Abstract
Objective
To identify structural and neurochemical properties that underlie functional connectivity impairments of the primary motor cortex (PMC) and how these relate to clinical findings in amyotrophic lateral sclerosis (ALS).Methods
52 patients with ALS and 52 healthy controls, matched for age and sex, were enrolled from 5 centres across Canada for the Canadian ALS Neuroimaging Consortium study. Resting-state functional MRI, diffusion tensor imaging and magnetic resonance spectroscopy data were acquired. Functional connectivity maps, diffusion metrics and neurometabolite ratios were obtained from the analyses of the acquired multimodal data. A clinical assessment of foot tapping (frequency) was performed to examine upper motor neuron function in all participants.Results
Compared with healthy controls, the primary motor cortex in ALS showed reduced functional connectivity with sensory (T=5.21), frontal (T=3.70), temporal (T=3.80), putaminal (T=4.03) and adjacent motor (T=4.60) regions. In the primary motor cortex, N-acetyl aspartate (NAA, a neuronal marker) ratios and diffusion metrics (mean, axial and radial diffusivity, fractional anisotropy (FA)) were altered. Within the ALS cohort, foot tapping frequency correlated with NAA (r=0.347) and white matter FA (r=0.537). NAA levels showed associations with disturbed functional connectivity of the motor cortex.Conclusion
In vivo neurochemistry may represent an effective imaging marker of impaired motor cortex functional connectivity in ALS.Free full text
Original research
Motor cortex functional connectivity is associated with underlying neurochemistry in ALS
Abstract
Objective
To identify structural and neurochemical properties that underlie functional connectivity impairments of the primary motor cortex (PMC) and how these relate to clinical findings in amyotrophic lateral sclerosis (ALS).
Methods
52 patients with ALS and 52 healthy controls, matched for age and sex, were enrolled from 5 centres across Canada for the Canadian ALS Neuroimaging Consortium study. Resting-state functional MRI, diffusion tensor imaging and magnetic resonance spectroscopy data were acquired. Functional connectivity maps, diffusion metrics and neurometabolite ratios were obtained from the analyses of the acquired multimodal data. A clinical assessment of foot tapping (frequency) was performed to examine upper motor neuron function in all participants.
Results
Compared with healthy controls, the primary motor cortex in ALS showed reduced functional connectivity with sensory (T=5.21), frontal (T=3.70), temporal (T=3.80), putaminal (T=4.03) and adjacent motor (T=4.60) regions. In the primary motor cortex, N-acetyl aspartate (NAA, a neuronal marker) ratios and diffusion metrics (mean, axial and radial diffusivity, fractional anisotropy (FA)) were altered. Within the ALS cohort, foot tapping frequency correlated with NAA (r=0.347) and white matter FA (r=0.537). NAA levels showed associations with disturbed functional connectivity of the motor cortex.
Conclusion
In vivo neurochemistry may represent an effective imaging marker of impaired motor cortex functional connectivity in ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is an adult-onset neurodegenerative disorder. ALS manifests with an impaired ability to perform motor tasks (eg, walking, eating or breathing) due to the degeneration of upper motor neurons (UMNs) in the primary motor cortex (PMC) and lower motor neurons in the brainstem and spinal cord. Clinical signs of ALS include muscular atrophy, fasciculations, hyperreflexia, weakness and spasticity.1 Of these, hyperreflexia, weakness and spasticity are signs of UMN impairment. Task and resting-state functional MRI (fMRI)2–6 studies have shown impairments in synchronous activity (ie, functional connectivity (FC)) occurring within and between motor and extramotor brain regions. Studies using diffusion tensor imaging (DTI) and voxel-based morphometry have respectively shown evidence of altered microstructure of the corticospinal tract7 and atrophy in the precentral gyrus.8 Magnetic resonance spectroscopy (MRS) studies have shown alterations in neurochemical levels in the PMC in patients with ALS when compared with healthy controls.9 These independent observations of UMN impairment, assessed in conjunction, could potentially provide deeper insights into ALS pathophysiology. Previous analyses of multiple MRI techniques have revealed associations between impaired cortical structure and function.10–14 However, there is no adequate understanding of the association between the functional and anatomical (structure and neurochemistry) properties of affected UMNs inherent to the PMC in ALS.
To address this gap in the literature, a multimodal approach was employed. fMRI, MRS and DTI data were analysed to assess FC, neurochemical and microstructural properties of the PMC. It was hypothesised that in ALS (1) there is altered FC of the PMC with the rest of the brain, (2) these FC alterations relate to underlying structural and/or neurochemical deficits and (3) these FC alterations are associated with clinical measures of UMN impairment. An extensive investigational approach was thus applied to evaluate FC of the PMC across different brain regions and to evaluate the relationship between FC and altered structural, neurochemical and clinical measures.
Methods
Study design and participants
A prospective, multicentre MRI study was conducted at academic hospitals affiliated with universities at five centres located in Edmonton (University of Alberta), Calgary (University of Calgary), Montreal (McGill University), Toronto (University of Toronto) and Vancouver (University of British Columbia), as part of the Canadian ALS Neuroimaging Consortium (CALSNIC).15
Participant demographics and clinical characteristics are shown in table 1 (and detailed in the Results section). Fifty-two patients with ALS were recruited from multidisciplinary ALS clinics at all centres. All patients met diagnostic criteria for clinically possible, probable-lab supported, probable or definite ALS according to El Escorial criteria.16 Patients were not included in the study if they had a symptom duration more than 5 years, did not complete imaging acquisition for either of the MRI sequences in this study, or presented with comorbid frontotemporal dementia (FTD) or other neurological conditions. Fifty-two healthy controls matched for age, sex and number of years of education, without a history of neurological or psychiatric conditions were also recruited into the study. See online supplemental file 1 for a breakdown of participant numbers by site. Foot tapping frequencies (number of taps per 10
years, did not complete imaging acquisition for either of the MRI sequences in this study, or presented with comorbid frontotemporal dementia (FTD) or other neurological conditions. Fifty-two healthy controls matched for age, sex and number of years of education, without a history of neurological or psychiatric conditions were also recruited into the study. See online supplemental file 1 for a breakdown of participant numbers by site. Foot tapping frequencies (number of taps per 10 s) were recorded for all participants bilaterally and averaged. This clinical variable was selected based on the midline localisation of the MRS region of interest, encompassing the foot region of the motor homunculus bilaterally. Therefore, the right/left foot tapping frequencies were averaged to obtain a single representative measure of UMN function.
s) were recorded for all participants bilaterally and averaged. This clinical variable was selected based on the midline localisation of the MRS region of interest, encompassing the foot region of the motor homunculus bilaterally. Therefore, the right/left foot tapping frequencies were averaged to obtain a single representative measure of UMN function.
Table 1
Participant demographics and clinical characteristics
| Participant characteristics | ALS | Healthy controls | p-value |
| Number of participants | 52 | 52 | |
| Sex (n): male/female | 34/18 | 25/27 | NS |
| Age (years) | |||
 Mean±SD Mean±SD | 58.4±10.0 | 54.9±9.8 | NS |
 Median (range) Median (range) | 57.5 (33.0–78.0) | 56.0 (29.0–69.0) | |
| Education (number of years) | |||
 Mean±SD Mean±SD | 15.4±4.0 | 16.6±3.2 | NS |
 Median (range) Median (range) | 15 (4–28) | 16.25 (11–28) | |
| Onset (n) | |||
 Limb/bulbar Limb/bulbar | 42/10 | – | |
| El Escorial clinical diagnosis category (n) | |||
 Definite ALS Definite ALS | 11 | – | |
 Probable ALS Probable ALS | 19 | – | |
 Probable ALS-lab supported Probable ALS-lab supported | 9 | – | |
 Possible ALS Possible ALS | 12 | – | |
| Revised ALS Functional Rating Scale Score (/48) | |||
 Mean±SD Mean±SD | 38.8±5.2 | – | |
 Median (range) Median (range) | 40 (22–47) | – | |
| Symptom duration (months) | |||
 Mean±SD Mean±SD | 26.7±14.6 | – | |
 Median (range) Median (range) | 21.5 (7.8–57.4) | – | |
Foot tapping frequencies (taps/10 s) s) | |||
 Mean±SD Mean±SD | 24±15 | 40±8 | <0.001 |
 Median (range) Median (range) | 23 (0–64) | 40 (24–62) | |
| Edinburgh Cognitive and Behavioral ALS Screen total (/136) | |||
 Mean±SD Mean±SD | 102.8±17.7 | 113.3±10.8 | <0.001 |
 Median (range) Median (range) | 106 (53–127) | 114 (62–134) |
ALS, amyotrophic lateral sclerosis.
Supplementary data
Image acquisition
Imaging data were acquired on 3T Siemens scanners in Edmonton (Prisma) and Montreal (Tim Trio) using 20-channel and 32-channel receiving head coils, respectively; on 3T General Electric Healthcare (Discovery MR750) scanners in Calgary and Toronto using 12-channel and 8-channel receiving head coils, respectively; and on 3T Philips Achieva scanner in Vancouver using an 8-channel head coil receiver.
A multicentre harmonised scanning protocol, adjusted for scanner variances, was employed to acquire T1-weighted (T1w) anatomical MRI, resting-state fMRI (rs-fMRI) of neuronal activity (measured indirectly by the blood oxygenation level-dependent effect), DTI and single-voxel MRS data. For anatomical localisation and normalisation, a high-resolution 3D T1w scan of the whole brain was acquired. A magnetisation-prepared rapid gradient-echo imaging sequence (MPRAGE; repetition time, TR=2300 ms; echo time, TE=3.43 ms; inversion time, TI=900 ms; flip angle=9°; field of view, FOV=256 mm×256 mm) was used to acquire T1w data with an isotropic resolution of 1
mm) was used to acquire T1w data with an isotropic resolution of 1 mm3. MRS data were acquired using a single voxel data acquisition protocol. As described previously,17 anatomical landmarks were used to place the MRS voxel in the left/right foot region of the PMC, centred symmetrically along the midline. A stimulated echo acquisition mode sequence (STEAM) was performed to acquire water suppressed spectra from the PMC with the following specifications: TR=3000 ms; TE=160 ms; mixing time, TM=40 ms; two acquisitions of 32 signal averages each. Whole-brain 3D T2*-weighted rs-fMRI data was acquired using an echo-planar imaging (EPI) pulse sequence with an isotropic resolution of 3.5
mm3. MRS data were acquired using a single voxel data acquisition protocol. As described previously,17 anatomical landmarks were used to place the MRS voxel in the left/right foot region of the PMC, centred symmetrically along the midline. A stimulated echo acquisition mode sequence (STEAM) was performed to acquire water suppressed spectra from the PMC with the following specifications: TR=3000 ms; TE=160 ms; mixing time, TM=40 ms; two acquisitions of 32 signal averages each. Whole-brain 3D T2*-weighted rs-fMRI data was acquired using an echo-planar imaging (EPI) pulse sequence with an isotropic resolution of 3.5 mm3 and the following specifications: TR=2200 ms; FOV=224×224×64 matrix; 40 slices, 192 trains; acquisition time=~7
mm3 and the following specifications: TR=2200 ms; FOV=224×224×64 matrix; 40 slices, 192 trains; acquisition time=~7 min. Participants were instructed to lie still with their eyes closed during the rs-fMRI scan. A 2D spin-echo, single-shot, EPI pulse sequence was used for the acquisition of diffusion-weighted images axially with the following specifications: TR=10
min. Participants were instructed to lie still with their eyes closed during the rs-fMRI scan. A 2D spin-echo, single-shot, EPI pulse sequence was used for the acquisition of diffusion-weighted images axially with the following specifications: TR=10 000 ms; TE=90 ms; flip angle=90°; 70 slices; b0 images=5; diffusion gradient directions=30; steady magnetisation, b0=1000
000 ms; TE=90 ms; flip angle=90°; 70 slices; b0 images=5; diffusion gradient directions=30; steady magnetisation, b0=1000 s/mm2; voxel size=2
s/mm2; voxel size=2 mm3. The parameters mentioned above are for the Siemens systems. MRI data acquired on the General Electric and Philips systems were harmonised to the Siemens data with slight differences specific to the scanner manufacturer.15 Harmonisation of MRI parameters was further ensured by scanning a set of participants two times at each scanner to ascertain test–retest and multicentre reliability.18
mm3. The parameters mentioned above are for the Siemens systems. MRI data acquired on the General Electric and Philips systems were harmonised to the Siemens data with slight differences specific to the scanner manufacturer.15 Harmonisation of MRI parameters was further ensured by scanning a set of participants two times at each scanner to ascertain test–retest and multicentre reliability.18
Image processing
See figure 1 for an overview of the processing pipeline used in this study. The details of data processing for the different MRI modalities are discussed in the following sections.
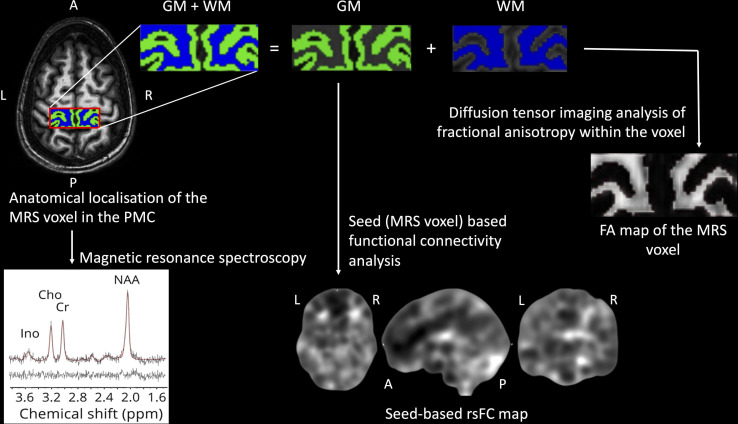
Overview of the processing pipeline. Spectral signatures of neurometabolites of interest were obtained from the primary motor cortex (PMC) (magnetic resonance spectroscopy (MRS)) voxel. The MRS voxel underwent segmentation into grey matter (GM) and white matter (WM) tissue classes, which respectively underwent analyses of functional connectivity and diffusion tensor imaging. FA, fractional anisotropy; rsFC, resting-state functional connectivity.
Magnetic resonance spectroscopy
MRS is an imaging technique that has been important to the study of neurological disorders. Metabolites such as N-acetyl aspartate (NAA) and NAA glutamate (NAAG), collectively referred to as total NAA (tNAA) moieties, provide an important marker of neuronal integrity.9 Ratios of tNAA to creatine (Cr), choline (Cho) or a combination (Cr+Cho) have been consistently shown to be reduced in the PMC and corticospinal tract regions, as well as in other extramotor brain regions.9 Based on the consistency of observations of PMC tNAA neurochemistry in the literature, the neurometabolite ratios of interest in this study were tNAA/Cr and tNAA/Cho. As described in a previous study,17 the proton MRS spectra from the PMC underwent fitting using LCModel (V.6.1). Spectra were excluded from further analysis if visual inspection showed data corruption or if an SD greater than 15% was obtained from the spectral fit. The peak areas for the NAA and NAAG metabolites were summed from the combined grey and white matter (WM) tissue classes within the MRS voxel to quantify the total amount of N-acetyl aspartyl moieties (tNAA) and expressed as ratios to Cr and Cho for statistical analyses.
Generation of grey matter (GM) and white matter (WM) segments within the MRS region of interest
The whole-brain T1w anatomical image underwent segmentation into the GM, WM, and cerebrospinal fluid tissue classes using a standard voxel-based morphometry pipeline (http://dbm.neuro.uni-jena.de/vbm8/). The obtained whole-brain segments for the GM and WM tissue classes underwent voxel-wise matrix multiplication with the MRS voxel to generate GM and WM segments for the PMC region of interest, which were used in further analyses in the study (figure 1). Prior to this multiplication, the T1w and MRS voxel images of all included participants were inspected manually to ascertain accuracy in voxel-matching and to ensure that the subsequent FC and DTI analyses were performed in the exact anatomical region from which the MRS spectra were obtained.
Diffusion tensor imaging
As described in a previous study,19 the DTI data were processed using ExploreDTI (V.4.8.6). First, a visual quality check was performed to assess for scan quality, head motion, signal artefacts. Preprocessing steps included corrections for temporal signal drift using quadratic model, Gibbs ringing artefacts (five non-diffusion-weighted images, lambda=100, iterations=100, step size=0.01), head motion and eddy current-induced geometric distortions. In particular, a non-rigid registration of the DTI data to the respective T1w MRI data was performed for each participant to correct for EPI distortions. Following this, voxel-wise maps were calculated for four diffusion metrics: fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD) and axial diffusivity (AD). Subsequently, average values for these diffusion measures were estimated for the WM tissue classes within the PMC using the ‘fslmeants’ function in FSL (https://fsl.fmrib.ox.ac.uk/fsl).
FC analysis for rs-fMRI
The rs-fMRI images were preprocessed using the functional connectivity toolbox (CONN) based on the Statistical Parametric Mapping (SPM) software (V.12, www.fil.ion.ucl.ac.uk/spm). The first four points in the time series of collected images were removed for every participant to account for the approach to steady state magnetisation. The time series data for each participant were corrected for differences in image slice acquisition times, followed by realignment for head motion within each imaging session and across imaging sessions using a six-parameter rigid body transformation algorithm. The mean of the multislice rs-fMRI data (temporally averaged across the time series) was coregistered to the T1w anatomical MRI data, and the resulting transformation matrix was applied to all time points of the rs-fMRI data within each session. Maps of FC were calculated in the ‘native’ anatomical space prior to transformation. Temporal correlations were performed voxel wise between the rs-fMRI signal time course of the PMC and other GM brain voxels to generate FC maps, using the PMC as a seed. A binary cut-off threshold was set at a Pearson correlation coefficient r≥0.2520 to identify strongly correlated voxel pairs, as a proxy to brain regions depicting high levels of functional integration. The FC maps generated in the individual native space underwent normalisation to the Montreal Neurological Institute template21 space and smoothing with a Gaussian smoothing kernel of 8
to identify strongly correlated voxel pairs, as a proxy to brain regions depicting high levels of functional integration. The FC maps generated in the individual native space underwent normalisation to the Montreal Neurological Institute template21 space and smoothing with a Gaussian smoothing kernel of 8 mm full width at half-maximum.
mm full width at half-maximum.
Rs-fMRI, MRS and DTI statistical analyses
Statistical plan
All generalised linear models (GLM) used the available software routines in SPM12 and included CALSNIC centre of data acquisition as a ‘factor’ in the statistical model. Significance for all analyses in SPM12 was set at a cluster threshold of k=25 voxels (obtained after performing Monte-Carlo simulations for 5000 iterations and plocal=0.001, pglobal=0.05 using a code developed in-house6). Significance for all analyses in SPSS V.26 was set at p<0.05.
The following sections describe the statistical analyses performed in this study:
Group differences in resting-state FC: A univariate full factorial GLM was used in SPM12 to examine group differences in FC of the PMC with other brain regions. Diagnosis was included as a factor in the GLM in addition to the CALSNIC centre of data acquisition. Age, sex and number of years of education had no influence on FC differences, so these variables were removed from the model to allow for an increase in the degrees of freedom.
Group differences in diffusion and neurochemical measures: A univariate analysis of variance full factorial model was used in SPSS to examine group differences in the average GM and WM diffusion metrics (FA, MD, RD, AD) and neurochemical levels (tNAA/Cr, tNAA/Cho) within the PMC across the two groups. Site-wise scatterplots of the data were generated to test for any outliers in the data to be eliminated in further statistical analyses.
Relationship between diffusion and neurochemical measures and UMN dysfunction: The structural and neurochemical measures which revealed significant between-group differences underwent bivariate Pearson’s correlational analyses with UMN measures in SPSS for the patient group. This was performed to identify the structural and neurochemical measures that were related to clinical UMN dysfunction. The imaging measures showing correlations with clinical variables were used in further analyses (see the next section).
Relationship between structural and neurochemical measures and FC differences in ALS: The structural and neurochemical measures showing significant correlations with UMN dysfunction were modelled as independent variables in a univariate full factorial GLM including CALSNIC centre of data acquisition as a factor. Subsequent bivariate correlational analyses were performed in SPM12 to examine the associations between these imaging measures and FC differences.
Relationship between UMN dysfunction and FC differences in ALS: Foot tapping was modelled as an independent variable in the univariate full factorial model in SPM12 (from statistical analyses section 1) and correlational analyses were performed to examine the relationship with FC differences.
Relationship between structural, neurochemical and functional characteristics and the rate of functional decline: The revised ALS Functional Rating Scale (ALSFRS-R), a 12-question self-administered questionnaire (maximum score=48), was used to calculate the rate of functional decline in patients with ALS using the formula (48-ALSFRS-R Score)/symptom duration. To ascertain the relationship between the rate of functional decline and MRI measures in the PMC, two sets of correlations were performed:
Bivariate Pearson’s correlational analyses between spectroscopy, diffusion measures and disease progression rate (SPSS).
Group-wise FC and disease progression rate (SPM12).
Results
Demographic and clinical characteristics of the sample
All patients in our study had a confirmed diagnosis of ALS and were matched with healthy controls on the basis of age, sex and years of education. Patients had an average symptom duration of 26.7 months and a mean ALSFRS-R Score of 38.8/48, with 42 patients having limb-onset ALS. Foot tapping frequencies were significantly reduced in ALS (mean±SD=24±15) compared with healthy controls (mean±SD=40±8). The Edinburgh Cognitive and Behavioral ALS Screen total scores were significantly reduced in patients (mean±SD=103±18) compared with healthy controls (mean±SD=113±11) (table 1). In terms of UMN involvement at the level of the lower limbs, as assessed on neurological examination, hyperreflexia was observed in 48/52 patients, spasticity was observed in 21/52 patients and the Babinski sign was observed in 15/52 patients at the time of data acquisition.
Group differences in resting state FC
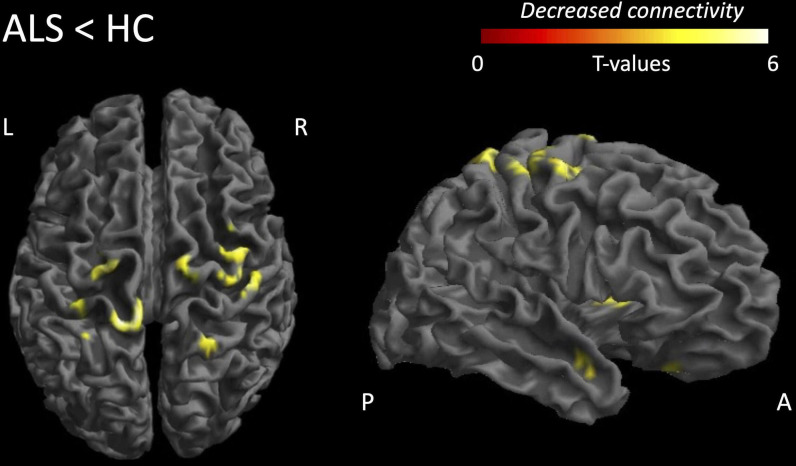
The PMC of patients with ALS showed reduced FC with regions in adjacent bilateral premotor cortices and supplementary motor areas (cluster extent, k=48, T=4.50), bilateral primary sensory cortices (k=95, T=5.21), right putamen (k=34, T=4.03), right temporal pole (k=38, T=3.80) and right inferior frontal gyrus (k=34, T=3.70) (figure 2 and table 2).
Table 2
Differences in resting-state FC
| Brain region | Brodmann area | Number of voxels in the cluster | Peak MNI coordinates | T-value | ||
| x | y | z | ||||
| Reduced FC in ALS (contrast ALS<HC) | ||||||
| B primary sensory cortex | BA 1 | 95 | −12 | −40 | 77 | 5.21 |
| B premotor+supplementary motor area | BA 6 | 48 | 36 | −4 | 59 | 4.60 |
| R putamen | – | 34 | 30 | 5 | 2 | 4.03 |
| R temporal pole | BA 38 | 38 | 45 | 8 | −25 | 3.80 |
| R inferior frontal gyrus | BA 47 | 34 | 18 | 20 | −28 | 3.70 |
| Increased FC in ALS (contrast ALS>HC) | ||||||
 No suprathreshold clusters No suprathreshold clusters | ||||||
T-values and coordinates in MNI standard space are reported (T; x,y,z).
ALS, amyotrophic lateral sclerosis; B, bilateral; BA, Brodmann area; FC, functional connectivity; HC, healthy controls; MNI, Montreal Neurological Institute; R, right.
Group differences in diffusion and neurochemical measures in the PMC
Neurochemical ratios in the PMC were reduced for tNAA/Cr (p<0.001) and tNAA/Cho (p=0.017) in ALS when compared with healthy controls. For the DTI measures, FA (p<0.001), MD (p<0.001) and WM (p=0.022) values were increased in the WM (table 3).
Table 3
Summary of structural and neurochemical measures in the PMC
| Imaging metric | Mean±SE | p-value | |
| ALS | HC | ||
| Neurochemical ratios | |||
 tNAA/Cr tNAA/Cr | 1.88±0.03 | 2.05±0.03 | <0.001 |
 tNAA/Cho tNAA/Cho | 2.44±0.14 | 2.94±0.14 | 0.017 |
| Diffusion in PMC WM | |||
 FA FA | 0.38±0.00 | 0.41±0.00 | <0.001 |
 MD MD | (0.80±0.01) | (0.77±0.01) | <0.001 |
 RD RD | (0.64±0.01) | (0.6±0.01) | 0.022 |
 AD AD | (1.12±0.01) | (1.12±0.01) | NS |
MD, RD, AD are ×10−3 mm2/s. The boldened p-values indicate statistically significant group differences.
AD, axial diffusivity; Cho, choline; Cr, creatine; FA, fractional anisotropy; MD, mean diffusivity; PMC, primary motor cortex; RD, radial diffusivity; tNAA, total NAA moieties; WM, white matter.
Relationship of diffusion and neurochemical measurements with UMN function in ALS
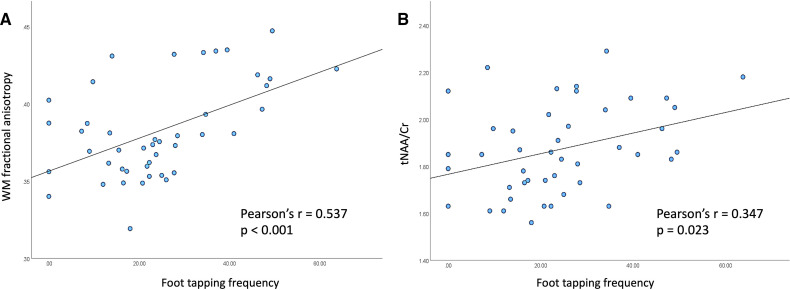
Foot tapping frequencies showed positive significant correlations with FA values in the WM (figure 3A, r=0.537, p<0.001) and tNAA/Cr levels (figure 3B, r=0.347, p=0.023) in the PMC of the ALS cohort.
Relationship between diffusion and neurochemical measures and FC differences
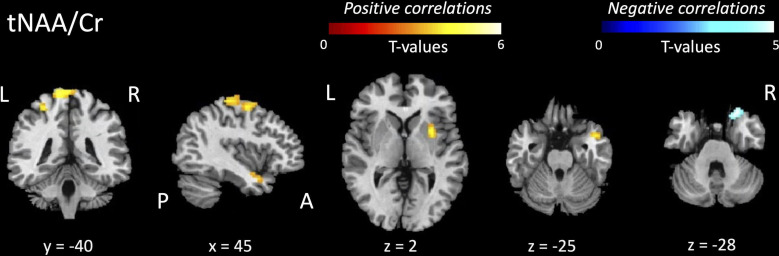
Despite WM FA showing stronger associations with clinical UMN impairment compared with tNAA/Cr, significant positive and negative associations of FC alterations were observed with tNAA/Cr and not WM FA values (figure 4 and table 4).
Table 4
Regional associations of reduced functional connectivity with primary motor cortex neurochemistry and FA
| Group comparison | Brain region (Brodmann area) | Brodmann area | Type of association | T -value |
| Associations between reduced FC and tNAA/Cr ratios | ||||
| ALS<HC | L primary sensory cortex | BA 1 | Positive | 5.09 |
| R primary motor cortex | BA 4 | 4.55 | ||
| B premotor+supplementary motor area | BA 6 | 4.44 | ||
| R putamen | – | 4.10 | ||
| R temporal pole | – | 3.82 | ||
| ALS<HC | R inferior frontal gyrus | BA 47 | Negative | −4.12 |
| Associations between reduced FC and WM FA—no suprathreshold clusters | ||||
T values and coordinates in MNI standard space are reported (T; x, y, z).
ALS, amyotrophic lateral sclerosis; B, bilateral; Cr, creatine; FA, fractional anisotropy; FC, functional connectivity; L, left; R, right; tNAA, total N-acetyl aspartate moieties.
Relationship between UMN function and FC reductions
There were no associations between foot tapping frequencies and FC alterations.
Relationship between structural, neurochemical and FC measures and the rate of functional decline in ALS
There were no associations between rate of functional decline and structural or neurochemical measures of the PMC. A positive association was observed between the rate of functional decline and FC of the PMC with the right dorsal anterior cingulate cortex (BA 32; k=343; T=4.49) and the left thalamus (k=51; T=4.40). A negative association was observed between the rate of functional decline and FC of the PMC with the right ventral anterior cingulate cortex (BA 24; k=244; T=4.95), left dorsolateral prefrontal cortex (BA 9; k=28; T=3.92) and right ventral posterior cingulate cortex (BA 23; k=25; T=3.62). When comparing ALS and HC cohorts, neither of these brain regions showed alterations in FC with the PMC.
Discussion
The present study sought to identify the effects of underlying diffusion and neurochemical deficits on PMC FC, and how these effects relate to clinical impairment in ALS. The main findings of the study are that FC of the PMC is reduced with multiple regions of the brain, the structural and neurochemical deficits in the PMC are associated with UMN dysfunction, and impaired PMC FC is related to altered neurochemistry but not WM microstructure.
The majority of studies on rs-fMRI in ALS have reported heterogeneous findings of altered FC within and between different resting-state networks, specifically the default mode and sensorimotor networks.6 22–24 In whole-brain rs-fMRI studies that assessed other measures of resting brain function (eg, regional homogeneity, fractional amplitude of low frequency fluctuations, etc), altered function was identified in the motor and extramotor brain regions.4 5 In region-of-interest-based studies on resting-state FC, reduced FC between the right and left motor cortices,25 altered FC of the motor cortex with other motor regions (superior parietal lobule, thalamus, basal ganglia, cerebellum)26 and altered FC of the motor cortex with extramotor regions (superior frontal and temporal cortices)14 have been reported. The findings from the current study (table 2) are congruent with these findings in the ALS literature.
Multimodal MRI has been previously used to identify the neurobiological changes underlying impaired cerebral function. Such studies have revealed structural–functional congruence in impairment between motor connectivity and WM structure27–29 that relates to clinical measures10 25 (eg, ALSFRS-R Scores, disease progression rates). These studies assessed the co-occurrence of functional and structural but not neurochemical alterations—an investigation that has been conducted for the first time in the present study. Additionally, none of the aforementioned studies considered the somatotopic organisation of the PMC in their interpretation of FC alterations in terms of clinical outcome measures. In the current study, the seed region was strategically defined in the foot region of the motor homunculus. The subsequent finding of the association between FC alterations and neurochemical concentrations in the PMC (table 4) is important for enhanced understanding of the diverse nature of ALS disease pathology.
Previously, structural–functional decoupling and the heterogeneity of its occurrence has been postulated in multiple disease pathologies. Decoupling, in the context of cerebral connectivity, refers to the dissociation between functional and structural connectivity abnormalities in a disease-specific state.30 31 In other words, decoupling simply refers to the co-occurring and/or sequential alterations in brain function and structure. For example, brain structural alterations preceding functional changes have been implicated cross-sectionally in major depressive disorder,32 in Parkinson’s disease with no cognitive impairment,33 and longitudinally in multiple sclerosis.34 In contrast, functional alterations have been shown to precede structural changes in Alzheimer’s disease.35 In patients with idiopathic Parkinson’s disease, differential atrophy patterns in hippocampal subfields have been reported to precede their phenotypic diagnostic conversion to Parkinson’s disease dementia,36 suggesting that structural changes occur before impairment of clinically defined cognitive functioning. In this study, no such evidence of sequential structure–function decoupling was observed.
Instead, we observed neurochemical-functional decoupling in relation to UMN impairment. To our best knowledge, there is no fMRI evidence of neurochemical–functional decoupling in neurodegenerative disorders in humans. Of the three imaging measures investigated (FC, FA and tNAA/Cr), FA and tNAA/Cr levels were found to correlate strongly with clinical UMN impairment, but not FC. Furthermore, the relationship between FC reductions and tNAA/Cr concentrations, but not of FC reductions and WM FA, could suggest that in terms of imaging identifiers of clinical impairment, alterations in neurochemical properties of the PMC might be an earlier occurrence in ALS pathophysiology and be more sensitive towards probing the functional underpinnings of ALS. The present work also observed changes in the underlying brain tissue microstructure which correlated with clinical impairment. However, the lack of associations between these microstructural changes and FC alterations suggests that neurochemical changes might even precede structural changes and translate to alterations in brain function and clinical outcomes. However, such chronology of cerebral changes cannot be confidently inferred through a cross-sectional study, presenting the need to perform a longitudinal characterisation of cerebral changes.
The present study benefits from a harmonised and multimodal MRI acquisition protocol across different ALS populations in Canada. This enables us to study different patient cohorts with varying clinical phenotypes of disease pathology across a multicentre cohort. This can provide a better understanding of the core neuronal processes that underlie cortical dysfunction in ALS. Additionally, harmonised imaging protocols can help reduce MRI system-related variance and increase statistical power.37 Another advantage of the present study is a localised, hypothesis-driven approach to uncover the diffusion and neurochemical signatures of altered function. This lowers the possibility of interpreting ALS disease pathology incorrectly by eliminating erroneous observations.
Heterogeneity of ALS, in terms of its clinical presentation and the underlying neurobiology, poses a challenge for scientific study of this complex disorder. Different genetic variants correlate with different clinical presentations of ALS.38 A limitation of the current study is that genetic information was not available for all patients. However, the present study controlled for heterogeneity to some extent by including patients who had a symptom duration of no more than 5 years38 and who did not previously receive a diagnosis of other neurological conditions such as FTD. Approximately 50% of patients presenting to a clinic with an El Escorial designation of suspected ALS can present with varying cognitive and behavioural impairments.39 Notably, there was no control for cognitive impairment as, even in patients presenting primarily with motor symptoms, brain regions outside the motor network could be affected.40 Another limitation of this study could be the lack of control for the potential pharmacodynamic impact of Riluzole therapy, a glutamate agonist, on in vivo imaging features. Previously, Riluzole therapy has been shown to prolong survival41 and in a small cohort to improve the concentrations of NAA after approximately 3 weeks of treatment42; however, the long-term effects on NAA are unknown.
In conclusion, this study has shown that reduced FC of the motor cortex in ALS is linked to the local concentrations of NAA. This highlights the importance of assessment of in vivo neurochemistry as an early pathophysiological marker of PMC functional changes in the characterisation of ALS disease pathology. Based on the findings from this study, it could be helpful to include MRS of NAA moieties within the PMC in relevant research protocols and in the investigation of patients with suspected ALS. However, we recognise that MRS can be more logistically challenging in a clinical setting because of the technical expertise required by the MRI system operator to accurately prescribe the MRS voxel. Future research studies could explore the functional, neurochemical and structural dynamics of the PMC, longitudinally and also in relation to Riluzole therapy. Such studies could aim to explore in greater depth neurochemical contributions to functional impairment and cortical excitability, for example, with the use of MRS to quantify levels of excitatory and inhibitory neurotransmitters, as well as techniques such as transcranial magnetic stimulation and positron emission tomography to quantify cortical motor neuron excitation and glucose metabolism, respectively.
Footnotes
Twitter: @avyarthanadey
Contributors: AD analysed and interpreted data and drafted the manuscript for intellectual content. CCL acquired data and revised the manuscript for intellectual content. AI, DT and OS analysed data and revised the manuscript for intellectual content. DK acquired data and revised the manuscript for intellectual content. PS contributed to MRI acquisition and revised the manuscript for intellectual content. CH contributed to the design of the MRS protocol, analysed data and revised the manuscript for intellectual content. CB contributed to the study design and revised the manuscript for intellectual content. LK, RF, LZ, SG, AG and HB acquired data and revised the manuscript for intellectual content. SK contributed to the design and conceptualisation of the study, acquired, analysed and interpreted the data, revised the manuscript for intellectual content, and is responsible for the overall content as guarantor.
Funding: CALSNIC is funded by the Canadian Institutes of Health Research fund (grant/award number: 123534), the ALS Society of Canada, Brain Canada and the Shelly Mrkonjic Research Fund.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. Requests for data should be made to the corresponding author, SK, who is the director of CALSNIC.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Health Research Ethics Board, University of Alberta: Pro00036028. Conjoint Health Research Ethics Board, University of Calgary: REB13-0651. Research Ethics Office, Sunnybrook Health Sciences Centre: 445-2013. Research Ethics Board, The Neuro, McGill University: NEU-13-016. Office of Research Ethics, University of British Columbia: H16-00528. Participants gave informed consent to participate in the study before taking part.
References
Full text links
Read article at publisher's site: https://doi.org/10.1136/jnnp-2022-329993
Read article for free, from open access legal sources, via Unpaywall:
https://jnnp.bmj.com/content/jnnp/early/2022/11/15/jnnp-2022-329993.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/138462138
Article citations
Metabolite profile in hereditary spastic paraplegia analyzed using magnetic resonance spectroscopy: a cross-sectional analysis in a longitudinal study.
Front Neurosci, 18:1416093, 13 Aug 2024
Cited by: 0 articles | PMID: 39193522 | PMCID: PMC11347332
Cortical microstructural abnormalities in amyotrophic lateral sclerosis: a gray matter-based spatial statistics study.
Quant Imaging Med Surg, 14(8):5774-5788, 16 Jul 2024
Cited by: 0 articles | PMID: 39144033 | PMCID: PMC11320503
Potential of neuroimaging as a biomarker in amyotrophic lateral sclerosis: from structure to metabolism.
J Neurol, 271(5):2238-2257, 17 Feb 2024
Cited by: 0 articles | PMID: 38367047
Review
Biomarkers in amyotrophic lateral sclerosis: current status and future prospects.
Nat Rev Neurol, 19(12):754-768, 10 Nov 2023
Cited by: 9 articles | PMID: 37949994
Review
Transcutaneous auricular vagus nerve stimulation on upper limb motor function with stroke: a functional near-infrared spectroscopy pilot study.
Front Neurosci, 17:1297887, 21 Nov 2023
Cited by: 2 articles | PMID: 38075278 | PMCID: PMC10702495
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Involvement of the dentate nucleus in the pathophysiology of amyotrophic lateral sclerosis: A multi-center and multi-modal neuroimaging study.
Neuroimage Clin, 28:102385, 16 Aug 2020
Cited by: 17 articles | PMID: 32871387 | PMCID: PMC7476068
Role of diffusion tensor imaging or magnetic resonance spectroscopy in the diagnosis and disability assessment of amyotrophic lateral sclerosis.
J Neurol Sci, 348(1-2):206-210, 06 Dec 2014
Cited by: 11 articles | PMID: 25524526
Differential involvement of corticospinal tract (CST) fibers in UMN-predominant ALS patients with or without CST hyperintensity: A diffusion tensor tractography study.
Neuroimage Clin, 14:574-579, 22 Feb 2017
Cited by: 17 articles | PMID: 28337412 | PMCID: PMC5349615
MRI biomarkers and neuropsychological assessments of hippocampal and parahippocampal regions affected by ALS: A systematic review.
CNS Neurosci Ther, 30(2):e14578, 01 Feb 2024
Cited by: 3 articles | PMID: 38334254 | PMCID: PMC10853901
Review Free full text in Europe PMC
Funding
Funders who supported this work.
ALS Society of Canada (1)
Grant ID: N/A
CIHR (1)
Grant ID: 123534
Canadian Institutes of Health Research (1)
Grant ID: 123534
Fondation Brain Canada (1)
Grant ID: N/A
Shelly Mrkonjic Research Fund (1)
Grant ID: N/A

 1,2, for the Canadian ALS Neuroimaging Consortium (CALSNIC)
1,2, for the Canadian ALS Neuroimaging Consortium (CALSNIC)