A secondary infection with virus of a heterologous serotype increases the risk of developing severe dengue hemorrhagic fever (7). To understand the molecular basis of a protective DENV antibody response, it is critical not only to map the epitopes of strongly neutralizing human monoclonal antibodies (hMAbs) but also to characterize the polyclonal neutralizing antibody responses to viruses of all the four serotypes after natural infection. This knowledge is critical for evaluating antibody responses to vaccination and improved second-generation vaccine design.
The DENV envelope (E) glycoprotein is required for viral binding and entry into cells (8, 9). E protein is also the main target of neutralizing antibodies (10). The four serotypes (DENV1 to DENV4) have variations of 25% to 40% in the amino acid sequence of the E protein (11, 12). The E protein monomer consists of three domains (envelope protein domain I [EDI], EDII, and EDIII), and two of these protomers form head-to-tail dimers in viral particles. Three dimers lie parallel to each other in the particles, forming a raft (13, 14), and 30 of these rafts are arranged in a herringbone pattern on the mature virion.
Unlike the DENV neutralizing antibody response in mice, which principally targets simple epitopes in EDIII (15, 16), nearly all human neutralizing antibodies target complex quaternary structure E protein epitopes that are displayed on intact dengue virions but not on soluble forms of E protein after natural infections (17, 18). Epitopes of human type-specific neutralizing antibodies against DENV1, DENV2, and DENV3 have been mapped to quaternary structure epitopes that span different E protein molecules (18,–21).
DENV4 has been less studied than the other serotypes. Kostyuchenko and colleagues recently solved a near-atomic-resolution structure of DENV4 (22). DENV4 surface E molecules are tightly packed compared to those of other serotypes, which may explain the more rigid and stable nature of DENV4. DENV4 type-specific and neutralizing MAb 5H2 was isolated from a chimpanzee infected with DENV4 (23). 5H2 binds to an epitope on the lateral ridge of EDI (23, 24). However, the epitopes targeted by DENV4-specific antibodies in humans have not been characterized. The goal of the current study was to map the antigenic sites on DENV4 recognized by antibodies in people exposed to primary DENV4 infections or a monovalent live attenuated DENV4 vaccine.
RESULTS
DENV4 is neutralized by type-specific antibodies in human immune sera.
To study the properties of serum polyclonal DENV4 neutralizing human antibodies, we assembled a panel of blood samples from people previously exposed to DENV4 infections or people who had received a monovalent live attenuated DENV4 vaccine (Table 1) (25, 26).
TABLE 1
Panel of DENV immune sera
| Serum type | Serum | Yr of infection | Location of infection | Time between infection and blood draw (yrs) | Reciprocal of Neut50 titers against DENV1 to DENV4a | |||
|---|---|---|---|---|---|---|---|---|
| DENV1 | DENV2 | DENV3 | DENV4 | |||||
| DENV4 immune sera | 002 | 1994 | Guatemala | 15 | <20 | <20 | 29 | 77 |
| 102 | 2007 | Honduras | 2 | <20 | <20 | 41 | 159 | |
| 112 | 2001 | Nicaragua | 2 | 128 | 346 | 175 | 1,639 | |
| 07/333 | Unknown | Thailand | Unknown | <20 | 153 | 367 | >1,280 | |
| 06/302 | Unknown | Thailand | Unknown | <20 | 32 | 60 | >1,280 | |
| 06/105 | Unknown | Thailand | Unknown | <20 | 26 | 91 | 685 | |
| DENV2 immune serum | 08/90 | Unknown | Thailand | Unknown | 20 | >1,280 | 60 | 32 |
| DENV3 immune serum | 118 | 2009 | Nicaragua | 1 | 60 | 32 | 980 | 76 |
| DENV4 vaccine immune serab | 256.03.36 | <20 | <20 | <20 | 142 | |||
| 256.03.38 | <20 | <20 | <20 | 148 | ||||
| 256.03.57 | <20 | <20 | <20 | 144 | ||||
| 256.03.68 | <20 | <20 | <20 | 988 | ||||
Individuals exposed to DENV have specific antibodies in circulation as well as DENV-specific memory B cells (MBCs). Some of the DENV-specific antibodies in circulation bind only to viruses of the serotype of the previous infection (type specific), while others cross-react with two or more serotypes. Using serum samples from people exposed to DENV4 natural infections or a monovalent live attenuated DENV4 vaccine, we performed antibody depletion studies to determine the relative contributions of serotype cross-reactive or type-specific antibodies to DENV4 neutralization. Polystyrene beads coated with the homotypic (DENV4) or heterotypic (DENV2) DENV serotypes were incubated with the immune serum samples to deplete specific populations of antibodies. Depletion of antibodies was confirmed by enzyme-linked immunosorbent assay (ELISA) before using the samples in DENV4 neutralization assays. Depleting the DENV4 immune serum samples with the homotypic DENV4 antigen led to the removal of nearly all the DENV-specific (serotype cross-reactive and DENV4 type-specific) antibodies in the samples. As anticipated, depletions performed with DENV4 antigen led to a large drop in levels of DENV4 neutralizing antibodies in both DENV4 infection and vaccine sera (Fig. 1A and andCC and Table 2). Depleting DENV4 immune sera with heterotypic DENV2 antigen led to the removal of serotype cross-reactive but not of DENV4 type-specific antibodies. There was minimal to no reduction in neutralization of DENV4 in sera depleted with DENV2 antigen (Fig. 1B and andDD and Table 2). The control depletions for subject 112 yielded different neutralization titers in the homotypic and heterotypic depletions (1,263 versus 773). This could have been because these control depletions were performed in separate experiments using different lots of the serum aliquots from the same individual. However, the trends remained the same for the neutralization titers against all four serotypes, with DENV4 titers being the highest in both cases.
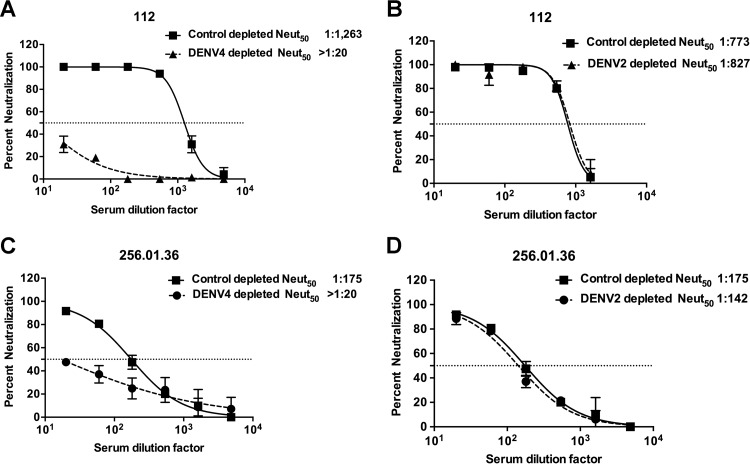
DENV4 is neutralized by type-specific antibodies in human primary infection immune sera or vaccine immune sera. Primary DENV4-immune serum sample 112 (A and B) and DENV4 NIH monovalent vaccine serum sample 256.01.36 (C and D) were depleted of antibodies binding to DENV4 antigen (A and C) or DENV2 antigen (B and D). Control depletions were performed using bovine serum albumin as an antigen. Results presented here for antibody depletions are representative of data obtained with three primary DENV4 immune sera and four DENV4 monovalent vaccine sera (Table 2). Results are from 2 technical replicates in one experiment. Each point represents the mean neutralization value from the two replicates, and the error bars depict the standard deviations.
TABLE 2
DENV4 neutralization by human immune sera depleted of DENV binding antibodiesa
| Serum type | Sample ID | Homotypic depletionb | Heterotypic depletionc | ||||
|---|---|---|---|---|---|---|---|
| Control depleted (Neut50) | DENV4 depleted (Neut50) | % loss of neutralization (mean ± SD) | Control depleted (Neut50) | DENV2 depleted (Neut50) | % loss of neutralization (mean ± SD) | ||
| DENV4 immune sera | 002 | 58 | <20 | 100 | 82 | 84 | 0 |
| 102 | 112 | 43 | 62 | 113 | 98 | 13 | |
| 112 | 1,263 | <20 | 100 | 773 | 827 | 0 | |
| DENV4 vaccine immune sera | 256.03.36 | 175 | <20 | 100 | 175 | 142 | 19 |
| 256.03.38 | 61 | <20 | 100 | 61 | 83 | 0 | |
| 256.03.57 | 53 | <20 | 100 | 98 | 75 | 23 | |
| 256.03.68 | 425 | 160 | 62 | 409 | 271 | 34 | |
Overall, these results demonstrate that type-specific antibodies were primarily responsible for DENV4 neutralization in serum samples collected following natural infection or monovalent DENV4 vaccination.
Isolation of DENV4 neutralizing human monoclonal antibodies.
To further characterize the B cell response to DENV4 infection, we transformed B cells from two DENV4-immune individuals (subjects 002 and 112) and isolated hMAbs, as previously described (27). The transformed B cell culture supernatants were screened for binding to DENV4. On the basis of the number of positive wells and the number of transformed B cells tested (determined by average colony counts in transformed wells), the frequencies of DENV-specific B cells in circulation were estimated to be 0.19% and 0.20% of transformable B cells for subjects 002 and 112, respectively. Previously, it was reported that there may be a long-term set point frequency of about 0.1% to 0.2% DENV-specific B cells in the circulating memory B cell pool following DENV infection (28), and the frequencies of 0.19% and 0.20% in the two subjects studied here are consistent with those previous estimates. Note that, even though the frequencies of DENV4-specific B cells determined for subjects 002 and 112 were similar, their serum titers were different. As mentioned above, the B cell response to primary DENV infection is a mix of DENV serotype cross-reactive and type-specific antibodies. Moreover, a relatively small fraction of the virus-specific B cells produce neutralizing Abs (29,–31). The serum neutralizing Abs are derived from long-lived plasma cells (LLPCs) in the bone marrow and not the memory B cell compartment. Previous studies have also reported a weak and inconsistent correlation between DENV-specific or other viral antigen-specific antibody titers in the serum and the frequency of antigen-specific memory B cells in the blood (32,–34).
We also determined the DENV serotype specificity of all the positive B cell culture supernatants from subject 112. Of the 34 DENV antigen-reactive supernatants, antibodies in 32% bound only to DENV4 (type-specific) and in 68% bound to two or more serotypes (cross-reactive). From the EBV-transformed B cell lines secreting DENV antigen-reactive antibodies, we isolated 8 human B cell hybridoma cell lines, as previously described (28). Two cell lines, designated D4-126 and D4-131, secreted DENV4 type-specific and neutralizing hMAbs with Neut50 values (defined as the dilution factor required to neutralize 50% of infection) of 0.54 μg/ml and 0.43 μg/ml, respectively (Fig. 2A). Both of these antibodies were isolated from subject 002. To characterize the binding properties of D4-126 or D4-131 hMAbs further, we performed binding assays with whole DENV4 virions, recombinant E (rE) or rEDIII proteins of DENV4, and increasing concentrations of hMAb D4-126 or D4-131. The two hMAbs bound to whole DENV4 particles similarly (Fig. 2B). HMAb D4-126 did not bind to rE protein, whereas D4-131 exhibited low levels of binding to rE protein at high concentrations (>10 μg/ml) (Fig. 2B). The hMAbs did not bind to rEDIII protein (Fig. 2B). These studies revealed that neutralizing hMAb D4-126 or D4-131 bound best to intact DENV4 virions. However, it is also possible that MAb D4-126 or D4-131 could bind to E dimers formed from rE monomers at higher concentrations as demonstrated by the D4-131 MAb binding to rE at higher concentrations.
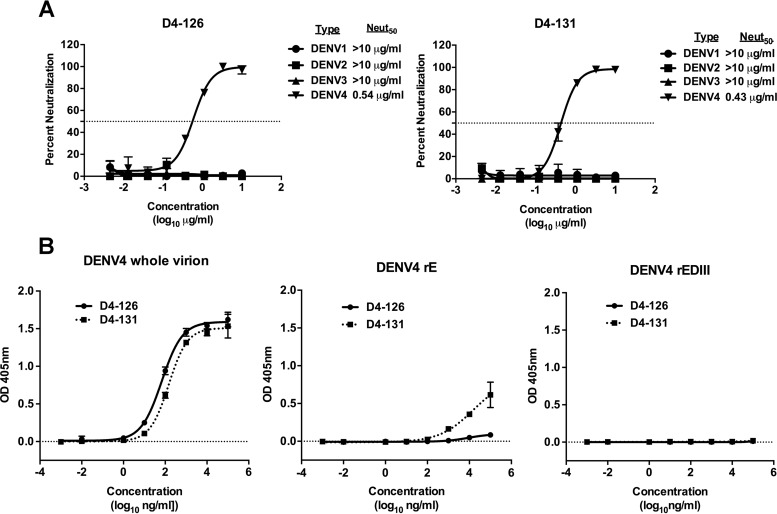
DENV4 neutralizing hMAb D4-126 or D4-131 preferentially binds to epitopes displayed on intact virions. (A) U937+DC-SIGN flow cytometry-based neutralization assays were conducted to determine the neutralization of DENV1 to DENV4 by D4-126 or D4-131. Results are from 2 technical replicates in an experiment. Each point represents the mean neutralization value from the two replicates, and the error bars depict the standard deviations. Neutralization experiments were repeated at least twice. (B) Binding to DENV4 whole virions, recombinant E protein ectodomain (rE), and recombinant E protein domain III (rEDIII) by D4-126 or D4-131 was tested in an ELISA. Results are from 2 technical replicates in one experiment. Each point represents the mean from the two replicates, and the error bars depict the standard deviations OD, optical density.
Additionally, these antibodies were not cross-reactive to the Zika virus (H/PF/2013 and MR 766 strains) and remained type specific to DENV4.
hMAb neutralization of different DENV4 strains.
To determine if hMAbs D4-126 and D4-131 neutralized diverse strains of DENV4, we used a panel of recombinant isogenic DENV4 expressing the E protein from different DENV4 genotypes and laboratory strains (Table 3). The hMAbs equally neutralized all variants tested, except for a Cambodia 2010 genotype I (GI) strain, which was not neutralized by D4-126 and was neutralized less efficiently by D4-131 than by other strains tested (Fig. 3).
TABLE 3
Characteristics of DENV4 strains used in this study
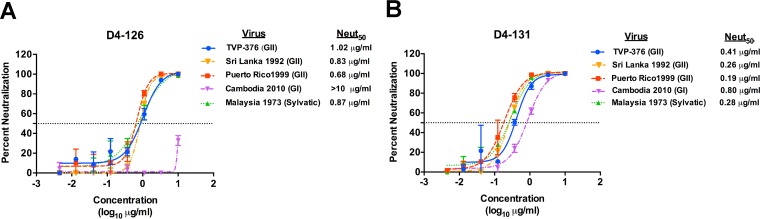
Neutralization of different DENV4 strains by hMAb D4-126 or D4-131. U937+DC-SIGN flow cytometry-based neutralization assays were conducted to compare the abilities of D4-126 (A) and D4-131 (B) hMAbs to neutralize different laboratory-adapted or clinical strains of DENV4. The MAbs neutralized all variants except for the Cambodia 2010 genotype I strain, which was not neutralized by D4-126 and was neutralized less efficiently by D4-131 than by the other strains. Results are from 2 technical replicates in one experiment. Each point represents the mean neutralization value from the two replicates, and the error bars depict the standard deviations.
Mapping the epitopes of DENV4 neutralizing hMAbs.
Human DENV1, DENV2, and DENV3 type-specific neutralizing antibodies often bind to quaternary structure epitopes centered on the EDI/II hinge (17,–19, 21, 38) and/or the EDIII region (2, 20, 21, 39, 40). Recently, we demonstrated that it is possible to recover recombinant chimeric DENVs displaying E protein domains or epitopes from viruses of two different serotypes (38, 39). We used a rDENV4 strain with a mutated EDI/II hinge region and EDII region (rDENV4/3) to map the binding sites of hMAbs D4-126 and D4-131 (Fig. 4A and andB).B). The recombinant virus has 25 amino acid replacements at different positions that are highlighted in green (Fig. 4A and andB)B) (unpublished data). We did not detect any binding or neutralizing activity for the hMAb D4-126 or D4-131 with the rDENV4/3, indicating that the DENV4 EDI/II hinge residues are part of the epitope recognized by these MAbs (Fig. 4C and andDD).
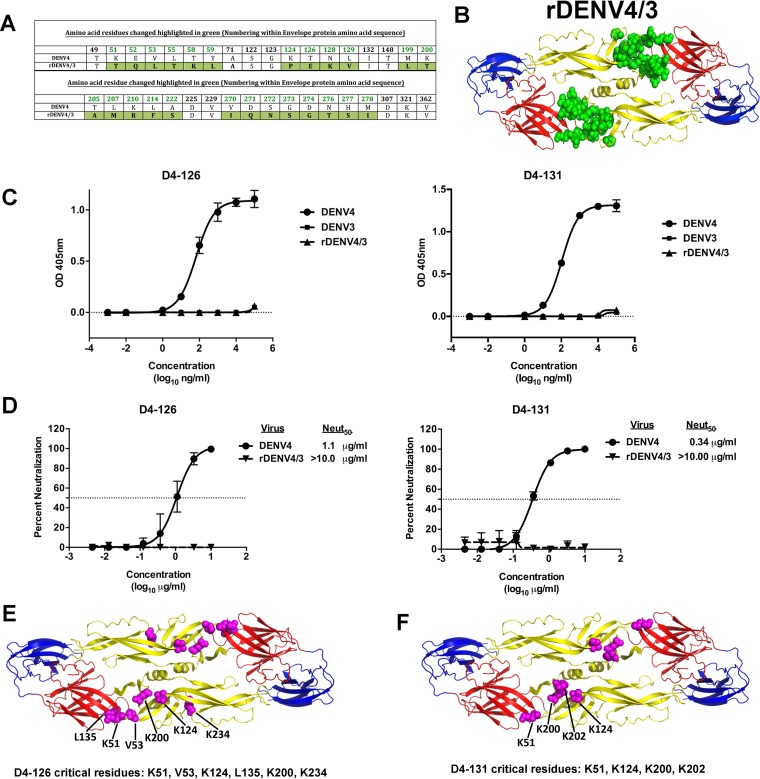
The EDI/II hinge region is important for the binding and neutralization of DENV4 by hMAbs D4-126 and D4-131. (A) Amino acid alignment of DENV4 and rDENV4/3 E protein sequence in the EDI/II hinge; EDII regions with mutated residues are colored in green. (B) Recombinant DENV4 containing EDI/II hinge; EDII residues from DENV3 (rDENV4/3) used to map the epitopes of hMAbs D4-126 and D4-131 are shown. The figure depicts a model of the DENV4 E protein dimer, with the mutated residues colored in green. (C) Binding of hMAbs D4-126 and D4-131 to DENV4, DENV3, or rDENV4/3. (D) DENV4 and rDENV4/3 neutralization by hMAbs D4-126 and D4-131. Results are from 2 technical replicates in an experiment. Each point represents the mean from the two replicates, and the error bars depict the standard deviations. Mapping of the critical residues for D4-126 and D4-131 was performed by shotgun mutagenesis by screening for loss of MAb binding to a DENV4 alanine scan mutation array. Critical residues are visualized as magenta spheres on the DENV E dimer structure (PDB identifier [ID] 1OAN). (E) Six critical residues were identified for D4-126 in the E protein DII and DI/II hinge region. (F) Four critical residues were identified for D4-131 in the E protein DII and DI/II hinge region.
As an alternative approach to mapping the epitopes of D4-126 and D4-131, both hMAbs were screened by shotgun mutagenesis against a comprehensive mutation library in which nearly every residue within the precursor membrane (prM) and E was individually mutated to alanine, as described previously (35). Residues were identified as critical to binding of the DENV4 hMAb if they did not bind to the DENV4 hMAb but did bind to other conformation-dependent MAbs. Six amino acids (K51, V53, K124, L135, K200, and K234) in the EDI/II hinge and EDII regions were critical for binding of D4-126 (Fig. 4E). K51, V53, K124, and K200 were among the residues that were altered in rDENV4/3. Four amino acids (K51, K124, K200, and K202) within the EDI/II hinge and EDII region were critical for binding of D4-131 (Fig. 4F). K51, K124, and K200 were among the residues that were altered in rDENV4/3. These data validated our observations that the EDI/II hinge region residues are critical for binding of D4-126 and D4-131 and also indicated that the epitopes differ slightly between the two hMAbs.
DENV4 neutralizing hMAbs define epitopes targeted by serum antibodies in DENV4-immune individuals.
HMAbs D4-126 and D4-131 were isolated from circulating memory B cells. Serum antibodies are generally thought to derive from secretion of long-lived plasma cells (LLPCs) residing in the bone marrow. To determine if DENV4 polyclonal serum neutralizing antibodies in immune sera also targeted the D4-131 and D4-126 epitopes, we performed competition-binding assays with DENV4 immune serum samples and labeled DENV4-specific MAbs. As depicted in Fig. 5, DENV4 immune sera effectively blocked the binding of each of the MAbs, whereas DENV2 or DENV3 immune sera had marginal effects on MAb binding. The magnitude of the DENV4 immune responses (based on their neutralization titers for DENV4) in serum samples correlated with the ability to block 50% of the binding of D4-126 or D4-131. These findings indicated that there are antibodies in DENV4 polyclonal immune serum samples that bind to sites similar to those that overlap tightly with the epitopes recognized by hMAbs D4-126 and D4-131.
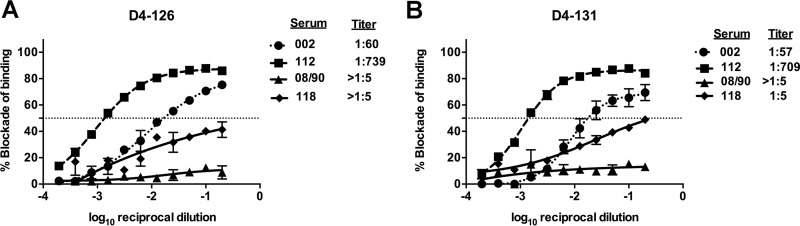
DENV4 immune sera block the binding of hMAb D4-126 or D4-131 to DENV4. Blockade of binding assays was performed with DENV immune sera for MAb D4-126 (A) or MAb D4-131 (B). Blockade assays were performed using 2 primary DENV4 immune sera (002 and 112), a primary DENV2 immune serum (08/90), or a primary DENV3 immune serum (118). The epitope-specific response in the serum samples was calculated by determining the serum dilution factor that led to a 50% reduction in hMAb binding to DENV4. Each value depicts the mean blockade of binding from two replicates in an experiment. Each point represents the mean from the two replicates, and the error bar depicts the standard deviation.
To further assess the epitope specificity of functionally neutralizing antibodies in DENV4 sera collected following infection or experimental vaccination, we performed neutralization assays with rDENV4/3, which had lost the epitopes recognized by hMAbs D4-126 and D4-131. Neutralization assays using the rDENV4/3 strain were performed with sera from six subjects with prior natural DENV4 infection and sera from 4 subjects previously immunized with a DENV4 live attenuated vaccine. The percent loss of neutralization was calculated as follows: percent loss of neutralization = 100 − [(rDENV4/3 Neut50/DENV4 Neut50) × 100] (Table 4). Sera from both types of immune donors neutralized the rDENV4/3 strain less efficiently than sera from wild-type (WT) DENV4, although to differing extents. The mean percentages of loss in neutralization with rDENV4/3 in both naturally infected immune sera and vaccine sera were significant (P = 0.005 and P = 0.002, respectively) by one-sample t test. Of the six DENV4 postinfection sera tested, those from three subjects (002, 102, and 07/333) showed a >60% loss in neutralization against rDENV4/3, while the remaining infection sera displayed a more modest or no detectable reduction in neutralization (Fig. 6A). All of the vaccine sera tested also showed a >60% loss in neutralization against the rDENV4/3 strain (Fig. 6B). These results suggested that the EDI/II hinge region is a target of polyclonal type-specific DENV4 neutralizing antibodies.
TABLE 4
Percent loss in neutralization of polyclonal DENV4 immune and vaccine sera against rDENV4/3a
| Serum type | Serum sample | Reciprocal of Neut50 titers against DENV4 and rDENV4/3 | % loss in neutralization | |
|---|---|---|---|---|
| DENV4 | rDENV4/3 | |||
| DENV4 immune sera | 002 | 115 | 44 | 62 |
| 102 | 153 | 31 | 80 | |
| 112 | 1,746 | 1,609 | 8 | |
| 07/333 | 1,256 | 399 | 69 | |
| 06/302 | 1,660 | 1,058 | 37 | |
| 06/105 | 399 | 204 | 49 | |
| DENV4 vaccine immune sera | 256.03.36 | 562 | 178 | 69 |
| 256.03.38 | 255 | 5 | 98 | |
| 256.03.57 | 153 | 5 | 97 | |
| 256.03.68 | 1,167 | 276 | 76 | |
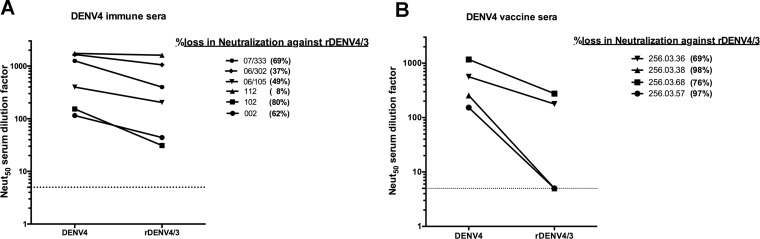
The DENV4 EDI/II hinge region is a target of neutralizing antibodies in people exposed to DENV4 infection or to an experimental vaccine. A U937+DC-SIGN flow cytometry-based neutralization assay was performed with primary DENV4 infection sera (A) or DENV4 monovalent vaccine sera (B) and WT DENV4 or rDENV4/3. The 50% neutralization (Neut50) titers were calculated and plotted. Values corresponding to loss of neutralization against rDENV4/3 for each sample are indicated. Samples that did not block 50% of infection at the highest concentration were assigned a value of 5. Results are from 2 technical replicates in one experiment. Each point represents the mean neutralization value from the two replicates.
DISCUSSION
Sustained humoral immunity depends on LLPCs to maintain protective levels of antibody in serum and on MBCs, which comprise a subset of cells poised to expand and adapt in response to subsequent exposure to the infecting pathogen (41). In this study, we characterized the properties of MBC- and LLPC-derived human antibodies that neutralize DENV4. Although people exposed to DENV4 infections developed serotype cross-reactive and type-specific antibodies, our results show that the type-specific antibodies were the principal determinants of neutralization of DENV4. Using MAbs isolated from the MBCs of people exposed to DENV4, we identified epitopes centered on the EDI/II hinge as the target of DENV4 neutralizing antibodies. In people exposed to DENV4 infections or a live attenuated vaccine candidate, both MBC- and LLPC-derived polyclonal neutralizing antibodies also recognized epitopes centered on the EDI/II hinge of DENV4.
Substantial progress has been made in understanding the epitopes targeted by human antibodies that neutralize DENV serotypes 1, 2, and 3 (2, 18,–21, 30), whereas serotype 4 is relatively less studied. The two DENV4 neutralizing MAbs whose results are reported in this study were sensitive to changes in or near the EDI/II hinge region. The hinge region plays a critical role in the conformational change that the E protein undergoes at low pH to fuse to the endosomal membrane allowing viral uncoating and the release of viral RNA into the cellular cytoplasm (42). Because these epitopes are located in this region, it is reasonable to hypothesize that these DENV4 hMAbs act by preventing conformational changes in the E protein required for fusion, entry, and initiation of a productive viral infection.
As reported previously with DENV3 natural isolates (43), some DENV4 epitopes targeted by neutralizing mouse MAbs differ in potency depending on the DENV4 genotype (35). We evaluated if hMAb D4-126 and D4-131 effectively neutralized different strains of DENV4. All strains studied here were neutralized well, except for one GI strain (Cambodia 2010) that was resistant and partially resistant to hMAb D4-126 and D4-131, respectively. Three of the 16 amino acids differing between the E proteins of Sri Lanka 1992 (GII) and the Cambodian 2010 (GI) in EDII (L122S, T203K, and H233Y) overlapped with the region identified by shotgun mutagenesis as representing the binding sites of D4-126 and D4-131. We propose that natural variation between DENV4 strains leads to poor or altered binding of D4-126 and D4-131 and neutralization escape. Moreover, recent studies demonstrated that the virion particles of some DENV strains flex and “breathe” more than those of other strains, which can also lead to better exposure of partially hidden epitopes (35, 44, 45). Differences in amino acid sequences outside the main footprints of D4-126 and D4-131 also may alter epitope exposure indirectly and contribute to strain-specific differences in neutralization sensitivity.
The LLPC-derived polyclonal serum antibodies likely provide the first line of defense against reinfection in vivo. Our studies using blockade of antibody binding demonstrated that the DENV4 polyclonal immune sera contained antibodies that specifically blocked the binding of MAbs D4-126 and D4-131 to their epitopes. Additionally, a recombinant DENV4 strain missing the D4-126 and D4-131 epitopes was less sensitive than WT DENV4 to neutralization by DENV4 infection or vaccine immune sera. These results establish that the regions/epitopes defined using MAbs are targets of the LLPC-derived polyclonal serum antibody response as well. In some individuals, a fraction or most of the serum DENV4 neutralizing antibody response was unaffected by EDI/II hinge mutations, indicating that other regions and epitopes are likely involved in DENV4 neutralization. Chimpanzee DENV4 type-specific strongly neutralizing MAb 5H2 is directed to the EDI region (23). Cockburn et al. demonstrated that at least a portion of the antibodies in DENV4 convalescent patient sera bound to epitopes on DI that overlapped with that of the MAb 5H2 footprint (24). Hence, our findings, while providing good insights into the DENV4 neutralizing antibody responses, clearly emphasize the need for further analyses of the polyclonal serum neutralizing antibody responses using a larger panel of DENV4 immune sera. There is also a need to finely map the epitopes of the other neutralizing antibodies against DENV4 to draw broader and more generalizable conclusions about the DENV4 serum neutralizing antibody responses.
In summary, we propose that the EDI/II hinge region is a target of DENV4 neutralizing human antibodies in both the MBC and LLPC compartments. The EDI/II hinge region is also a target of human type-specific antibodies that neutralize DENV1 and DENV3. Our observations have implications for understanding the mechanisms of DENV4 neutralization, evaluating new vaccine candidates, and developing next-generation vaccines.
MATERIALS AND METHODS
Cells.
Aedes albopictus C6/36 cells (American Type Culture Collection; CRL-1660) were maintained in minimal essential medium (MEM; Gibco) at 32°C. Vero cells (American Type Culture Collection; CCL-81) were maintained in Dulbecco's modified Eagle's medium-F12 (DMEM-F12) at 37°C. A human monocyte lymphoma cell line (U937) (American Type Culture Collection; CRL-1593.2) ectopically expressing dendritic cell (DC)-specific intercellular adhesion molecule-3-grabbing nonintegrin DC-SIGN (U937+DC-SIGN) (46, 47) was maintained in RPMI 1640 (Gibco) medium supplemented with 50 mM beta-mercaptoethanol at 37°C. U937+ DC-SIGN cells were kindly provided by the laboratory of Mark Heise at the University of North Carolina, Chapel Hill. All growth and maintenance media used were supplemented with 5% fetal bovine serum (FBS), 100 U/ml penicillin, 100 mg/ml streptomycin, 0.1 mM nonessential amino acids (Gibco), and 2 mM glutamine. All cells were incubated in the presence of 5% CO2. The 5% FBS was reduced to 2% to make infection medium for each cell line.
Ethics statement.
Convalescent DENV immune blood samples were collected from travelers visiting countries in which dengue is endemic. The protocol for obtaining dengue immune blood samples was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill (protocol 08-0895). Written informed consent was obtained from all subjects before their participation in the study.
DENV immune sera.
DENV serum samples used in this study were obtained from the previously existing dengue traveler collections as mentioned above. The samples were not from acute clinical patient samples and hence were not PCR confirmed. DENV4 immune sera were also obtained from people who had received a live attenuated monovalent DENV4 vaccine (25, 26) developed by the US National Institutes of Health (NIH) and were provided by Anna Durbin and Stephen Whitehead. All samples were coded and analyzed anonymously.
Viruses, rE, and rEDIII.
DENV1 (American genotype; strain West Pac74), DENV2 (Asian genotype; strain S-16803), DENV3 (Asian genotype; strain CH-53489), and DENV4 (American genotype; strain TVP-376) (provided by Robert Putnak, Walter Reed Army Institute of Research, Silver Spring, MD) were used for both binding enzyme-linked immunosorbent assays (ELISAs) and neutralization assays. All viruses used in the neutralization assays were grown in C6/36 Aedes albopictus mosquito cells at 32°C, as previously described (48). DENV4 was purified in our laboratory as previously described (16). Purified live DENV2 was purchased from Microbix Biosystems, Inc. (Mississauga, Ontario, Canada). Recombinant envelope (rE) proteins (80% of E protein) from each of the four serotypes were produced within our laboratory or purchased from Hawaii Biotech, Inc. (49). Recombinant EDIII proteins were produced within our laboratory as described previously (50).
Depletion of DENV4-specific antibodies from human immune sera collected from subjects with prior DENV4 infection or vaccination.
Purified DENV was absorbed onto 4.5-μm-diameter Polybead polystyrene microspheres (Polysciences, Inc.) at a bead (microliters) to ligand (micrograms) ratio of 5:2. Polystyrene beads were washed three times with 0.1 M borate buffer (pH 8.5) and incubated with the relevant purified DENV (DENV4 for homotypic depletions and DENV2 for heterotypic depletions) overnight at room temperature (RT). Control beads were incubated overnight with an equivalent amount of bovine serum albumin (BSA). The control and virus-adsorbed beads were blocked with BSA (10 mg/ml)–borate buffer for 30 min at RT three times and washed four times with phosphate-buffered saline (PBS). DENV4 immune sera from naturally infected individuals or NIH vaccine candidate recipients were depleted of virus-specific antibodies by incubating the samples with virus-adsorbed beads for 1 h at 37°C with end-over-end mixing. Samples were subjected to at least three sequential rounds of depletions before successful removal of the respective antibodies was confirmed by ELISA. The ability of the depleted samples to neutralize viruses of all of the four serotypes was tested after the confirmation ELISA.
Generation of DENV4-specific MAbs.
Previously cryopreserved peripheral blood mononuclear cells (PBMCs) isolated from blood samples collected as part of the dengue traveler studies were thawed rapidly in a 37°C water bath and washed prior to transformation with Epstein-Barr virus (EBV) contained in the clarified supernatants from cultures of B95.8 cells (ATCC VR-1492) and incubated with CpG and additional supplements, as described previously (27, 51). Cultures were incubated at 37°C with 5% CO2 for 10 days prior to screening for DENV4-reactive cell lines with ELISA. The minimal frequency of DENV4-reactive B cells was estimated on the basis of the number of wells with DENV4-reactive supernatants compared to the total number of lymphoblastoid cell line (LCL) colonies in the transformation plates as follows: (number of wells with DENV4-reactive supernatants)/(number of LCL colonies in the plate). Cells from wells with supernatants reacting in the DENV4 capture ELISA were subjected to cytofusion, as previously described (27, 51). Following cytofusion, hybridomas were selected for growth in hypoxanthine-aminopterin-thymidine (HAT) medium containing ouabain. Wells containing hybridomas producing DENV4-reactive antibodies were cloned biologically by 3 rounds of limiting dilution plating. Once clonal, the cell lines were used to produce MAb immunoglobulin G (IgG) in cell supernatants, using serum-free medium, followed by protein G column purification.
Virus, rE, and rEDIII ELISA.
Equivalent quantities of DENV (as previously titrated by ELISA) were captured by anti-E mouse MAb 4G2, or rE proteins were directly coated (rE, 100 ng/well; rEDIII, 200 ng/well) on ELISA plates overnight at 4°C (39). Plates were blocked with 3% (vol/vol) normal goat serum (Gibco, Thermo Fisher, USA)–Tris-buffered saline (TBS)–0.05% (vol/vol) Tween 20 (blocking buffer). Primary antibodies were diluted serially to generate a range of concentrations. Alkaline phosphatase-conjugated secondary antibodies were used to detect binding of primary antibodies with p-nitrophenyl phosphate substrate, and reaction color changes were quantified by spectrophotometry.
Blockade of binding assays.
Assays for blockade of binding were performed as described previously (52, 53). Briefly, DENV4 was captured using mouse anti-E MAb 4G2 and was blocked as described above. Serial dilutions of DENV serum were added to the DENV4-coated plates and incubated at 37°C for 1 h. The plates were washed, and alkaline phosphatase-conjugated DENV4 hMAbs were added and incubated at 37°C for 1 h. P-nitrophenyl phosphate substrate was added, and reaction color changes were quantified by spectrophotometry. Percentages of blockade of binding were calculated as follows: [100 − (optical density of sample/optical density of control) × 100].
Flow cytometry-based U937+DC-SIGN neutralization assay.
Neutralization potentials of the DENV4 immune sera and hMAbs were measured using a flow cytometry-based neutralization assay with U937+DC-SIGN cells as previously described (17). Briefly, virus and antibody mixtures or sera were incubated for 1 h at 37°C, prior to the addition of U937+DC-SIGN cells. After 2 h of incubation, cells were washed twice with infection media. Cells then were fixed and permeabilized 24 h after infection and probed with 2H2 (anti-prM MAb) conjugated to Alexa-Fluor 488, and the percentage of infected cells was determined using a Guava flow cytometer (FC) (EMD Millipore). Stained cells were analyzed to calculate 50% neutralization titers.
Construction of rDENV strains.
rDENV4/3 was generated as previously described (39). Briefly, the DENV4 genome (Sri Lanka 1992; GenBank accession no. KJ160504.1) (36) was split into 4 separate plasmids (plasmids A, B, C, and D), allowing production of genomic cDNA. Plasmids were digested, and genome fragments were ligated together into a full-length cDNA genome from which RNA transcripts were derived. These transcripts were electroporated into cells, and cell culture supernatant containing viable virus was harvested. Virus was passaged two times on C6/36 cell monolayer cultures and stored at −80°C. To generate rDENV, the nucleotide sequence of the E glycoprotein was changed to alter the amino acid residues. rDENV4/3 contains EDI/II hinge residues (with 25 amino acids altered) from DENV3 (Fig. 4A) (Sri Lanka 1989, designated UNC3001; NCBI accession no. JQ411814.1) (43).
Generation of DENV4 strains displaying diverse E glycoproteins.
In order to examine genetic diversity within a serotype, a panel of near-isogenic rDENV4 was generated by replacing the E gene of WT genotype II infectious clone virus (Sri Lanka 1992; accession no. KJ160504.1) (36) with E glycoprotein genes representing diverse strains within DENV4. Subgenomic A plasmids encoding E protein genes only were synthesized (all other proteins were encoded by genes based on DENV Sri Lanka 1992) representing genotype I (GI) (Cambodia 2010; NCBI accession no. KF543272.1) (V. Duong, S. Lay, and P. Buchy, unpublished data) or genotype II (GII) (Puerto Rico 1999; NCBI accession no. FJ882599.1), available in GenBank database (http://www.ncbi.nlm.nih.gov/nuccore/228539113) or a sylvatic E protein sequence (Malaysia 1973; NCBI accession no. JF262780) (37). Recombinant subgenomic A plasmids were synthesized, and then viral assembly and rescue were performed as described above for generation of rDENV4.
Shotgun mutagenesis epitope mapping.
Shotgun mutagenesis epitope mapping was performed as described previously (35). Briefly, a DENV4 prM-E protein expression construct was subjected to high-throughput alanine scanning mutagenesis to generate a comprehensive mutation library (with each residue mutated to alanine and alanine residues mutated to serine). Expression plasmids encoding the mutant proteins (97% coverage) were generated and arrayed into 384-well plates. Mutants were transfected into HEK-293T cells (American Type Culture Collection CRL-3216) and allowed to express for 22 h. Cells were fixed in 4% (vol/vol) paraformaldehyde (Electron Microscopy Sciences) and permeabilized with 0.1% (wt/vol) saponin (Sigma)–PBS containing calcium and magnesium. Cells were stained with purified anti-DENV4 hMAbs (D4-126 and D4-131) diluted in 10% normal goat serum (NGS; Sigma)–0.1% saponin (pH 9.0). Antibody binding was detected using Alexa Fluor 488-conjugated secondary antibody (Jackson ImmunoResearch Laboratories)–10% NGS (Sigma)–0.1% saponin. Cells were washed three times with PBS supplemented with 0.1% saponin, 1 mM MgCl2, and CaCl2 followed by two washes in PBS. The mean cellular fluorescence was detected using a high-throughput flow cytometer (HTFC; Intellicyt). Mutations were identified as critical to the hMAb epitope if they did not bind the test hMAb but did bind other conformation-dependent MAbs. This counter-screen strategy facilitated the exclusion of E mutants that were locally misfolded or had expression defects (54).
ACKNOWLEDGMENTS
We thank all the dengue-immune travelers who participated in the study. We also thank members of the de Silva laboratory for their assistance.
This research was supported by funding from U.S. National Institutes of Health grants R01 AI107731, (principal investigator [PI], Aravinda M. de Silva), R01 AI125198 (PI, Aravinda M. de Silva), U19 AI109761 (PI, Ralph S. Baric), and P01 AI106695 (PI, Eva Harris) and the Bill and Melinda Gates Foundation (PI, Anna P. Durbin).
REFERENCES
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.02041-16
Read article for free, from open access legal sources, via Unpaywall:
https://jvi.asm.org/content/jvi/91/5/e02041-16.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/jvi.02041-16
Article citations
Dengue virus 4/2 envelope domain chimeric virus panel maps type-specific responses against dengue serotype 2.
mBio, 14(5):e0081823, 06 Oct 2023
Cited by: 0 articles | PMID: 37800919 | PMCID: PMC10653845
Blockade-of-Binding Activities toward Envelope-Associated, Type-Specific Epitopes as a Correlative Marker for Dengue Virus-Neutralizing Antibody.
Microbiol Spectr, 11(4):e0091823, 06 Jul 2023
Cited by: 2 articles | PMID: 37409936 | PMCID: PMC10433959
A live dengue virus vaccine carrying a chimeric envelope glycoprotein elicits dual DENV2-DENV4 serotype-specific immunity.
Nat Commun, 14(1):1371, 13 Mar 2023
Cited by: 7 articles | PMID: 36914616 | PMCID: PMC10009830
In Silico Comparative Analysis of Predicted B Cell Epitopes against Dengue Virus (Serotypes 1-4) Isolated from the Philippines.
Vaccines (Basel), 10(8):1259, 05 Aug 2022
Cited by: 1 article | PMID: 36016147 | PMCID: PMC9415047
Vaccine-induced antibodies to contemporary strains of dengue virus type 4 show a mechanistic correlate of protective immunity.
Cell Rep, 39(10):110930, 01 Jun 2022
Cited by: 3 articles | PMID: 35675766 | PMCID: PMC9326871
Go to all (35) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Nucleotide Sequences (Showing 6 of 6)
- (3 citations) ENA - KJ160504
- (2 citations) ENA - JF262780
- (2 citations) ENA - KF543272
- (2 citations) ENA - FJ882599
- (1 citation) ENA - KC963424
- (1 citation) ENA - JQ411814
Show less
Protein structures in PDBe
-
(1 citation)
PDBe - 1OANView structure
Protocols & materials
Related Immune Epitope Information - Immune Epitope Database and Analysis Resource
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A new quaternary structure epitope on dengue virus serotype 2 is the target of durable type-specific neutralizing antibodies.
mBio, 6(5):e01461-15, 13 Oct 2015
Cited by: 66 articles | PMID: 26463165 | PMCID: PMC4620467
Analysis of Individuals from a Dengue-Endemic Region Helps Define the Footprint and Repertoire of Antibodies Targeting Dengue Virus 3 Type-Specific Epitopes.
mBio, 8(5):e01205-17, 19 Sep 2017
Cited by: 9 articles | PMID: 28928210 | PMCID: PMC5605938
Beyond Neutralizing Antibody Levels: The Epitope Specificity of Antibodies Induced by National Institutes of Health Monovalent Dengue Virus Vaccines.
J Infect Dis, 220(2):219-227, 01 Jun 2019
Cited by: 16 articles | PMID: 30895307 | PMCID: PMC6581895
The human antibody response to dengue virus infection.
Viruses, 3(12):2374-2395, 25 Nov 2011
Cited by: 196 articles | PMID: 22355444 | PMCID: PMC3280510
Review Free full text in Europe PMC
Funding
Funders who supported this work.
HHS | National Institutes of Health (4)
Grant ID: AI109761 (Project 1)
Grant ID: R01 AI107731
Grant ID: AI125198
Grant ID: PO1 AI106695 (Eva Harris)
NIAID NIH HHS (5)
Grant ID: U19 AI109761
Grant ID: P30 AI050410
Grant ID: R01 AI107731
Grant ID: P01 AI106695
Grant ID: R01 AI125198
 b,c,d and
b,c,d and 




