Semin Immunol. Author manuscript; available in PMC 2019 Feb 1.
Published in final edited form as:
PMCID: PMC5866202
NIHMSID: NIHMS928408
Neutrophils and PMN-MDSCs: their biological role and interaction with stromal cells
,1,2 ,3 ,1,2 and 1,2,3
Jie Zhou
1Institute of Human Virology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, China
2Key Laboratory of Tropical Disease Control, Chinese Ministry of Education, Guangzhou, China
Yulia Nefedova
3The Wistar Institute, Philadelphia, PA, USA, 19104
Aihua Lei
1Institute of Human Virology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, China
2Key Laboratory of Tropical Disease Control, Chinese Ministry of Education, Guangzhou, China
Dmitry Gabrilovich
1Institute of Human Virology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, China
2Key Laboratory of Tropical Disease Control, Chinese Ministry of Education, Guangzhou, China
3The Wistar Institute, Philadelphia, PA, USA, 19104
1Institute of Human Virology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, China
2Key Laboratory of Tropical Disease Control, Chinese Ministry of Education, Guangzhou, China
3The Wistar Institute, Philadelphia, PA, USA, 19104
Address for correspondence: Dmitry Gabrilovich, The Wistar Institute, 3601 Spruce Str., Rm. 118, Philadelphia, PA, 19104;
gro.ratsiw@hcivolirbagdThe publisher's final edited version of this article is available at
Semin ImmunolSee other articles in PMC that
cite the published article.
Abstract
Neutrophils and polymorphonucler myeloid-derived suppressor cells (PMN-MDSC) share origin and many morphological and phenotypic features. However, they have different biological role. Neutrophils are one of the major mechanisms of protection against invading pathogens, whereas PMN-MDSC have immune suppressive activity and restrict immune responses in cancer, chronic infectious disease, trauma, sepsis, and many other pathological conditions. Although in healthy adult individuals, PMN-MDSC are not or barely detectable, in patients with cancer and many other diseases they accumulate at various degree and co-exist with neutrophils. Recent advances allow for better distinction of these cells and better understanding of their biological role. Accumulating evidence indicates PMN-MDSC as pathologically activated neutrophils, with important role in regulation of immune responses. In this review, we provide an overview on the definition and characterization of PMN-MDSCs and neutrophils, their pathological significance in a variety of diseases, and their interaction with other stromal components.
Keywords: myeloid-derived suppressor cells, neutrophils, fibroblasts, cancer, infectious diseases
1. Introduction
Myeloid-derived suppressor cells (MDSC) are a heterogeneous population of pathologically activated myeloid precursors and relatively immature myeloid cells that accumulate under many pathological conditions [1, 2]. Currently, MDSC could be further divided into two major subsets: polymorphonuclear (PMN)-MDSC and monocytic (M)-MDSC. PMN-MDSC share many morphological and phenotypic characteristics of neutrophils, whereas M-MDSC are similar to monocytes [3, 4]. In both mice and humans PMN-MDSC represent the most abundant population of MDSC. In recent years an important biological role of PMN-MDSCs has emerged. These cells have been implicated in control of immune responses and their clinical relevance has been demonstrated in cancer and other pathologic conditions. In this review, we discuss main features of PMN-MDSC vis-a-vis neutrophils and their significance in cancer and infectious diseases.
2. Neutrophil differentiation
Neutrophils are the most abundant type of granulocytes [5, 6]. The development of neutrophils occurs in the bone marrow (BM) and involves several defined steps including common myeloid progenitors, granulocyte–monocyte myeloid progenitors, myeloblasts, promyelocytes, myelocytes, metamyelocytes, band neutrophils and, finally, segmented neutrophils [7, 8]. The granules formed during neutrophil maturation serve as a reservoir of anti-microbial factors and enzymes, such as myeloperoxidase (MPO), neutrophil elastase (NE), defensins, cathelicidins and matrix metalloproteinase (MMP), which protect hosts from infections and promote the resolution of inflammation [9, 10]. After generation in the BM and brief period of circulation in peripheral blood, neutrophils migrate to the tissues. This migration is regulated by chemokines released by activated endothelial cells, fibroblasts, macrophages, and various products of microorganisms in case of infection. Apoptotic neutrophils are cleared primarily by resident tissue macrophages [6, 11, 12].
Granulocyte-colony stimulating factor (G-CSF, CSF3) has been identified as a key regulator in neutrophil development [8, 13, 14]. G-CSF receptor is expressed during granulocyte differentiation from early progenitors to mature neutrophils. However, G-CSF is not absolutely required for granulocytopoiesis as G-CSF deficient mice have around 25% of residual granulocytopoiesis and can produce fully differentiated neutrophils [15]. Other factors, such as granulocyte–macrophage-colony stimulating factor (GM-CSF, CSF2), interleukin 6 (IL-6), and c-kit ligand have been implicated in neutrophil development [16–18]. Retention of neutrophils in the BM largely depends on chemokine receptor CXCR4. Deletion of CXCR4 led to release of neutrophils from the BM into circulation [19]. In contrast, chemokine receptor CXCR2, which functions through its ligands CXCL1/CXCL2, promotes the release of neutrophils from the BM [20]. G-CSF has been shown to downregulate the expression of CXCR4 and its ligand CXCL12, leading to CXCR2/CXCL2-mediated mobilization of neutrophils into circulation [21].
3. PMN-MDSCs and neutrophils: how to distinguish these cells?
Neutrophils and PMN-MDSC have the same origin and follow the same differentiation pathway described above. They are also phenotypically similar. In mice, these cells have a phenotype of CD11b+Ly6G+Ly6Clow; in humans, they are defined as CD14−CD11b+CD15+(CD66b+) cells [2, 22]. Human neutrophils and PMN-MDSC can be separated by gradient centrifugation using 1.077 g/mL density gradient media [23]. Neutrophils are high density cells, whereas PMN-MDSC are enriched in a low-density mononuclear cell fraction. Although gradient centrifugation is actively used for evaluation of PMN-MDSC in clinical studies, there are some limitations in this method that should be considered. Specifically, a number of cells in a low-density fraction can be represented by activated neutrophils rather than PMN-MDSC. Moreover, some PMN-MDSC can be located in a high-density fraction. This may potentially lead to miscalculation of the proportion of these cells under pathological conditions. A proportion of PMN-MDSC may also be affected by mishandling of peripheral blood samples including their prolonged storage or freezing [24]. Therefore, the time before sample processing and flow cytometric analysis of PMN-MDSC should be minimized.
Despite phenotypic similarity, differences in the expression level of some surface markers have been reported between neutrophils and PMN-MDSC. Thus, mouse PMN-MDSC express higher levels of CD115 and CD244 than neutrophils [25]. However, due to heterogeneity of PMN-MDSC these markers have limited value for defining these cell populations. Recently, lectin type oxidized LDL receptor 1 (LOX-1), highly expressed on human PMN-MDSCs, has been shown to distinguish these cells from neutrophils in peripheral blood and tumor tissues of patients with a variety of cancers [26]. LOX-1 could potentially serve as a marker for identification of PMN-MDSC in cancer patients; however, more studies are needed to confirm it.
PMN-MDSC, but not neutrophils, are immunosuppressive [22, 27]. In addition to directly inhibiting T-cell function, PMN-MDSC can inhibit the activity and function of other myeloid cells and NK cells [28, 29]. In contrast, neutrophils are generally involved in the activation, regulation and effector functions of other myeloid and lymphoid cells (rev. in [5]).
PMN-MDSC and neutrophils have different molecular and biochemical characteristics [3, 22]. Quantitative proteomics of murine MDSC determined that these cells constitute a distinct myeloid population characterized by a “kinase signature” and well-defined “interactome” [30, 31]. Whole-transcriptome analysis revealed a clear difference between PMN-MDSC from tumor-bearing mice and neutrophils from tumor-free mice [25, 32]. PMN-MDSC had a higher expression of genes involved in the cell cycle, autophagy, G-protein signaling, and CREB pathway, whereas neutrophils had an elevated expression of genes associated with NF-κB signaling and lymphotoxin-β receptor signaling [25]. Further analysis confirmed that neutrophils have substantially higher basal levels of phosphorylated c-Jun, p38, JNK, and ERK1/2 than PMN-MDSC [25]. Activated neutrophils expressed significantly higher level of TNF-α as compared to PMN-MDSC [25]. In head and neck cancer patients, Brandau et al. identified a subpopulation of PMN-MDSC with reduced release of IL-8 in response to LPS as compared to mature neutrophils [33]. Some genes encoding chemokines and their receptors associated with migration were differentially expressed in PMN-MDSC and neutrophils. For instance, neutrophils had an increased levels of CXCL4 and CXCL12 and reduced expression of CCL3, CCL4, and CXCL2 as compared to PMN-MDSC [32]. PMN-MDSCs from cancer patients showed markedly reduced chemotaxis toward tumor conditioned medium and lower expression of chemokine receptors CXCR1 and CXCR2, which are necessary for neutrophil extravasation from the bloodstream and subsequent tissue infiltration [33].
A number of signaling pathways have been implicated in regulation of PMN-MDSC development and function. Activation of STAT3 was shown to be responsible for accumulation of MDSC. Therefore, upregulation of this transcription factor is considered to be a hallmark of PMN-MDSC in humans and mice [34]. Downregulation of interferon related factor (IRF)-8, a member of the IRF family, has been closely associated with PMN-MDSC expansion in mice [35–37]. It has been recently reported that tumor growth was accompanied by a selective expansion of IRF8low granulocyte progenitors with increased capability of differentiation into PMN-MDSC [38]. Up-regulation of C/EBPβ transcription factor, a member of a family of basic-region-leucine zipper transcriptional factors, was associated with MDSC expansion [39]. The most prominent factors implicated in PMN-MDSC suppressive activity on T lymphocytes so far include arginase, reactive oxygen species (ROS), and prostaglandin E2 (PGE2) [40–42]. Changes in oxidative phosphorylation and glycolysis in tumors have also been associated with MDSC function. In vitro models, increase in glycolysis rate was concurrent with an increased arginase 1 activity in MDSC [43]. Recently, tumor-infiltrating MDSC have been shown to preferentially utilize fatty acid β oxidation as a primary source of energy [44].
Endoplasmic reticulum (ER) stress response has recently emerged as an important mechanism involved in regulation of pathologic activation of MDSC and thus critical for their functions. MDSC from tumor-bearing mice and cancer patients demonstrated a significantly increased ER stress response than neutrophils and monocytes from tumor-free hosts [45]. Experimental induction of ER stress enhanced the immunosuppressive capacity of tumor-infiltrating MDSC; this effect was mediated through upregulation of ARG1, NOS2, and NOX2 expression [26]. MDSC isolated from tumors established in C/EBP homologous protein (CHOP)-deficient mice demonstrated reduced expression of phospho-STAT3 and decreased production of IL-6 and arginase 1, leading to decreased immunosuppressive activity in these cells [46]. Increased spliced X-box binding protein 1 (sXBP1), a member of another pathway of ER stress response, was observed in human LOX-1+ PMN-MDSC as compared to LOX-1− neutrophils [26]. Moreover, the induction of ER stress in neutrophils isolated from healthy donors converted them into potent immune suppressive cells [26]. Several other mechanisms of MDSC-mediated immune suppression include activation of regulatory T cells, increased expression of immune suppressive cytokines transforming growth factor β (TGF-β) and IL-10, sequestration of cysteine, and decreased expression of L-selectin by T cells among others [47].
4. Biological significance of neutrophils and PMN-MDSC interaction with stromal cells
The interaction of neutrophils with endothelial or epithelial cells has been viewed as “a double-edged sword”. On the one side, communication between these cells is essential for neutrophil migration and subsequent antimicrobial function. On the other side, their interaction causes tissue damage [20, 48].
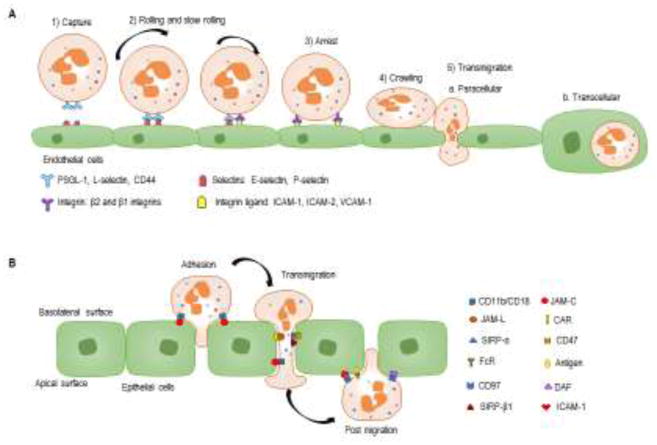
Mature neutrophils migrate to the sites of tissue inflammation or infection through the vasculature, primarily post-capillary venules, in a well-defined sequential process referred to as neutrophil recruitment [49–52]. A clear example of the complex interaction between neutrophil and stromal cellular components such as the endothelial cells and epithelial cells were shown in . Neutrophil recruitment cascade involves multiple interactions of the neutrophil receptors with their ligands expressed on activated endothelium. The classical neutrophil recruitment cascade consists of the following steps: capturing, rolling, firm arrest, crawling, and transmigration. Transmigration occurs between endothelial cells (paracellularly) or through endothelial cells (transcellularly). While paracellular transmigration is the prevalent type, transcellular transmigration occurs in case of high intracellular adhesion molecule (ICAM)-1 expression by endothelial cells [53]. In comparison with transendothelial migration, transepithelial migration of neutrophils occurs only paracellularly. The molecular mechanisms of the neutrophil recruitment have been well defined and reviewed elsewhere [20]. During recruitment process, neutrophils release the content of their granules and produce ROS and cytokines, which together induce junction dissociation, leading to the loss of barrier integrity and consequently, to increased neutrophil transendothelial migration [54]. Neutrophil-derived proteinase 3 was found to play an important role in protecting endothelial cells from protease-activated receptor-1-induced permeability changes that occur during thrombotic and inflammatory events [55]. In contrast, heparin-binding protein released by neutrophils can induce distinct changes in endothelial cell barrier integrity through binding to proteoglycans on the cell surface [56]. Neutrophil-derived ROS and myeloperoxidase (MPO) also affect endothelial cell integrity, reducing barrier function [53, 57, 58]. It was recently shown that MPO promotes intestinal epithelial injury by inhibiting restitutive responses [59]. Thus, interactions of neutrophils with endothelial or epithelial cells during inflammation or infection can have a significant effect on the host barrier functions. There is no evidence suggesting that PMN-MDSC use different mechanisms for their interaction with endothelial and epithelial cells. In line with that, PMN-MDSC have a high level of ROS production, MPO and MMP expression, suggesting these cells possess an active machinery for transendothelial migration.
Sequential steps of neutrophil migration on endothelial and epithelial cellsA. A neutrophil migration on endothelial cells consists of the following steps: 1) binding of neutrophils to endothelial cells depends on the transient interaction of P- and E-selectins with their ligands, such as P-selection glycoprotein ligand (PSGL)-1, L-selectin and CD44; 2) rolling and slow rolling along the vessel wall depend on selectins and integrins (β2 and β1 integrins); 3) the interaction between activated integrins and their ligands (primarily ICAM-1 and ICAM-2) results in the firm neutrophil arrest on the endothelium; 4) crawling of neutrophils follows the chemokine gradient along the endothelium, which leads them to the preferential sites of transmigration; 5) transmigration of neutrophils via endothelial cell-cell junctions (paracellular transmigration) or through the endothelium (transcellular transmigration). B. For neutrophil migration across epithelia, the process contains three sequential steps: adhesion, migration, and post-migration stage. Neutrophil transepithelial migration starts with adhesion of the neutrophils to the basolateral epithelial membrane, which is supported by ligation of CD11b/CD18 on the neutrophil surface to several molecules on the epithelial surface including fucosylated glycoproteins, JAM-C. After adhesion, neutrophils crawl along the epithelial cell membrane through sequential binding to several epithelial cell surface molecules, such as CD47 (binding to SIRPα). Tight junction between neutrophil and epithelium requires binding of JAML to epithelial CAR. After neutrophils completely go through the epithelial monolayer, they adhere to the apical epithelial surface mediated by the binding of the FcR to apical antigens, binding of CD11b/CD18 to ICAM-1, and likely binding of DAF to CD97.
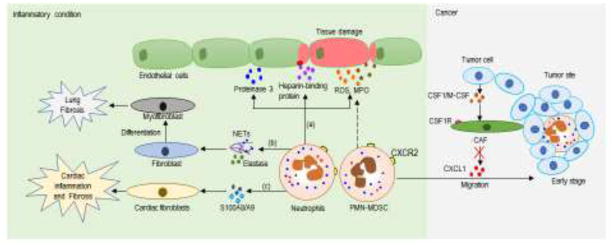
Interaction of neutrophils with fibroblasts has also been extensively studied under inflammatory conditions. Once activated by inflammatory stimuli, neutrophils release extracellular DNA referred to neutrophil extracellular traps (NETs)which are able to promote differentiation and function of fibroblasts leading to fibrosis [60]. In another study, neutrophil elastase has been shown to promote myofibroblast differentiation and lung fibrosis [61]. In a model of angiotensin II induced-cardiovascular injury, S100A8/A9 released by neutrophils activated cardiac fibroblasts to trigger inflammation and cardiac injury [62].
Recently, cellular network controlling PMN-MDSC migration to tumor site has been elucidated. Tumor cells inhibited release of CXCL1 and other neutrophil chemokines by carcinoma-associated fibroblasts (CAF) via production of CSF1/M-CSF[63]. This restricted migration of neutrophils and PMN-MDSC to tumor site probably evolved as a protection of tumors from infiltration by classically activated neutrophils in early stages of tumor development. Therapeutic targeting of CSF1R signaling with small molecules or antibody resulted in substantial increase in PMN-MDSC infiltration. As a result, the antitumor effect of CSF1R inhibitors was blunted. However, combination of CSF1R and CXCR2 inhibitors, which block PMN-MDSC migration to the tumor site, substantially reduced tumor progression [63]. Taken together, interactions of neutrophils with endothelial cells, epithelial cells or fibroblasts play an important role under pathological conditions and therefore, targeting key molecules that control barrier integrity and/or neutrophil function could be therapeutically beneficial ().
Neutrophils/PMN-MDSC crosstalk with stromal cells under pathological conditionsUnder inflammatory conditions, neutrophils crosstalk with endothelial cells and fibroblasts involves: (a) neutrophils release soluble factors, which on one hand protect endothelial cells like proteinase 3; on the other hand, they produce heparin-binding protein, ROS and MPO that damage the barrier integrity. (b) neutrophils release NETs or elastase to promote fibroblast differentiate into myofibroblasts, leading to lung fibrosis; (c) or they release S100A8/A9 proteins to activate cardiac fibroblasts and causes cardiac inflammation and fibrosis. PMN-MDSC, which have a high level of ROS and MPO, may also contribute to the tissue damage during their trafficking. In cancers, tumor cells inhibit the release of CXCL1 and other neutrophil chemokines by carcinoma-associated fibroblasts via production of CSF1/M-CSF, resulting in the impaired migration of neutrophils and PMN-MDSC. NETs: neutrophil extracellular traps. CAF: carcinoma-associated fibroblasts.
5. Neutrophils and PMN-MDSC in cancer
Neutrophils are traditionally considered as inflammatory immune cells and inflammation plays an important role in tumor development. High levels of neutrophils are closely associated with disease progression and poor clinical outcome. In particular, the neutrophil-to-lymphocyte ratio (NLR) and neutrophilia were independent prognostic markers in many types of cancers [64–66]. However, neutrophils were also reported to have an antitumor activity. Tumor-associated neutrophils (TANs) display plasticity and their transcriptional reprogramming could be modulated by distinct tumor microenvironment signaling [67, 68]. The roots of this controversy may lie in the nature of the cells studied. Early reports that focused on the role of mouse neutrophils in cancer did not distinguish neutrophils from PMN-MDSC. This is especially important in experiments with tumor-bearing mice where most of the neutrophils were represented by bona-fide PMN-MDSC. Unfortunately, a number of studies did not evaluate immune suppressive activity and other functions of myeloid cells and therefore, it is impossible to make a conclusion of whether these cells are either neutrophils or PMN-MDSC. Below, a role of neutrophils and PMN-MDSC in cancer is discussed.
5.1. Antitumor activity of neutrophils
There is sufficient evidence indicating that neutrophils may have antitumor activity [69, 70]. Hypoxia in the tumors can induce expression of CXCL1, CXCL2, and CXCL5 to recruit neutrophils [71]. Upregulation of the hepatocyte growth factor receptor c-MET on neutrophils by endothelial-derived TNF caused these cells to produce nitric oxide (NO), which had cytotoxic effects on cancer cells [72]. Recently, it was reported that inhibition c-MET impaired the recruitment of neutrophils into tumors and draining lymph nodes in response to cytotoxic immunotherapies. In the absence of c-MET inhibition, neutrophils recruited to T cell-inflamed microenvironments rapidly acquired immunosuppressive properties, restraining T cell expansion and effector functions [73]. Anti-metastatic effect of neutrophils was mediated by hydrogen peroxide or thrombospondin 1, but the latter was degraded by NE and cathepsin G during inflammation [74–77]. Following antibody-mediated tumor therapy, neutrophils are activated via their Fc receptors and release mediators with direct tumoricidal activity [69]. In cancer patients, several studies demonstrated that an infiltration of tumor by neutrophils was associated with better survival [63, 78]. The number of MPO-positive neutrophils was an independent favorable prognostic factor in patients with colorectal cancer in another study; however, no significant correlation was found with CD15 expression [79]. Thus, neutrophils may have an antitumor activity. However, the vast majority of studies demonstrated a role for neutrophils in cancer promotion. PMN-MDSC, however, are always considered to exert a pro-tumorigenic effect.
5.2. Protumorigenic function of neutrophils and PMN-MDSC
Accumulating evidence has demonstrated that neutrophils play an important role in both initiation and progression of tumors [7, 80]. Deletion of CXCR2-positive neutrophils in inflammation-induced mouse models of cancer inhibited neutrophil trafficking and prevented tumor initiation [81, 82]. This was further supported by deleting neutrophils using anti-Ly6G antibodies to prevent tumorigenesis in different tumor models [81, 83–85]. Recruitment of neutrophils by PGE2 after epithelial damage could also promote tumor development [86]. Besides their role in tumor initiation, neutrophils are also involved in regulation of cancer progression, including tumor cell growth, invasion, angiogenesis, and metastasis [87, 88]. However, it is unclear whether neutrophils or PMN-MDSC were analyzed in these studies. Co-transfer of isolated tumor neutrophils (likely PMN-MDSC) and cancer cell lines increased tumor growth and angiogenesis [89]. Another study found that neutrophils (likely PMN-MDSC) can also promote tumor growth through converting senescent cancer cells into proliferating cancer cells via IL-1 receptor antagonist [90]. Neutrophils and PMN-MDSC could enhance angiogenesis through the production of MMP9, prokineticin 2 (PROK2, also known as BV8) and vascular endothelial growth factor (VEGF) [7, 91–93]. In melanoma, ultraviolet radiation led to release of high mobility group box 1 from keratinocytes, which recruited neutrophils. These neutrophils induced migration of cancer cells towards endothelial cells leading to enhanced metastasis [94]. Furthermore, neutrophils (likely PMN-MDSC) could enhance tumor metastasis by priming organ-specific pre-metastatic niche [95, 96]. For example, Yan et al. found increased neutrophil numbers in the lungs of mammary adenocarcinoma-bearing mice before tumor cell arrival and these cells produced pro-inflammatory cytokines and MMP9 [95]. Neutrophils also capture circulating cancer cells by direct interactions using the cell surface molecules or by releasing NETs, which were associated with increased formation of metastases [84, 97]. Inhibition of NETs could decrease adhesion of lung carcinoma cells and formation of metastases [98].
Characterization of PMN-MDSC in recent years demonstrated that protumorigenic activity of neutrophils could be in fact attributed to PMN-MDSC. These cells play an essential role in tumor development and progression [99, 100]. In addition to well-established immune suppressive activity, PMN-MDSC have been found to promote tumor angiogenesis through the production of soluble factors such as MMP9, BV8 and VEGF [89, 101–103]. Blockade of MDSC infiltration of tumor site resulted in the inhibition of tumor angiogenesis [104]. Furthermore, PMN-MDSC were able to acquire a proangiogenic activity after homing into tumor microenvironment or during exposure to tumor-conditioned medium ex vivo [89, 105].
PMN-MDSC were directly implicated in the promotion of tumors metastasis. In 4T1 model of breast cancer, the high level of PMN-MDSC correlated with increased bone metastasis, and co-injection of 4T1 cells and MDSC promoted lung metastasis [106]. In another study, Wei et al. found that inhibition PMN-MDSC differentiation in BM by using phytochemical polyacetylenes drastically suppresses tumor metastasis [107]. A more recent study showed that exosomes miR-126a released from MDSC induced by doxorubicin treatment promotes breast tumor lung metastasis [108]. Furthermore, MDSC are significantly increased in lungs of mice bearing mammary adenocarcinomas before tumor cell arrival [95], suggesting a role of MDSC in establishing premetastatic niche. Chemokines CXCL1, CXCL2, and CXCL5, which bind to the same receptor, CXCR2, have been shown to recruit MDSC to the tumor site [109] or to the premetastatic niche [55]. Another study also found that PMN-MDSC recruitment to the premetastatic niche relies on hypoxic tumor cell–derived monocyte chemotactic protein-1 [110].
Recently, PMN-MDSC have been shown to contribute to epithelial-mesenchymal transition (EMT) and “stemness” of tumor cells. For instance, coculture of MDSC with tumor cells induced a stem-like phenotype in tumor cells and enhanced their ability to metastasize in vivo [111]. In the ret-oncogene transgenic mouse model of spontaneous melanoma, PMN-MDSC were recruited to the tumor site and expressed hepatocyte growth factor and TGF-β. This led to EMT of primary melanoma cells [112].
5.3. Clinical relevance of neutrophils and PMN-MDSC in cancer
Despite the dual roles of neutrophils in cancer, an ever-increasing number of clinical evidence indicates NLR as negative predictor and mostly supports the notion that neutrophils promote, rather than inhibit, cancer progression [113]. In prostate cancer, NLR was identified as a predictive marker for overall survival in a cohort of 1688 patients [114]. Other studies also found an association between elevated NLR and poor survival in gastric cancer patients [115], non-metastatic renal cell carcinoma [62] and lung cancer patients [116]. A recent meta-analysis revealed that NLR is a potential prognostic biomarker in patients with ovarian cancer [117]. Collectively, these studies indicate a close association between the count of neutrophils in peripheral blood and an adverse prognosis in cancer patients. However, the contribution of PMN-MDSC to the overall pool of neutrophils, which vary between patients and cancer types, may contribute to the variability of the results.
In contrast to NLR, the prognostic and predictive power of TAN is more variable. One study suggested a positive correlation of TAN with outcome in gastric cancer patients [63] while other studies reported a negative correlation with patient outcome in renal cancer [118] and melanoma [119]. Meanwhile, there is also report showing no correlation of TAN with patient outcome in lung cancer [120]. These discrepancies may represent the result of the variability of PMN-MDSC contribution to the overall pool of TAN.
In recent years, phenotypic identification of PMN-MDSC in patients’ peripheral blood allowed for evaluation of their prognostic value. PMN-MDSC represent a reliable predictor of negative outcome and response to therapy. Studies showed an association of high PMN-MDSC numbers with poor outcomes [121–123]. PMN-MDSC frequencies correlated with the presence of tumor metastasis [124, 125]. In head and neck cancer patients, the outcome of pre-operative cetuximab treatment can be predicted by PMN-MDSC numbers, which decreased in the responder group and unchanged in non-responders [126]. Taken together, these studies highlight the potential use of PMN-MDSC in assessing the prognosis of cancer patients. However, many studies in this field still face inconsistent and arbitrary PMN-MDSC phenotyping, which impairs the use of PMN-MDSC in immune monitoring. Recent effort in clarification and unification phenotypic criteria for defining these cells in cancer should help in resolving this issue [127].
Thus, in cancer a population of neutrophils is represented by two distinct groups: bona-fide classically activated neutrophils and pathologically activated PMN-MDSC. Neutrophils may or may not display antitumor activity, whereas PMN-MDSC are universally in support of tumor progression.
6. The role of PMN-MDSCs and neutrophils in sepsis and infectious diseases
Sepsis is a systemic inflammatory response associated with uncontrolled immune activation [128, 129]. Some studies demonstrated that high levels of activated neutrophils were able to clear effectively bacteria in early sepsis [130–132] and reduced neutrophil numbers and function resulted in increased susceptibility to secondary infections [133]. However, delayed apoptosis of neutrophil is associated with tissue damage and aggravating inflammation in sepsis [134]. In addition, in polymicrobial sepsis the migration of neutrophils to the site of infection could be suppressed by TLR2 signaling via downregulation of CXCR2 [68]. The function of neutrophils in the liver during sepsis is to provide protection against bacterial dissemination by releasing NETs [135]. Parker et al. suggested that NET-associated MPO directly killed bacteria in the presence of hydrogen peroxide [136]. However, some bacteria can avoid capture and cell death by expressing nucleases degrading NETs [137].
The major function of MDSC in sepsis is immune suppression [138–141]. In a mouse model of polymicrobial sepsis, MDSC expansion was induced via MyD88 signaling and could effectively inhibit CD8+ T-cell responses [142]. Transfer of MDSC also inhibited T-cell proliferation and improved the survival rate of septic mice [143]. In these mice, PMN-MDSC defined as CD11b+CD48− cells, displayed immune suppression via inducible (i)NOS [144]. In patients with sepsis, PMN-MDSC were primarily induced by Gram-positive pathogens [145], and MDSC-mediated immune suppression was dependent on production of either ROS [145] or arginase 1 [146, 147].
During bacterial and fungal infections, neutrophils are dramatically increased in the circulation and tissues, and their reduction, due to genetic defects or chemotherapy, increases susceptibility of the host to numerous microbial infections [148–150]. However, excessive neutrophil infiltration into tissues has also been shown to result in tissue damage [151]. For instance, neutrophils displayed both protective and pathologic functions in tuberculosis (TB) caused by M. tuberculosis. Neutrophils could control the growth of M. tuberculosis [152] and their depletion resulted in an elevated severity of TB [153]. However, recent studies showed that severe TB in humans correlated with neutrophil abundance and lymphocyte deficiency [154], and excessive recruitment of neutrophils to the lungs could lead to pulmonary necrosis [155]. Depletion of neutrophils resulted in reduced lung tissue pathology in TB [156]. Mice infected with mycobacteria had an increased infiltration of lungs by MDSC. MDSC were able to phagocytose mycobacteria but did not clear this pathogen, thus acting as a shelter for intracellular bacteria [157]. Depletion of MDSC resulted in an increased T cell levels, reduced bacterial burden, and attenuated the disease [157], whereas MDSC accumulation was associated with progress and severity of TB [158]. PMN-MDSC inhibit T-cell responses in active TB. A steep drop in these cells was observed following anti-TB therapy, suggesting that they are potential biomarkers for monitoring efficacy of anti-TB treatment [159]. However, work of Chavez-Galan demonstrated that, in a mouse model of acute pleural TB, PMN-MDSC can attenuate excessive inflammation caused by infection, thus displaying a protective role [160].
Similarly, increasing evidence indicates that neutrophils play a dual role in antiviral immunity [161]. Most of the studies clearly demonstrate a protective role of neutrophils in a large array of infections [162–169]. However, excessive neutrophil activation results in production of pro-inflammatory mediators and negative impact on the host [170, 171].
Accumulation of PMN-MDSC has been reported in a variety of infectious diseases, including viral, bacterial, parasitic, and fungal infections [172, 173]. Human immunodeficiency virus type 1 (HIV-1) infection promotes MDSC expansion to facilitate viral replication [174]. Vollbrecht et al. [175] reported that untreated HIV patients had increased numbers of PMN-MDSC, which contributed to the impaired T-cell responses [176, 177]. Interestingly, Qin et al. found substantially increased proportion of M-MDSC, but not PMN-MDSC in HIV patients, which inhibited T cell responses via arginase-1 [178]. In hepatitis B virus (HBV) infected patients, PMN-MDSC expanded transiently in the acute phase of resolving HBV infection, while in persistent infection, arginase-1 positive PMN-MDSC were elevated [179]. MDSC from chronically HBV-infected patients inhibited CD8+ T cell responses through PD-1-induced IL-10 production [180]. In a mouse model of HBV infection, immune suppressive MDSC were also identified [163, 181]. An increase in immune suppressive MDSC has also been found in other viral infections, including hepatitis C virus[182], adenovirus [183] influenza A virus [184, 185] and vaccinia [186].
Bacterial infection can also cause the abundant generation of PMN-MDSC. For example, large numbers of Gram-positive and negative bacteria have been shown to induce PMN-MDSC in vitro and in vivo [187–196].
Collectively, these studies demonstrate that while neutrophils have a potent role in clearing pathogens, PMN-MDSC inhibit adaptive immune responses. The challenge is to develop approaches to translate these findings into the clinic.
7. Conclusion and perspectives
PMN-MDSC emerged as critical negative regulator of immune responses under many pathologic conditions and major partner of mesenchymal cells in promotion of tumor metastases. The distinction between PMN-MDSC and neutrophils has been debated for many years. These cells are phenotypically and morphologically similar. The main feature of PMN-MDSC that separates them from neutrophils is their immunosuppressive activity. Recently, more data have emerged indicating that these cells could be distinguished based on genomic, proteomic, and biochemical characteristics. PMN-MDSC could be considered as pathologically activated neutrophils. It appears that at any given moment patients with cancer and various chronic infections have population of classically activated neutrophils with protective functions and PMN-MDSC that promote tumor progression and immune suppression in cancer, infectious diseases and other pathologies. The therapeutic targeting of PMN-MDSC is highly promising. However, it success will depend on the development of highly selective therapeutic approaches that would shift the balance towards classical neutrophils.
Acknowledgments
This work was supported by NIH grants CA084488 and CA100062 (to DG) and CA196788 (to YN).
Abbreviation
| BM | bone marrow |
| BV8 | prokineticin 2 |
| CHOP | C/EBP homologous protein |
| EMT | epithelial-mesenchymal transition |
| ER | endoplasmic reticulum |
| G-CSF | granulocyte colony stimulating factor |
| GM-CSF | granulocyte–macrophage-colony stimulating factor |
| HBV | hepatitis B virus |
| HIV-1 | human immunodeficiency virus |
| ICAM-1(-2) | intracellular adhesion molecule-1 (-2) |
| IL-6, (IL-8, IL-10) | interleukin 6, (interleukin-8, interleukin-10) |
| IRF8 | interferon related factor 8 |
| M-CSF | macrophage colony stimulating factor |
| MDSC | myeloid-derived suppressor cells |
| MMP | matrix metalloproteinase |
| MPO | myeloperoxidase |
| NE | neutrophil elastase |
| NETs | neutrophil extracellular traps |
| NLR | neutrophil-to-lymphocyte ratio |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| LOX-1 | lectin type oxidized LDL receptor 1 |
| PGE2 | prostaglandin E2 |
| PMN | polymorphonuclear cells |
| ROS | reactive oxygen species |
| sXBP1 | spliced X-box binding protein 1 |
| TAN | Tumor associated neutrophils |
| TB | tuberculosis |
| TGF-β | transforming growth factor β |
| TNF- α | tumor necrosis factor α |
| VEGF | vascular endothelial growth factor |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
1.
Ost M, Singh A, Peschel A, Mehling R, Rieber N, Hartl D. Myeloid-Derived Suppressor Cells in Bacterial Infections. Frontiers in Cellular and Infection Microbiology. 2016;6:37. [Europe PMC free article] [Abstract] [Google Scholar]2.
Brandau S, Moses K, Lang S. The kinship of neutrophils and granulocytic myeloid-derived suppressor cells in cancer: Cousins, siblings or twins? Seminars in Cancer Biology. 2013;23(3):171–182. [Abstract] [Google Scholar]5.
Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nature Reviews Immunology. 2011;11(8):519–531. [Abstract] [Google Scholar]6.
Kobayashi SD, DeLeo FR. Role of neutrophils in innate immunity: a systems biology-level approach, Wiley interdisciplinary reviews. Systems biology and medicine. 2009;1(3):309–33. [Europe PMC free article] [Abstract] [Google Scholar]7.
Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nature reviews. Cancer. 2016;16(7):431–46. [Abstract] [Google Scholar]8.
Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33(5):657–70. [Abstract] [Google Scholar]9.
Hager M, Cowland JB, Borregaard N. Neutrophil granules in health and disease. Journal of internal medicine. 2010;268(1):25–34. [Abstract] [Google Scholar]10.
Borregaard N, Sorensen OE, Theilgaard-Monch K. Neutrophil granules: a library of innate immunity proteins. Trends in immunology. 2007;28(8):340–5. [Abstract] [Google Scholar]11.
Furze RC, Rankin SM. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22(9):3111–9. [Europe PMC free article] [Abstract] [Google Scholar]12.
Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22(3):285–94. [Abstract] [Google Scholar]13.
Roberts AW. G-CSF: a key regulator of neutrophil production, but that’s not all! Growth factors (Chur, Switzerland) 2005;23(1):33–41. [Abstract] [Google Scholar]14.
Bugl S, Wirths S, Muller MR, Radsak MP, Kopp HG. Current insights into neutrophil homeostasis. Annals of the New York Academy of Sciences. 2012;1266:171–8. [Abstract] [Google Scholar]15.
Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler KJ, Basu S, Zhan YF, Dunn AR. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84(6):1737–1746. [Abstract] [Google Scholar]16.
Molineux G, Migdalska A, Szmitkowski M, Zsebo K, Dexter TM. The effects on hematopoiesis of recombinant stem cell factor (ligand for c-kit) administered in vivo to mice either alone or in combination with granulocyte colony-stimulating factor. Blood. 1991;78(4):961–6. [Abstract] [Google Scholar]17.
Liu F, Poursine-Laurent J, Wu HY, Link DC. Interleukin-6 and the granulocyte colony-stimulating factor receptor are major independent regulators of granulopoiesis in vivo but are not required for lineage commitment or terminal differentiation. Blood. 1997;90(7):2583–90. [Abstract] [Google Scholar]18.
Seymour JF, Lieschke GJ, Grail D, Quilici C, Hodgson G, Dunn AR. Mice lacking both granulocyte colony-stimulating factor (CSF) and granulocyte-macrophage CSF have impaired reproductive capacity, perturbed neonatal granulopoiesis, lung disease, amyloidosis, and reduced long-term survival. Blood. 1997;90(8):3037–49. [Abstract] [Google Scholar]19.
Eash KJ, Means JM, White DW, Link DC. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood. 2009;113(19):4711–4719. [Europe PMC free article] [Abstract] [Google Scholar]20.
Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120(7):2423–31. [Europe PMC free article] [Abstract] [Google Scholar]21.
Kim HK, De La Luz Sierra M, Williams CK, Gulino AV, Tosato G. G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood. 2006;108(3):812–20. [Europe PMC free article] [Abstract] [Google Scholar]22.
Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nature communications. 2016;7:12150. [Europe PMC free article] [Abstract] [Google Scholar]23.
Moses K, Brandau S. Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Seminars in Immunology. 2016;28(2):187–196. [Abstract] [Google Scholar]24.
Kotsakis A, Harasymczuk M, Schilling B, Georgoulias V, Argiris A, Whiteside TL. Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J Immunol Methods. 2012;381(1–2):14–22. [Europe PMC free article] [Abstract] [Google Scholar]25.
Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91(1):167–81. [Europe PMC free article] [Abstract] [Google Scholar]26.
Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, Tcyganov E, Hashimoto A, Nefedova Y, Lin C, Partlova S, Garfall A, Vogl DT, Xu X, Knight SC, Malietzis G, Lee GH, Eruslanov E, Albelda SM, Wang X, Mehta JL, Bewtra M, Rustgi A, Hockstein N, Witt R, Masters G, Nam B, Smirnov D, Sepulveda MA, Gabrilovich DI. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016;1(2) [Europe PMC free article] [Abstract] [Google Scholar]28.
Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Seminars in cancer biology. 2012;22(4):275–81. [Europe PMC free article] [Abstract] [Google Scholar]29.
Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179(2):977–83. [Abstract] [Google Scholar]30.
Gato M, Blanco-Luquin I, Zudaire M, de Morentin XM, Perez-Valderrama E, Zabaleta A, Kochan G, Escors D, Fernandez-Irigoyen J, Santamaria E. Drafting the proteome landscape of myeloid-derived suppressor cells. Proteomics. 2016;16(2):367–78. [Abstract] [Google Scholar]31.
Gato-Canas M, Martinez de Morentin X, Blanco-Luquin I, Fernandez-Irigoyen J, Zudaire I, Liechtenstein T, Arasanz H, Lozano T, Casares N, Chaikuad A, Knapp S, Guerrero-Setas D, Escors D, Kochan G, Santamaria E. A core of kinase-regulated interactomes defines the neoplastic MDSC lineage. Oncotarget. 2015;6(29):27160–75. [Europe PMC free article] [Abstract] [Google Scholar]32.
Fridlender ZG, Sun J, Mishalian I, Singhal S, Cheng G, Kapoor V, Horng W, Fridlender G, Bayuh R, Worthen GS, Albelda SM. Transcriptomic Analysis Comparing Tumor-Associated Neutrophils with Granulocytic Myeloid-Derived Suppressor Cells and Normal Neutrophils. PloS one. 2012;7(2) [Europe PMC free article] [Abstract] [Google Scholar]33.
Brandau S, Trellakis S, Bruderek K, Schmaltz D, Steller G, Elian M, Suttmann H, Schenck M, Welling J, Zabel P, Lang S. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol. 2011;89(2):311–7. [Abstract] [Google Scholar]35.
Mattei F, Schiavoni G, Sestili P, Spadaro F, Fragale A, Sistigu A, Lucarini V, Spada M, Sanchez M, Scala S, Battistini A, Belardelli F, Gabriele L. IRF-8 controls melanoma progression by regulating the cross talk between cancer and immune cells within the tumor microenvironment. Neoplasia. 2012;14(12):1223–35. [Europe PMC free article] [Abstract] [Google Scholar]36.
Paschall AV, Zhang R, Qi CF, Bardhan K, Peng L, Lu G, Yang J, Merad M, McGaha T, Zhou G, Mellor A, Abrams SI, Morse HC, 3rd, Ozato K, Xiong H, Liu K. IFN Regulatory Factor 8 Represses GM-CSF Expression in T Cells To Affect Myeloid Cell Lineage Differentiation. J Immunol. 2015;194(5):2369–79. [Europe PMC free article] [Abstract] [Google Scholar]37.
Waight JD, Netherby C, Hensen ML, Miller A, Hu Q, Liu S, Bogner PN, Farren MR, Lee KP, Liu K, Abrams SI. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest. 2013;123(10):4464–78. [Europe PMC free article] [Abstract] [Google Scholar]38.
Netherby CS, Messmer MN, Burkard-Mandel L, Colligan S, Miller A, Cortes Gomez E, Wang J, Nemeth MJ, Abrams SI. The Granulocyte Progenitor Stage Is a Key Target of IRF8-Mediated Regulation of Myeloid-Derived Suppressor Cell Production. Journal of immunology. 2017;198(10):4129–4139. [Europe PMC free article] [Abstract] [Google Scholar]39.
Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32(6):790–802. [Abstract] [Google Scholar]40.
Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202(7):931–9. [Europe PMC free article] [Abstract] [Google Scholar]41.
Donkor MK, Lahue E, Hoke TA, Shafer LR, Coskun U, Solheim JC, Gulen D, Bishay J, Talmadge JE. Mammary tumor heterogeneity in the expansion of myeloid-derived suppressor cells. Int Immunopharmacol. 2009;9(7–8):937–48. [Abstract] [Google Scholar]42.
Mao Y, Poschke I, Wennerberg E, Pico de Coana Y, Egyhazi Brage S, Schultz I, Hansson J, Masucci G, Lundqvist A, Kiessling R. Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res. 2013;73(13):3877–87. [Abstract] [Google Scholar]43.
Hammami I, Chen J, Murschel F, Bronte V, De Crescenzo G, Jolicoeur M. Immunosuppressive activity enhances central carbon metabolism and bioenergetics in myeloid-derived suppressor cells in vitro models. BMC cell biology. 2012;13:18. [Europe PMC free article] [Abstract] [Google Scholar]44.
Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, Valle LD, Trillo-Tinoco J, Maj T, Zou W, Rodriguez PC, Ochoa AC. Inhibition of Fatty Acid Oxidation Modulates Immunosuppressive Functions of Myeloid-Derived Suppressor Cells and Enhances Cancer Therapies. Cancer Immunol Res. 2015;3(11):1236–47. [Europe PMC free article] [Abstract] [Google Scholar]45.
Condamine T, Kumar V, Ramachandran IR, Youn JI, Celis E, Finnberg N, El-Deiry WS, Winograd R, Vonderheide RH, English NR, Knight SC, Yagita H, McCaffrey JC, Antonia S, Hockstein N, Witt R, Masters G, Bauer T, Gabrilovich DI. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J Clin Invest. 2014;124(6):2626–39. [Europe PMC free article] [Abstract] [Google Scholar]46.
Thevenot PT, Sierra RA, Raber PL, Al-Khami AA, Trillo-Tinoco J, Zarreii P, Ochoa AC, Cui Y, Del Valle L, Rodriguez PC. The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity. 2014;41(3):389–401. [Europe PMC free article] [Abstract] [Google Scholar]49.
Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature reviews. Immunology. 2007;7(9):678–89. [Abstract] [Google Scholar]50.
Schmidt S, Moser M, Sperandio M. The molecular basis of leukocyte recruitment and its deficiencies. Molecular immunology. 2013;55(1):49–58. [Abstract] [Google Scholar]53.
Jerke U, Rolle S, Purfurst B, Luft FC, Nauseef WM, Kettritz R. beta2 integrin-mediated cell-cell contact transfers active myeloperoxidase from neutrophils to endothelial cells. J Biol Chem. 2013;288(18):12910–9. [Europe PMC free article] [Abstract] [Google Scholar]55.
Kuckleburg CJ, Newman PJ. Neutrophil proteinase 3 acts on protease-activated receptor-2 to enhance vascular endothelial cell barrier function. Arteriosclerosis, thrombosis and vascular biology. 2013;33(2):275–84. [Europe PMC free article] [Abstract] [Google Scholar]56.
Gautam N, Olofsson AM, Herwald H, Iversen LF, Lundgren-Akerlund E, Hedqvist P, Arfors KE, Flodgaard H, Lindbom L. Heparin-binding protein (HBP/CAP37): a missing link in neutrophil-evoked alteration of vascular permeability. Nat Med. 2001;7(10):1123–7. [Abstract] [Google Scholar]57.
Boueiz A, Hassoun PM. Regulation of endothelial barrier function by reactive oxygen and nitrogen species. Microvasc Res. 2009;77(1):26–34. [Abstract] [Google Scholar]58.
Pitanga TN, de Aragao Franca L, Rocha VC, Meirelles T, Borges VM, Goncalves MS, Pontes-de-Carvalho LC, Noronha-Dutra AA, dos-Santos WL. Neutrophil-derived microparticles induce myeloperoxidase-mediated damage of vascular endothelial cells. BMC cell biology. 2014;15:21. [Europe PMC free article] [Abstract] [Google Scholar]59.
Slater TW, Finkielsztein A, Mascarenhas LA, Mehl LC, Butin-Israeli V, Sumagin R. Neutrophil Microparticles Deliver Active Myeloperoxidase to Injured Mucosa To Inhibit Epithelial Wound Healing. Journal of immunology (Baltimore, Md: 1950) 2017;198(7):2886–2897. [Europe PMC free article] [Abstract] [Google Scholar]60.
Chrysanthopoulou A, Mitroulis I, Apostolidou E, Arelaki S, Mikroulis D, Konstantinidis T, Sivridis E, Koffa M, Giatromanolaki A, Boumpas DT, Ritis K, Kambas K. Neutrophil extracellular traps promote differentiation and function of fibroblasts. The Journal of pathology. 2014;233(3):294–307. [Abstract] [Google Scholar]61.
Gregory AD, Kliment CR, Metz HE, Kim KH, Kargl J, Agostini BA, Crum LT, Oczypok EA, Oury TA, Houghton AM. Neutrophil elastase promotes myofibroblast differentiation in lung fibrosis. Journal of leukocyte biology. 2015;98(2):143–52. [Europe PMC free article] [Abstract] [Google Scholar]62.
Wu Y, Li Y, Zhang C, XA, Wang Y, Cui W, Li H, Du J. S100a8/a9 released by CD11b+Gr1+ neutrophils activates cardiac fibroblasts to initiate angiotensin II-Induced cardiac inflammation and injury. Hypertension. 2014;63(6):1241–50. [Abstract] [Google Scholar]63.
Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, Hashimoto A, Vonteddu P, Behera R, Goins MA, Mulligan C, Nam B, Hockstein N, Denstman F, Shakamuri S, Speicher DW, Weeraratna AT, Chao T, Vonderheide RH, Languino LR, Ordentlich P, Liu Q, Xu X, Lo A, Puré E, Zhang C, Loboda A, Sepulveda MA, Snyder LA, Gabrilovich DI. Cancer-Associated Fibroblasts Neutralize the Anti-tumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumors. Cancer Cell. 2017 in press. [Europe PMC free article] [Abstract] [Google Scholar]64.
Templeton AJ, Pezaro C, Omlin A, McNamara MG, Leibowitz-Amit R, Vera-Badillo FE, Attard G, de Bono JS, Tannock IF, Amir E. Simple prognostic score for metastatic castration-resistant prostate cancer with incorporation of neutrophil-to-lymphocyte ratio. Cancer. 2014;120(21):3346–52. [Abstract] [Google Scholar]65.
Keizman D, Gottfried M, Ish-Shalom M, Maimon N, Peer A, Neumann A, Rosenbaum E, Kovel S, Pili R, Sinibaldi V, Carducci MA, Hammers H, Eisenberger MA, Sella A. Pretreatment neutrophil-to-lymphocyte ratio in metastatic castration-resistant prostate cancer patients treated with ketoconazole: association with outcome and predictive nomogram. The oncologist. 2012;17(12):1508–14. [Europe PMC free article] [Abstract] [Google Scholar]66.
Keizman D, Ish-Shalom M, Huang P, Eisenberger MA, Pili R, Hammers H, Carducci MA. The association of pre-treatment neutrophil to lymphocyte ratio with response rate, progression free survival and overall survival of patients treated with sunitinib for metastatic renal cell carcinoma. European journal of cancer (Oxford, England: 1990) 2012;48(2):202–8. [Europe PMC free article] [Abstract] [Google Scholar]67.
Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2013;228(7):1404–12. [Abstract] [Google Scholar]68.
Elpek KG, Cremasco V, Shen H, Harvey CJ, Wucherpfennig KW, Goldstein DR, Monach PA, Turley SJ. The tumor microenvironment shapes lineage, transcriptional, and functional diversity of infiltrating myeloid cells. Cancer Immunol Res. 2014;2(7):655–67. [Europe PMC free article] [Abstract] [Google Scholar]69.
Brandau S, Dumitru CA, Lang S. Protumor and antitumor functions of neutrophil granulocytes. Semin Immunopathol. 2013;35(2):163–76. [Abstract] [Google Scholar]70.
Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–31. [Abstract] [Google Scholar]71.
Blaisdell A, Crequer A, Columbus D, Daikoku T, Mittal K, Dey SK, Erlebacher A. Neutrophils Oppose Uterine Epithelial Carcinogenesis via Debridement of Hypoxic Tumor Cells. Cancer Cell. 2015;28(6):785–799. [Europe PMC free article] [Abstract] [Google Scholar]72.
Finisguerra V, Di Conza G, Di Matteo M, Serneels J, Costa S, Thompson AA, Wauters E, Walmsley S, Prenen H, Granot Z, Casazza A, Mazzone M. MET is required for the recruitment of anti-tumoural neutrophils. Nature. 2015;522(7556):349–53. [Europe PMC free article] [Abstract] [Google Scholar]73.
Glodde N, Bald T, van den Boorn-Konijnenberg D, Nakamura K, O’Donnell JS, Szczepanski S, Brandes M, Eickhoff S, Das I, Shridhar N, Hinze D, Rogava M, van der Sluis TC, Ruotsalainen JJ, Gaffal E, Landsberg J, Ludwig KU, Wilhelm C, Riek-Burchardt M, Muller AJ, Gebhardt C, Scolyer RA, Long GV, Janzen V, Teng MWL, Kastenmuller W, Mazzone M, Smyth MJ, Tuting T, Holzel M. Reactive Neutrophil Responses Dependent on the Receptor Tyrosine Kinase c-MET Limit Cancer Immunotherapy. Immunity. 2017;47(4):789–802 e9. [Abstract] [Google Scholar]75.
Lopez-Lago MA, Posner S, Thodima VJ, Molina AM, Motzer RJ, Chaganti RS. Neutrophil chemokines secreted by tumor cells mount a lung antimetastatic response during renal cell carcinoma progression. Oncogene. 2013;32(14):1752–60. [Europe PMC free article] [Abstract] [Google Scholar]76.
Catena R, Bhattacharya N, El Rayes T, Wang S, Choi H, Gao D, Ryu S, Joshi N, Bielenberg D, Lee SB, Haukaas SA, Gravdal K, Halvorsen OJ, Akslen LA, Watnick RS, Mittal V. Bone marrow-derived Gr1+ cells can generate a metastasis-resistant microenvironment via induced secretion of thrombospondin-1. Cancer Discov. 2013;3(5):578–89. [Europe PMC free article] [Abstract] [Google Scholar]77.
El Rayes T, Catena R, Lee S, Stawowczyk M, Joshi N, Fischbach C, Powell CA, Dannenberg AJ, Altorki NK, Gao D, Mittal V. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc Natl Acad Sci U S A. 2015;112(52):16000–5. [Europe PMC free article] [Abstract] [Google Scholar]78.
Sconocchia G, Zlobec I, Lugli A, Calabrese D, Iezzi G, Karamitopoulou E, Patsouris ES, Peros G, Horcic M, Tornillo L, Zuber M, Droeser R, Muraro MG, Mengus C, Oertli D, Ferrone S, Terracciano L, Spagnoli GC. Tumor infiltration by FcgammaRIII (CD16)+ myeloid cells is associated with improved survival in patients with colorectal carcinoma. International journal of cancer. 2011;128(11):2663–72. [Europe PMC free article] [Abstract] [Google Scholar]79.
Droeser RA, Hirt C, Eppenberger-Castori S, Zlobec I, Viehl CT, Frey DM, Nebiker CA, Rosso R, Zuber M, Amicarella F, Iezzi G, Sconocchia G, Heberer M, Lugli A, Tornillo L, Oertli D, Terracciano L, Spagnoli GC. High myeloperoxidase positive cell infiltration in colorectal cancer is an independent favorable prognostic factor. PloS one. 2013;8(5):e64814. [Europe PMC free article] [Abstract] [Google Scholar]80.
Treffers LW, Hiemstra IH, Kuijpers TW, van den Berg TK, Matlung HL. Neutrophils in cancer. Immunol Rev. 2016;273(1):312–28. [Abstract] [Google Scholar]81.
Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJB, Sansom OJ. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. 2012;122(9):3127–3144. [Europe PMC free article] [Abstract] [Google Scholar]82.
Katoh H, Wang DZ, Daikoku T, Sun HY, Dey SK, DuBois RN. CXCR2-Expressing Myeloid-Derived Suppressor Cells Are Essential to Promote Colitis-Associated Tumorigenesis. Cancer Cell. 2013;24(5):631–644. [Europe PMC free article] [Abstract] [Google Scholar]83.
Shang K, Bai YP, Wang C, Wang Z, Gu HY, Du X, Zhou XY, Zheng CL, Chi YY, Mukaida N, Li YY. Crucial Involvement of Tumor-Associated Neutrophils in the Regulation of Chronic Colitis-Associated Carcinogenesis in Mice. Plos One. 2012;7(12) [Europe PMC free article] [Abstract] [Google Scholar]84.
Spicer JD, McDonald B, Cools-Lartigue JJ, Chow SC, Giannias B, Kubes P, Ferri LE. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. 2012;72(16):3919–27. [Abstract] [Google Scholar]85.
Tazawa H, Okada F, Kobayashi T, Tada M, Mori Y, Une Y, Sendo F, Kobayashi M, Hosokawa M. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. Am J Pathol. 2003;163(6):2221–32. [Europe PMC free article] [Abstract] [Google Scholar]86.
Antonio N, Bonnelykke-Behrndtz ML, Ward LC, Collin J, Christensen IJ, Steiniche T, Schmidt H, Feng Y, Martin P. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 2015;34(17):2219–36. [Europe PMC free article] [Abstract] [Google Scholar]87.
Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, Stolz DB, Land SR, Marconcini LA, Kliment CR, Jenkins KM, Beaulieu KA, Mouded M, Frank SJ, Wong KK, Shapiro SD. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16(2):219–23. [Europe PMC free article] [Abstract] [Google Scholar]88.
Tazzyman S, Barry ST, Ashton S, Wood P, Blakey D, Lewis CE, Murdoch C. Inhibition of neutrophil infiltration into A549 lung tumors in vitro and in vivo using a CXCR2-specific antagonist is associated with reduced tumor growth. Int J Cancer. 2011;129(4):847–58. [Abstract] [Google Scholar]89.
Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6(4):409–21. [Abstract] [Google Scholar]90.
Di Mitri D, Toso A, Chen JJ, Sarti M, Pinton S, Jost TR, D’Antuono R, Montani E, Garcia-Escudero R, Guccini I, Da Silva-Alvarez S, Collado M, Eisenberger M, Zhang Z, Catapano C, Grassi F, Alimonti A. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature. 2014;515(7525):134–7. [Abstract] [Google Scholar]91.
Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103(33):12493–8. [Europe PMC free article] [Abstract] [Google Scholar]93.
Shojaei F, Singh M, Thompson JD, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci U S A. 2008;105(7):2640–5. [Europe PMC free article] [Abstract] [Google Scholar]94.
Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D, Kohlmeyer J, Riesenberg S, van den Boorn-Konijnenberg D, Homig-Holzel C, Reuten R, Schadow B, Weighardt H, Wenzel D, Helfrich I, Schadendorf D, Bloch W, Bianchi ME, Lugassy C, Barnhill RL, Koch M, Fleischmann BK, Forster I, Kastenmuller W, Kolanus W, Holzel M, Gaffal E, Tuting T. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014;507(7490):109–13. [Abstract] [Google Scholar]95.
Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, Geng Y, Gray JW, Moses HL, Yang L. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70(15):6139–49. [Europe PMC free article] [Abstract] [Google Scholar]97.
Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013 [Europe PMC free article] [Abstract] [Google Scholar]98.
Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. The Journal of clinical investigation. 2013 [Europe PMC free article] [Abstract] [Google Scholar]101.
Kusmartsev S, Eruslanov E, Kubler H, Tseng T, Sakai Y, Su Z, Kaliberov S, Heiser A, Rosser C, Dahm P, Siemann D, Vieweg J. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J Immunol. 2008;181(1):346–53. [Abstract] [Google Scholar]102.
Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, Ho C, Ross J, Tan M, Carano RA, Meng YG, Ferrara N. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450(7171):825–31. [Abstract] [Google Scholar]103.
Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, Bou-Reslan H, Kallop D, Weimer R, Ludlam MJ, Kaminker JS, Modrusan Z, van Bruggen N, Peale FV, Carano R, Meng YG, Ferrara N. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010;107(50):21248–55. [Europe PMC free article] [Abstract] [Google Scholar]104.
Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, Chen SH. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111(1):219–28. [Europe PMC free article] [Abstract] [Google Scholar]105.
Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol. 2011;11(7):856–61. [Europe PMC free article] [Abstract] [Google Scholar]106.
Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13(1):23–35. [Europe PMC free article] [Abstract] [Google Scholar]107.
Wei WC, Lin SY, Lan CW, Huang YC, Lin CY, Hsiao PW, Chen YR, Yang WC, Yang NS. Inhibiting MDSC differentiation from bone marrow with phytochemical polyacetylenes drastically impairs tumor metastasis. Sci Rep. 2016;6:36663. [Europe PMC free article] [Abstract] [Google Scholar]108.
Deng Z, Rong Y, Teng Y, Zhuang X, Samykutty A, Mu J, Zhang L, Cao P, Yan J, Miller D, Zhang HG. Exosomes miR-126a released from MDSC induced by DOX treatment promotes lung metastasis. Oncogene. 2017;36(5):639–651. [Europe PMC free article] [Abstract] [Google Scholar]109.
Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, Shono Y, Kitabatake M, Kakimi K, Mukaida N, Matsushima K. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008;111(12):5457–66. [Abstract] [Google Scholar]110.
Sceneay J, Chow MT, Chen A, Halse HM, Wong CS, Andrews DM, Sloan EK, Parker BS, Bowtell DD, Smyth MJ, Moller A. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72(16):3906–11. [Abstract] [Google Scholar]111.
Cui TX, Kryczek I, Zhao L, Zhao E, Kuick R, Roh MH, Vatan L, Szeliga W, Mao Y, Thomas DG, Kotarski J, Tarkowski R, Wicha M, Cho K, Giordano T, Liu R, Zou W. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity. 2013;39(3):611–21. [Europe PMC free article] [Abstract] [Google Scholar]112.
Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, Kato M, Prevost-Blondel A, Thiery JP, Abastado JP. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS biology. 2011;9(9):e1001162. [Europe PMC free article] [Abstract] [Google Scholar]113.
Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. [Abstract] [Google Scholar]114.
Zhang GM, Zhu Y, Ma XC, Qin XJ, Wan FN, Dai B, Sun LJ, Ye DW. Pretreatment Neutrophil-to-Lymphocyte Ratio: A Predictor of Advanced Prostate Cancer and Biochemical Recurrence in Patients Receiving Radical Prostatectomy. Medicine (Baltimore) 2015;94(41):e1473. [Europe PMC free article] [Abstract] [Google Scholar]115.
Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol. 2012;82(3):296–309. [Abstract] [Google Scholar]116.
Yu Y, Qian L, Cui J. Value of neutrophil-to-lymphocyte ratio for predicting lung cancer prognosis: A meta-analysis of 7,219 patients. Mol Clin Oncol. 2017;7(3):498–506. [Europe PMC free article] [Abstract] [Google Scholar]117.
Chen S, Zhang L, Yan G, Cheng S, Fathy AH, Yan N, Zhao Y. Neutrophil-to-Lymphocyte Ratio Is a Potential Prognostic Biomarker in Patients with Ovarian Cancer: A Meta-Analysis. Biomed Res Int. 2017;2017:7943467. [Europe PMC free article] [Abstract] [Google Scholar]118.
Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27(28):4709–17. [Abstract] [Google Scholar]119.
Jensen TO, Schmidt H, Moller HJ, Donskov F, Hoyer M, Sjoegren P, Christensen IJ, Steiniche T. Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer. 2012;118(9):2476–85. [Abstract] [Google Scholar]120.
Carus A, Ladekarl M, Hager H, Pilegaard H, Nielsen PS, Donskov F. Tumor-associated neutrophils and macrophages in non-small cell lung cancer: no immediate impact on patient outcome. Lung Cancer. 2013;81(1):130–7. [Abstract] [Google Scholar]121.
Yang G, Shen W, Zhang Y, Liu M, Zhang L, Liu Q, Lu HH, Bo J. Accumulation of myeloid-derived suppressor cells (MDSCs) induced by low levels of IL-6 correlates with poor prognosis in bladder cancer. Oncotarget. 2017;8(24):38378–38388. [Europe PMC free article] [Abstract] [Google Scholar]122.
Li X, Xing YF, Lei AH, Xiao Q, Lin ZH, Hong YF, Wu XY, Zhou J. Neutrophil count is associated with myeloid derived suppressor cell level and presents prognostic value of for hepatocellular carcinoma patients. Oncotarget. 2017;8(15):24380–24388. [Europe PMC free article] [Abstract] [Google Scholar]123.
Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60(10):1419–30. [Europe PMC free article] [Abstract] [Google Scholar]124.
Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. [Europe PMC free article] [Abstract] [Google Scholar]125.
Achberger S, Aldrich W, Tubbs R, Crabb JW, Singh AD, Triozzi PL. Circulating immune cell and microRNA in patients with uveal melanoma developing metastatic disease. Mol Immunol. 2014;58(2):182–6. [Europe PMC free article] [Abstract] [Google Scholar]126.
Li J, Srivastava RM, Ettyreddy A, Ferris RL. Cetuximab ameliorates suppressive phenotypes of myeloid antigen presenting cells in head and neck cancer patients. J Immunother Cancer. 2015;3:54. [Europe PMC free article] [Abstract] [Google Scholar]127.
Mandruzzato S, Brandau S, Britten CM, Bronte V, Damuzzo V, Gouttefangeas C, Maurer D, Ottensmeier C, Burg SH, Welters MJP, Walter S. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: results from an interim study. Cancer Immunology Immunotherapy. 2016;65(2):161–169. [Europe PMC free article] [Abstract] [Google Scholar]128.
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent J-L, Ramsay G International Sepsis Definitions C. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive care medicine. 2003;29(4):530–8. [Abstract] [Google Scholar]129.
Viale P, Tedeschi S, Scudeller L, Attard L, Badia L, Bartoletti M, Cascavilla A, Cristini F, Dentale N, Fasulo G, Legnani G, Trapani F, Tumietto F, Verucchi G, Virgili G, Berlingeri A, Ambretti S, De Molo C, Brizi M, Cavazza M, Giannella M. Infectious Diseases Team for the Early Management of Severe Sepsis and Septic Shock in the Emergency Department. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2017;65(8):1253–1259. [Abstract] [Google Scholar]130.
Alves-Filho JC, de Freitas A, Spiller F, Souto FO, Cunha FQ. The role of neutrophils in severe sepsis. Shock (Augusta, Ga) 2008;30(Suppl 1):3–9. [Abstract] [Google Scholar]131.
Craciun FL, Schuller ER, Remick DG. Early enhanced local neutrophil recruitment in peritonitis-induced sepsis improves bacterial clearance and survival. Journal of immunology (Baltimore, Md: 1950) 2010;185(11):6930–8. [Europe PMC free article] [Abstract] [Google Scholar]132.
Kasten KR, Muenzer JT, Caldwell CC. Neutrophils are significant producers of IL-10 during sepsis. Biochemical and biophysical research communications. 2010;393(1):28–31. [Europe PMC free article] [Abstract] [Google Scholar]133.
Delano MJ, Thayer T, Gabrilovich S, Kelly-Scumpia KM, Winfield RD, Scumpia PO, Cuenca AG, Warner E, Wallet SM, Wallet MA, O’Malley KA, Ramphal R, Clare-Salzer M, Efron PA, Mathews CE, Moldawer LL. Sepsis Induces Early Alterations in Innate Immunity That Impact Mortality to Secondary Infection. Journal of Immunology. 2011;186(1):195–202. [Europe PMC free article] [Abstract] [Google Scholar]134.
Paunel-Gorgulu A, Kirichevska T, Logters T, Windolf J, Flohe S. Molecular mechanisms underlying delayed apoptosis in neutrophils from multiple trauma patients with and without sepsis. Molecular medicine (Cambridge, Mass) 2012;18:325–35. [Europe PMC free article] [Abstract] [Google Scholar]135.
McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell host & microbe. 2012;12(3):324–33. [Abstract] [Google Scholar]136.
Parker H, Albrett AM, Kettle AJ, Winterbourn CC. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. Journal of Leukocyte Biology. 2012;91(3):369–376. [Abstract] [Google Scholar]137.
Berends ETM, Horswill AR, Haste NM, Monestier M, Nizet V, von Koeckritz-Blickwede M. Nuclease Expression by Staphylococcus aureus Facilitates Escape from Neutrophil Extracellular Traps. Journal of Innate Immunity. 2010;2(6):576–586. [Europe PMC free article] [Abstract] [Google Scholar]138.
Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, Heyworth PG, Efron PA, Moldawer LL. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med. 2011;17(3–4):281–92. [Europe PMC free article] [Abstract] [Google Scholar]139.
Janols H, Bergenfelz C, Allaoui R, Larsson AM, Ryden L, Bjornsson S, Janciauskiene S, Wullt M, Bredberg A, Leandersson K. A high frequency of MDSCs in sepsis patients, with the granulocytic subtype dominating in gram-positive cases. J Leukoc Biol. 2014;96(5):685–93. [Abstract] [Google Scholar]140.
Brudecki L, Ferguson DA, McCall CE, El Gazzar M. Myeloid-derived suppressor cells evolve during sepsis and can enhance or attenuate the systemic inflammatory response. Infect Immun. 2012;80(6):2026–34. [Europe PMC free article] [Abstract] [Google Scholar]141.
Mathias B, Delmas AL, Ozrazgat-Baslanti T, Vanzant EL, Szpila BE, Mohr AM, Moore FA, Brakenridge SC, Brumback BA, Moldawer LL, Efron PA. C.I.R.C.I. and the Sepsis, Human Myeloid-derived Suppressor Cells are Associated With Chronic Immune Suppression After Severe Sepsis/Septic Shock. Ann Surg. 2017;265(4):827–834. [Europe PMC free article] [Abstract] [Google Scholar]142.
Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204(6):1463–74. [Europe PMC free article] [Abstract] [Google Scholar]143.
Derive M, Bouazza Y, Alauzet C, Gibot S. Myeloid-derived suppressor cells control microbial sepsis. Intensive Care Med. 2012;38(6):1040–9. [Abstract] [Google Scholar]144.
Jia B, Zhao C, Li G, Kong Y, Ma Y, Wang Q, Wang B, Zeng H. A Novel CD48-Based Analysis of Sepsis-Induced Mouse Myeloid-Derived Suppressor Cell Compartments. Mediators of Inflammation. 2017 [Europe PMC free article] [Abstract] [Google Scholar]145.
Janols H, Bergenfelz C, Allaoui R, Larsson A-M, Ryden L, Bjornsson S, Janciauskiene S, Wullt M, Bredberg A, Leandersson K. A high frequency of MDSCs in sepsis patients, with the granulocytic subtype dominating in gram-positive cases. Journal of Leukocyte Biology. 2014;96(5):685–693. [Abstract] [Google Scholar]146.
Darcy CJ, Minigo G, Piera KA, Davis JS, McNeil YR, Chen Y, Volkheimer AD, Weinberg JB, Anstey NM, Woodberry T. Neutrophils with myeloid derived suppressor function deplete arginine and constrain T cell function in septic shock patients. Critical Care. 2014;18(4) [Europe PMC free article] [Abstract] [Google Scholar]147.
Uhel F, Azzaoui I, Gregoire M, Pangault C, Dulong J, Tadie J-M, Gacouin A, Camus C, Cynober L, Fest T, Le Tulzo Y, Roussel M, Tarte K. Early Expansion of Circulating Granulocytic Myeloid-derived Suppressor Cells Predicts Development of Nosocomial Infections in Patients with Sepsis. American journal of respiratory and critical care medicine. 2017;196(3):315–327. [Abstract] [Google Scholar]148.
Antachopoulos C, Roilides E. Cytokines and fungal infections. British journal of haematology. 2005;129(5):583–596. [Abstract] [Google Scholar]149.
Choi KS, Dumler JS. Early induction and late abrogation of respiratory burst in A-phagocytophilum - Infected neutrophils. In: Hechemy KE, AvsicZupanc T, Childs JE, Raoult DA, editors. Rickettsiology: Present and Future Directions. 2003. pp. 488–493. [Abstract] [Google Scholar]150.
Waldorf AR. Pulmonary defense mechanisms against opportunistic fungal pathogens. Immunology series. 1989;47:243–71. [Abstract] [Google Scholar]151.
Butterfield TA, Best TM, Merrick MA. The dual roles of neutrophils and macrophages in inflammation: A critical balance between tissue damage and repair. Journal of Athletic Training. 2006;41(4):457–465. [Europe PMC free article] [Abstract] [Google Scholar]152.
Brown AE, Holzer TJ, Andersen BR. Capacity of human neutrophils to kill Mycobacterium tuberculosis. The Journal of infectious diseases. 1987;156(6):985–9. [Abstract] [Google Scholar]153.
Pedrosa J, Saunders BM, Appelberg R, Orme IM, Silva MT, Cooper AM. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infection and Immunity. 2000;68(2):577–583. [Europe PMC free article] [Abstract] [Google Scholar]154.
Panteleev AV, Nikitina IY, Burmistrova IA, Kosmiadi GA, Radaeva TV, Amansahedov RB, Sadikov PV, Serdyuk YV, Larionova EE, Bagdasarian TR, Chernousova LN, Ganusov VV, Lyadova IV. Severe Tuberculosis in Humans Correlates Best with Neutrophil Abundance and Lymphocyte Deficiency and Does Not Correlate with Antigen-Specific CD4 T-Cell Response. Front Immunol. 2017;8:963. [Europe PMC free article] [Abstract] [Google Scholar]155.
Almeida FM, Ventura TLB, Amaral EP, Ribeiro SCM, Calixto SD, Manhaes MR, Rezende AL, Souzal GS, de Carvalho IS, Silva EC, da Silva JA, Carvalho ECQ, Kritski AL, Lasunskaia EB. Hypervirulent Mycobacterium tuberculosis strain triggers necrotic lung pathology associated with enhanced recruitment of neutrophils in resistant C57BL/6 mice. PloS one. 2017;12(3) [Europe PMC free article] [Abstract] [Google Scholar]156.
Yeremeev V, Linge I, Kondratieva T, Apt A. Neutrophils exacerbate tuberculosis infection in genetically susceptible mice. Tuberculosis. 2015;95(4):447–451. [Abstract] [Google Scholar]157.
Knaul JK, Jorg S, Oberbeck-Mueller D, Heinemann E, Scheuermann L, Brinkmann V, Mollenkopf HJ, Yeremeev V, Kaufmann SH, Dorhoi A. Lung-residing myeloid-derived suppressors display dual functionality in murine pulmonary tuberculosis. American journal of respiratory and critical care medicine. 2014;190(9):1053–66. [Abstract] [Google Scholar]158.
Tsiganov EN, Verbina EM, Radaeva TV, Sosunov VV, Kosmiadi GA, Nikitina IY, Lyadova IV. Gr-1dimCD11b+ immature myeloid-derived suppressor cells but not neutrophils are markers of lethal tuberculosis infection in mice. Journal of immunology (Baltimore, Md: 1950) 2014;192(10):4718–27. [Europe PMC free article] [Abstract] [Google Scholar]159.
El Daker S, Sacchi A, Tempestilli M, Carducci C, Goletti D, Vanini V, Colizzi V, Lauria FN, Martini F, Martino A. Granulocytic myeloid derived suppressor cells expansion during active pulmonary tuberculosis is associated with high nitric oxide plasma level. PloS one. 2015;10(4):e0123772. [Europe PMC free article] [Abstract] [Google Scholar]160.
Chavez-Galan L, Vesin D, Uysal H, Blaser G, Benkhoucha M, Ryffel B, Quesniaux VFJ, Garcia I. Transmembrane Tumor Necrosis Factor Controls Myeloid-Derived Suppressor Cell Activity via TNF Receptor 2 and Protects from Excessive Inflammation during BCG-Induced Pleurisy. Frontiers in Immunology. 2017;8 [Europe PMC free article] [Abstract] [Google Scholar]161.
Galani IE, Andreakos E. Neutrophils in viral infections: Current concepts and caveats. Journal of Leukocyte Biology. 2015;98(4):557–564. [Abstract] [Google Scholar]162.
Fujisawa H, Tsuru S, Taniguchi M, Zinnaka Y, Nomoto K. Protective mechanisms against pulmonary infection with influenza virus. I. Relative contribution of polymorphonuclear leukocytes and of alveolar macrophages to protection during the early phase of intranasal infection. The Journal of general virology. 1987;68(Pt 2):425–32. [Abstract] [Google Scholar]163.
Kong X, Sun R, Chen Y, Wei H, Tian Z. gamma delta T Cells Drive Myeloid-Derived Suppressor Cell-Mediated CD8(+) T Cell Exhaustion in Hepatitis B Virus-Induced Immunotolerance. Journal of Immunology. 2014;193(4):1645–1653. [Abstract] [Google Scholar]164.
Tumpey TM, Garcia-Sastre A, Taubenberger JK, Palese P, Swayne DE, Pantin-Jackwood MJ, Schultz-Cherry S, Solorzano A, Van Rooijen N, Katz JM, Basler CF. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: Functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. Journal of Virology. 2005;79(23):14933–14944. [Europe PMC free article] [Abstract] [Google Scholar]165.
Tate MD, Deng Y-M, Jones JE, Anderson GP, Brooks AG, Reading PC. Neutrophils Ameliorate Lung Injury and the Development of Severe Disease during Influenza Infection. Journal of Immunology. 2009;183(11):7441–7450. [Abstract] [Google Scholar]166.
Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JPY. The NLRP3 Inflammasome Mediates In Vivo Innate Immunity to Influenza A Virus through Recognition of Viral RNA. Immunity. 2009;30(4):556–565. [Europe PMC free article] [Abstract] [Google Scholar]167.
Thomas PG, Dash P, Aldridge JR, Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti T-D. The Intracellular Sensor NLRP3 Mediates Key Innate and Healing Responses to Influenza A Virus via the Regulation of Caspase-1. Immunity. 2009;30(4):566–575. [Europe PMC free article] [Abstract] [Google Scholar]168.
Zhou JH, Stohlman SA, Hinton DR, Marten NW. Neutrophils promote mononuclear cell infiltration during viral-induced encephalitis. Journal of Immunology. 2003;170(6):3331–3336. [Abstract] [Google Scholar]169.
Tumpey TM, Chen S-H, Oakes JE, Lausch RN. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. Journal of Virology. 1996;70(2):898–904. [Europe PMC free article] [Abstract] [Google Scholar]170.
Seki M, Kohno S, Newstead MW, Zeng X, Bhan U, Lukacs NW, Kunkel SL, Standiford TJ. Critical Role of IL-1 Receptor-Associated Kinase-M in Regulating Chemokine-Dependent Deleterious Inflammation in Murine Influenza Pneumonia. Journal of Immunology. 2010;184(3):1410–1418. [Europe PMC free article] [Abstract] [Google Scholar]171.
Stacey MA, Marsden M, Pham NT, Clare S, Dolton G, Stack G, Jones E, Klenerman P, Gallimore AM, Taylor PR, Snelgrove RJ, Lawley TD, Dougan G, Benedict CA, Jones SA, Wilkinson GW, Humphreys IR. Neutrophils recruited by IL-22 in peripheral tissues function as TRAIL-dependent antiviral effectors against MCMV. Cell host & microbe. 2014;15(4):471–83. [Europe PMC free article] [Abstract] [Google Scholar]174.
Garg A, Spector SA. HIV Type 1 gp120-Induced Expansion of Myeloid Derived Suppressor Cells Is Dependent on Interleukin 6 and Suppresses Immunity. Journal of Infectious Diseases. 2014;209(3):441–451. [Europe PMC free article] [Abstract] [Google Scholar]175.
Vollbrecht T, Roider J, Stirner R, Tufman A, Huber RM, Bogner JR, Lechner A, Bourquin C, Draenert R. Chronic progressive HIV-1 infection is associated with elevated levels of myeloid-derived suppressor cells. Retrovirology. 2012;9 [Abstract] [Google Scholar]176.
Tumino N, Turchi F, Meschi S, Lalle E, Bordoni V, Casetti R, Agrati C, Cimini E, Montesano C, Colizzi V, Martini F, Sacchi A. In HIV-positive patients, myeloid-derived suppressor cells induce T-cell anergy by suppressing CD3 zeta expression through ELF-1 inhibition. Aids. 2015;29(18):2397–2407. [Abstract] [Google Scholar]177.
Tumino N, Bilotta MT, Pinnetti C, Ammassari A, Antinori A, Turchi F, Agrati C, Casetti R, Bordoni V, Cimini E, Abbate I, Capobianchi MR, Martini F, Sacchi A. Granulocytic Myeloid-Derived Suppressor Cells Increased in Early Phases of Primary HIV Infection Depending on TRAIL Plasma Level. Jaids-Journal of Acquired Immune Deficiency Syndromes. 2017;74(5):575–582. [Abstract] [Google Scholar]178.
Qin A, Cai W, Pan T, Wu K, Yang Q, Wang N, Liu Y, Yan D, Hu F, Guo P, Chen X, Chen L, Zhang H, Tang X, Zhou J. Expansion of Monocytic Myeloid-Derived Suppressor Cells Dampens T Cell Function in HIV-1-Seropositive Individuals. Journal of Virology. 2013;87(3):1477–1490. [Europe PMC free article] [Abstract] [Google Scholar]179.
Pallett LJ, Gill US, Quaglia A, Sinclair LV, Jover-Cobos M, Schurich A, Singh KP, Thomas N, Das A, Chen A, Fusai G, Bertoletti A, Cantrell DA, Kennedy PT, Davies NA, Haniffa M, Maini MK. Metabolic regulation of hepatitis B immunopathology by myeloid-derived suppressor cells. Nat Med. 2015;21(6):591–600. [Europe PMC free article] [Abstract] [Google Scholar]180.
Huang A, Zhang B, Yan W, Wang B, Wei H, Zhang F, Wu L, Fan K, Guo Y. Myeloid-derived suppressor cells regulate immune response in patients with chronic hepatitis B virus infection through PD-1-induced IL-10. Journal of immunology (Baltimore, Md: 1950) 2014;193(11):5461–9. [Abstract] [Google Scholar]181.
Chen S, Akbar SMF, Abe M, Hiasa Y, Onji M. Immunosuppressive functions of hepatic myeloid-derived suppressor cells of normal mice and in a murine model of chronic hepatitis B virus. Clinical and experimental immunology. 2011;166(1):134–142. [Abstract] [Google Scholar]182.
Goh CC, Roggerson KM, Lee H-C, Golden-Mason L, Rosen HR, Hahn YS. Hepatitis C Virus-Induced Myeloid-Derived Suppressor Cells Suppress NK Cell IFN-gamma Production by Altering Cellular Metabolism via Arginase-1. Journal of Immunology. 2016;196(5):2283–2292. [Europe PMC free article] [Abstract] [Google Scholar]184.
De Santo C, Salio M, Masri SH, Lee LY-H, Dong T, Speak AO, Porubsky S, Booth S, Veerapen N, Besra GS, Groene H-J, Platt FM, Zambon M, Cerundolo V. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. Journal of Clinical Investigation. 2008;118(12):4036–4048. [Europe PMC free article] [Abstract] [Google Scholar]185.
Jeisy-Scott V, Davis WG, Patel JR, Bowzard JB, Shieh W-J, Zaki SR, Katz JM, Sambhara S. Increased MDSC Accumulation and Th2 Biased Response to Influenza A Virus Infection in the Absence of TLR7 in Mice. PloS one. 2011;6(9) [Europe PMC free article] [Abstract] [Google Scholar]187.
Heim CE, Vidlak D, Scherr TD, Hartman CW, Garvin KL, Kielian T. IL-12 Promotes Myeloid-Derived Suppressor Cell Recruitment and Bacterial Persistence during Staphylococcus aureus Orthopedic Implant Infection. Journal of Immunology. 2015;194(8):3861-+. [Europe PMC free article] [Abstract] [Google Scholar]188.
Heim CE, Vidlak D, Scherr TD, Kozel JA, Holzapfel M, Muirhead DE, Kielian T. Myeloid-Derived Suppressor Cells Contribute to Staphylococcus aureus Orthopedic Biofilm Infection. Journal of Immunology. 2014;192(8):3778–3792. [Europe PMC free article] [Abstract] [Google Scholar]189.
Peng K-T, Hsieh C-C, Huang T-Y, Chen P-C, Shih H-N, Lee MS, Chang P-J. Staphylococcus aureus biofilm elicits the expansion, activation and polarization of myeloid-derived suppressor cells in vivo and in vitro. PloS one. 2017;12(8) [Europe PMC free article] [Abstract] [Google Scholar]190.
du Plessis N, Loebenberg L, Kriel M, von Groote-Bidlingmaier F, Ribechini E, Loxton AG, van Helden PD, Lutz MB, Walzl G. Increased Frequency of Myeloid-derived Suppressor Cells during Active Tuberculosis and after Recent Mycobacterium tuberculosis Infection Suppresses T-Cell Function. American journal of respiratory and critical care medicine. 2013;188(6):724–732. [Abstract] [Google Scholar]191.
Knaul JK, Joerg S, Oberbeck-Mueller D, Heinemann E, Scheuermann L, Brinkmann V, Mollenkopf H-J, Yeremeev V, Kaufmann SHE, Dorhoi A. Lung-Residing Myeloid-derived Suppressors Display Dual Functionality in Murine Pulmonary Tuberculosis. American journal of respiratory and critical care medicine. 2014;190(9):1053–1066. [Abstract] [Google Scholar]192.
Oz HH, Zhou B, Voss P, Careyic M, Schroth C, Frey N, Rieber N, Hector A, Hartl D. Pseudomonas aeruginosa Airway Infection Recruits and Modulates Neutrophilic Myeloid-Derived Suppressor Cells. Frontiers in Cellular and Infection Microbiology. 2016;6 [Europe PMC free article] [Abstract] [Google Scholar]193.
Poe SL, Arora M, Oriss TB, Yarlagadda M, Isse K, Khare A, Levy DE, Lee JS, Mallampalli RK, Chan YR, Ray A, Ray P. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal immunology. 2013;6(1):189–199. [Europe PMC free article] [Abstract] [Google Scholar]194.
Sander LE, Sackett SD, Dierssen U, Beraza N, Linke RP, Mueller M, Blander JM, Tacke F, Trautwein C. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. Journal of Experimental Medicine. 2010;207(7):1453–1464. [Europe PMC free article] [Abstract] [Google Scholar]195.
Cuenca AG, Cuenca AL, Winfield RD, Joiner DN, Gentile L, Delano MJ, Kelly-Scumpia KM, Scumpia PO, Matheny MK, Scarpace PJ, Vila L, Efron PA, LaFace DM, Moldawer LL. Novel Role for Tumor-Induced Expansion of Myeloid-Derived Cells in Cancer Cachexia. Journal of Immunology. 2014;192(12):6111–6119. [Europe PMC free article] [Abstract] [Google Scholar]196.
Dietlin TA, Hofman FM, Lund BT, Gilmore W, Stohlman SA, van der Veen RC. Mycobacteria-induced Gr-1+ subsets from distinct myeloid lineages have opposite effects on T cell expansion. J Leukoc Biol. 2007;81(5):1205–12. [Abstract] [Google Scholar]