Abstract
Free full text

Brain MRI Findings in Severe COVID-19: A Retrospective Observational Study
Abstract
Background
Brain MRI parenchymal signal abnormalities have been in association with SARS-CoV-2.
Purpose
Describe the neuroimaging findings (excluding ischemic infarcts) in patients with severe COVID-19 infection.
Methods
This was a retrospective study of patients evaluated from March 23th, 2020 to April 27th, 2020 at 16 hospitals. Inclusion criteria were: (i) positive nasopharyngeal or lower respiratory tract reverse transcriptase-polymerase chain reaction assays; (ii) severe COVID infection defined as requirement for hospitalization and oxygen therapy; (iii) neurologic manifestations; (iv) abnormal brain MRI. Exclusion criteria were patients with missing or non-contributory data regarding brain MRI or a brain MRI showing ischemic infarcts, cerebral venous thrombosis, or chronic lesions unrelated to the current event. Categorical data were compared using Fisher exact test. Quantitative data were compared using Student’s t-test or Wilcoxon test. A p-value lower than 0.05 was considered significant.
Results
Thirty men (81%) and 7 women (19%) met inclusion criteria, with a mean age of 61+/- 12 years (range: 8-78). The most common neurologic manifestations were alteration of consciousness (27/37, 73%), pathological wakefulness when the sedation was stopped (15/37, 41%), confusion (12/37, 32%), and agitation (7/37, 19%). The most frequent MRI findings were: signal abnormalities located in the medial temporal lobe in 16/37 (43%, 95% CI 27-59%) patients, non-confluent multifocal white matter hyperintense lesions on FLAIR and diffusion sequences, with variable enhancement, with associated hemorrhagic lesions in 11/37 patients (30%, 95% CI 15-45%), and extensive and isolated white matter microhemorrhages in 9/37 patients (24%, 95% CI 10-38%). A majority of patients (20/37, 54%) had intracerebral hemorrhagic lesions with a more severe clinical presentation: higher admission rate in intensive care units, 20/20 patients, 100% versus 12/17 patients, 71%, p=0.01; development of the acute respiratory distress syndrome in 20/20 patients, 100% versus 11/17 patients, 65%, p=0.005. Only one patient was positive for SARS-CoV-2 RNA in the cerebrospinal fluid.
Conclusion
Patients with severe COVID-19 and without ischemic infarcts had a wide range of neurologic manifestations that were be associated with abnormal brain MRIs. Eight distinctive neuroradiological patterns were described.
Introduction
SARS-CoV-2 is the seventh member of the family of coronaviruses (CoVs) that infect humans (1) and induces COVID-19 disease. Human CoVs (HCoVs) have neuroinvasive capacities and may be neurovirulent by two main mechanisms (2-4): viral replication into glial or neuronal cells of the brain, or autoimmune reaction with a misdirected host immune response (5). Thus, a few cases of acute encephalitis-like syndromes with hCoVs were reported in the past two decades (5-8). Concerning COVID-19, current data on central nervous system (CNS) involvement is uncommon but growing (9-17), demonstrating the high frequency of neurological symptoms.
However, the delineation of a large cohort of confirmed brain MRI parenchymal signal abnormalities (excluding ischemic infarcts) related to COVID-19 has never been performed, and the underlying pathophysiological mechanisms remain unknown. The purpose of this current study was to describe the neuroimaging findings (excluding ischemic infarcts) in patients with severe COVID-19 and report the clinico-biological profile of these patients.
Material & Methods
This retrospective observational national multicenter study was initiated by the French Society of Neuroradiology (SFNR) in collaboration with neurologists, intensivists, and infectious disease specialists, and brought together 16 hospitals. The study was approved by the ethical committee of Strasbourg University Hospital (CE-2020-37) and was in accordance with the 1964 Helsinki Declaration and its later amendments. Due to the emergency in the context of COVID-19 pandemic responsible for acute respiratory and neurological manifestations pandemic, the requirement for patients’ written informed consent was waived.
Patient cohort
Consecutive patients with COVID-19 infection and neurologic manifestations who underwent brain MRI were included from March 23th, 2020, to April 27th, 2020, in 16 French centers, including 11 university hospitals and 5 general hospitals. Inclusion criteria were: (i) diagnosis of COVID-19 based on possible exposure history or symptoms clinically compatible, validated with a detection of SARS-CoV-2 by reverse transcriptase-polymerase chain reaction (RT-PCR) assays on the nasopharyngeal, throat or lower respiratory tract swabs; (ii) severe COVID-19 infection defined as requirement for hospitalization and oxygen therapy; (iii) neurologic manifestations; (iv) abnormal brain MRI with acute/subacute abnormalities. Exclusion criteria were: (i) patients with missing or non-contributory data (lack of sequences, numerous artifacts) regarding brain MRI; (ii) a brain MRI showing ischemic infarcts, cerebral venous thrombosis, or chronic lesions unrelated to the current event.
Clinical and laboratory data were extracted from the patients’ electronic medical records in the Hospital Information System. Only laboratory analysis within three days before the brain MRI were considered. In the case of redundancy of the tests, the worst value has been kept. Clinical and biological data were reviewed by two neurologists (J.D.S., and M.A. with 25 and 15 years of clinical expertise on neurology, respectively), and by one virologist (S.F-K). They participated to the elaboration of the study design, the interpretation of the data, and to manuscript editing. When available, all electroencephalogram (EEG) were reviewed by one expert neurologist (C.B.) and classified into five groups (normal, under sedation, nonspecific, encephalopathy or seizures).
Virological assessment
Quantitative real-time RT-PCR tests for SARS-CoV-2 nucleic acid were performed on nasopharyngeal or lower respiratory tract swabs, and cerebrospinal fluid (CSF). Primer and probe sequences target two regions on the RdRp gene and are specific to SARS-CoV-2. Assay sensitivity is around 10 copies/reaction (in house-method, Institut Pasteur, Paris, France) (18).
Brain MRI protocols
Imaging studies were conducted either on 1.5- or 3-Tesla MRI. The multicenter nature of the study and the various clinical setups did not allow standardization of sequences. The most frequently sequences performed were 3D T1 weighted spin-echo MRI with and without contrast enhancement, diffusion-weighted imaging (DWI), gradient-echo T2 or Susceptibility-weighted imaging, and 2D or 3D FLAIR after administration of gadolinium-based contrast agent.
MRI interpretation
After anonymization, images were presented to readers with our GE Picture Archiving and Communication System (General Electric, Milwaukee, WI, USA). After review of MRI studies by three neuroradiologists (S.K., F.C., and F.L. with 20, 25, and 9 years of experience in neuroradiology, respectively) who were blinded to all patient data, brain MRI findings were divided by consensus into eight groups: (a) unilateral FLAIR and/or diffusion hyperintensities located in medial temporal lobe; (b) FLAIR and diffusion ovoid hyperintense lesion located in the central part of the splenium of the corpus callosum; (c) non-confluent multifocal white matter (WM) hyperintense lesions on FLAIR and diffusion, with variable enhancement; (d) non-confluent multifocal WM hyperintense lesions on FLAIR and diffusion, with variable enhancement, associated with hemorrhagic lesions; (e) acute necrotizing encephalopathy (ANE) (9) when symmetric thalamic lesions (edema, petechial hemorrhage, and necrosis), with variable involvement of the brainstem, internal capsule, putamen, cerebral and cerebellar WM; (f) extensive and isolated WM microhemorrhages; (g) extensive and confluent supratentorial WM FLAIR hyperintensities; (h) FLAIR hyperintense lesions involving both middle cerebellar peduncles. Patients could have had more than one pattern.
Statistical analysis
Data were described using frequency and proportion (n, %) for categorical variables, using mean, median, interquartile range, and range for quantitative data. In a second step, patients with hemorrhagic lesions were gathered into a single group called « patients with hemorrhagic complications », to look for clinico-biological differences between the two populations. Categorical data were compared using Fisher exact test. Quantitative data were compared using Student’s t-test or Wilcoxon test. A p-value lower than 0.05 was considered significant.
Results
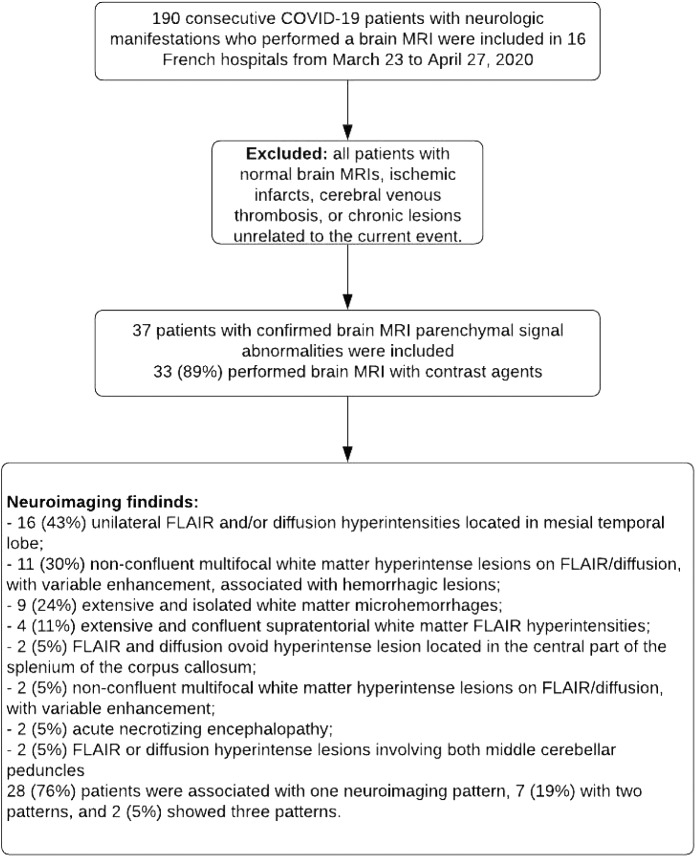
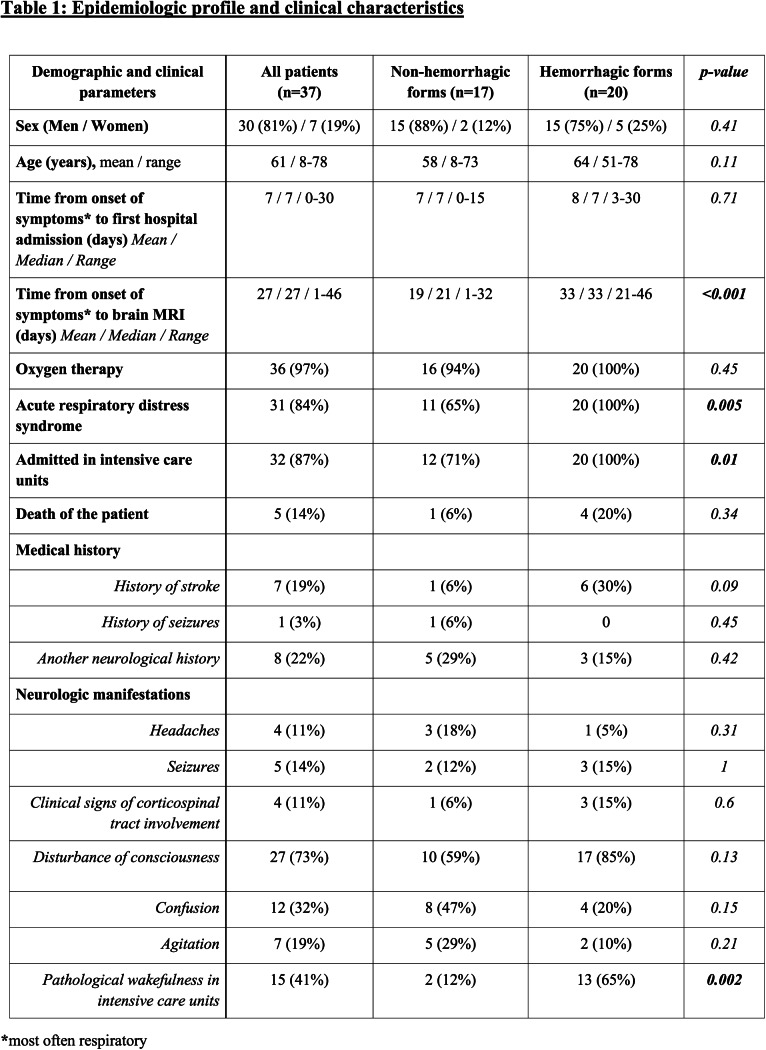
Between March 23th, 2020, and April 27th, 2020, 190 consecutive patients with COVID-19 infection and neurologic manifestations, performed a brain MRI in 16 hospitals. Among them, were excluded all patients with normal brain MRI, ischemic infarcts, cerebral venous thrombosis or chronic lesions unrelated to the current event. A total of 37 patients with COVID-19 infection were finally included in this study (figure 1). The average age of the patients was 61 +/- 12 years with 30 men and 7 women included (table 1). The majority of our patients (32/37, 87%) were admitted to Intensive Care Units (ICUs) because of acute respiratory failure. The most frequent neurologic manifestations were alteration of consciousness (27/37, 73%), pathological wakefulness after sedation (15/37, 41%), confusion (12/37, 32%), and agitation (7/37, 19%).
Table 1:
Epidemiologic profile and clinical characteristics
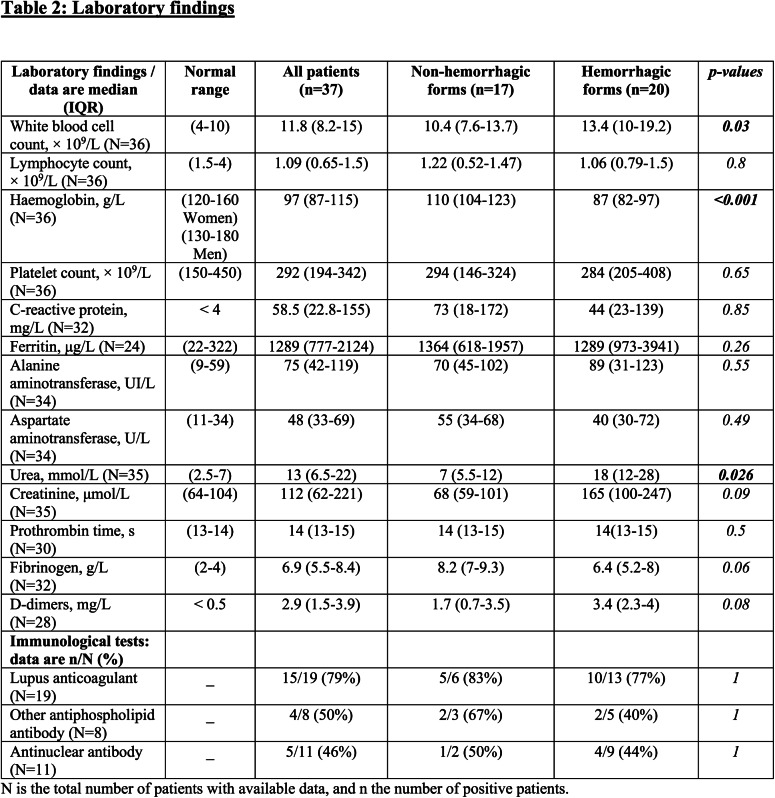
Among the 26 EEG performed, 2 (8%) were considered as normal, 6 (23%) were realized under sedation, 10 (39%) showed nonspecific findings, 7 (27%) were classified as encephalopathy, and 1 (4%) case of seizures was also described. At the end of the study, the mortality rate was 14%. The blood counts of patients showed leukocytosis, lymphopenia, and anemia. Patients had elevated serum levels of C-reactive protein, ferritin, alanine aminotransferase, aspartate aminotransferase, urea, creatinine, fibrinogen, and D-dimers (table 2). Fifteen out of the 19 patients (79%) studied for the presence of a lupus anticoagulant were positive.
Table 2:
Laboratory findings
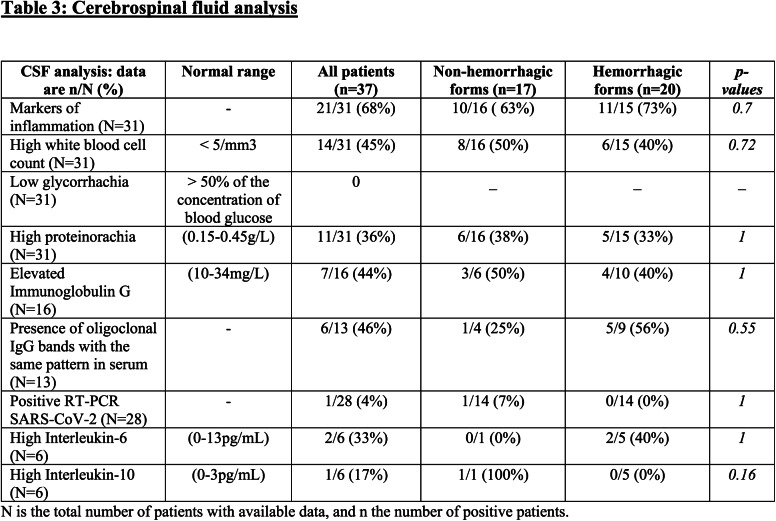
Thirty-one patients underwent a lumbar puncture, and among them, 21/31(68%) had increased markers of inflammation (high white blood cell count, and/or high proteinorachia, and/or elevated immunoglobulin G). One patient demonstrated the presence of SARS-CoV-2 on RT-PCR. High levels of interleukin-6 were found in 2 out of 6 patients (table 3).
Table 3:
Cerebrospinal fluid analysis
Neuroimaging findings
The results of MRI findings are summarized in figure 1. Among the 37 patients included, 28/37 (76%) were associated with one neuroimaging pattern, 7/37 (19%) with two patterns, and 2/37 (5%) showed three patterns (figures 1--6).6). The most frequent neuroimaging findings were: signal abnormalities located in the medial temporal lobe in 16/37 (43%, 95%IC 27-59%) patients (figure 2), non-confluent multifocal WM hyperintense lesions on FLAIR and diffusion, with variable enhancement, associated with hemorrhagic lesions in 11/37 (30%, 95%IC 15-45%) patients (figure 3), and in 9/37 (24%, 95%IC 10-38%) patients extensive and isolated WM microhemorrhages were detected (figure 4).
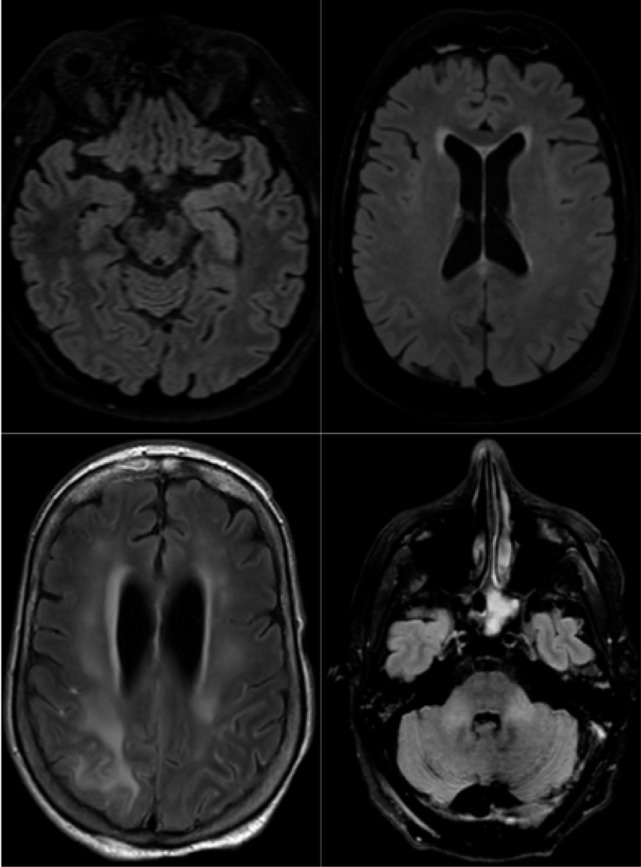
Axial FLAIR in four different COVID-19 patients. A) 58-year old man with impaired consciousness: FLAIR hyperintensities located in the left medial temporal lobe. B) 66-year old man with impaired consciousness: FLAIR ovoid hyperintense lesion located in the central part of the splenium of the corpus callosum. C) 71-year old woman with pathological wakefulness after sedation: extensive and confluent supratentorial white matter FLAIR hyperintensities (arrows). Association with leptomeningeal enhancement (stars) D) 61-year old man with confusion: hyperintense lesions involving both middle cerebellar peduncles.
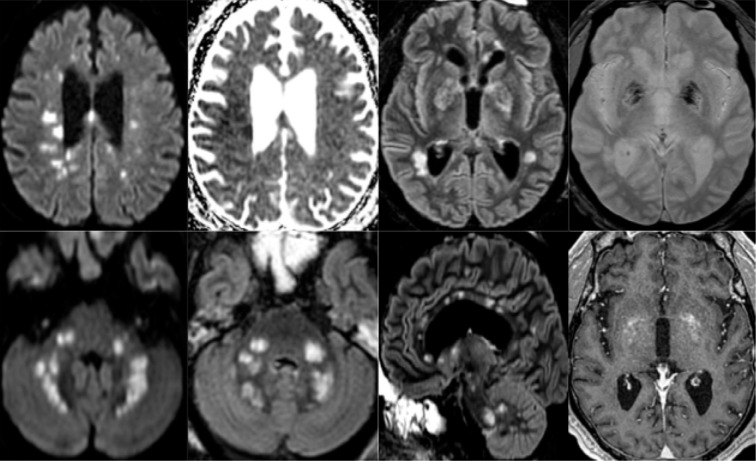
65-year old man with pathological wakefulness after sedation. Non-confluent multifocal white matter hyperintense lesions on FLAIR and diffusion, with variable enhancement, and hemorrhagic lesions. Axial Diffusion (A, B), Apparent Diffusion Coefficient (ADC) map (C), axial FLAIR (D, E), sagittal FLAIR (F), axial Susceptibility weighted imaging (SWI) (G), and postcontrast T1 weighted MR images (H). Multiple nodular hyperintense Diffusion and FLAIR lesions localized in the white matter including the corpus callosum (F). Some of them (white arrow) are associated with reduced ADC corresponding to cytotoxic edema (C). Other lesions are located next to the lenticular nucleus (cross) (E, G, H), with hemorrhagic changes (G), and enhancement after contrast administration.
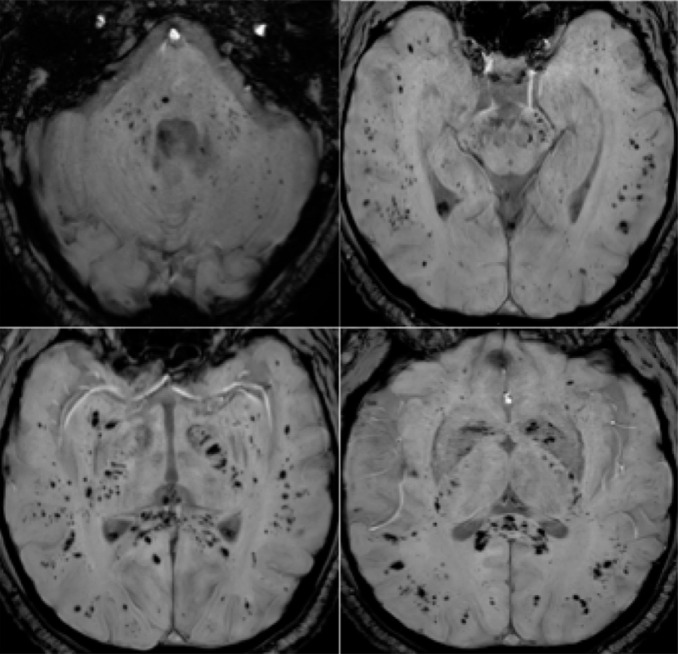
57-year old man with pathological wakefulness after sedation. Extensive and isolated white matter microhemorrhages. Axial Susceptibility weighted imaging (SWI) (A, B, C, D): multiple microhemorrhages mainly affecting the subcortical white matter, corpus callosum, internal capsule, and cerebellar peduncles.
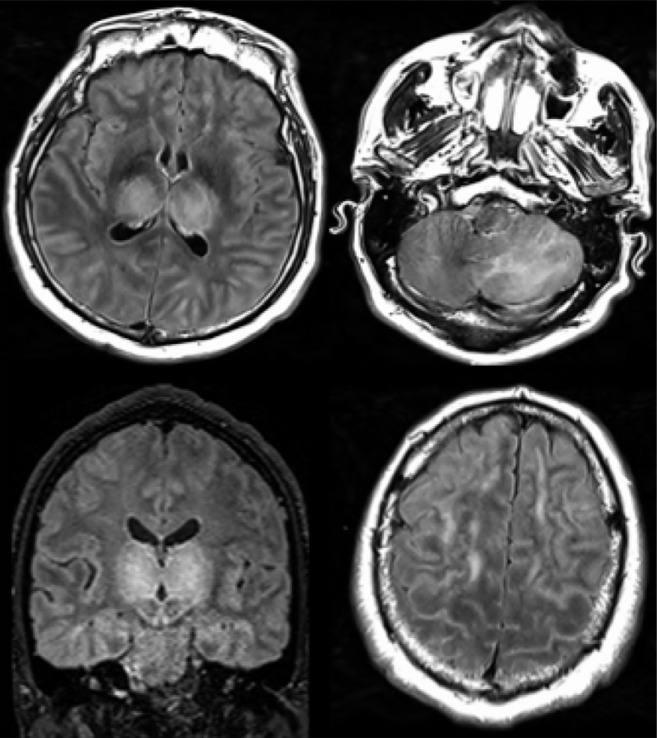
51-year old man with impaired consciousness. Acute necrotizing encephalopathy. Axial FLAIR (A, C, D), and coronal FLAIR (B): bilateral FLAIR hyperintensity (cross) in both thalami (A, B), associated with involvement of the cerebellar (C), and cerebral (D) white matter (arrows).
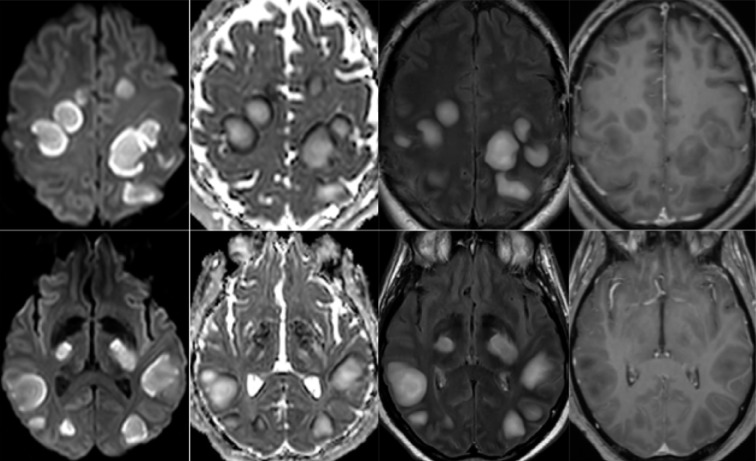
54-year old man with pathological wakefulness after sedation. Non-confluent multifocal white matter hyperintense lesions on FLAIR and diffusion, with variable enhancement. Axial Diffusion (A, B), Apparent Diffusion Coefficient (ADC) map (C, D), axial postcontrast FLAIR (E, F), and postcontrast T1 weighted MR images (G, H). Multiple nodular hyperintense Diffusion and FLAIR subcortical and corticospinal tracts lesions, with very mild mass effect on adjacent structures. The lesions present a center with an elevation of ADC corresponding to vasogenic edema and a peripheral ring of reduced ADC corresponding to cytotoxic edema (C, D). After contrast administration, small areas of very mild enhancement are detected (G, H).
Comparison of patient groups with and without hemorrhagic lesions
The comparison between patients with and without intracerebral hemorrhagic lesions shows that the hemorrhagic complications were more frequently associated with ICU admission (20/20, 100% versus 12/17, 71%, p=0.01), with acute respiratory distress syndrome (ARDS) (20/20, 100% versus 11/17, 65%, p=0.005) and with pathological wakefulness when sedative therapies were stopped (13/20, 65% versus 2/17, 12%, p=0.002). The time between the onset of symptoms (most often respiratory) to brain MRI was longer for patients with intracerebral hemorrhagic lesions (mean duration of 33 days versus 19 days, p<0.001). Leukocytosis (median of 13.4 x 109/L versus 10.4 x 109/L, p=0.03), anemia (median of 87 g/L versus 110 g/L, p<0.001), and renal dysfunction (urea’s median of 18mmol/L versus 7mmol/L, p=0.026) were more pronounced in the case of hemorrhagic lesions.
Discussion
Among the eight groups of brain MRI features classification, three main neuroradiological patterns appeared more frequently in patient with severe COVID-19: signal abnormalities located in the medial temporal lobe, non-confluent multifocal WM hyperintense lesions on FLAIR and diffusion with variable enhancement, associated with hemorrhagic lesions, and extensive and isolated WM microhemorrhages. The presence of hemorrhage was frequent, and the detection is of clinical importance as it was associated with worse respiratory, neurological, and biological status. Nevertheless, the underlying mechanism of brain abnormalities remains unsolved, and the direct implication of SARS-CoV-2 is not clear as only one patient was positive for SARS-CoV-2 RNA in the CSF.
Unilateral FLAIR and/or diffusion hyperintensities located in medial temporal lobe were frequent and have been previously reported in one patient with COVID-19 (10). The latter is frequently observed in case of infectious encephalitis (especially with some viruses like Herpes simplex virus, Human herpesvirus 6, or Epstein-Barr virus) or in association with autoimmune limbic encephalitis (19).
Non-confluent multifocal WM hyperintense lesions on FLAIR and diffusion, with variable enhancement, which could be associated with hemorrhagic lesions, have rarely been reported in patients with COVID-19 (20). The latter presentation is close to what can be observed on brain MRIs in case of an inflammatory demyelinating disease such as acute disseminated encephalomyelitis (ADEM) or acute hemorrhagic leukoencephalitis. However, these two latter diagnoses cannot only be retained on the radiological presentation without the typical CSF analysis or clinical presentation (21,22). Several putative mechanisms underlying neurological consequences of COVID-19 are evoked and among them immunological parainfectious processes (23). The immunologic assumption is also reinforced by a recent neuropathological study which described ADEM-like lesions in the subcortical WM in a patient with severe COVID-19 (24).
Extensive and isolated WM microhemorrhages pattern was recently described in 7 critically ill patients with COVID-19 (12) and in the neuropathology study above mentioned (24). A similar pattern was recently described in one case (25) with disseminated intravascular coagulation. However, according to the criteria endorsed by the International Society on Thrombosis and Haemostasis (27), when they were available, no case of disseminated intravascular coagulation was present in our cohort. Its precise pathophysiology remains uncertain and will require further studies. Radmanesh et al. (12) evoking the assumptions of hypoxia or small vessel vasculitis.
A small number of patients presented extensive and confluent supratentorial WM FLAIR hyperintensities (figure 2), as previously described by Kandemirli et al. (11) and Radmanesh et al. (12). Its precise pathophysiology remains unclear: viral encephalitis (not supported by CSF analysis) or post-infectious demyelinating diseases, as previously mentioned, may be evoked. Since most of our patients were admitted to ICUs for an ARDS, more general assumptions may be considered, such as delayed post-hypoxic leukoencephalopathy (27), metabolic or toxic encephalopathy, and posterior reversible encephalopathy syndrome (PRES). This last hypothesis is in accordance with recently published non-hemorrhagic and hemorrhagic PRES in patients with COVID-19 (28).
Even if this national neuroimaging cohort remains unique, our study has several limitations, mainly due to his retrospective design. The main limitation is that certain laboratory data were missing for some patients, notably the immunological tests. Moreover, patients’ outcomes were not always known at the time of this communication. Thus, the mortality rate is probably underestimated in our cohort.
In conclusion, in this multi-institutional study, we report 37 patients with COVID-19 and abnormal brain MRIs (excluding ischemic infarcts). Three main neuroradiological patterns could be distinguished, and the presence of hemorrhage was associated with worse clinical status. SARS-CoV-2 RNA was detected in the CSF only in one patient, and the underlying mechanisms of brain involvement remain unclear. Imaging and neurological follow up has to be undertaken in order to evaluate the prognosis of these patients.
*S.K. and F.L. contributed equally to the work.
Conflicts of Interest: All authors declare no competing interests.
Abbreviations:
- ADEM
- Acute disseminated encephalomyelitis
- ANE
- Acute necrotizing encephalopathy
- ARDS
- Acute respiratory distress syndrome
- CNS
- Central nervous system
- CoV
- Coronavirus
- CSF
- Cerebrospinal fluid
- EEG
- Electroencephalogram
- FLAIR
- Fluid-attenuated inversion recovery
- HCoV
- Human coronavirus
- ICU
- Intensive care unit
- PRES
- Posterior reversible encephalopathy syndrome
- RT-PCR
- Reverse transcriptase-polymerase chain reaction
- SARS-CoV-2
- Severe acute respiratory syndrome coronavirus 2
- WM
- White matter
References
Full text links
Read article at publisher's site: https://doi.org/10.1148/radiol.2020202222
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc7301613?pdf=render
Citations & impact
Impact metrics
Article citations
Brain structures as potential mediators of the causal effect of COVID 19 on migraine risk.
Sci Rep, 14(1):27895, 13 Nov 2024
Cited by: 0 articles | PMID: 39537835 | PMCID: PMC11560959
Quantitative susceptibility mapping at 7 T in COVID-19: brainstem effects and outcome associations.
Brain, awae215, 07 Oct 2024
Cited by: 0 articles | PMID: 39375207 | PMCID: PMC7616766
Structural and functional brain markers of cognitive impairment in healthcare workers following mild SARS-CoV-2 infection during the original stream.
Brain Commun, 6(5):fcae340, 30 Sep 2024
Cited by: 0 articles | PMID: 39416878 | PMCID: PMC11481020
Causal effect of COVID-19 on longitudinal volumetric changes in subcortical structures: A mendelian randomization study.
Heliyon, 10(17):e37193, 30 Aug 2024
Cited by: 0 articles | PMID: 39296245 | PMCID: PMC11408012
Decreased Cerebral Creatine and N-Acetyl Aspartate Concentrations after Severe COVID-19 Infection: A Magnetic Resonance Spectroscopy Study.
J Clin Med, 13(14):4128, 15 Jul 2024
Cited by: 0 articles | PMID: 39064167 | PMCID: PMC11277668
Go to all (260) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Retrospective Observational Study of Brain MRI Findings in Patients with Acute SARS-CoV-2 Infection and Neurologic Manifestations.
Radiology, 297(3):E313-E323, 17 Jul 2020
Cited by: 104 articles | PMID: 32677875 | PMCID: PMC7370354
Neurologic and neuroimaging findings in patients with COVID-19: A retrospective multicenter study.
Neurology, 95(13):e1868-e1882, 17 Jul 2020
Cited by: 143 articles | PMID: 32680942
Early postmortem brain MRI findings in COVID-19 non-survivors.
Neurology, 95(14):e2016-e2027, 16 Jun 2020
Cited by: 166 articles | PMID: 32546654
Clinical Characteristics and Morbidity Associated With Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit.
JAMA Netw Open, 3(6):e2012270, 01 Jun 2020
Cited by: 390 articles | PMID: 32543702 | PMCID: PMC7298606
Review Free full text in Europe PMC

 *
*