Abstract
Free full text

The role of the microbiota in myelopoiesis during homeostasis and inflammation
Abstract
Abstract The microbiota engages in the development and maintenance of the host immune system. The microbiota affects not only mucosal tissues where it localizes but also the distal organs. Myeloid cells are essential for host defense as first responders of the host immune system. Their generation, called myelopoiesis, is regulated by environmental signals, including commensal microbiota. Hematopoietic stem and progenitor cells in bone marrow can directly or indirectly sense microbiota-derived signals, thereby giving rise to myeloid cell lineages at steady-state and during inflammation. In this review, we discuss the role of commensal microorganisms in the homeostatic regulation of myelopoiesis in the bone marrow. We also outline the effects of microbial signals on myelopoiesis during inflammation and infection, with a particular focus on the development of innate immune memory. Studying the relationship between the microbiota and myelopoiesis will help us understand how the microbiota regulates immune responses at a systemic level beyond the local mucosa.
Introduction
The microbiota, a community of microorganisms consisting of bacteria, fungi, archaea and viruses, inhabits the human body. The microbiota provides a wide variety of health benefits to the host, including assisting digestion, synthesizing vitamins and essential amino acids, protecting against infectious pathogens and regulating energy homeostasis (1–10). In addition, the microbiota plays a pivotal role in the development and ‘training’ of the host immune system (11–14). It is becoming apparent that the impact of the microbiota is restricted to not only local immunity but also systemic immunity (15–20).
Myeloid cells serve as the first line of defense against invading pathogens by eliminating and preventing the spread of pathogens and alerting the adaptive immune response (21). Granulocytes and monocytes, the major myeloid lineage immune cells, arise from hematopoietic stem cells (HSCs) in the bone marrow (BM) and enter systemic blood circulation as they mature (22). Tissue-resident macrophages develop from circulating monocytes or embryonic precursors. Tissue-resident macrophages are maintained by local self-renewal in adulthood in peripheral tissues, such as the skin, liver, lungs and heart. On the other hand, in some tissues, such as the intestine, macrophages are continuously replenished by circulating monocytes (23–28). Since myeloid cells in circulation have a short lifespan, the BM acts as a reservoir of differentiated myeloid cells and their precursors, releasing them rapidly upon host alert to maintain a constant number of circulating myeloid cells (29). During systemic inflammation or infection, BM myelopoiesis is markedly increased to replenish peripheral myeloid cells; this is referred to as emergency or demand-adapted myelopoiesis (30). Hence, a better understanding of the regulatory mechanisms of BM myelopoiesis could help strengthen host immune defenses and regulate innate immune responses during disease.
Since hematopoietic stem and progenitor cells (HSPCs) in BM express several Toll-like receptors (TLRs) at steady-state and during inflammation, their ligands, such as microbe-associated molecular patterns (MAMPs), can act directly on HSPCs to promote proliferation and differentiation into the myeloid cell lineage (31–34). In addition, HSPCs are regulated by cytokines and growth factors secreted by immune and non-immune cells in the BM niche and peripheral tissues (35–41). In this context, accumulating evidence suggests that the microbiota controls BM myelopoiesis beyond the local mucosal immune responses. In this review, we discuss the role and mechanisms of the microbiota in myelopoiesis in the BM, both under homeostasis and during disease.
The role of the gut microbiota in regulating myelopoiesis
The role of microbiota in the regulation of BM myelopoiesis was examined in mice treated with antibiotics and in mice raised under germ-free (GF) conditions. Depletion of the commensal microbiota by broad-spectrum antibiotics treatment influences the homeostasis of HSPCs in the BM. HSPCs (lineage− Sca-1+ c-Kit + [LSK]), hematopoietic stem cells (HSCs; LSK CD150+ CD48− CD34− Flk2−) and multipotent progenitors (MPPs; LSK CD150+/− CD48+/− CD34+ Flk2+/−) are significantly decreased by antibiotic treatment. Although antibiotic treatment does not affect the populations of common myeloid progenitors (CMPs; lineage− Sca1− c-Kit+ IL-7Ra− CD34+ CD16/CD32−) and granulocyte-monocyte progenitors (GMPs; lineage− Sca1− c-Kit+ CD41− CD16/CD32+), the treatment significantly decreases granulocytes in BM (42). Other studies showed that antibiotic treatment reduces GMPs in BM and reduces monocyte-dendritic cell (DC) progenitors (MDPs; lineage− c-Kit+ CD105− CD115+ Ly6C−), committed monocyte progenitors (cMoPs; lineage− c-Kit+ CD105− CD115+ Ly6C+), monocytes and macrophages in the spleen (43, 44).
The impaired BM myelopoiesis is also observed in GF mice. GMPs, granulocytes, monocytes and macrophages in the BM and the spleen are significantly fewer in GF mice compared with specific pathogen-free (SPF) mice (42, 44, 45). However, in peripheral blood, the numbers of granulocytes and monocytes in antibiotic-treated and GF mice are comparable with those in SPF mice (42, 45). Furthermore, it has been shown that there is a higher influx rate of granulocytes into the blood and a higher number of myeloid cells in the spleen of SPF mice compared with GF mice (45). Thus, the equal rate of myeloid cell influx and removal (by migration to peripheral tissues) in blood possibly results in a similar number of myeloid cells in the peripheral blood of SPF and microbiota-depleted mice (45). In addition, higher numbers of basophil precursors and circulating basophils are observed in the BM and peripheral blood, respectively, in GF and antibiotic-treated mice, compared with SPF mice (46).
The composition of the microbiota is also known to be associated with the changes in myelopoiesis. Mice from pet stores have different bacterial communities compared with SPF mice, with higher Proteobacteria and lower Verrucomicrobia, although there are no differences in species richness (47). In addition, serological assays revealed that pet-store mice harbor several pathogens, including mouse hepatitis virus and murine norovirus. As a result, pet-store mice exhibit a higher number of granulocytes, DCs and monocytes in the spleen and peripheral blood (47–49). Co-housing SPF mice with pet-store mice for 60 days allows the acquisition of pet-store microbes, thereby resulting in a marked increase of neutrophils and monocytes in peripheral blood in SPF mice (47).
Moreover, exposure of laboratory-bred mice to a natural environment (so-called “re-wilding”) leads to the elevation of granulocytes in the blood and mesenteric lymph nodes (mLN) through colonization by environmental microbes enriched with fungi (50). Similarly, co-housing of mice raised in SPF facilities with those born and raised in conventional facilities (likely having a more-complexed microbiota) leads to the expansion of GMPs in BM and increased circulating CD11b+ myeloid cells (51). In conventional mice, genes related to cell survival pathways, including TLR, Wnt and NF-kB signaling, were highly expressed in long-term HSCs (LT-HSCs) and GMPs in BM. Interestingly, mice reconstituted with BM from conventional mice exhibited an increased LSK cell reconstitution and CD11b+ myeloid cell differentiation, suggesting that conventional microbiota imprints a myeloid bias on the HSPCs (51).
A high-fat diet (HFD) intake causes an increase in HSCs and myeloid progenitors (52, 53). Given that mice colonized with the microbiota from HFD-fed mice display increased LSK, MPPs, and myeloid progenitors, it is plausible that HFD-induced gut dysbiosis mediates myeloid lineage skewing of HSCs (52).
Microbial control of myeloid cells in host defense and repair
Several studies showed that increased microbiota-driven myelopoiesis positively correlates with resistance to pathogen infection. For example, GF mice, which harbor fewer myeloid cells, display decreased clearance of pathogens compared with mice colonized with three species of bacteria (45). Similarly, GF mice are more susceptible to infection with Listeria monocytogenes or Staphylococcus aureus than SPF mice are, with higher bacterial loads in the spleen and liver during infection (44). Given that myeloid cells, such as neutrophils and macrophages, are major cell types responsible for early pathogen clearance during infection (54, 55), reduced clearance of pathogens in GF mice is due to fewer myeloid cells. In this context, it was reported that the capacity of bacterial clearance by CD11b+ myeloid cells is comparable between GF and SPF mice (44). These results suggest that the microbiota confers protection to the host by promoting myelopoiesis (increased number of myeloid cells) rather than by enhancing the bactericidal capacities of myeloid cells.
In addition to the presence or absence of microbiota, the complexity of the microbiota may also influence myelopoiesis and subsequent host defense against pathogen infection. For example, natural transfer of the microbiota from pet store mice into SPF mice by co-housing results in enhanced resistance to Listeria infection (47). Furthermore, offspring of ex-GF mice re-wilded by reconstitution with ileocecal contents from wild mice, acquire enhanced resistance to lethal influenza A virus infection compared with offspring born from SPF mice (56).
Furthermore, commensal microbiota-driven myelopoiesis has a crucial role in tissue repair. The depletion of the microbiota by antibiotic treatment results in a decrease in myeloid cells in the heart and leads to impaired tissue repair after cardiac infarction, resulting in higher mortality rates (57). Reconstitution of the gut microbiota or administration of a Lactobacillus-based probiotic cocktail restores myeloid cell accumulation in the heart and improves survival through the up-regulation of short-chain fatty acids (SCFAs), which are microbial metabolites (57). More specifically, the administration of propionate, one of the SCFAs, promoted the infiltration of CX3CR1+ monocytic cells in the heart (57). In mice with dextran sulfate sodium (DSS)-induced colitis, commensal Bacteroides species promote the differentiation of MPPs towards Ly6C+ and Ly6G+ myeloid cells with the expression of immunosuppressive genes in the mLNs, which contributes to gut tissue repair during colitis (58).
Moreover, several studies showed that depletion of the microbiota is associated with an increased risk of allergic disease (46, 59, 60). One possible mechanism suggested by Hill et al. is that microbiota depletion leads to an increase in BM basophil precursors and circulating basophils, which hypersensitize Th2 cell responses to allergens (46). Moreover, Maslowski et al. showed that SCFAs act on neutrophils and eosinophils through G-protein-coupled receptor 43 (GPR43) and suppress the production of inflammatory mediators and subsequent inflammation in colitis, arthritis and asthma (61).
Emerging evidence has demonstrated that myeloid cells acquire memory-like abilities upon primary stimulation, thereby altering non-specific responses to secondary stimuli (62). This acquisition of a memory-like phenotype in innate immune cells process is termed ‘trained immunity’ (63). In various diseases, trained immunity confers protection on the host. One arm of trained immunity is to develop immune tolerance to prevent excessive immune reactions against secondary stimuli. For example, exposure to lipopolysaccharide (LPS; a ligand of TLR4) causes epigenetic reprogramming in monocytes and macrophages, whereby these cells acquire a hyporesponsive phenotype (e.g. produce a lesser amount of pro-inflammatory cytokines) (64, 65).
Another arm of trained immunity is to enhance innate immune responses involved in host defense. It has been reported that microbial stimuli, including a fungus-derived microbial molecule β-glucan or Mycobacterium bovis bacillus Calmette–Guérin (BCG), induce epigenetic changes in BM myeloid progenitors or peripheral myeloid cells and enhance innate immune responses to secondary stimuli (64, 66–68). It has been reported that LPS and BCG induce acetylation of histone 3 lysine 27 (H3K27) at different parts of promoter and enhancer regions, leading to different gene expression patterns (64). Thus, distinct patterns of epigenetic gene regulation may determine how the host cells acquire silenced or enhanced innate immune responses. However, the precise mechanisms by which different stimuli induce distinct patterns of epigenetic reprogramming remain elusive.
Although the lifespan of myeloid cells is generally short, the memory of trained innate immune cells can be maintained for several months (62). Imprinting this relatively long-term innate immune memory can be explained by the training of HSPCs. The injection of β-glucan enhances myelopoiesis and transcriptional changes in HSCs through activation of IL-1β and granulocyte-macrophage CSF (GM-CSF) signaling, thus playing a protective role against secondary LPS challenge and myeloablative chemotherapy (68, 69). Type I interferon (type I IFN) signaling mediates β-glucan-induced trained immunity with transcriptomic and epigenetic changes of GMPs and training neutrophils toward an anti-tumor phenotype (68). BCG vaccination induces epigenetic modifications and transcriptional changes in HSCs, leading to enhanced myelopoiesis and subsequent defense against infection (70).
Vaccination with an engineered fungal pathogen Cryptococcus neoformans induces a long-term memory phenotype in pulmonary and splenic DCs with enhanced pro-inflammatory transcripts and histone modifications (71). Respiratory adenoviral infection induces a memory phenotype in alveolar macrophages independently of BM progenitors and exerts protective effects against subsequent bacterial infection (72). Thus, infection with pathogenic microorganisms induces trained immunity in myeloid cells.
However, little is known as yet about the contribution of the gut microbiota to the development of trained immunity. In this regard, it is reported that exogenous administration of gut commensal bacteria can induce trained immunity, although the conditions do not fully reflect the physiological exposure to the gut microbiota. For example, oral administration of Lactobacillus plantarum increases neutrophils in the spleen and monocytes in the lymph nodes, conferring host resistances to Leptospira interrogans-induced renal inflammation and fibrosis (73). BM-derived DCs (BMDCs) from Lactobacillus johnsonii-treated mice produce IFN-β (a type I IFN) but display decreased expression of maturation markers, such as MHC-II, CD80 and CD86, after respiratory syncytial virus (RSV) challenge. Adoptive transfer of trained BMDCs alleviates RSV-induced airway inflammation (74). Of note, preincubation with plasma from L. johnsonii-treated mice results in the induction of trained immunity in BMDCs, suggesting that microbial metabolites present in the plasma may be involved in modifying progenitor cell functions (74). Similarly, BMDCs from mice colonized with segmented filamentous bacteria (SFB) have enhanced IL-23 production and migration to the gut, and their transfer protects mice from Entamoeba histolytica infection (75).
Microbe-induced myelopoiesis in the pathogenesis of inflammatory disease
As discussed, myelopoiesis facilitates pathogen clearance. On the other hand, myelopoiesis can also cause adverse outcomes, such as a cytokine storm, during infection. It has been reported that some infections cause septic shock with hyperactivation of myeloid cells and subsequent development of cytokine storms. For example, mice colonized with the microbiota from pet-store mice display more exaggerated, lethal myeloid-derived cytokine responses than normal SPF mice during sepsis (47). Moreover, antibiotic-treated and GF mice are less sensitive to cytokine storms than SPF mice are because of reduced myelopoiesis and decreased accumulation of inflammatory monocytes (43). Also, the microbiota promotes the senescence of neutrophils with increased pro-inflammatory activity. Therefore, SPF mice are more susceptible to LPS-induced septic shock than antibiotic-treated mice are (76).
Several studies demonstrated that myelopoiesis as a part of innate immune training also contributes to the pathogenesis of non-infectious inflammatory diseases. A low-grade chronic inflammation that develops with aging is called inflammaging (77). Chronic inflammation is a risk factor for aging-associated diseases, such as cardiovascular diseases, diabetes, chronic kidney disease, cancer and dementia (78). Thevarnanjan et al. found that old SFP mice, compared with young mice, exhibit increased intestinal permeability and subsequently elevated bacterial components and inflammatory mediators in peripheral blood. As a result, the baseline activation of immune cells in the peripheral blood is increased in old mice. In contrast, under GF conditions, such age-associated inflammatory changes do not occur, suggesting the crucial role of the commensal microbiota in age-related chronic inflammation (79). Inflammaging is associated with a myeloid bias in HSCs (80–82). Recently, it has been reported that the microbiota mediates the aging-associated myeloid bias via IL-1 signaling (83). LT-HSCs from aged GF mice do not show myeloid-biased differentiation upon transplantation. Moreover, antibiotic treatment can reverse the myeloid bias in HSCs in aged mice (83).
Leukocytosis is commonly seen in the acute phase of stroke, which correlates with the disease severity, adverse clinical outcomes, risk of recurrent ischemic stroke and disability (84–87). Courties et al. reported that ischemic stroke causes skewing of hematopoiesis toward the myeloid lineage with an increase in HSCs, GMPs, neutrophils and monocytes, and a decrease in common lymphoid progenitors in BM, 3 days after onset of stroke (88). Intriguingly, impaired gut permeability is observed in mice after stroke induction, leading to bacterial translocation to the lung, liver and spleen (89). This result indicates the possible contribution of gut microbiota in myelopoiesis in stroke.
Chronic colitis caused by the adoptive transfer of T cells is known to increase the expansion of LSK cells in the BM and GMPs in the BM and spleen, which is involved in the exacerbation of colitis (90). Interestingly, GMPs with a high proliferative potential also reside in the colonic mucosa, indicating that these cells supply inflammatory myeloid cells locally during colitis (90). Although the administration of GM-CSF or G-CSF leads to increased LSK cells and GMPs in the BM, neither treatment expands GMPs in the spleen and colonic mucosa. This result suggests that the expansion of GMPs in peripheral tissues is mediated by other factors (90). In addition, it has been reported that recirculating HSPCs gives rise to myeloid cells locally upon stimulation with TLR ligands (91). This study raises the possibility that the microbial components provided by the gut microbiota may induce extramedullary myelopoiesis in the colonic mucosa.
Myelopoiesis is a key feature of pathogenic trained immunity, and such inflammatory memory is associated with an increased risk of inflammatory comorbidities. For example, experimental periodontitis exacerbates arthritis and vice versa via trained myeloid cells (92). Pathogenic trained immunity is also induced by the consumption of a Western diet. Western-diet feeding induces myelopoiesis with epigenomic modifications, thereby leading to long-lasting systemic inflammation even after switching to a normal diet. Such inflammatory training by a Western diet detrimentally affects the progression of cardiovascular disease (93). Given the evidence that a Western diet alters the composition of the gut microbiota (94, 95), it is possible that the microbiota modified by the diet contributes to the induction of this phenotype.
Myelopoiesis regulation by microbe- and host-derived mediators
Myelopoiesis is regulated by various microbial components and host mediators induced by the microbes in both homeostatic and pathogenic conditions (30, 45). We will summarize such mediators below.
Microbe-associated molecular patterns
Oral administration of heat-killed Escherichia coli or autoclaved cecal contents of SPF mice to GF mice increases neutrophils and monocytes in the BM (44), suggesting that MAMPs promote the differentiation of myeloid cells in the BM. Balmer et al. further verified that injection of heat-inactivated serum from SPF mice induces MyD88-dependent expansion of BM myeloid cells. This result suggests that circulating heat-resistant TLR ligands (presumably derived from the gut microbiota) may regulate the myeloid cell differentiation and homeostasis in the BM (Fig. 1) (45). Consistently, in the absence of MyD88 (in Myd88−/− mice), microbiota colonization does not increase BM GMPs and myeloid cells (45, 96). Furthermore, it has been reported that activation of MyD88 signaling in B cells by commensal microbiota leads to basophil precursor proliferation. A study suggested that limited IgE production from B cells by the microbiota reduces the expression of IL-3R in basophil precursors which in part regulates basophil expansion (46).
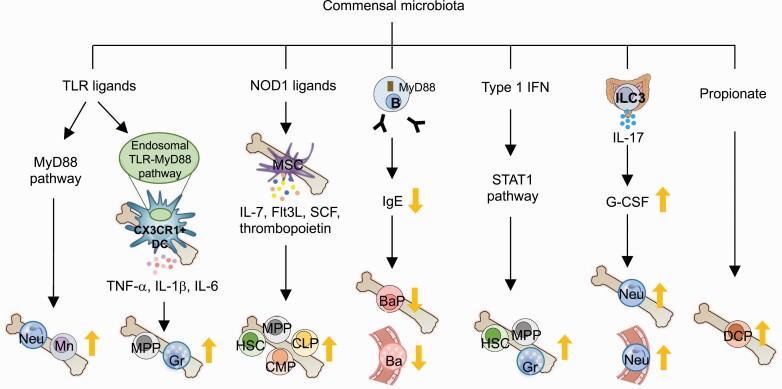
The mechanisms of microbiota effects on myelopoiesis. The commensal microbiota regulates hematopoiesis, especially increasing myelopoiesis. MAMPs, cytokines and growth factors induced by microbes, as well as short-chain fatty acids produced by the microbiota induce myelopoiesis in bone marrow. Ba, basophil; BaP, basophil precursor; CLP, common lymphoid progenitor; CMP, common myeloid progenitor; DC, dendritic cell; DCP, dendritic cell precursor; Flt3L, Flt3 ligand; G-CSF, granulocyte colony-stimulating factor; Gr, granulocyte; HSC, hematopoietic stem cell; ILC3, group 3 innate lymphoid cell; Mn, monocyte; MPP, multi-potent progenitor; MSC, mesenchymal stromal cell; Neu, neutrophil; SCF, stem cell factor; TLR, Toll-like receptor; type I IFN, type I interferon.
Interestingly, a higher level of bacterial DNA is detected in SPF BM cells, exclusively CX3CR1+ mononuclear cells, under steady-state conditions compared with GF mice (97). CX3CR1+ cells that sense MAMPs via endosomal TLRs regulate steady-state myelopoiesis by inflammatory cytokine production (97).
In addition, the NOD1 pathway is involved in steady-state myelopoiesis. The oral administration of NOD1 ligands restores the number of HSCs, MPPs, CMPs and granulocytes in the BM of GF and antibiotic-treated mice (98, 99). Activation of NOD1 induces cytokine secretion, such as IL-7, Flt3L, stem cell factor (SCF) and thrombopoietin, by BM mesenchymal stromal cells (MSCs), which promotes the proliferation of LSK cells and CMPs but not HSPCs (98). Also, NOD1 stimulation in neutrophils enhances their bactericidal functions (100).
Listeria monocytogenes infection induces expansion of monocyte precursors and monocytes in a MyD88-dependent manner (101). Candida albicans infection enhances the differentiation of macrophages and monocyte-derived DCs through the activation of TLR2 signaling in lineage− progenitor cells (102). Also, it has been demonstrated that LPS and CpG DNA promote the differentiation of GMPs and MDPs, respectively (103). Since HSPCs express various TLRs (34), TLR agonists can directly induce myeloid-biased differentiation of HSPCs. In fact, stimulation of LSK cells with TLR2 or TLR4 agonists drives myeloid differentiation in a MyD88-dependent manner in vitro (31). Moreover, CMPs can directly sense TLR7 agonists, thereby leading to their differentiation toward myeloid cells (104).
Short-chain fatty acids
SCFAs, including acetate, propionate and butyrate, are metabolites produced by the gut microbiota through the fermentation of dietary fibers. SCFAs have various biological functions and play a pivotal role in maintaining hemostasis in the gastrointestinal tract, including regulating the function of myeloid cells (105). It has become evident that SCFAs can affect myelopoiesis. Butyrate treatment increases Ly6C− patrolling monocytes and interstitial macrophages in the lung tissue and in vitro treatment promotes the differentiation of BM cells into macrophages (106). Propionate administration increases DC precursors and modulates DC function. As a result, DCs in mice treated with propionate exhibit an impaired ability to promote Th2 cell differentiation, resulting in reduced allergic airway inflammation (15).
Cytokines and growth factors
Mice deficient in the transcription factor STAT1 or type I IFN have reduced numbers of LSK cells and granulocytes in the BM compared with wild-type mice (42, 99). Given that antibiotic treatment does not show any further effects on LSK cells and granulocytes in these mice, it is conceivable that commensal microbiota-mediated homeostatic myelopoiesis is regulated by STAT1 and type I IFN signaling (42, 99). Consistently, phosphorylation of STAT1 and the expression of type I IFNs and IFN-γ in myeloid cell progenitors are markedly reduced when the microbiota is depleted by antibiotics (43). However, analysis of HSPCs under inflammatory conditions requires some caution in cell markers. For example, although Sca-1 is a representative marker for identifying myeloid progenitors and hematopoietic stem progenitors, Sca-1 is upregulated in an IFN-dependent manner during inflammation. Thus, Sca-1 is not a definitive marker for HSPC analysis in inflammatory conditions (107, 108). Alternatively, CD86 has been proposed as a stable marker for analyzing bona-fide myelopoiesis under inflammation (107).
In addition to cytokines, the expression of G-CSF and M-CSF, central regulators of BM myelopoiesis, is decreased by the antibiotic treatment (43). Activation of group 3 innate lymphoid cells (ILC3s) by the gut microbiota results in the production of IL-17 by ILC3s. The produced IL-17, in turn, increases plasma G-CSF levels, thereby increasing neutrophils in the systemic circulation and BM (109).
Besides homeostatic myelopoiesis, cytokines induced during infection and inflammation promote pathologic myelopoiesis. Acute intestinal infection with Toxoplasma gondii induces IFN-γ secretion by BM-resident NK cells, which is attributed to transcriptional changes in monocyte progenitors in BM (110). Serum amyloid A (SAA), induced during inflammation, increases G-CSF in monocytes and macrophages via TLR2, which has been shown to bind SAA (111), and thereby leads to the expansion of neutrophils in blood (112). During sepsis, B cells produce IL-3 and promote hematopoiesis and myelopoiesis, increased numbers of HSPCs, CMPs and GMPs in BM, which further fuel a cytokine storm (113).
Conclusions
Signaling from microbes is essential for fine-tuning myelopoiesis at steady-state and during emergent conditions. At steady-state, the commensal microbiota is an important regulator of homeostatic myelopoiesis. Differentiated myeloid cells in the intestinal mucosa contribute to host defense against invading external pathogens. Moreover, the microbiota remotely regulates myelopoiesis in the BM, which is also pivotal in preparing against various pathological insults, such as infection or tumor development, in both intestinal and extra-intestinal organs (114). Thus, gut dysbiosis may increase the risk of various diseases by impairing local and systemic myeloid cell pools and functions. On the other hand, foreign invaders (pathogens) may serve as significant inducers of myelopoiesis in emergency conditions, such as during infections. Baseline myelopoiesis induced by commensal microbiota can be protective or detrimental during pathogen infections.
Although the increase in myeloid cells and enhancement of their bactericidal functions promote the eradication of infectious pathogens, the excessive activation of myeloid cells may lead to adverse outcomes, such as cytokine storms. However, the mechanisms by which myelopoiesis causes pathological reactions (adverse events) beyond the homeostatic (host defense) response remain incompletely understood. Further studies are required to fine-tune pathogen-induced myelopoiesis to a level that does not exceed the level of host defense.
Contributor Information
Yeji Kim, Division of Gastroenterology and Hepatology, Department of Internal Medicine, University of Michigan, Ann Arbor, MI 48109, USA.
Nobuhiko Kamada, Division of Gastroenterology and Hepatology, Department of Internal Medicine, University of Michigan, Ann Arbor, MI 48109, USA. Laboratory of Microbiology and Immunology, WPI Immunology Frontier Research Center, Osaka University, Suita, Osaka 565-0871, Japan,
Funding
This work was supported by the National Institutes of Health (DK108901, DK119219, AI142047, DK125087); Japan Agency for Medical Research and Development (AMED) PRIME (JP21gm6310023) (to N.K.).
Conflicts of interest statement: the authors declare no conflicts of interest.
References
Articles from International Immunology are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/intimm/dxad002
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/intimm/article-pdf/35/6/267/50408120/dxad002.pdf
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/141768709
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Microbiota-myeloid cell crosstalk beyond the gut.
J Leukoc Biol, 100(5):865-879, 07 Sep 2016
Cited by: 48 articles | PMID: 27605211 | PMCID: PMC6608064
Review Free full text in Europe PMC
Emerging Principles in Myelopoiesis at Homeostasis and during Infection and Inflammation.
Immunity, 50(2):288-301, 01 Feb 2019
Cited by: 81 articles | PMID: 30784577
Review
Trained Immunity and Cardiometabolic Disease: The Role of Bone Marrow.
Arterioscler Thromb Vasc Biol, 41(1):48-54, 19 Nov 2020
Cited by: 13 articles | PMID: 33207931 | PMCID: PMC7769996
Review Free full text in Europe PMC
Emergency myelopoiesis in solid cancers.
Br J Haematol, 205(3):798-811, 23 Jul 2024
Cited by: 0 articles | PMID: 39044285
Review
Funding
Funders who supported this work.
Japan Agency for Medical Research and Development (1)
Grant ID: JP22gm6310023
NIDDK NIH HHS (3)
Grant ID: R01 DK119219
Grant ID: R01 DK125087
Grant ID: R01 DK108901
National Institutes of Health (4)
Grant ID: DK119219
Grant ID: DK108901
Grant ID: AI142047
Grant ID: DK125087





